A Multi-Level Study on the Anti-Lung Cancer Mechanism of Peiminine, a Key Component of Fritillaria ussuriensis Maxim.: Integrating Quality Analysis, Network Pharmacology, Bioinformatics Analysis, and Experimental Validation
Abstract
1. Introduction
2. Results
2.1. Determination of the Content of Peiminine in Fritillaria ussuriensis Maxim. Using UPLC-MS/MS
2.1.1. Method Validation Results
2.1.2. Sample Analysis
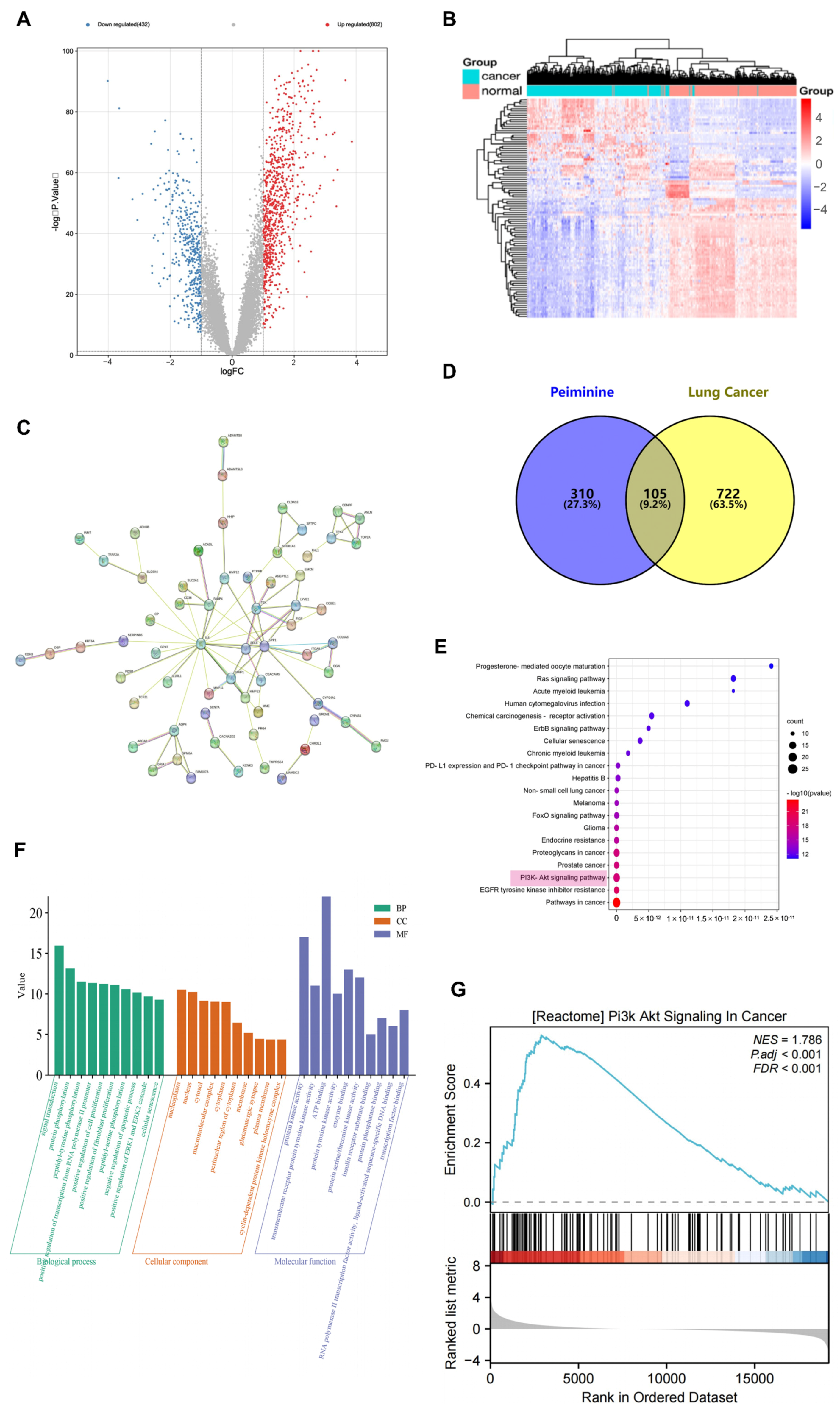
2.2. Target Screening for Peiminine and Lung Cancer
2.3. PPI Network Analysis
2.4. GO Analysis, KEGG Analysis, and GSEA
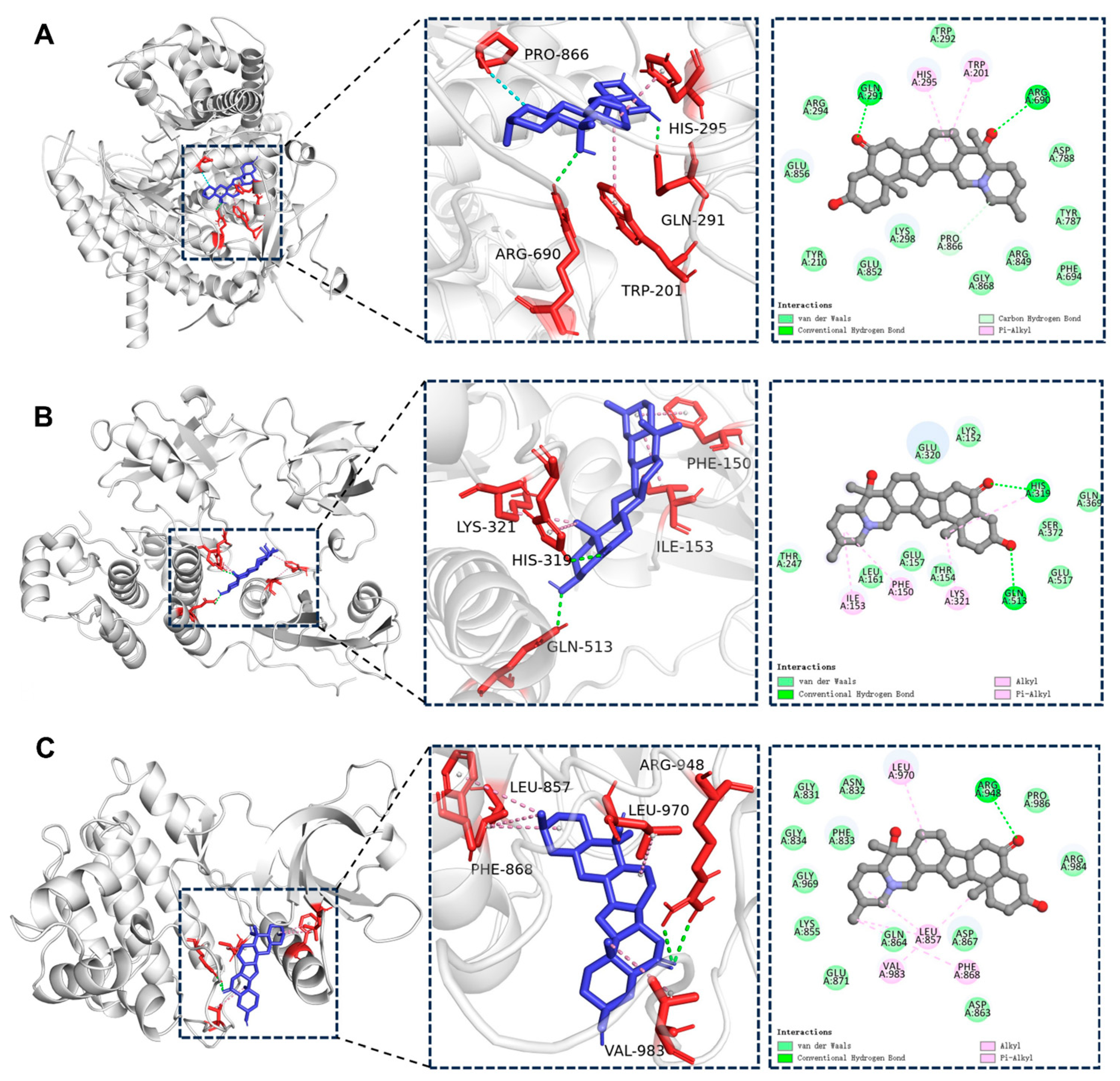
2.5. Molecular Docking Results
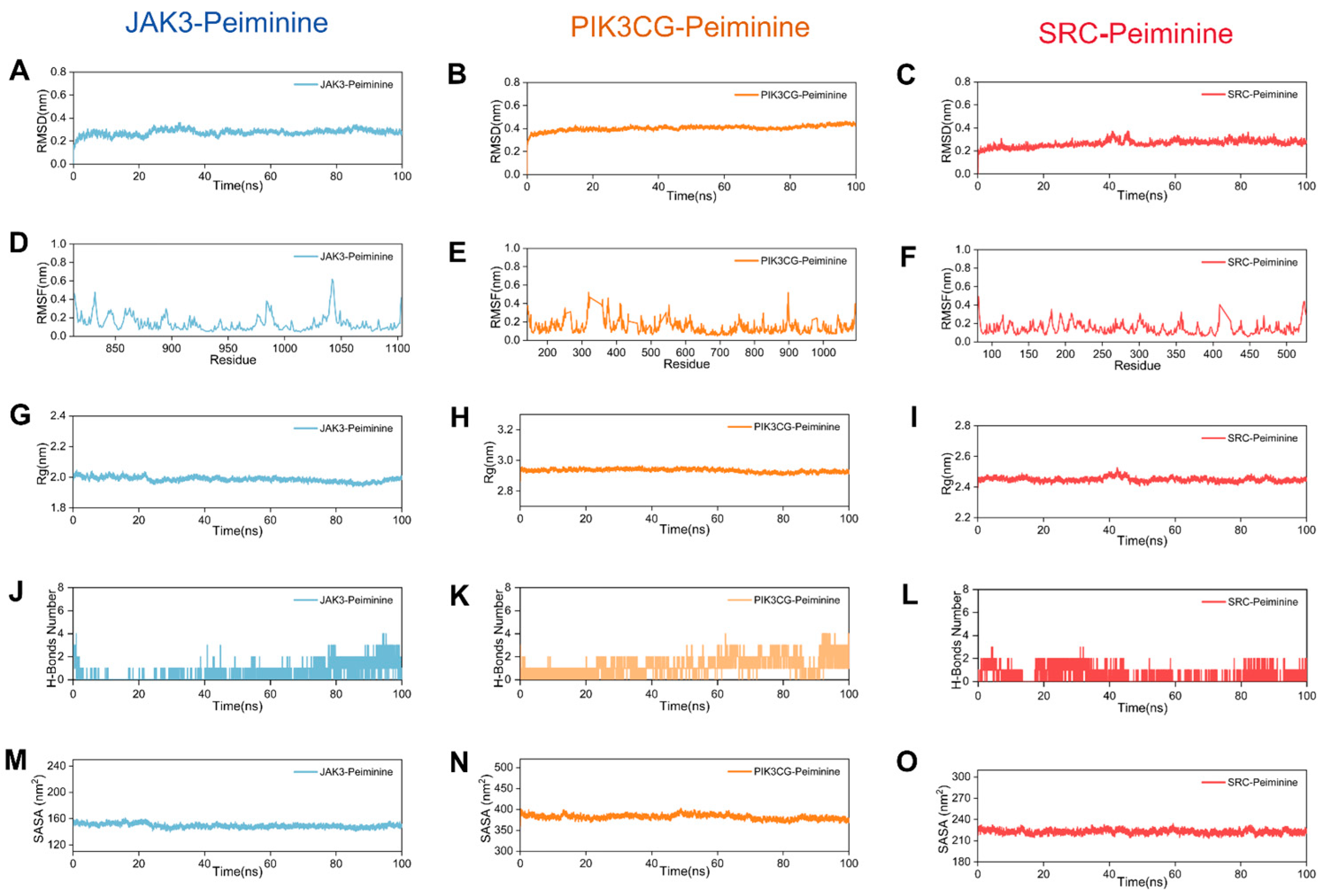
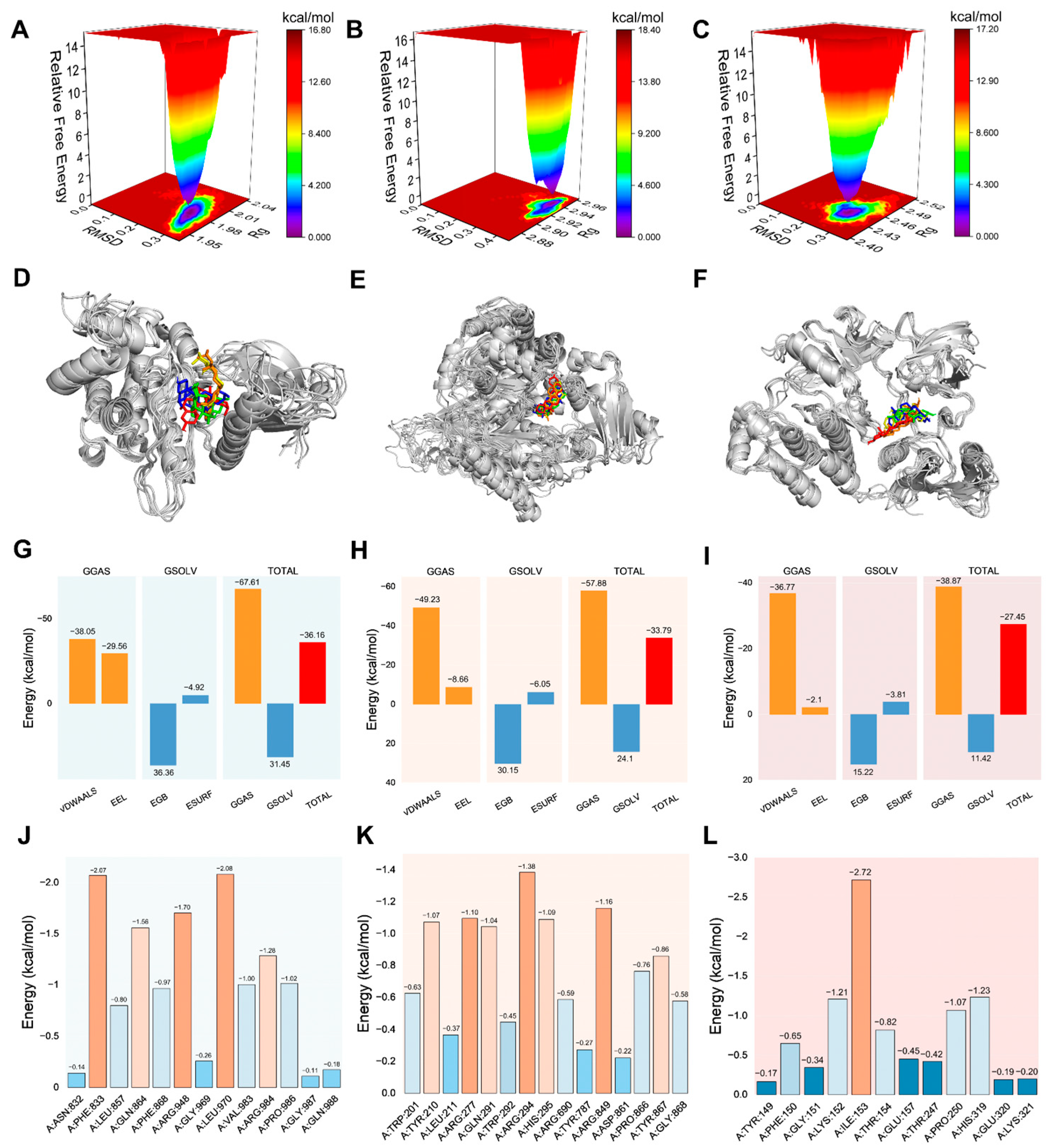
2.6. MD Analysis
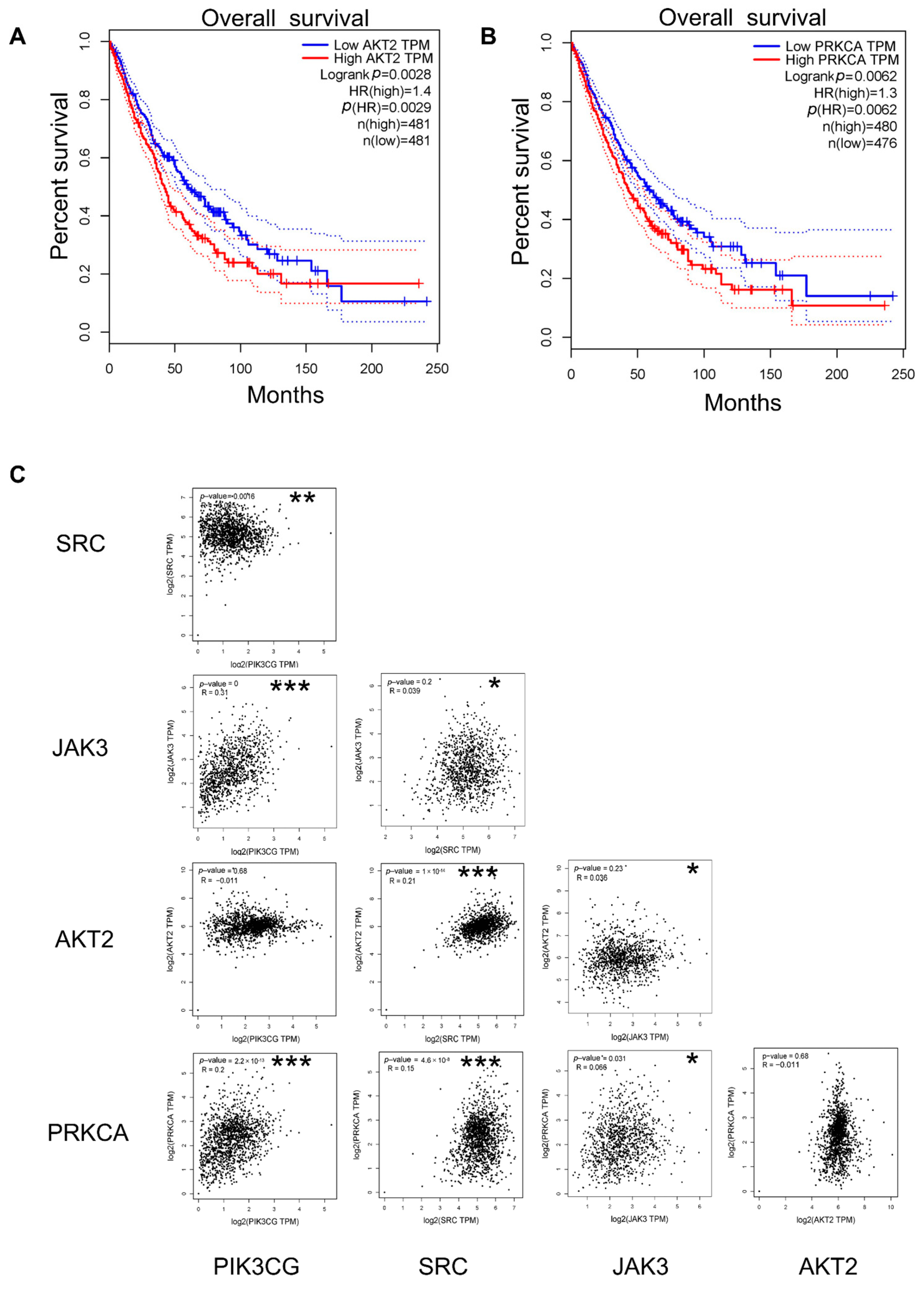
2.7. Survival, Expression, and Correlation Analysis
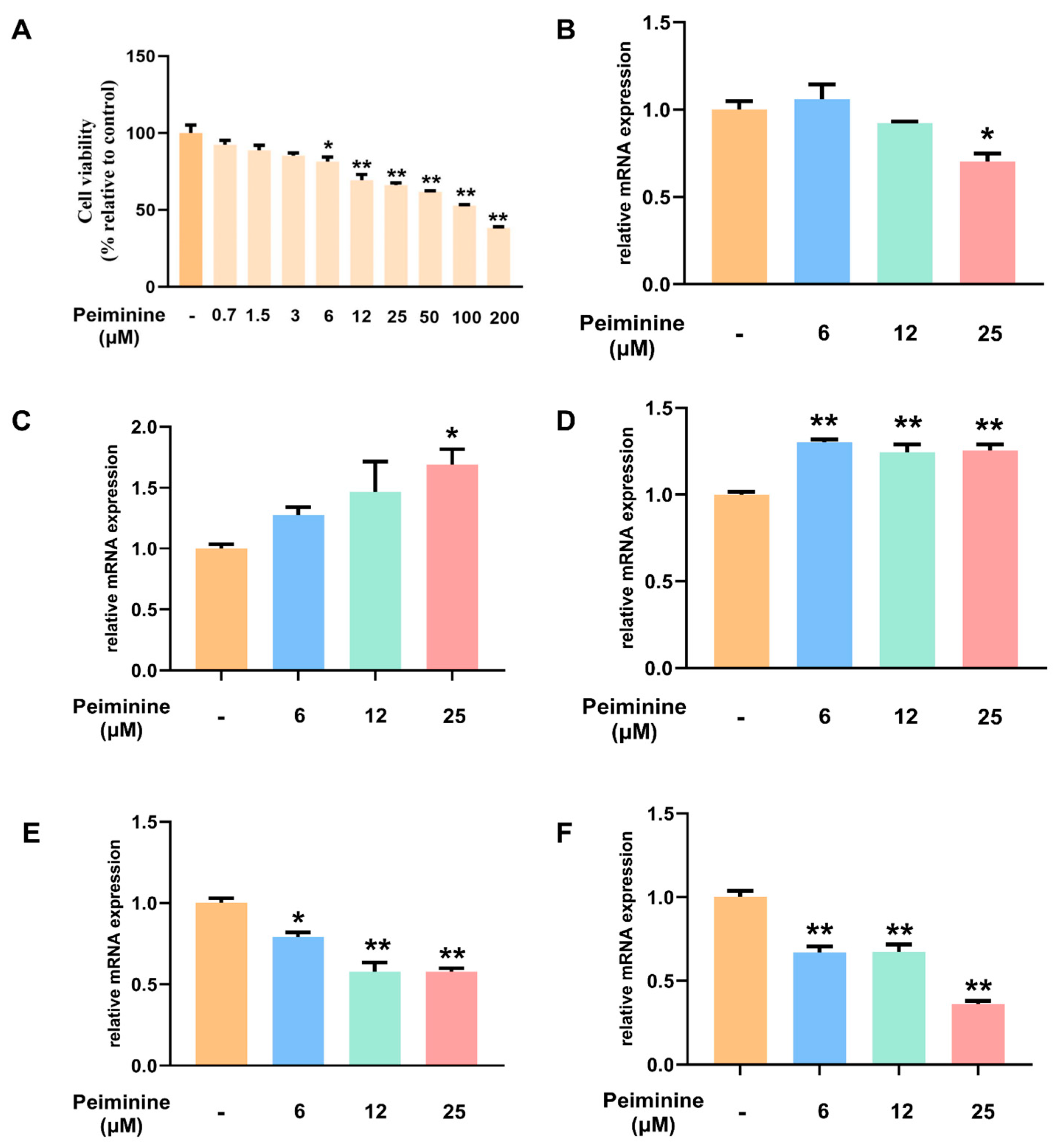
2.8. Effects of Peiminine on the Viability of H1299 Cells
2.9. Transcriptional Regulation of the PI3K–Akt Pathway and Apoptosis Pathway by Peiminine in H1299 Cells
3. Discussion
4. Materials and Methods
4.1. Determination of Peiminine Content in Fritillaria by UHPLC–MS/MS Method
4.1.1. Preparation of Reference Substance Solution
4.1.2. Preparation of Test Solution
4.1.3. UHPLC–MS/MS: Instruments and Conditions
4.1.4. Method Validation
4.1.5. Content Determination
4.2. Network Pharmacology
4.2.1. Target Collection for Peiminine
4.2.2. Target Collection for Lung Cancer from Databases
4.2.3. Target Collection for Lung Cancer from Expression Databases
4.2.4. PPI Network Construction
4.2.5. Pathway and Functional Enrichment Analysis
4.3. GSEA
4.4. Molecular Docking
4.5. Molecular Dynamics Simulation
4.6. Survival Analysis
4.7. Correlation and Expression Analysis
4.8. Validation on H1299 Cells
4.8.1. Cell Culture and Peiminine Administration
4.8.2. Isolation of RNA and Reverse Transcription–Polymerase Chain Reaction
