The Role of Temperature and Subphase Components in Shaping Selected Physicochemical Properties of the Phosphatidylinositol Monolayer
Abstract
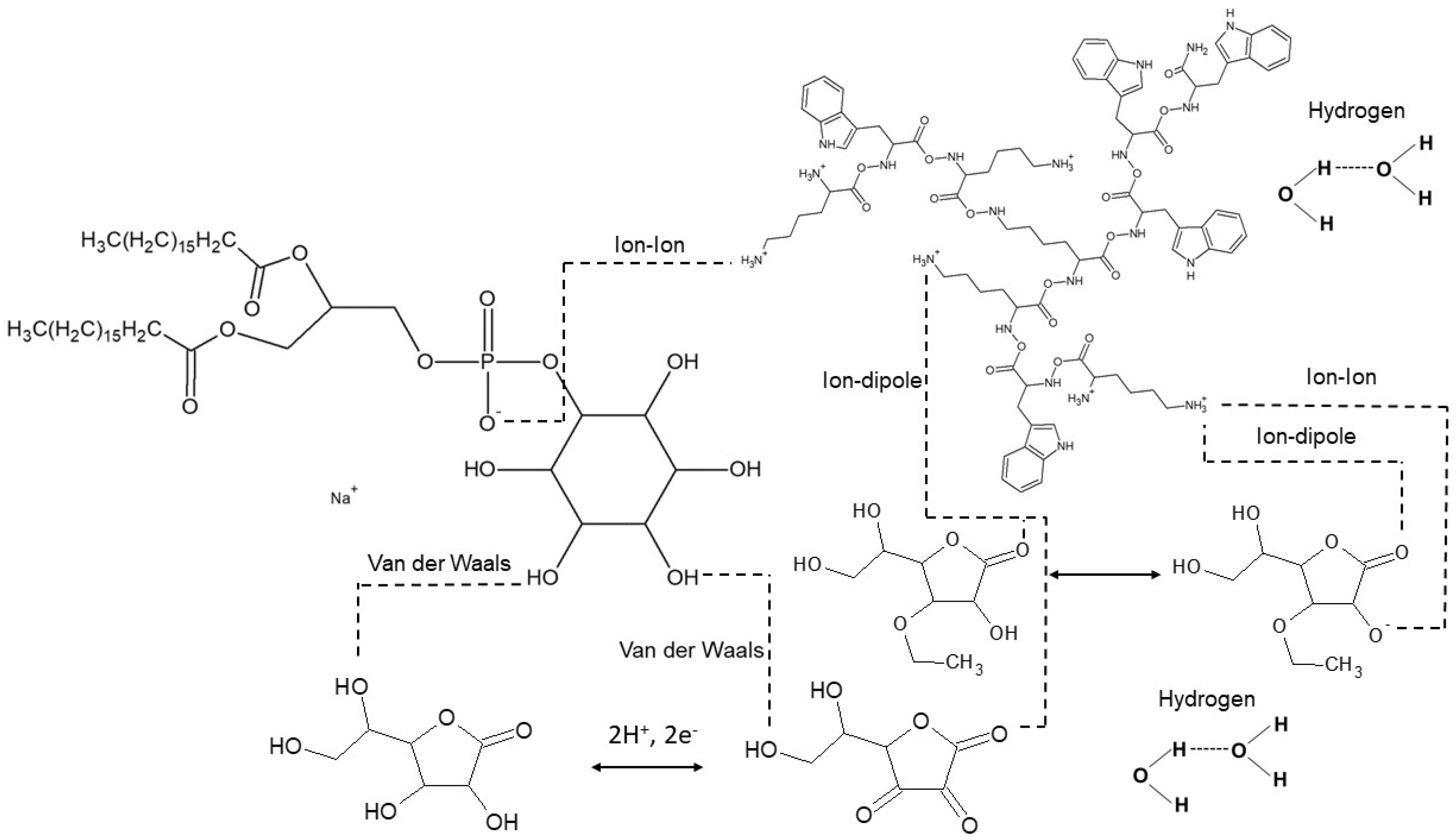
1. Introduction
2. Results and Discussion
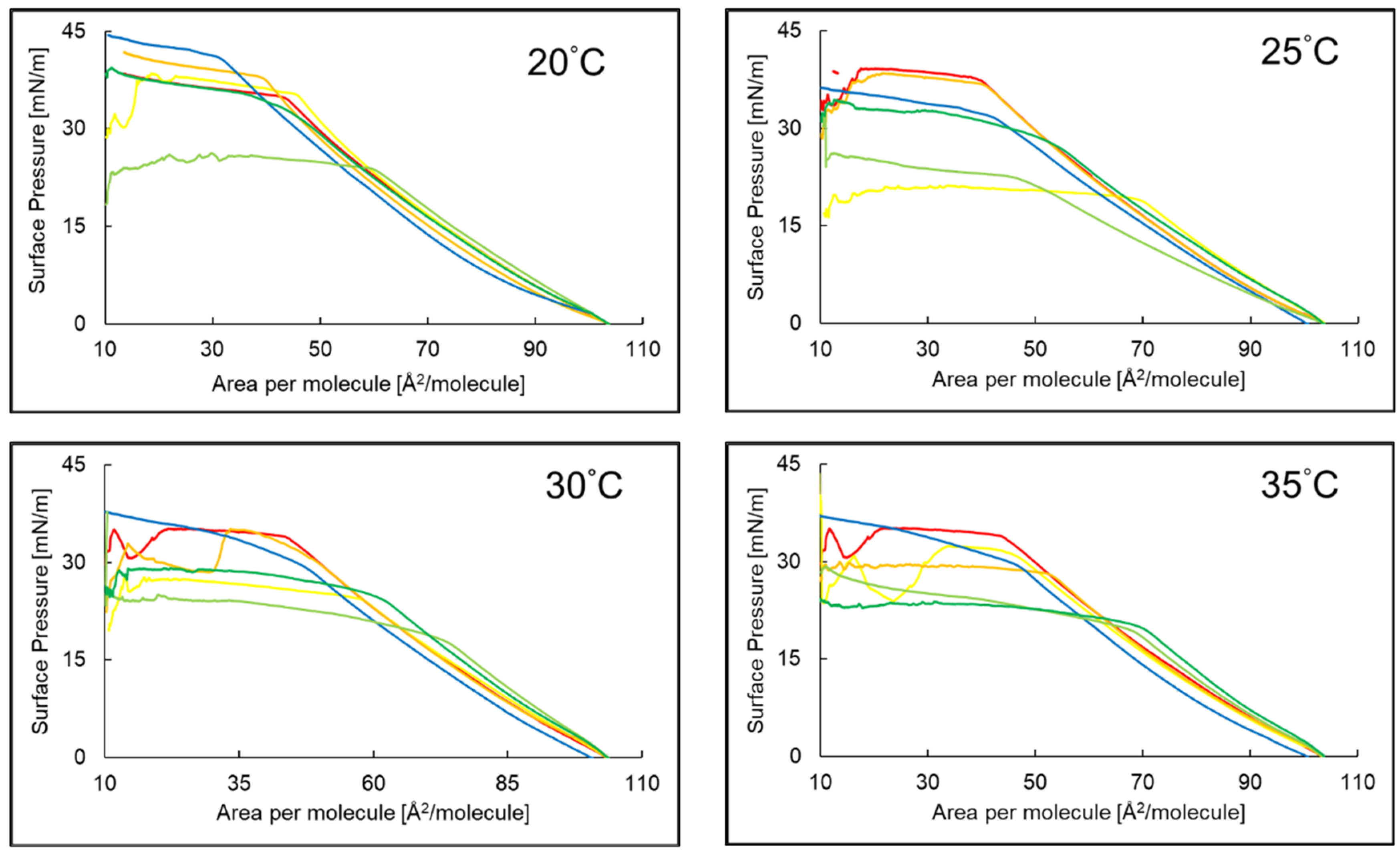
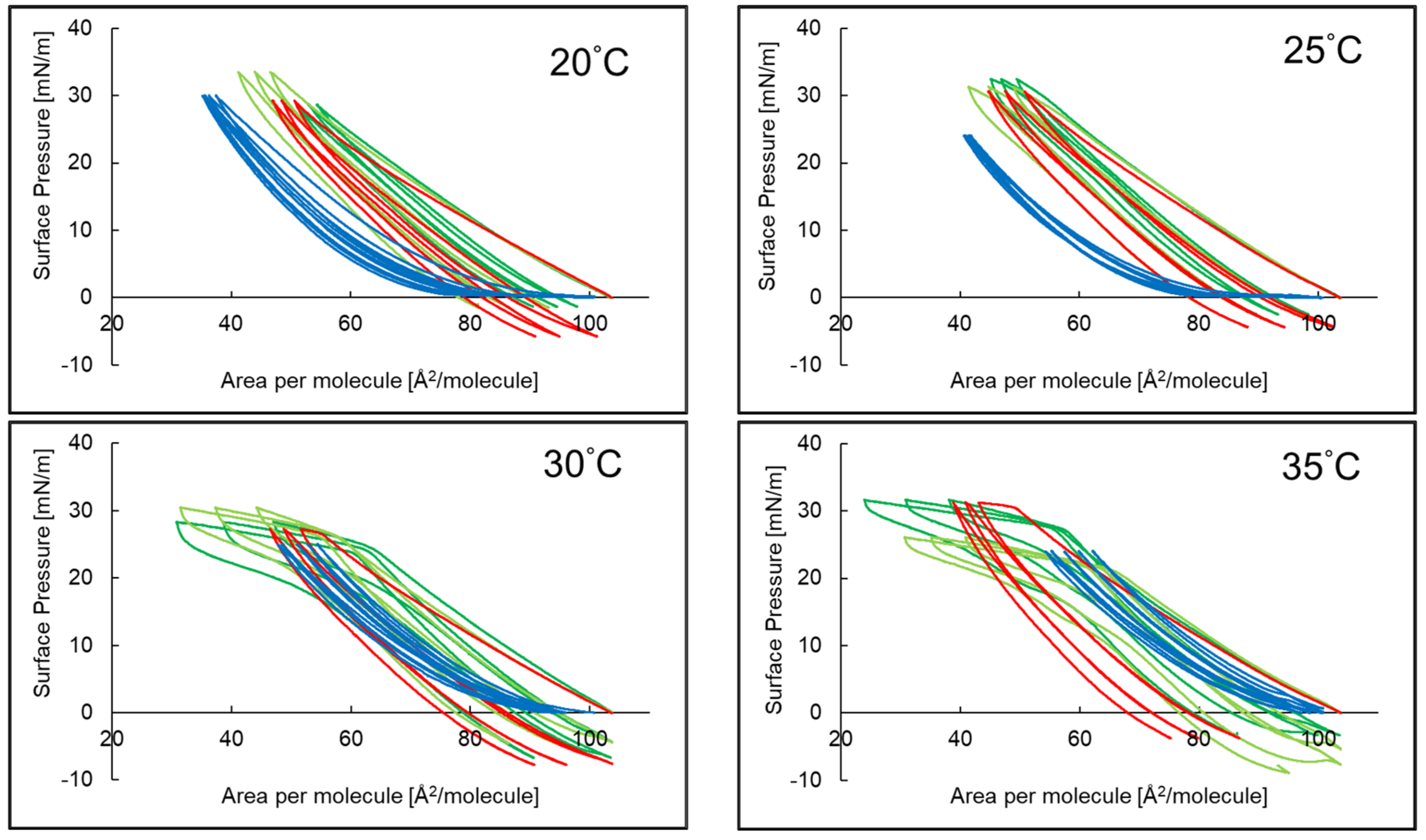
2.1. Compression Isotherms of PI Monolayer Assessed over Aqueous Subphase with Peptide and AA or EAA
