Stress-Induced Cholesterol Metabolic Dysregulation and Differentiation Trajectory Shift in Oligodendrocytes Synergistically Drive Demyelination
Abstract
1. Introduction
2. Results
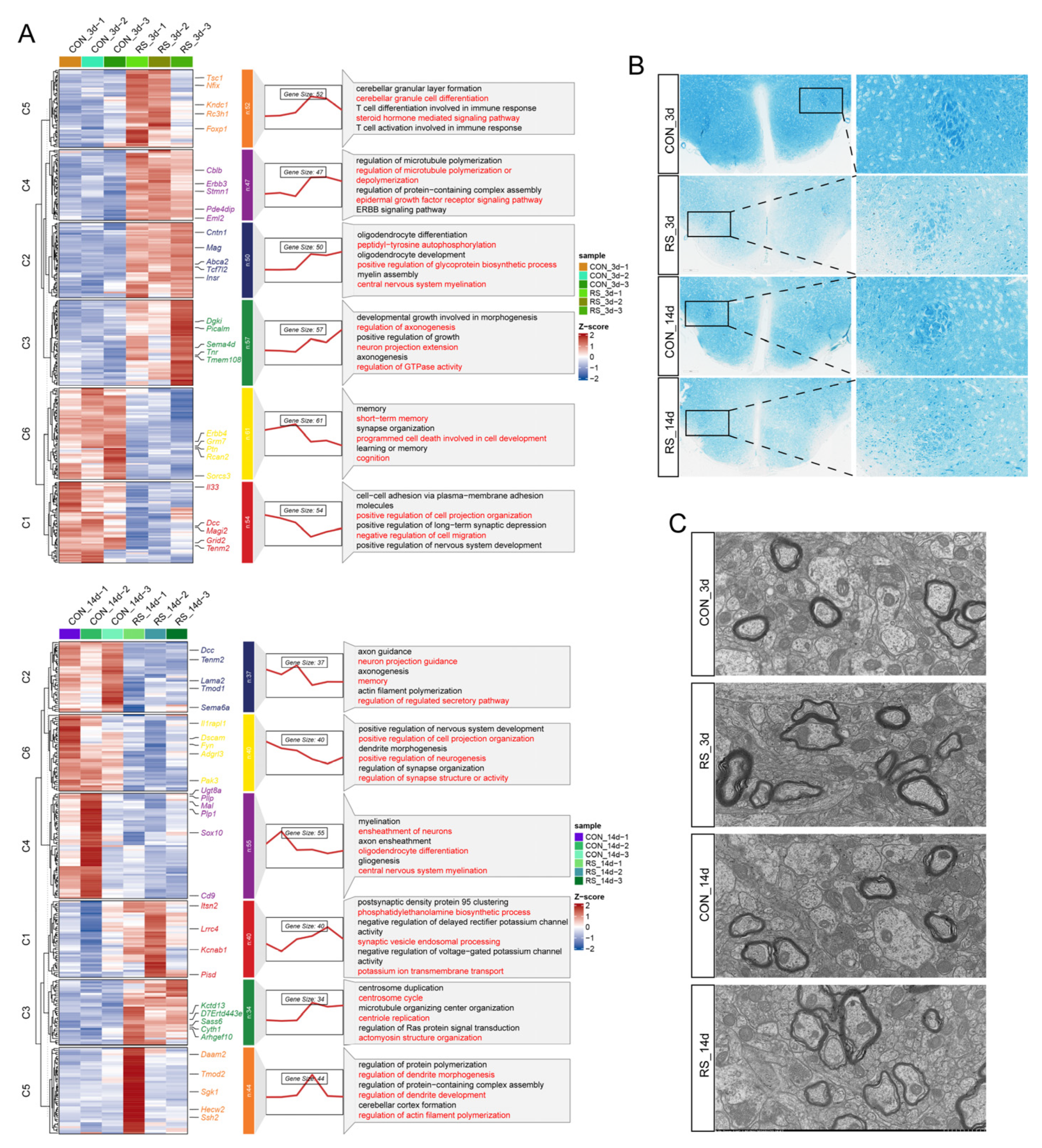
2.1. Stress-Induced OLG Cholesterol Dysregulation Is Associated with Demyelination
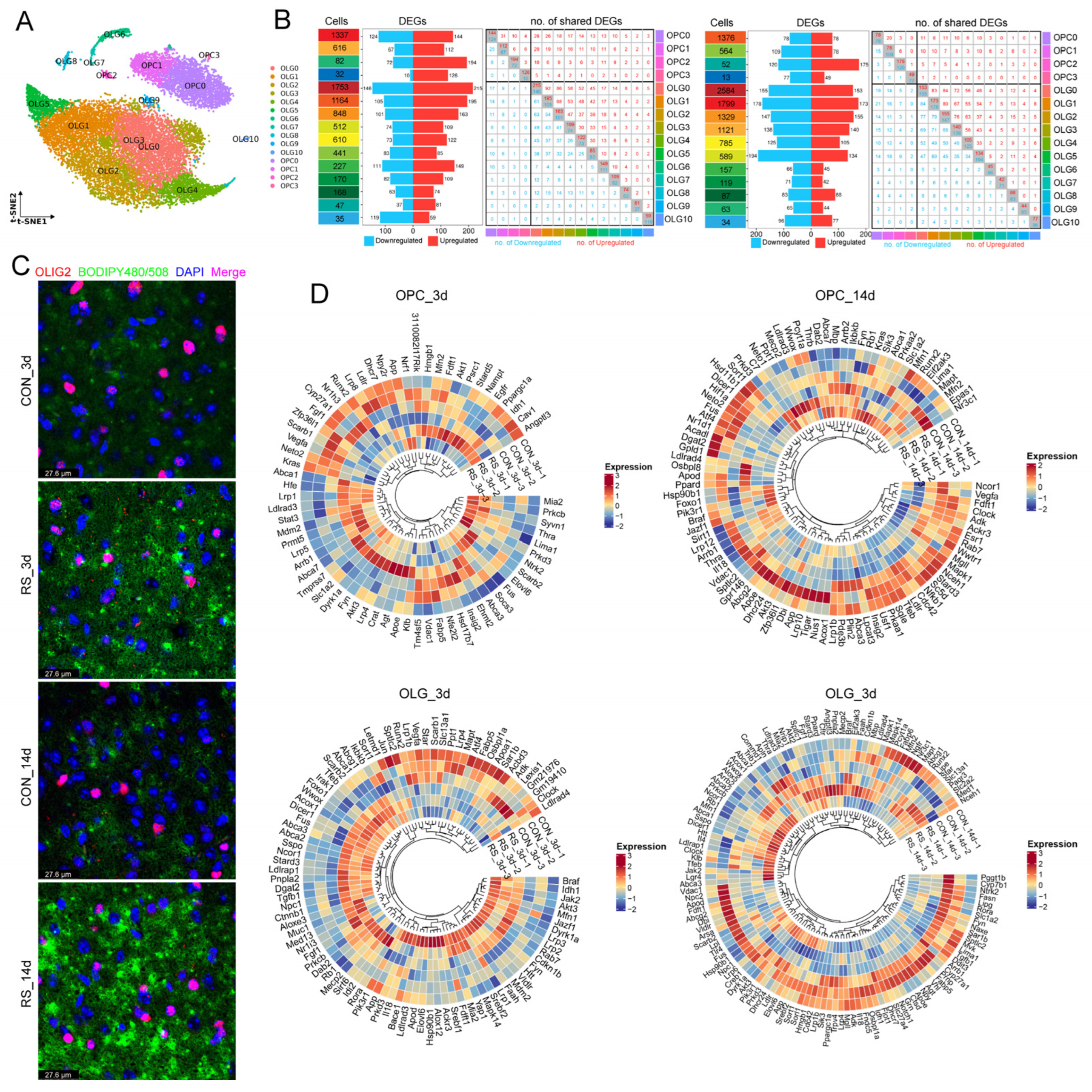
2.2. AMPK Alleviates Stress-Induced OLG Cholesterol Dysregulation and Demyelination
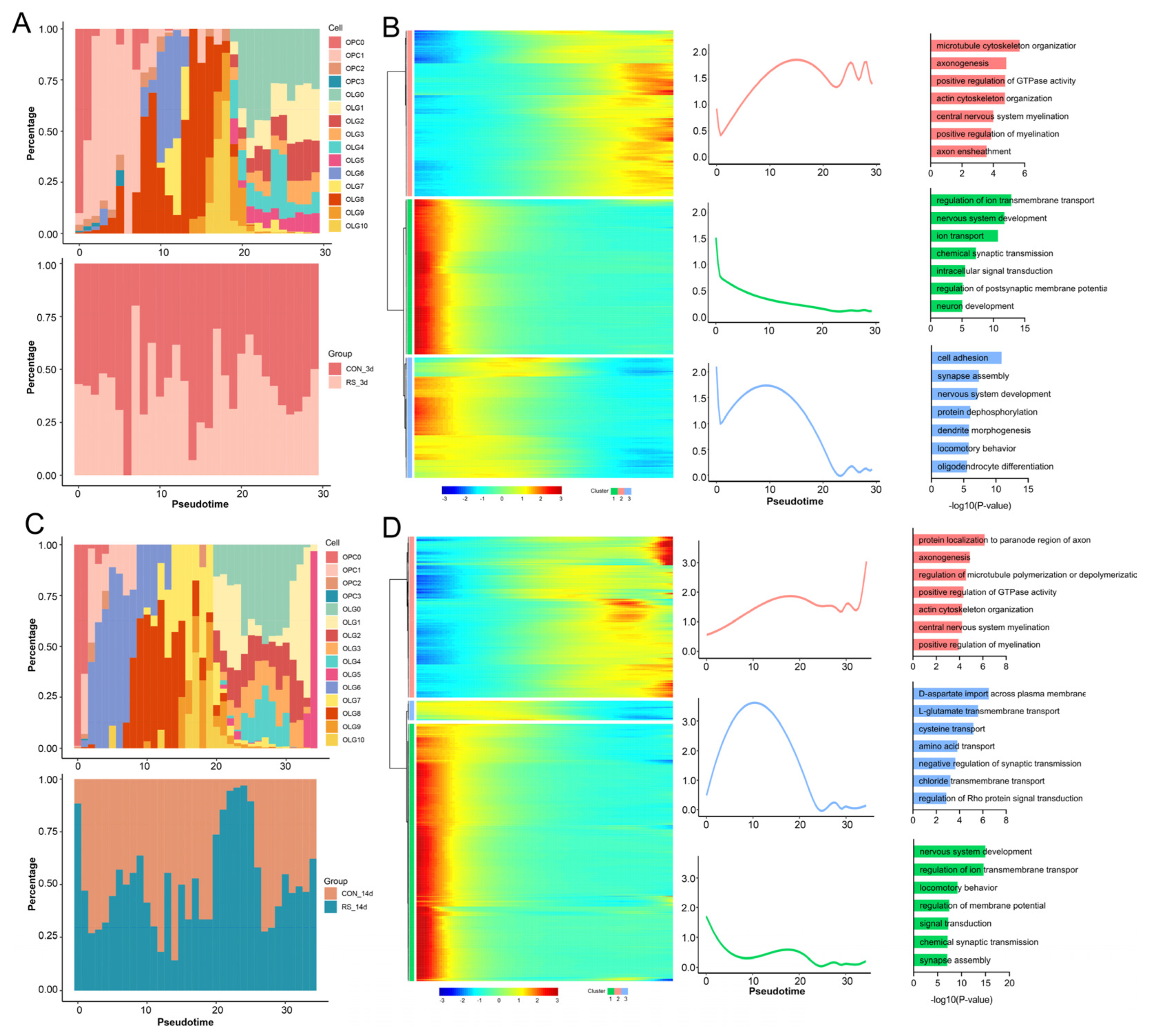
2.3. Stress-Induced Impairment of OLG and OPC Differentiation Exacerbates Demyelination
2.4. Identification of Immune Subpopulations of OLGs
3. Discussion
4. Materials and Methods
4.1. Establishment of the Stress Experimental Model
4.2. HE Staining
4.3. LFB Myelin Staining
4.4. IF Staining
4.5. TEM
4.6. Western Blotting
4.7. Hypothalamic snRNA-seq
4.8. Bioinformatics Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Agostino, D.; Wu, Y.; Daskalopoulou, C.; Hasan, M.T.; Huisman, M.; Prina, M. Global trends in the prevalence and incidence of depression: A systematic review and meta-analysis. J. Affect. Disord. 2021, 281, 235–243. [Google Scholar] [CrossRef]
- Ho, T.C.; Sisk, L.M.; Kulla, A.; Teresi, G.I.; Hansen, M.M.; Wu, H.; Gotlib, I.H. Sex differences in myelin content of white matter tracts in adolescents with depression. Neuropsychopharmacology 2021, 46, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, P.; Chen, Y.; Nie, J.; Yuan, T.; Wong, A.H.; Liu, F. The Eph receptor A4 plays a role in demyelination and depression-related behavior. J. Clin. Investig. 2022, 132, 152187. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Maitra, M.; Tanti, A.; Suderman, M.; Theroux, J.; Davoli, M.A.; Perlman, K.; Yerko, V.; Wang, Y.C.; Tripathy, S.J.; et al. Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat. Neurosci. 2020, 23, 771–781. [Google Scholar] [CrossRef]