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Chan, T.A.; Kroemer, G.; Wolchok, J.D.; López-Soto, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. 2018, 10, eaat7807. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Wolchok, J.D.; Bass, A.R. TNF in the era of immune checkpoint inhibitors: Friend or foe? Nat. Rev. Rheumatol. 2021, 17, 213–223. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, P.; Singh, P.K.; Singh, T.G.; Saini, B. Medicinal Potential of Heterocyclic Compounds from Diverse Natural Sources for the Management of Cancer. Mini Rev. Med. Chem. 2020, 20, 942–957. [Google Scholar] [CrossRef]
- Liu, D.M.; Cao, Z.X.; Yan, H.L.; Li, W.; Yang, F.; Zhao, W.J.; Diao, Q.C.; Tan, Y.Z. A new abietane diterpenoid from Ajuga ovalifolia var. calantha induces human lung epithelial A549 cell apoptosis by inhibiting SHP2. Fitoterapia 2020, 141, 104484. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Zhang, L.; Heng, Y.; Xu, T.; Zhang, Y.; Chen, X.; Hoffman, R.M.; Jia, L. Andrographolide Induces Noxa-Dependent Apoptosis by Transactivating ATF4 in Human Lung Adenocarcinoma Cells. Front. Pharmacol. 2021, 12, 680589. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, J.; Wang, S.; Wang, X.; Ou, Y.; Wei, D.; Li, X. Antitussive, expectorant and anti-inflammatory alkaloids from Bulbus Fritillariae Cirrhosae. Fitoterapia 2011, 82, 1290–1294. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Chen, X.; Xu, X.; Zhu, J.; Nie, L.; Long, X. Antitussive, expectorant and anti-inflammatory activities of four alkaloids isolated from Bulbus of Fritillaria wabuensis. J. Ethnopharmacol. 2012, 139, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.B.; Brinckmann, J.A.; Pei, S.J.; Luo, P.; Schippmann, U.; Long, X.; Bi, Y.F. High altitude species, high profits: Can the trade in wild harvested Fritillaria cirrhosa (Liliaceae) be sustained? J. Ethnopharmacol. 2018, 223, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, H.; Ren, Q.; Hu, H.; Yang, T.; Li, X. Natural drug sources for respiratory diseases from Fritillaria: Chemical and biological analyses. Chin. Med. 2021, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, A.A.; Syed, V. Inhibition of Transforming Growth Factor-β (TGF-β) Signaling by Scutellaria baicalensis and Fritillaria cirrhosa Extracts in Endometrial Cancer. J. Cell. Biochem. 2015, 116, 1797–1805. [Google Scholar] [CrossRef]
