Dynamics of IgM and IgA Antibody Response Profile Against Vibrio cholerae Toxins A, B, and P
Abstract
1. Introduction
2. Results
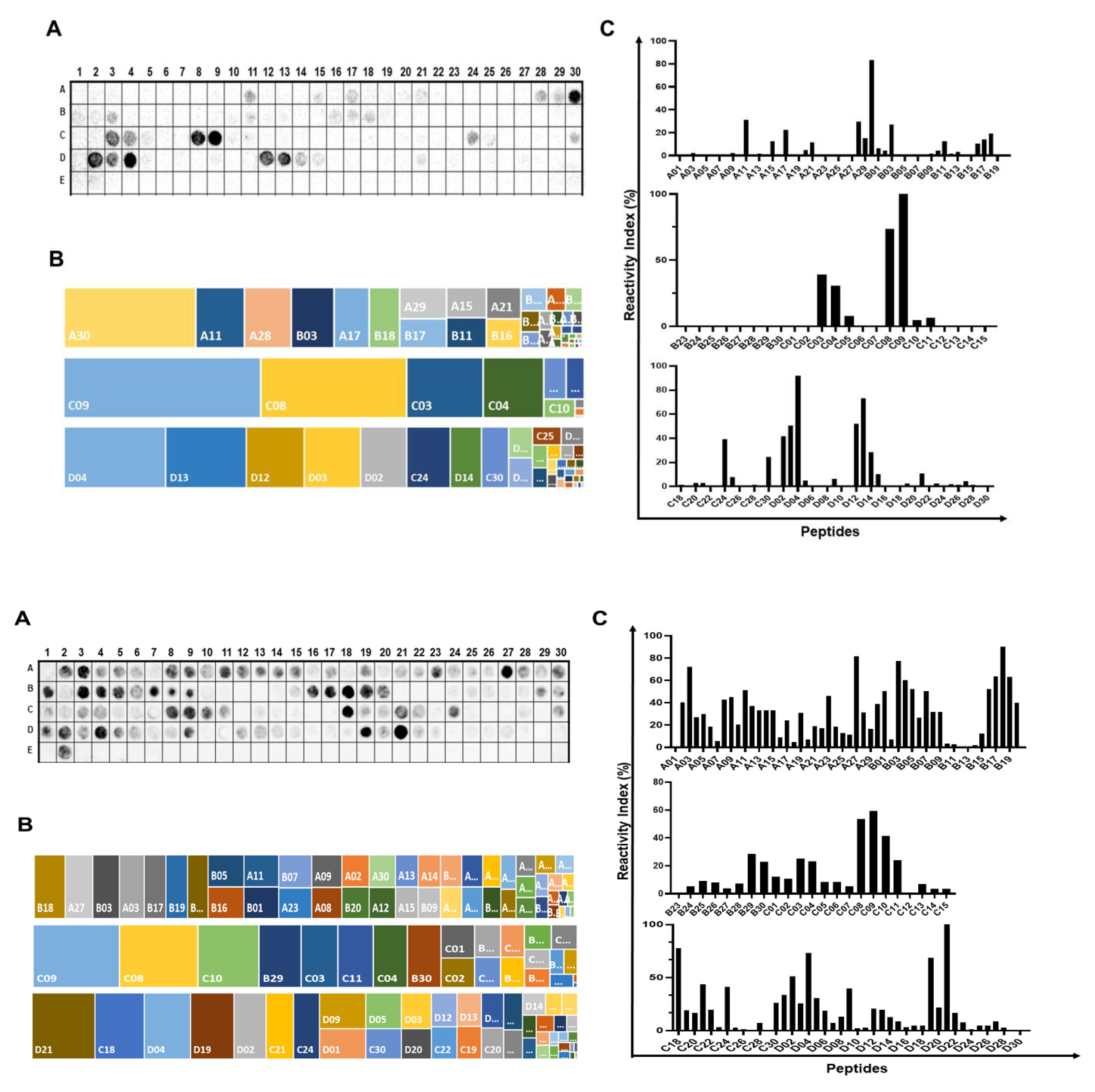
2.1. Identification of the Immunodominant IgA and IgM Epitopes in Cholera Toxin Subunits
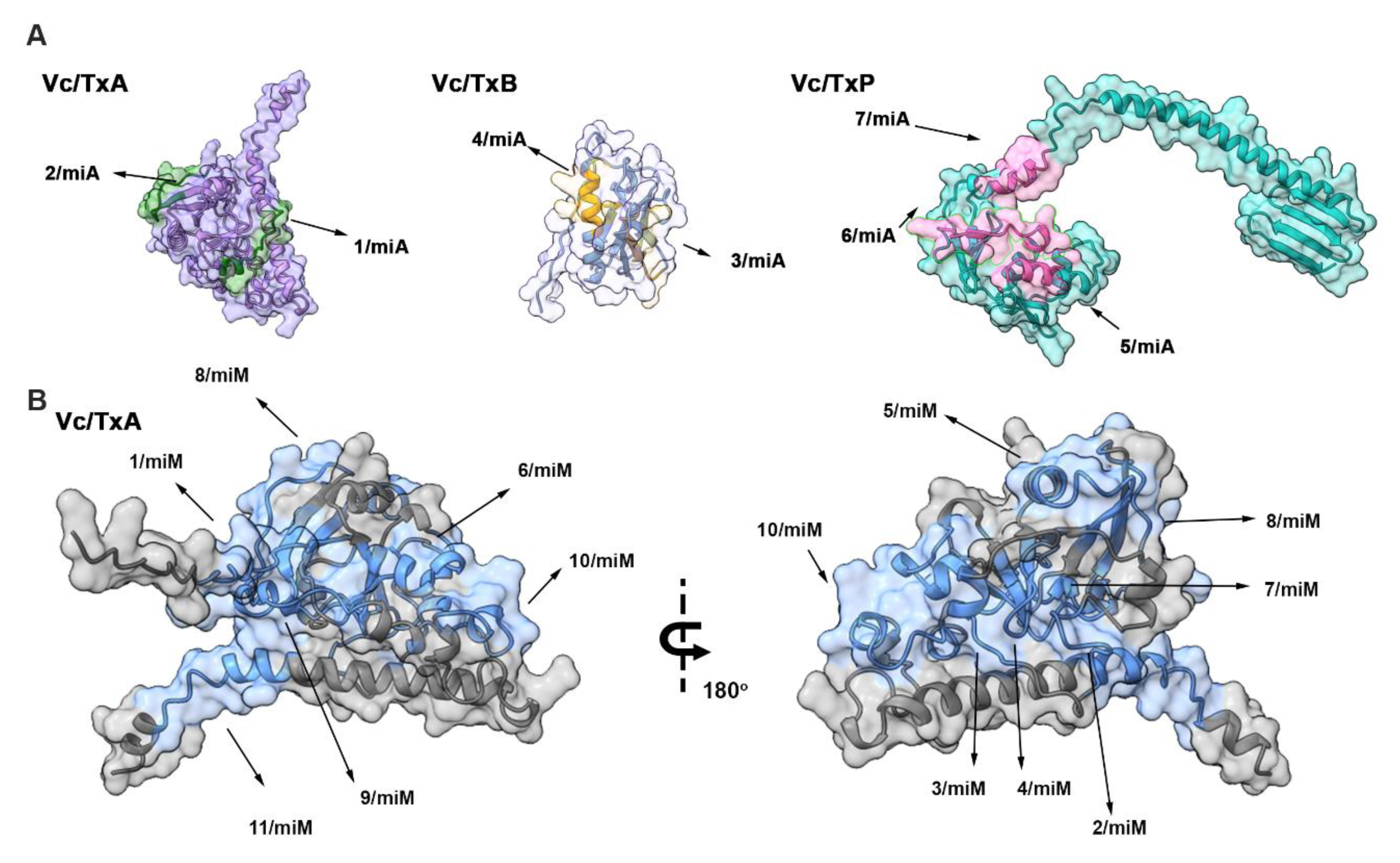
2.2. Spatial Localization of the Major IgA and IgM Epitopes Within the Three Chain of the Toxin
2.3. Spatial Distribution of the Reactive Epitopes of Enterotoxin A, B, and P
2.4. Specific and Cross-Immune IgA and IgM Epitopes
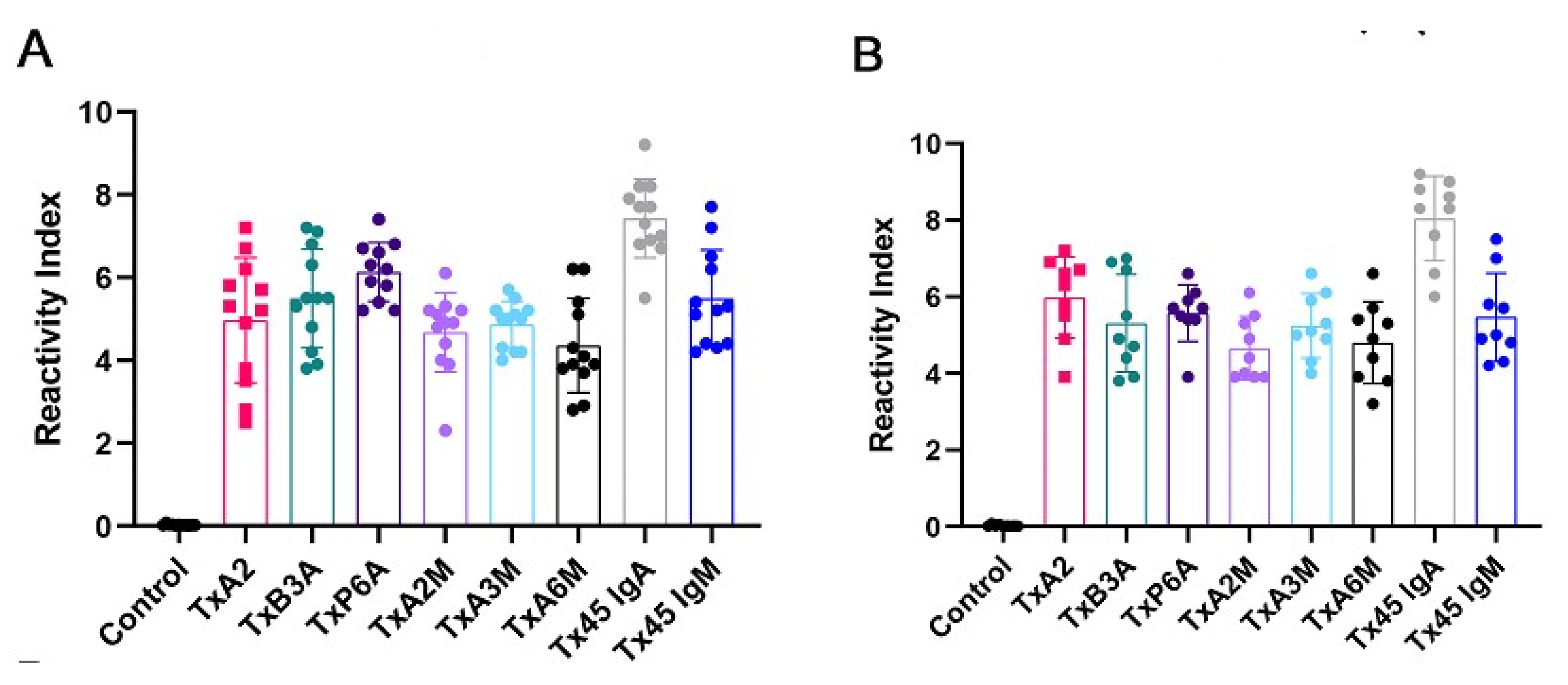
2.5. Reactivity of MAP4 and Chimeric Peptides via ELISA
3. Discussion
4. Materials and Methods
4.1. Immunization of Mice
4.2. Synthesis of the Cellulose Membrane-Bound Peptide Array
4.3. Screening of SPOT Membranes
4.4. Scanning and Measurement of Spot Signal Intensities
4.5. Preparation of Single and Multi-Antigen Peptides (MAPs)
4.6. Preparation of 45-mer Chimeric Peptides
4.7. In-House ELISA
4.8. Structural Localization of IgG Epitopes and Bioinformatics Tools
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, M.; Nelson, A.R.; Lopez, A.L.; Sack, D.A. Updated global burden of cholera in endemic countries. PLoS Negl. Trop. Dis. 2015, 9, e0003832. [Google Scholar] [CrossRef] [PubMed]
- Yuki, Y.; Nojima, M.; Hosono, O.; Tanaka, H.; Kimura, Y.; Satoh, T.; Imoto, S.; Uematsu, S.; Kurokawa, S.; Kashima, K.; et al. Oral MucoRice-CTB vaccine for safety and microbiota-dependent immunogenicity in humans: A phase 1 randomized trial. Lancet Microbe 2021, 2, e429–e440. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, L.; Grande, C.; Reid, P.C.; Hélaouët, P.; Edwards, M.; Höfle, M.G.; Brettar, I.; Colwell, R.R.; Pruzzo, C. Climate influence on vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc. Natl. Acad. Sci. USA 2016, 113, E5062–E5071. [Google Scholar] [CrossRef] [PubMed]
- Deeb, R.; Tufford, D.; Scott, G.I.; Moore, J.G.; Dow, K. Impact of climate change on Vibrio vulnificus abundance and exposure risk. Estuaries Coasts 2018, 41, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. Infections. Nat. Rev. Dis. Prim. 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Legros, D.; Partners of the Global Task Force on Cholerae Control. Global cholerae epidemiology: Opportunities to reduce the burden of cholera by 2030. J. Infect. Dis. 2018, 218, S137–S140, Erratum in J. Infect. Dis. 2019, 219, 509. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Cholerae; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/news-room/fact-sheets/detail/cholera?gadsource=1&gclid=EAIaIQobChMIqYXdhPW1hgMVgUVIAB0qbwipEAAYASAAEgJ1CfD_BwE (accessed on 21 March 2023).
- Banerjee, T.; Grabon, A.; Taylor, M.; Teter, K. cAMP-Independent activation of the unfolded protein response by cholera toxin. Infect. Immun. 2021, 89, e00447-20. [Google Scholar] [CrossRef]
- Clemens, J.D.; Nair, G.B.; Ahmed, T.; Qadri, F.; Holmgren, J. Cholerae. Lancet 2017, 390, 1539–1549. [Google Scholar] [CrossRef]