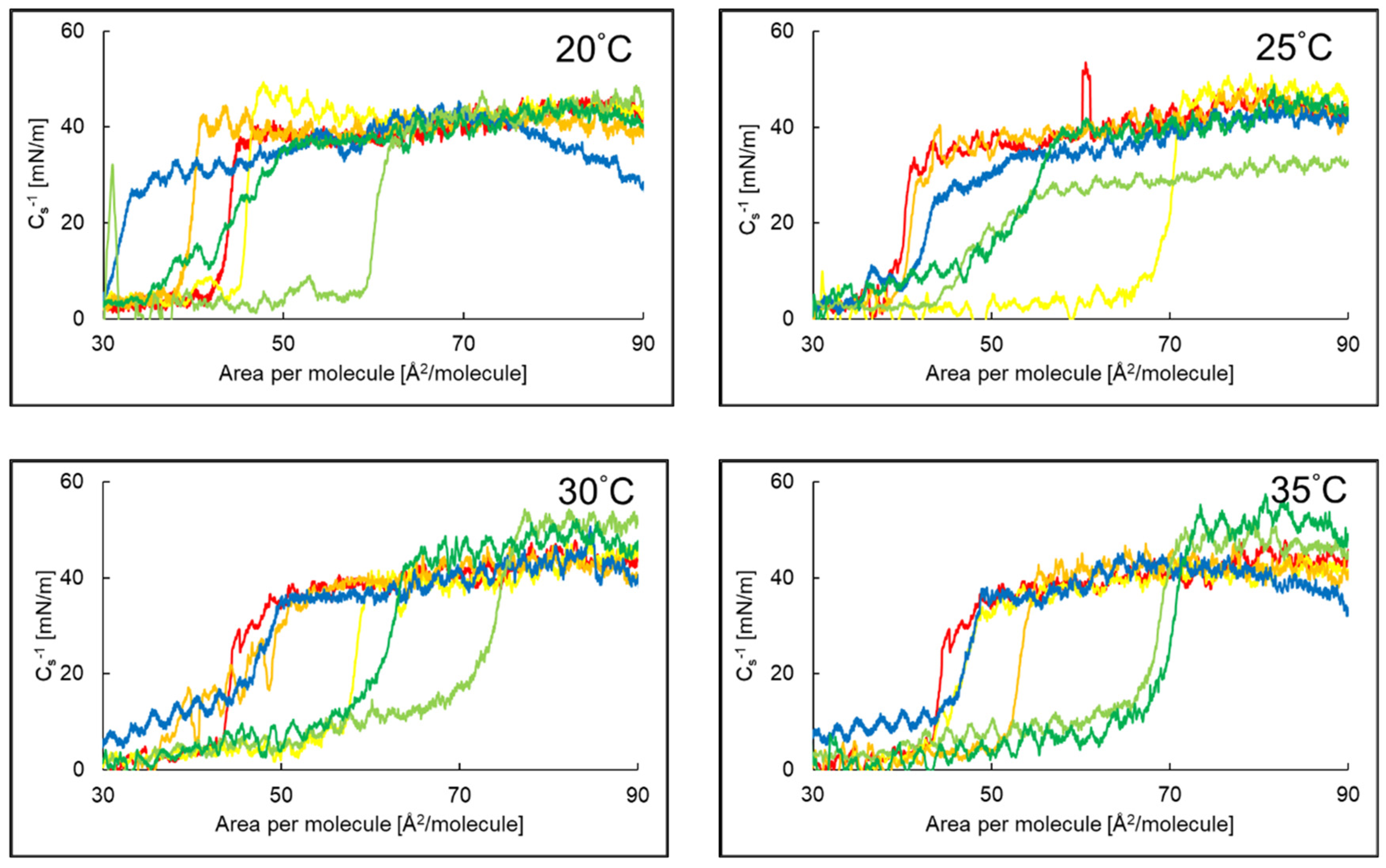
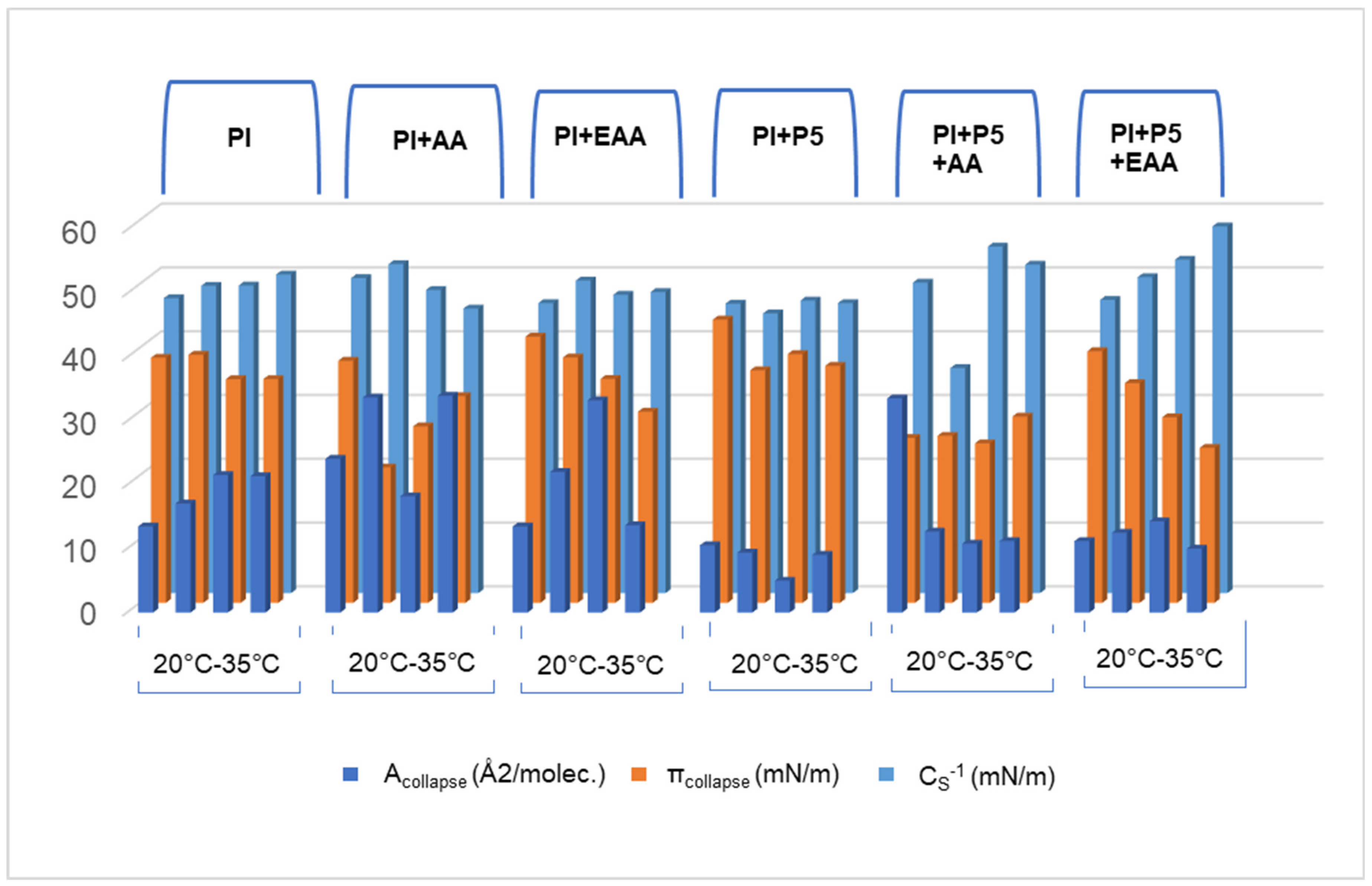
2.2. Compressibility Coefficient of PI Monolayer Assessed over Aqueous Subphase with Peptide, AA or EAA
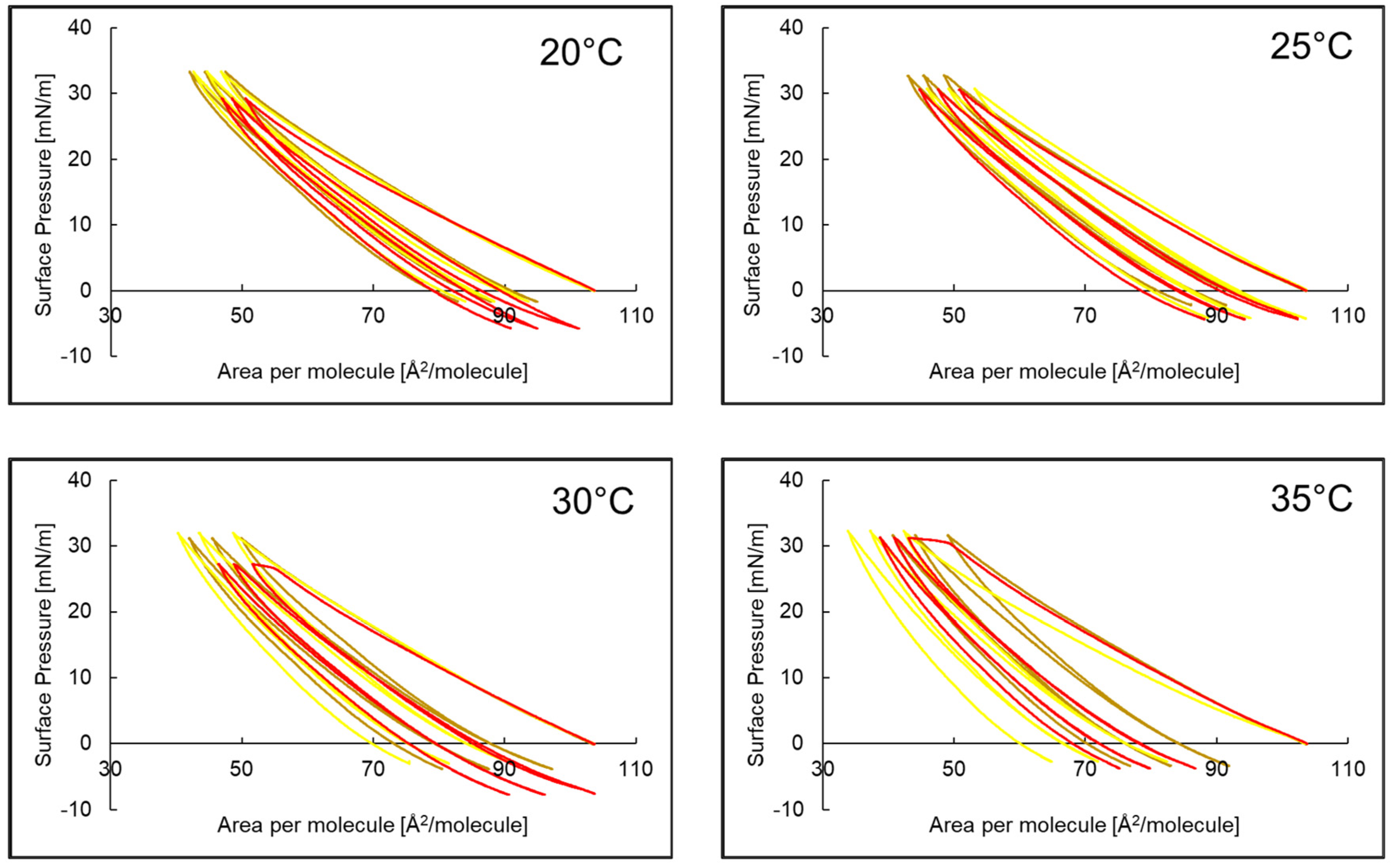
2.3. Compression and Expansion of PI Monolayer Assessed over Aqueous Subphase with Peptide, AA or EAA
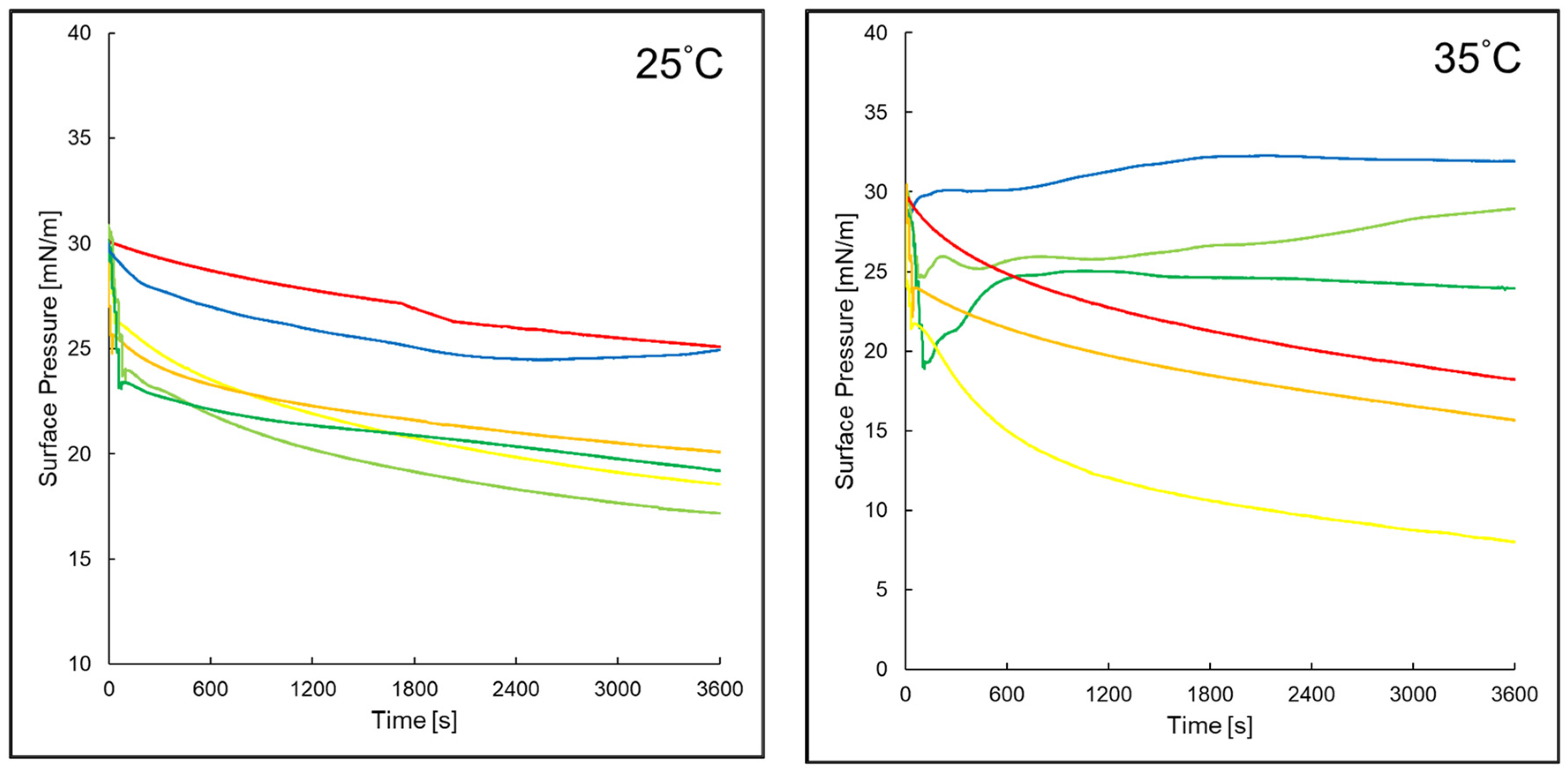
2.4. Surface Pressure of Evaluated Monolayers Versus Time in Assessed Systems