- Pae, H.O.; Oh, H.; Choi, B.M.; Oh, G.S.; Paik, S.G.; Jeong, S.; Hwang, K.M.; Yun, Y.G.; Chung, H.T. Differentiation-inducing effects of verticinone, an isosteroidal alkaloid isolated from the bulbus of Fritillaria ussuriensis, on human promyelocytic leukemia HL-60 cells. Biol. Pharm. Bull. 2002, 25, 1409–1411. [Google Scholar] [CrossRef][Green Version]
- Bhat, B.A.; Mir, W.R.; Alkhanani, M.; Almilaibary, A.; Mir, M.A. Network Pharmacology and Experimental Validation for Deciphering the Action Mechanism of Fritillaria cirrhosa D. Don Constituents in Suppressing Breast Carcinoma. J. Biomol. Struct. Dyn. 2024, 42, 13002–13022. [Google Scholar] [CrossRef]
- Cha, S.J.; Kim, S.S.; Shin, J.H.; Seo, S.R. Peiminine Exerts Its Anti-Acne Effects by Regulating the NF-κB Pathway. Antioxidants 2024, 13, 131. [Google Scholar] [CrossRef]
- Pai, M.; Er-Bu, A.; Wu, Y.; Ming, T.W.; Gaun, T.K.W.; Ye, B. Total alkaloids of bulbus of Fritillaria cirrhosa alleviate bleomycin-induced inflammation and pulmonary fibrosis in rats by inhibiting TGF-β and NF-κB signaling pathway. Food Nutr. Res. 2023, 67, 10-29219. [Google Scholar] [CrossRef]
- Du, B.; Cao, L.; Wang, K.; Miu, J.; Yao, L.; Xu, Z.; Song, J. Peiminine Attenuates Acute Lung Injury Induced by LPS Through Inhibiting Lipid Rafts Formation. Inflammation 2020, 43, 1110–1119. [Google Scholar] [CrossRef]
- Gu, H.; Wei, J. Peiminine regulates bone-fat balance by canonical Wnt/β-catenin pathway in an ovariectomized rat model. Phytother. Res. PTR 2023, 37, 2841–2853. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, R.; Bi, Y.; Xu, W.; Hao, M.; Liang, Y.; Li, Y.; Wang, H.; Zhang, J.; Xie, J.; et al. Peimenine unleashes therapeutic promise in urothelial bladder cancer: Inhibition of proliferation, induction of cell death and modulation of key pathways. Chem. Biol. Drug Des. 2024, 103, e14528. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Z.; Hu, H.; Yang, Y.; Xiao, C.; Xi, L.; Lu, J.; Tian, S.; Zhao, H. Synergistic antitumor effects of Peiminine and Doxorubicin on breast cancer through enhancing DNA damage via ZEB1. Biomed. Pharmacother. 2024, 173, 116353. [Google Scholar] [CrossRef]
- Yi, N.; Wang, L.; Jiang, Z.; Xu, G.; Li, L.; Zhang, Y.; Tan, Y. Peiminine triggers ferroptosis to inhibit breast cancer growth through triggering Nrf2 signaling. Tissue Cell 2024, 87, 102323. [Google Scholar] [CrossRef]
- Yagüe, E.; Sun, H.; Hu, Y. East Wind, West Wind: Toward the modernization of traditional Chinese medicine. Front. Neurosci. 2022, 16, 1057817. [Google Scholar] [CrossRef]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, F.; Yang, K.; Fang, S.; Bu, D.; Li, H.; Sun, L.; Hu, H.; Gao, K.; Wang, W.; et al. SymMap: An integrative database of traditional Chinese medicine enhanced by symptom mapping. Nucleic Acids Res. 2019, 47, D1110–D1117. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. In Cancer Epidemiology, Biomarkers & Prevention: A publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology; American Association for Cancer Research: Philadelphia, PA, USA, 2019; Volume 28, pp. 1563–1579. [Google Scholar] [CrossRef]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef]
- Senan, S.; Brade, A.; Wang, L.H.; Vansteenkiste, J.; Dakhil, S.; Biesma, B.; Martinez Aguillo, M.; Aerts, J.; Govindan, R.; Rubio-Viqueira, B.; et al. Proclaim: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Borjigin, G.; Wei, F.; Jiang, S.; Li, Q.; Yang, C. Extraction, Purification, Structural Characterization and Biological Activity of Polysaccharides from Fritillaria: A Review. Int. J. Biol. Macromol. 2023, 242, 124817. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Cui, Y.; Liu, C.; Ding, W.; Ren, S.; Dong, R.; Zhang, J. Development of a Quality Evaluation Method for Allii Macrostemonis Bulbus Based on Solid-Phase Extraction-High-Performance Liquid Chromatography-Evaporative Light Scattering Detection Chromatographic Fingerprinting, Chemometrics, and Quantitative Analysis of Multi-Components via a Single-Marker Method. Molecules 2024, 29, 4600. [Google Scholar] [CrossRef]