- Bourque, D.L.; Bhuiyan, T.R.; Genereux, D.P.; Rashu, R.; Ellis, C.N.; Chowdhury, F.; Khan, A.I.; Alam, N.H.; Paul, A.; Hossain, L.; et al. Analysis of the human mucosal response to cholerae reveals sustained activation of innate immune signaling pathways. Infect. Immun. 2018, 86, e00594-17. [Google Scholar] [CrossRef]
- Sánchez, J.; Holmgren, J. Cholera toxin-a foe & a friend. Indian J Med Res. 2011, 133, 153–163. [Google Scholar]
- Taylor, R.K.; Miller, V.L.; Furlong, D.B.; Mekalanos, J.J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 1987, 84, 2833–2837. [Google Scholar] [CrossRef] [PubMed]
- Waldor, M.K.; Colwell, R.; Mekalanos, J.J. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc. Natl. Acad. Sci. USA 1994, 91, 11388–11392. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, V.; Giron, J.A.; Silveira, W.D.; Kaper, J.B. The OmpU outer membrane protein, a potential adherence factor of Vibrio cholerae. Infect. Immun. 1995, 63, 4433–4438. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.K.; Sengupta, T.K.; Ghose, A.C. Major outer membrane proteins of Vibrio cholerae and their role in induction of protective immunity through inhibition of intestinal colonization. Infect. Immun. 1992, 60, 4848–4855. [Google Scholar] [CrossRef] [PubMed]
- Nandi, B.; Nandy, R.K.; Sarkar, A.; Ghose, A.C. Structural features, properties, and regulation of the outer-membrane protein W (OmpW) of Vibrio cholerae. Microbiology 2005, 151, 2975–2986. [Google Scholar] [CrossRef]
- Qadri, F.; Ali, M.; Chowdhury, F.; Khan, A.I.; Saha, A.; Khan, I.A.; Begum, Y.A.; Bhuiyan, T.R.; Chowdhury, M.I.; Uddin, M.J.; et al. Feasibility and effectiveness of oral cholerae vaccine in an urban endemic setting in Bangladesh: A cluster randomized open-label trial. Lancet 2015, 386, 1362–1371. [Google Scholar] [CrossRef]
- Bi, Q.; Ferreras, E.; Pezzoli, L.; Legros, D.; Ivers, L.C.; Date, K.; Qadri, F.; Digilio, L.; Sack, D.A.; Ali, M.; et al. Protection against cholera from killed whole-cell oral cholerae vaccines: A systematic review and meta-analysis. Oral cholerae vaccine working group of the global task force on cholerae control. Lancet Infect. Dis. 2017, 17, 1080–1088. [Google Scholar] [CrossRef]
- Peak, C.M.; Reilly, A.L.; Azman, A.S.; Buckee, C.O. Prolonging herd immunity to cholerae via vaccination: Accounting for human mobility and waning vaccine effects. PLoS Negl. Trop. Dis. 2018, 12, e0006257. [Google Scholar] [CrossRef]
- Royal, J.M.; Reeves, M.A.; Matoba, N. Repeated oral administration of a KDEL-tagged recombinant cholerae toxin B subunit effectively mitigates DSS colitis despite a robust immunogenic response. Toxins 2019, 11, 678. [Google Scholar] [CrossRef]
- Kabir, S. Critical analysis of compositions and protective efficacies of oral killed cholera vaccines. Clin. Vaccine Immunol. 2014, 21, 1195–1205. [Google Scholar] [CrossRef]
- Chen, W.H.; Cohen, M.B.; Kirkpatrick, B.D.; Brady, R.C.; Galloway, D.; Gurwith, M.; Hall, R.H.; Kessler, R.A.; Lock, M.; Haney, D.; et al. Single-dose live oral cholera vaccine CVD 103-HgR protects against human experimental infection with Vibrio cholerae El Tor. Clin. Infect. Dis. 2016, 62, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.J. Modern history of cholera vaccines and the pivotal role of ICDDR. Infect. Dis. 2021, 224, S742–S748. [Google Scholar] [CrossRef] [PubMed]