- Zhou, B.; Zhu, Z.; Ransom, B.R.; Tong, X. Oligodendrocyte lineage cells and depression. Mol. Psychiatry 2021, 26, 103–117. [Google Scholar] [CrossRef]
- Becker, L.J.; Fillinger, C.; Waegaert, R.; Journee, S.H.; Hener, P.; Ayazgok, B.; Humo, M.; Karatas, M.; Thouaye, M.; Gaikwad, M.; et al. The basolateral amygdala-anterior cingulate pathway contributes to depression-like behaviors and comorbidity with chronic pain behaviors in male mice. Nat. Commun. 2023, 14, 2198. [Google Scholar] [CrossRef]
- Huang, C.; Wu, Z.; Wang, D.; Qu, Y.; Zhang, J.; Jiang, R.; Xu, X.; Xu, X.; Wang, Y.; Liu, H.; et al. Myelin-associated oligodendrocytic basic protein-dependent myelin repair confers the long-lasting antidepressant effect of ketamine. Mol. Psychiatry 2024, 29, 1741–1753. [Google Scholar] [CrossRef]
- Zeng, T.; Li, J.; Xie, L.; Dong, Z.; Chen, Q.; Huang, S.; Xie, S.; Lai, Y.; Li, J.; Yan, W.; et al. Nrf2 regulates iron-dependent hippocampal synapses and functional connectivity damage in depression. J. Neuroinflamm. 2023, 20, 212. [Google Scholar] [CrossRef]
- Nestler, E.J.; Russo, S.J. Neurobiological basis of stress resilience. Neuron 2024, 112, 1911–1929. [Google Scholar] [CrossRef]
- Das Neves, S.P.; Delivanoglou, N.; Ren, Y.; Cucuzza, C.S.; Makuch, M.; Almeida, F.; Sanchez, G.; Barber, M.J.; Rego, S.; Schrader, R.; et al. Meningeal lymphatic function promotes oligodendrocyte survival and brain myelination. Immunity 2024, 57, 2328–2343. [Google Scholar] [CrossRef] [PubMed]
- Smolic, T.; Zorec, R.; Vardjan, N. Pathophysiology of Lipid Droplets in Neuroglia. Antioxidants 2021, 11, 22. [Google Scholar] [CrossRef]