- Rahman, M.A.; Jalouli, M.; Bhajan, S.K.; Al-Zharani, M.; Harrath, A.H.A. Comprehensive Review of Nanoparticle-Based Drug Delivery for Modulating PI3K/AKT/mTOR-Mediated Autophagy in Cancer. Int. J. Mol. Sci. 2025, 26, 1868. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.; Wymann, M.P.; Patrucco, E.; Tolosano, E.; Bulgarelli-Leva, G.; Marengo, S.; Rocchi, M.; Altruda, F. Analysis of the Murine Phosphoinositide 3-Kinase Gamma Gene. Gene 2000, 256, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.; Katanaev, V.L.; Garlanda, C.; Azzolino, O.; Pirola, L.; Silengo, L.; Sozzani, S.; Mantovani, A.; Altruda, F.; Wymann, M.P. Central Role for G Protein-coupled Phosphoinositide 3-Kinase Gamma in Inflammation. Science 2000, 287, 1049–1053. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, E.; Wei, L.; Zeng, H.; Qin, H.; Zhang, X.; Liao, M.; Chen, L.; Zhao, L.; Ruan, X.Z.; et al. The Fatty Acid Receptor CD36 Promotes HCC Progression through Activating Src/PI3K/AKT Axis-dependent Aerobic Glycolysis. Cell Death Dis. 2021, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Song, J.; Ruze, R.; Chen, Y.; Yin, X.; Wang, C.; Zhao, Y. SQLE Promotes Pancreatic Cancer Growth by Attenuating ER Stress and Activating Lipid Rafts-regulated Src/PI3K/Akt Signaling Pathway. Cell Death Dis. 2023, 14, 497. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, M.; Zou, X.; Mao, Y.; Niu, J.; Jiang, J.; Dong, T.; Shi, Y.; Yang, X.; Liu, P. CCL2-targeted Ginkgolic Acid Exerts Anti-glioblastoma Effects by Inhibiting the JAK3-STAT1/PI3K-AKT Signaling Pathway. Life Sci. 2022, 311, 121174. [Google Scholar] [CrossRef]
- Li, J.X.; Li, R.Z.; Sun, A.; Zhou, H.; Neher, E.; Yang, J.S.; Huang, J.M.; Zhang, Y.Z.; Jiang, Z.B.; Liang, T.L.; et al. Metabolomics and Integrated Network Pharmacology Analysis Reveal Tricin as the Active Anti-cancer Component of Weijing Decoction by Suppression of PRKCA and Sphingolipid Signaling. Pharmacol. Res. 2021, 171, 105574. [Google Scholar] [CrossRef]