- Wierzba, T.F. Oral cholera vaccines and their impact on the global burden of disease. Hum. Vaccines Immunother. 2019, 15, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Chowdhury, M.I.; Faruque, S.M.; Salam, M.A.; Ahmed, T.; Begum, Y.A.; Saha, A.; Al Tarique, A.; Seidlein, L.V.; Park, E.; et al. PXV Study Group, Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine 2007, 25, 231–238. [Google Scholar] [CrossRef]
- Khan, A.I.; Islam, M.T.; Khan, Z.H.; Tanvir, N.A.; Amin, M.A.; Khan, I.I.; Bhuiyan, A.T.M.R.H.; Hasan, A.S.M.M.; Islam, M.S.; Bari, T.I.A.; et al. Implementation and delivery of oral cholera vaccination campaigns in humanitarian crisis settings among Rohingya Myanmar nationals in Cox’s Bazar, Bangladesh. Vaccines 2023, 11, 843. [Google Scholar] [CrossRef]
- Song, K.R.; Lim, J.K.; Park, S.E.; Saluja, T.; Cho, S.I.; Wartel, T.A.; Lynch, J. Oral cholera vaccine efficacy and effectiveness. Vaccines 2021, 9, 1482. [Google Scholar] [CrossRef]
- Ali, M.; Qadri, F.; Kim, D.R.; Islam, M.T.; Im, J.; Ahmmed, F.; Khan, A.I.; Zaman, K.; Marks, F.; Kim, J.H.; et al. Effectiveness of a killed whole-cell oral cholera vaccine in Bangladesh: Further follow-up of a cluster-randomised trial. Lancet Infect. Dis. 2021, 21, 1407–1414. [Google Scholar] [CrossRef]
- Koelle, K.; Rodo, X.; Pascual, M.; Yunus, M.; Mostafa, G. Refractory periods and climate forcing in cholerae dynamics. Nature 2005, 436, 696–700. [Google Scholar] [CrossRef]
- Khatib, A.M.; Ali, M.; von Seidlein, L.; Kim, D.R.; Hashim, R.; Reyburn, R.; Ley, B.; Thriemer, K.; Enwere, G.; Hutubessy, R.; et al. Effectiveness of an oral cholerae vaccine in Zanzibar: Findings from a mass vaccination campaign and observational cohort study. Lancet Infect. Dis. 2012, 1, 837–844. [Google Scholar] [CrossRef]
- Ali, M.; Sur, D.; You, Y.A.; Kanungo, S.; Sah, B.; Manna, B.; Puri, M.; Wierzba, T.F.; Donner, A.; Nair, G.B.; et al. Herd protection by a bivalent killed whole-cell oral cholerae vaccine in the slums of Kolkata, India. Clin. Infect. Dis. 2013, 56, 1123–1131. [Google Scholar] [CrossRef]
- Holmgren, J. An update on cholerae immunity and current and future cholerae vaccines. Trop Med Infect Dis. 2021, 6, 64. [Google Scholar] [CrossRef]
- De-Simone, S.G.; Napoleão-Pêgo, P.; Gonçalves, P.S.; Provance-Jr, D.W.; Morel, C.M.; Silva, F.R. B cell epitope mapping of the Vibrio cholerae toxins A, B, and P. Int. J. Mol. Sci. 2023, 24, 531. [Google Scholar] [CrossRef]
- Pandey, S.; Malviya, G.; Chottova Dvorakova, M. Role of peptides in diagnostics. Int. J. Mol. Sci. 2021, 22, 8828. [Google Scholar] [CrossRef] [PubMed]
- Andresen, H.; Bier, F.F. Peptide microarrays for serum antibody diagnostics. Methods Mol. Biol. 2009, 509, 123–134. [Google Scholar] [CrossRef]