- Gibbons, A.S.; Udawela, M.; Jeon, W.J.; Seo, M.S.; Brooks, L.; Dean, B. The neurobiology of APOE in schizophrenia and mood disorders. Front. Biosci. 2011, 16, 962–979. [Google Scholar] [CrossRef]
- Blanchard, J.W.; Akay, L.A.; Davila-Velderrain, J.; von Maydell, D.; Mathys, H.; Davidson, S.M.; Effenberger, A.; Chen, C.; Maner-Smith, K.; Hajjar, I.; et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 2022, 611, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Pasquini, L.A. Galectin-3-Mediated Glial Crosstalk Drives Oligodendrocyte Differentiation and (Re)myelination. Front. Cell. Neurosci. 2018, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.A.; Millet, V.; Hoyos, H.C.; Giannoni, J.P.; Croci, D.O.; Marder, M.; Liu, F.T.; Rabinovich, G.A.; Pasquini, J.M. Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ. 2011, 18, 1746–1756. [Google Scholar] [CrossRef]
- Buynitsky, T.; Mostofsky, D.I. Restraint stress in biobehavioral research: Recent developments. Neurosci. Biobehav. Rev. 2009, 33, 1089–1098. [Google Scholar] [CrossRef]
- Lei, L.; Li, Y.; Li, M.; Xin, H.; Tian, X.; Zhang, Y.; Shi, W.; Cong, B. Pathological changes in the spleen of mice subjected to different time courses of restraint stress. Sci. Rep. 2024, 14, 13543. [Google Scholar] [CrossRef]
- Li, Z.; Chen, K.; Shao, Q.; Lu, H.; Zhang, X.; Pu, Y.; Sun, X.; He, H.; Cao, L. Nanoparticulate MgH2 ameliorates anxiety/depression-like behaviors in a mouse model of multiple sclerosis by regulating microglial polarization and oxidative stress. J. Neuroinflamm. 2023, 20, 16. [Google Scholar] [CrossRef]