- Li, Y.; Yang, G.; Li, Q.; Zhang, Y.; Zhang, S.; Zhou, T.; Wang, X.; Liu, F.; Miao, Z.; Qi, Y.; et al. Guiqi Baizhu Decoction Enhances Radiosensitivity in Non-Small Cell Lung Cancer by Inhibiting the HIF-1α/DNA-PKcs Axis-Mediated DNA Repair. Phytomedicine 2025, 140, 156591. [Google Scholar] [CrossRef]
- Macerelli, M.; Ganzinelli, M.; Gouedard, C.; Broggini, M.; Garassino, M.C.; Linardou, H.; Damia, G.; Wiesmüller, L. Can the Response to a Platinum-based Therapy Be Predicted by the DNA Repair Status in Non-small Cell Lung Cancer? Cancer Treat. Rev. 2016, 48, 8–19. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, D.; Shangguan, C.; Xu, C.; Li, S.; Li, Y.; Liu, Y.; Jiang, S. Altingia chinensis Petroleum Ether Extract Suppresses NSCLC via Induction of Apoptosis, Attenuation of EMT, and Downregulation of PI3K/Akt Pathway. Phytomedicine 2024, 135, 156218. [Google Scholar] [CrossRef]
- Kryczka, J.; Kryczka, J.; Czarnecka-Chrebelska, K.H.; Brzeziańska-Lasota, E. Molecular Mechanisms of Chemoresistance Induced by Cisplatin in NSCLC Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 8885. [Google Scholar] [CrossRef] [PubMed]
- Fadejeva, I.; Olschewski, H.; Hrzenjak, A. MicroRNAs as Regulators of Cisplatin-resistance in Non-small Cell Lung Carcinomas. Oncotarget 2017, 8, 115754–115773. [Google Scholar] [CrossRef]
- Tsvetkova, E.; Goss, G.D. Drug Resistance and Its Significance for Treatment Decisions in Non-small-cell Lung Cancer. Curr. Oncol. 2012, 19, S45–S51. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput.-Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Huang, S.; Cai, S.; Ling, L.; Zhang, W.; Xiao, H.; Yu, D.; Zhong, X.; Tao, P.; Luo, Y. Investigating the Molecular Mechanism of Traditional Chinese Medicine for the Treatment of Placental Syndromes by Influencing Inflammatory Cytokines Using Mendelian Randomization and Molecular Docking Technology. Front. Endocrinol. 2024, 14, 1290766. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A Web Server for Cancer and Normal Gene Expression Profiling and Interactive Analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