- Winkler, D.F.; Campbell, W.D. The spot technique: Synthesis and screening of peptide macroarrays on cellulose membranes. Methods Mol. Biol. 2008, 494, 47–70. [Google Scholar] [CrossRef]
- Naidu, A.; Lulu, S.S. Mucosal and systemic immune responses to Vibrio cholerae infection and oral cholerae vaccines (OCVs) in humans: A systematic review. Expert. Rev. Clin. Immunol. 2022, 18, 1307–1318. [Google Scholar] [CrossRef]
- Kanungo, S.; Azman, A.S.; Ramamurthy, T.; Deen, J.; Dutta, S. Cholerae. Lancet 2022, 399, 1429–1440. [Google Scholar] [CrossRef]
- Rask, C.; Fredriksson, M.; Lindblad, M.; Czerkinsky, C.; Holmgren, J. Mucosal and systemic antibody responses after peroral or intranasal immunization: Effects of conjugation to enterotoxin B subunits and/or of co-administration with free toxin as adjuvant. APMIS 2000, 108, 178–186. [Google Scholar] [CrossRef]
- Akter, A.; Kelly, M.; Charles, R.C.; Harris, J.B.; Calderwood, S.B.; Bhuiyan, T.R.; Biswas, R.; Xu, P.; Kováč, P.; Qadri, F.; et al. Parenteral vaccination with a cholera conjugate vaccine boosts vibriocidal and anti-OSP responses in mice previously immunized with an oral cholera vaccine. Am. J. Trop. Med. Hyg. 2021, 104, 2024–2030. [Google Scholar] [CrossRef]
- Montero, D.A.; Vidal, R.M.; Velasco, J.; George, S.; Lucero, Y.; Gómez, L.A.; Carreño, L.J.; García-Betancourt, R.; O’Ryan, M. Vibrio cholerae, classification, pathogenesis, immune response, and trends in vaccine development. Front Med (Lausanne) 2023, 10, 1155751. [Google Scholar] [CrossRef]
- Sánchez, J.; Holmgren, J. Cholerae toxin structure, gene regulation, and pathophysiological and immunological aspects. Cell. Mol. Life Sci. 2008, 65, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Baldauf, K.J.; Royal, J.M.; Hamorsky, K.T.; Matoba, N. Cholera toxin B: One subunit with many pharmaceutical applications. Toxins 2015, 7, 974–996. [Google Scholar] [CrossRef] [PubMed]
- Lycke, N.; Eriksen, L.; Holmgren, J. Protection against cholera toxin after oral immunization is thymus-dependent and associated with intestinal production of neutralizing IgA antitoxin. Scand. J. Immunol. 1987, 25, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Pedoussaut, S.; Delmas, A.; Milhaud, G.; Rivaille, P.; Gruaz-Guyon, A. Oral immunization with a free peptide from cholera toxin: Local protection and IgA production. Mol. Immunol. 1989, 26, 115–119. [Google Scholar] [CrossRef]
- Jacob, C.O.; Vaerman, J.P. Induction of rat secretory IgA antibodies against cholera toxin by a synthetic peptide. Immunology 1986, 59, 129–133. [Google Scholar]
- Delmas, A.; Gruaz-Guyon, A.; Pedoussaut, S.; Pierre, P.; Rivaille, P.; Vaerman, J.P. Neutralization of cholera toxin by rat IgA secretory antibodies induced by a free synthetic peptide. Biochem. Biophys. Res. Commun. 1989, 159, 707–712. [Google Scholar] [CrossRef]
- Sikora, A.E. Proteins secreted via the type II secretion system: Smart strategies of Vibrio cholerae to maintain fitness in different ecological niches. PLoS Pathog. 2013, 9, e1003126. [Google Scholar] [CrossRef]
- Schroeder, H.W., Jr.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef]