- Margoni, M.; Preziosa, P.; Rocca, M.A.; Filippi, M. Depressive symptoms, anxiety and cognitive impairment: Emerging evidence in multiple sclerosis. Transl. Psychiatry 2023, 13, 264. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Hu, A.; Deng, G.; Wei, J.; Li, Y.; Liu, Y.; Lu, X.; Qiu, Z.; Shi, X.; et al. Inhibition of ASGR1 decreases lipid levels by promoting cholesterol excretion. Nature 2022, 608, 413–420. [Google Scholar] [CrossRef]
- Townsend, L.K.; Steinberg, G.R. AMPK and the Endocrine Control of Metabolism. Endocr. Rev. 2023, 44, 910–933. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, J.; Liu, Q. Brain cell type-specific cholesterol metabolism and implications for learning and memory. Trends Neurosci. 2022, 45, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, B.; Popko, B. Molecular Control of Oligodendrocyte Development. Trends Neurosci. 2019, 42, 263–277. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, L.; Wang, L.; Liu, T.; Zheng, Y.; Si, L.; Ge, H.; Xu, H.; Xiao, L.; Wang, G. Single-nucleus transcriptomic analysis reveals the relationship between gene expression in oligodendrocyte lineage and major depressive disorder. J. Transl. Med. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Jakel, S.; Agirre, E.; Mendanha Falcao, A.; van Bruggen, D.; Lee, K.W.; Knuesel, I.; Malhotra, D.; Ffrench-Constant, C.; Williams, A.; Castelo-Branco, G. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2019, 566, 543–547. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Fitzner, D.; Bosch-Queralt, M.; Weil, M.; Su, M.; Sen, P.; Ruhwedel, T.; Mitkovski, M.; Trendelenburg, G.; Lutjohann, D.; et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 2018, 359, 684–688. [Google Scholar] [CrossRef]