3. Materials and Methods
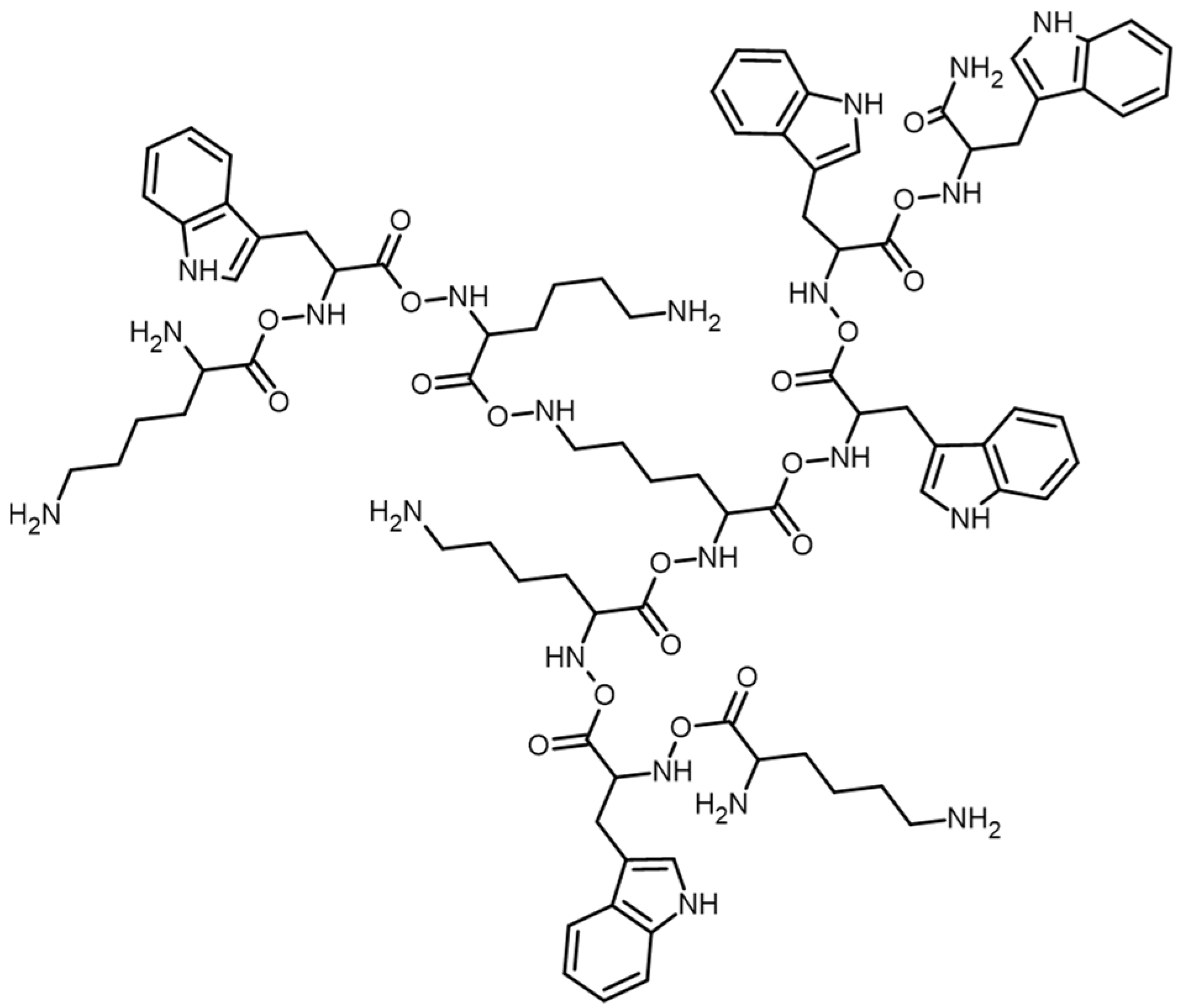
3.1. Synthesis and Characterization of the Peptides
Preparation of the Peptides and Purity and Structure of the Peptides
3.2. Langmuir Films
3.3. Compression Isotherms of Phosphatidylinositol on the Aqueous Subphase with P5 and AA or EAA
3.4. Hysteresis
3.5. Compressibility Coefficient of the Monolayer