- Kerr, M.A. The structure and function of human IgA. Biochem. J. 1990, 271, 285–296. [Google Scholar] [CrossRef]
- Steffen, U.; Koeleman, C.A.; Sokolova, M.V.; Bang, H.; Kleyer, A.; Rech, J.; Unterweger, H.; Schicht, M.; Garreis, F.; Hahn, J.; et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat. Commun. 2020, 11, 120. [Google Scholar] [CrossRef]
- Hansen, I.S.; Baeten, D.L.P.; den Dunnen, J. The inflammatory function of human IgA. Cell Mol. Life Sci. 2019, 76, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Bunker, J.J.; Bendelac, A. IgA Responses to Microbiota. Immunity 2018, 49, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.S.; Krabbendam, L.; Bernink, J.H.; Loayza-Puch, F.; Hoepel, W.; van Burgsteden, J.A.; Kuijper, E.C.; Buskens, C.J.; Bemelman, W.A.; Zaat, S.A.J.; et al. FcαRI co-stimulation converts human intestinal CD103+ dendritic cells into pro-inflammatory cells through glycolytic reprogramming. Nat. Commun. 2018, 28, 863. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Janda, A. Immunoglobulin isotype influences affinity and specificity. Proc. Natl. Acad. Sci. USA. 2012, 109, 12272–12273. [Google Scholar] [CrossRef]
- Janda, A.; Bowen, A.; Greenspan, N.S.; Casadevall, A. Ig Constant region effects on variable region structure and function. Front. Microbiol. 2016, 7, 22. [Google Scholar] [CrossRef]
- Garzón-Ospina, D.; Buitrago, S.P. Immunoglobulin heavy constant gamma gene evolution is modulated by both the divergent and birth-and-death evolutionary models. Primates 2022, 63, 611–625. [Google Scholar] [CrossRef]
- Mekalanos, J.; Collier, R.; Romig, W. Enzymic activity of cholerae toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J. Biol. Chem. 1979, 254, 5855–5861. [Google Scholar] [CrossRef]
- Mayo, S.; Royo, F.; Hau, J. Correlation between adjuvanticity and immunogenicity of cholerae toxin B subunit in orally immunized young chickens. APMIS 2005, 113, 284–287. [Google Scholar] [CrossRef]
- Price, G.A.; Holmes, R.K. Evaluation of TcpF-A2-CTB chimera and evidence of additive protective efficacy of immunizing with TcpF and CTB in the suckling mouse model of cholerae. PLoS ONE 2012, 7, e42434. [Google Scholar] [CrossRef]
- Maślanka, T.; Clapp, B.; Hoffman, C.; Robison, A.; Gregorczyk, I.; Pascual, D.W. Nasal vaccination of β7 integrin-deficient mice retains elevated IgA immunity. Immunol. Cell Biol. 2020, 98, 667–681. [Google Scholar] [CrossRef]
- Adachi, M.; Kurihara, Y.; Nojima, H.; Takeda-Shitaka, M.; Kamiya, K.; Umeyama, H. Interaction between the antigen and antibody is controlled by the constant domains: Normal mode dynamics of the HEL-HyHEL-10 complex. Protein Sci. 2003, 12, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Min, Q.; Xiong, E.; Heyman, B.; Wang, J.Y. Regulation of humoral immune responses and B cell tolerance by the IgM Fc receptor (FcμR). Adv. Exp. Med. Biol. 2020, 1254, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; An, S.J.; Jang, M.S.; Song, M.; Han, S.H. IgM specific to lipopolysaccharide of Vibrio cholerae is a surrogate antibody isotype responsible for serum vibriocidal activity. PLoS ONE 2019, 14, e0213507. [Google Scholar] [CrossRef] [PubMed]