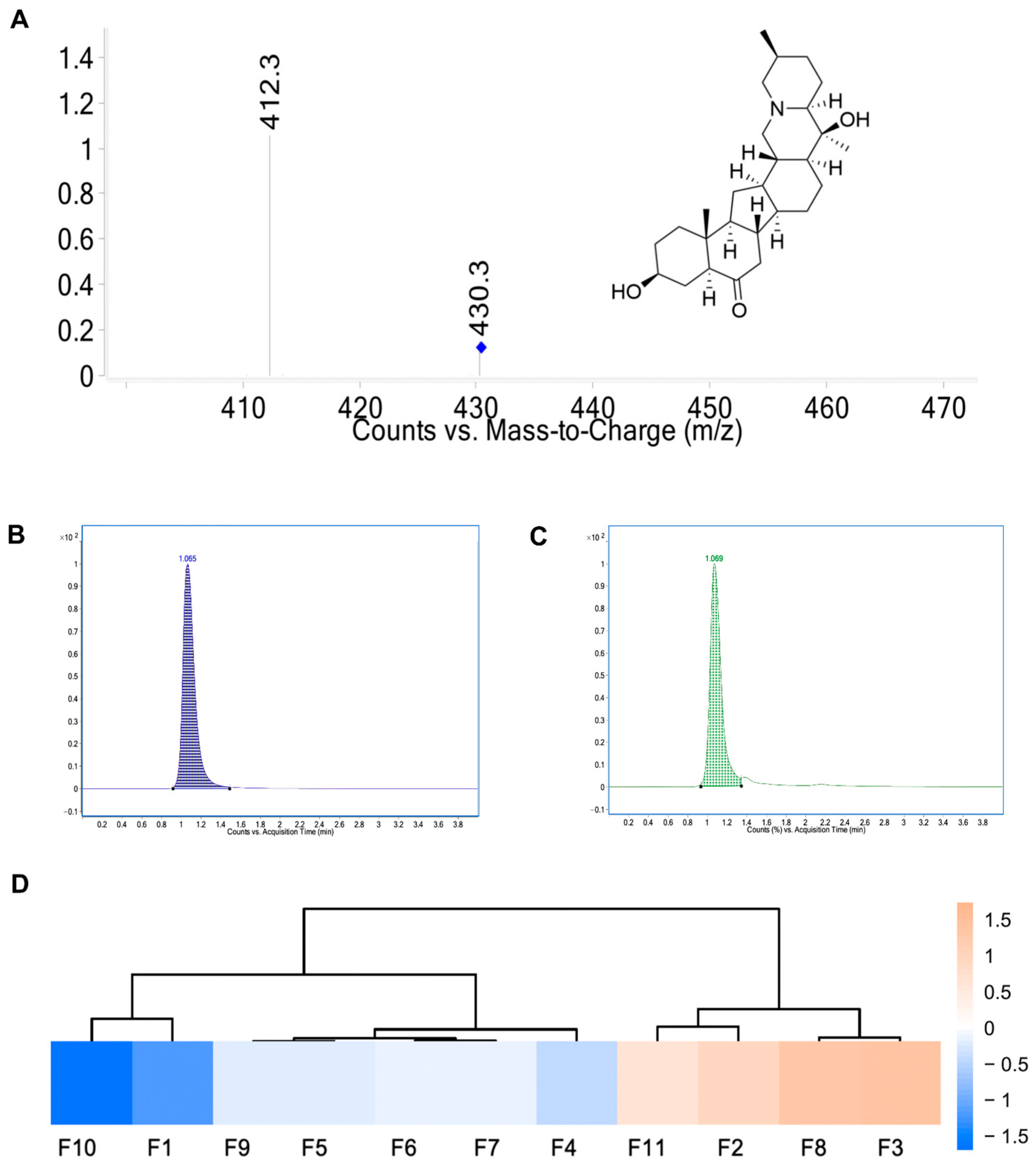

| Source | Content (µg) | |
|---|---|---|
| F1 | Tieli, Heilongjiang Province | 457.08 |
| F2 | Tanglinnongchang, Heilongjiang Province | 1023.88 |
| F3 | Mudanjiang, Heilongjiang Province | 1134.64 |
| F4 | Yanshou, Heilongjiang Province | 656.24 |
| F5 | Jilin Province | 711.88 |
| F6 | Hebei Province | 726.96 |
| F7 | Liaoning Province | 730.08 |
| F8 | Changbaishan, Jilin Province | 1110.72 |
| F9 | Shanghai | 711.88 |
| F10 | Hubei Province | 316.68 |
| F11 | Sichuan Province | 933.92 |
| Target | Uniprot ID | PDB ID | Binding Energy (kcal/mol) |
|---|---|---|---|
| PIK3CG | P48736 | 1E8Y | −10.1 |
| SRC | P12931 | 1FMK | −10.1 |
| JAK3 | P52333 | 5LWM | −9.9 |
| AKT3 | Q9Y243 | 2X18 | −9.8 |
| MDM2 | Q00987 | 5C5A | −9.8 |
| IGF1R | P08069 | 1P4O | −9.5 |
| MET | P08581 | 4R1V | −9.4 |
| CCNA2 | P20248 | 4EOJ | −9.3 |
| SIRT1 | Q96EB6 | 4KXQ | −9.3 |
| IGF1 | P05019 | 1TGR | −9.2 |
| PIK3R1 | P42336 | 7JIU | −9.2 |
| RARA | P10276 | 1DSZ | −9.0 |
| JAK2 | O60674 | 7LL4 | −9.0 |
| RARB | P10826 | 4DM6 | −9.0 |
| STAT3 | P40763 | 6NJS | −9.0 |
| PTPN11 | Q06124 | 3ZM1 | −8.9 |
| ERBB4 | Q15303 | 2R4B | −8.8 |
| KDM1A | O60341 | 7JK7 | −8.8 |
| MAPK14 | Q16539 | 3LFF | −8.8 |
| CDK4 | P11802 | 6P8E | −8.6 |
| AKT2 | P31751 | 1O6L | −8.4 |
| CDK2 | P24941 | 1GZ8 | −8.4 |
| MAP2K1 | Q02750 | 3EQC | −8.4 |
| CDK1 | P06493 | 6GU2 | −8.3 |
| EGFR | P00533 | 5UG9 | −8.3 |
| HDAC1 | Q13547 | 4BKX | −8.2 |
| HSP90AA1 | P07900 | 5J2X | −7.9 |
| Primer Name | Sequences (5’ to 3’) |
|---|---|
| Human actin F | TCCTTCCTGGGCATGGAGT |
| Human actin R | AGCACTGTGTTGGCGTACAG |
| Human PI3K F | GGTTTGGCCTGCTTTTGGAG |
| Human PI3K R | CCATTGCCTCGACTTGCCTA |
| Human AKT F | TCTATGGCGCTGAGATTGTG |
| Human AKT R | CTTAATGTGCCCGTCCTTGT |