- Liu, X.; Xin, D.E.; Zhong, X.; Zhao, C.; Li, Z.; Zhang, L.; Dourson, A.J.; Lee, L.; Mishra, S.; Bayat, A.E.; et al. Small-molecule-induced epigenetic rejuvenation promotes SREBP condensation and overcomes barriers to CNS myelin regeneration. Cell 2024, 187, 2465–2484. [Google Scholar] [CrossRef]
- Molusky, M.M.; Hsieh, J.; Lee, S.X.; Ramakrishnan, R.; Tascau, L.; Haeusler, R.A.; Accili, D.; Tall, A.R. Metformin and AMP Kinase Activation Increase Expression of the Sterol Transporters ABCG5/8 (ATP-Binding Cassette Transporter G5/G8) with Potential Antiatherogenic Consequences. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1493–1503. [Google Scholar] [CrossRef]
- Yu, C.; Takeda, M.; Soliven, B. Regulation of cell cycle proteins by TNF-alpha and TGF-beta in cells of oligodendroglial lineage. J. Neuroimmunol. 2000, 108, 2–10. [Google Scholar] [CrossRef]
- Duncan, I.D.; Radcliff, A.B.; Heidari, M.; Kidd, G.; August, B.K.; Wierenga, L.A. The adult oligodendrocyte can participate in remyelination. Proc. Natl. Acad. Sci. USA 2018, 115, E11807–E11816. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, E.; Trevisiol, A.; Saab, A.S.; Looser, Z.J.; Dibaj, P.; Ebrahimi, R.; Kusch, K.; Ruhwedel, T.; Mobius, W.; Jahn, O.; et al. Oligodendroglial fatty acid metabolism as a central nervous system energy reserve. Nat. Neurosci. 2024, 27, 1934–1944. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, S.; Volbracht, K.; Lundgaard, I.; Karadottir, R.T. Glutamate signalling: A multifaceted modulator of oligodendrocyte lineage cells in health and disease. Neuropharmacology 2016, 110, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef]
- Menard, C.; Hodes, G.E.; Russo, S.J. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 2016, 321, 138–162. [Google Scholar] [CrossRef]