- Freytag, L.C.; Clements, J.D. Mucosal adjuvants. Vaccine. 2005, 23, 1804–1813. [Google Scholar] [CrossRef]
- Keyt, B.A.; Baliga, R.; Sinclair, A.M.; Carroll, S.F.; Peterson, M.S. Structure, function, and therapeutic use of IgM antibodies. Antibodies 2020, 9, 53. [Google Scholar] [CrossRef]
- Li, Y.; Shen, H.; Zhang, R.; Ji, C.; Wang, Y.; Su, C.; Xiao, J. Immunoglobulin M perception by FcμR. Nature 2023, 615, 907–912. [Google Scholar] [CrossRef]
- Hiramoto, E.; Tsutsumi, A.; Suzuki, R.; Matsuoka, S.; Arai, S.; Kikkawa, M.; Miyazaki, T. The IgM pentamer is an asymmetric pentagon with an open groove that binds the AIM protein. Sci. Adv. 2018, 4, eaau1199. [Google Scholar] [CrossRef]
- Wang, Y.; Su, C.; Ji, C.; Xiao, J. CD5L associates with IgM via the J chain. Nat. Commun. 2024, 15, 8397. [Google Scholar] [CrossRef]
- Muzembo, B.A.; Kitahara, K.; Ohno, A.; Debnath, A.; Okamoto, K.; Miyoshi, S.I. Cholera rapid diagnostic tests for the detection of Vibrio cholerae O1: An updated meta-analysis. Diagnostics 2021, 11, 2095. [Google Scholar] [CrossRef]
- Debes, A.K.; Murt, K.N.; Waswa, E.; Githinji, G.; Umuro, M.; Mbogori, C.; Roskosky, M.; Ram, M.; Shaffer, A.; Sack, D.A. Laboratory and field evaluation of the Crystal VC-O1 cholera rapid diagnostic test. Am. J. Trop. Med. Hyg. 2021, 104, 2017–2023. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Islam, K.; Hossain, M.; Akter, N.J.; Alam, M.N.; Sultana, N.; Khanam, F.; Kelly, M.; Charles, R.C.; Kováč, P.; et al. Development of a new dipstick (Cholkit) for rapid detection of Vibrio cholerae O1 in acute watery diarrheal stools. PLoS Negl. Trop. Dis. 2018, 12, e0006286. [Google Scholar] [CrossRef] [PubMed]
- Heyman, B.; Shulman, M.J. Encyclopedia of Immunobiology; DeFranco, A.L., Ratcliffe, M.J.H., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–14. ISBN 012374282X/9780123742827. [Google Scholar]
- McGettigan, S.E.; Aira, L.E.; Kumar, G.; Ballet, R.; Butcher, E.C.; Baumgarth, N.; Debes, G.F. Secreted IgM modulates IL-10 expression in B cells. Nat Commun 2024, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- De-Simone, S.G.; Gomes, L.R.; Napoleão-Pêgo, P.; Lechuga, G.C.; Pina, J.C.; Silva, F.R. Identification of linear B epitopes liable for the protective immunity of diphtheria toxin. Vaccines 2021, 9, 313. [Google Scholar] [CrossRef]
- Muzembo, B.A.; Kitahara, K.; Debnath, A.; Okamoto, K.; Miyoshi, S.I. Accuracy of cholera rapid diagnostic tests: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.J.; Grembi, J.A.; Chao, D.L.; Andrews, J.R.; Alexandrova, L.; Rodriguez, P.H.; Ramachandran, V.V.; Sayeed, M.A.; Wamala, J.F.; Debes, A.K.; et al. Gold standard cholera diagnostics are tarnished by lytic bacteriophage and antibiotics. J. Clin. Microbiol. 2020, 58, e00412-20. [Google Scholar] [CrossRef]