| Human BCL2F | GAACTGGGGGAGGATTGTGG |
| Human BCL2 R | CATCCCAGCCTCCGTTATCC |
| Human BAX F | TCAGGATGCGTCCACCAAGAAG |
| Human BAX R | TGTGTCCACGGCGGCAATCATC |
| Human PTEN F | TAGAGGAGCCGTCAAATC |
| Human PTEN R | ATCAGAGTCAGTGGTGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Syed Faizan Ali, S.; Huang, X.; Wei, L.; Zhong, Y.; Shi, X.; Wu, X.; Gan, C.; Wang, Z.; Yang, C. A Multi-Level Study on the Anti-Lung Cancer Mechanism of Peiminine, a Key Component of Fritillaria ussuriensis Maxim.: Integrating Quality Analysis, Network Pharmacology, Bioinformatics Analysis, and Experimental Validation. Int. J. Mol. Sci. 2025, 26, 3506. https://doi.org/10.3390/ijms26083506
Yang Z, Syed Faizan Ali S, Huang X, Wei L, Zhong Y, Shi X, Wu X, Gan C, Wang Z, Yang C. A Multi-Level Study on the Anti-Lung Cancer Mechanism of Peiminine, a Key Component of Fritillaria ussuriensis Maxim.: Integrating Quality Analysis, Network Pharmacology, Bioinformatics Analysis, and Experimental Validation. International Journal of Molecular Sciences. 2025; 26(8):3506. https://doi.org/10.3390/ijms26083506
Chicago/Turabian StyleYang, Ziwen, Shah Syed Faizan Ali, Xinhui Huang, Lin Wei, Yinze Zhong, Xuepeng Shi, Xiaotian Wu, Chunli Gan, Zhibin Wang, and Chunjuan Yang. 2025. "A Multi-Level Study on the Anti-Lung Cancer Mechanism of Peiminine, a Key Component of Fritillaria ussuriensis Maxim.: Integrating Quality Analysis, Network Pharmacology, Bioinformatics Analysis, and Experimental Validation" International Journal of Molecular Sciences 26, no. 8: 3506. https://doi.org/10.3390/ijms26083506
APA StyleYang, Z., Syed Faizan Ali, S., Huang, X., Wei, L., Zhong, Y., Shi, X., Wu, X., Gan, C., Wang, Z., & Yang, C. (2025). A Multi-Level Study on the Anti-Lung Cancer Mechanism of Peiminine, a Key Component of Fritillaria ussuriensis Maxim.: Integrating Quality Analysis, Network Pharmacology, Bioinformatics Analysis, and Experimental Validation. International Journal of Molecular Sciences, 26(8), 3506. https://doi.org/10.3390/ijms26083506