- Kokkosis, A.G.; Madeira, M.M.; Mullahy, M.R.; Tsirka, S.E. Chronic stress disrupts the homeostasis and progeny progression of oligodendroglial lineage cells, associating immune oligodendrocytes with prefrontal cortex hypomyelination. Mol. Psychiatry 2022, 27, 2833–2848. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Shi, R.; Li, Y.; Zhang, G.; Feng, X.; Cong, J.; He, M.; An, Y.; Ma, R.; Shi, W.; et al. Stress-Induced Cholesterol Metabolic Dysregulation and Differentiation Trajectory Shift in Oligodendrocytes Synergistically Drive Demyelination. Int. J. Mol. Sci. 2025, 26, 3517. https://doi.org/10.3390/ijms26083517
Zhu W, Shi R, Li Y, Zhang G, Feng X, Cong J, He M, An Y, Ma R, Shi W, et al. Stress-Induced Cholesterol Metabolic Dysregulation and Differentiation Trajectory Shift in Oligodendrocytes Synergistically Drive Demyelination. International Journal of Molecular Sciences. 2025; 26(8):3517. https://doi.org/10.3390/ijms26083517
Chicago/Turabian StyleZhu, Weihao, Rui Shi, Yingmin Li, Guowei Zhang, Xiaowei Feng, Jingze Cong, Mengting He, Yuchuan An, Rufei Ma, Weibo Shi, and et al. 2025. "Stress-Induced Cholesterol Metabolic Dysregulation and Differentiation Trajectory Shift in Oligodendrocytes Synergistically Drive Demyelination" International Journal of Molecular Sciences 26, no. 8: 3517. https://doi.org/10.3390/ijms26083517
APA StyleZhu, W., Shi, R., Li, Y., Zhang, G., Feng, X., Cong, J., He, M., An, Y., Ma, R., Shi, W., & Cong, B. (2025). Stress-Induced Cholesterol Metabolic Dysregulation and Differentiation Trajectory Shift in Oligodendrocytes Synergistically Drive Demyelination. International Journal of Molecular Sciences, 26(8), 3517. https://doi.org/10.3390/ijms26083517