- Ramamurthy, T.; Das, B.; Chakraborty, S.; Mukhopadhyay, A.K.; Sack, D.A. Diagnostic techniques for rapidly detecting Vibrio cholerae O1/O139. Vaccine 2020, 38, A73–A82. [Google Scholar] [CrossRef]
- Chakraborty, S.; Velagic, M.; Connor, S. Development of a simple, rapid, and sensitive molecular diagnostic assay for cholera. PLoS Negl. Trop. Dis. 2023, 17, e0011113. [Google Scholar] [CrossRef]
- Zareitaher, T.; Sadat, T.; Seyed, A.S.; Gargari, L.M. Immunogenic efficacy of DNA and protein-based vaccine from a chimeric gene consisting of OmpW, TcpA, and CtxB, of Vibrio cholerae. Immunobiology 2022, 227, 152190. [Google Scholar] [CrossRef]
- Zereen, F.; Akter, S.; Sobur, M.A.; Hossain, M.T.; Rahman, M.T. Molecular detection of Vibrio cholerae from human stool collected from SK Hospital, Mymensingh, and their antibiogram. J. Adv. Vet. Anim. Res. 2019, 6, 451–455. [Google Scholar] [CrossRef]
- Pezzoli, L. Oral cholera vaccine working group of the global task force on cholera control. Global oral cholera vaccine use, 2013–2018. Vaccine 2020, 38, A132–A140. [Google Scholar] [CrossRef]
- Saif-Ur-Rahman, K.M.; Mamun, R.; Hasan, M.; Meiring, J.E.; Khan, M.A. Oral killed cholera vaccines for preventing cholera. Cochrane Database Syst. Rev. 2024, 1, CD014573. [Google Scholar] [CrossRef]
- Bishop, A.L.; Schild, S.; Patimalla, B.; Klein, B.; Camilli, A. Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility. Infect. Immun. 2010, 78, 4402–4420. [Google Scholar] [CrossRef] [PubMed]
- Charles, R.C.; Kelly, M.; Tam, J.M.; Akter, A.; Hossain, M.; Islam, K.; Biswas, R.; Kamruzzaman, M.; Chowdhury, F.; Khan, A.I.; et al. Humans surviving cholera develop antibodies against Vibrio cholerae O-specific polysaccharide that inhibit pathogen motility. mBio 2020, 11, e02847-20. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, R.C.; Adekunle, O.; Yu, H.; Cho, A.; Nyhoff, L.E.; Kelly, M.; Harris, J.B.; Bhuiyan, T.R.; Qadri, F.; Calderwood, S.B.; et al. Impact of immunoglobulin isotype and epitope on the functional properties of Vibrio cholerae O-specific polysaccharide-specific monoclonal antibodies. mBio 2021, 12, e03679-20. [Google Scholar] [CrossRef]
- Dias, E.R.; Durans, A.M.; Succar, B.B.; Pinto, L.A.L.T.; Lechuga, G.C.; Miguez, M.G.; Figueira-Mansur, J.; Argondizzo, A.P.C.; Bernardo, A.R.; Diniz, R.L.; et al. A multi-epitope protein for high-performance serodiagnosis of chronic Chagas Disease in ELISA and lateral flow platforms. Int. J. Mol. Sci. 2024, 25, 9811. [Google Scholar] [CrossRef]
- Silva, F.R.; Napoleão-Pêgo, P.; De-Simone, S.G. Identification of linear B epitopes of pertactin of Bordetella pertussis induced by immunization with whole and acellular vaccine. Vaccine 2014, 32, 6251–6258. [Google Scholar] [CrossRef]
- Moutsinas, G.; Shuaib, C.; Guo, W.; Jarvis, S. Graph hierarchy: A novel framework to analyze hierarchical structures in complex networks. Sci. Rep. 2021, 11, 13943. [Google Scholar] [CrossRef]
- De-Simone, S.G.; Napoleão-Pêgo, P.; Lechuga, G.C.; Carvalho, J.P.R.S.; Gomes, L.R.; Cardoso, S.V.; Morel, C.M.; Provance-Jr, D.W.; Silva, F.R.S. High-throughput IgG epitope mapping of tetanus neurotoxin: Implications for immunotherapy and vaccine design. Toxins 2023, 15, 239. [Google Scholar] [CrossRef]
- De-Simone, S.; Souza, A.L.A.; Melgarejo, A.R.; Aguiar, A.S.; Provance, D.W., Jr. Development of elisa assay to detect specific human IgE anti-therapeutic horse sera. Toxicon 2017, 138, 37–42. [Google Scholar] [CrossRef]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef]
- Jumper, J.; Evansm, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
| Protein Code | Code | aa | Sequence | Second Structure * | Peptide Search ** |
|---|---|---|---|---|---|
| P01555 | TxA-1A | 51 | RGTQMNINLYDHARG | C | E. coli |
| TxA-2A | 146–160 | YRVHFGVLDEQLHRN | C | Sp | |
| P01556 | TxB-3A | 66–75 | REMAIITFKN | C + H | Sp |
| TxB-4A | 81–90 | SQKKAIERMK | H | E. coli | |
| P29485 | TxP-5A | 31–45 | KPERLIGTPSIIQT | C + H | Sp |
| TxP-6A | 81–90 | AIKRTRDFLN | C + H | Sp | |
| TxP-7A | 126–135 | QKKSVKERIK | C + H | Various bacteria | |
| P01555 | TxA-1M | 11–25 | FLSSFSYANDDKLYR | C | Various bacteria |
| TxA-2M | 41–50 | MPRGQSEYFD | C | Sp | |
| TxA-3M | 56–64 | NINLYDHAR | C + H | Sp | |
| TxA-4M | 71–80 | VRHDDGYVST | C | E. coli | |
| TxA-5M | 91–105 | GQTILSGHSTYYIYV | C + H | Various bacteria | |
| TxA-6M | 111–125 | NMFNVNDVLGAYSPH | C | Sp | |
| TxA-7M | 131–145 | VSALGGIPYSQIYGW | C | Various bacteria | |
| TxA-8M | 151–160 | GVLDEQLHRN | C | Sp | |
| TxA-9M | 171–185 | RGYRDRYYSNLDIAP | C | Sp | |
| TxA-10M | 191–205 | GLAGFPPEHRAWREE | C | Sp | |
| TxA-11M | 236–250 | VKRQIFSGYQSDIDT | C + H | Sp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De-Simone, S.G.; Napoleão-Pêgo, P.; Lechuga, G.C.; Carvalho, J.P.R.S.; Cardozo, S.V.; Saisse, A.O.; Morel, C.M.; Provance, D.W., Jr.; da Silva, F.R. Dynamics of IgM and IgA Antibody Response Profile Against Vibrio cholerae Toxins A, B, and P. Int. J. Mol. Sci. 2025, 26, 3507. https://doi.org/10.3390/ijms26083507
De-Simone SG, Napoleão-Pêgo P, Lechuga GC, Carvalho JPRS, Cardozo SV, Saisse AO, Morel CM, Provance DW Jr., da Silva FR. Dynamics of IgM and IgA Antibody Response Profile Against Vibrio cholerae Toxins A, B, and P. International Journal of Molecular Sciences. 2025; 26(8):3507. https://doi.org/10.3390/ijms26083507
Chicago/Turabian StyleDe-Simone, Salvatore Giovanni, Paloma Napoleão-Pêgo, Guilherme Curty Lechuga, Joao Pedro Rangel Silva Carvalho, Sergian Vianna Cardozo, Alexandre Oliveira Saisse, Carlos Medicis Morel, David William Provance, Jr., and Flavio Rocha da Silva. 2025. "Dynamics of IgM and IgA Antibody Response Profile Against Vibrio cholerae Toxins A, B, and P" International Journal of Molecular Sciences 26, no. 8: 3507. https://doi.org/10.3390/ijms26083507
APA StyleDe-Simone, S. G., Napoleão-Pêgo, P., Lechuga, G. C., Carvalho, J. P. R. S., Cardozo, S. V., Saisse, A. O., Morel, C. M., Provance, D. W., Jr., & da Silva, F. R. (2025). Dynamics of IgM and IgA Antibody Response Profile Against Vibrio cholerae Toxins A, B, and P. International Journal of Molecular Sciences, 26(8), 3507. https://doi.org/10.3390/ijms26083507