3.6. Surface Pressure Changes over Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bataille, V.; Snieder, H.; MacGregor, A.J.; Sasieni, P.; Spector, T.D. The influence of genetics and environmental factors in the pathogenesis of acne: A twin study of acne in women. J. Investig. Dermatol. 2002, 119, 1317–1322. [Google Scholar] [PubMed]
- Cibula, D.; Hill, M.; Vohradnikova, O.; Kuzel, D.; Fanta, M.; Zivny, J. The role of androgens in determining acne severity in adult women. Br. J. Dermatol. 2000, 143, 399–404. [Google Scholar] [PubMed]
- Zouboulis, C.C. Acne and sebaceous gland function. Clin. Dermatol. 2004, 22, 360–366. [Google Scholar]
- Draelos, Z.D.; DiNardo, J.C. A re-evaluation of the comedogenicity concept. J. Am. Acad. Dermatol. 2006, 54, 507–512. [Google Scholar]
- Dessinioti, C.; Katsambas, A.D. The role of Propionibacterium acnes in acne pathogenesis: Facts and controversies. Clin. Dermatol. 2010, 28, 2–7. [Google Scholar]
- Bowe, W.P.; Joshi, S.S.; Shalita, A.R. Diet and acne. J. Am. Acad. Dermatol. 2010, 63, 124–141. [Google Scholar] [PubMed]
- Jović, A.; Marinović, B.; Kostović, K.; Čeović, R.; Basta-Juzbašić, A.; Bukvić Mokos, Z. The Impact of Pyschological Stress on Acne. Acta Dermatovenerol. Croat. 2017, 25, 1133–1141. [Google Scholar] [PubMed]
- Krutmann, J.; Moyal, D.; Liu, W.; Kandahari, S.; Lee, G.S.; Nopadon, N.; Xiang, L.F.; Seité, S. Pollution and acne: Is there a link? Clin. Cosmet. Investig. Dermatol. 2017, 10, 199–204. [Google Scholar]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.; Yang, H.; Sun, L. Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Updates 2023, 68, 100954. [Google Scholar] [CrossRef]
- Koo, H.B.; Seo, J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Li, J.; Koh, J.J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane Active Antimicrobial Peptides: Translating Mechanistic Insights to Design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef]
- Strøm, M.B.; Haug, B.E.; Skar, M.L.; Stensen, W.; Stiberg, T.; Svendsen, J.S. The pharmacophore of short cationic antibacterial peptides. J. Med. Chem. 2003, 46, 1567–1570. [Google Scholar] [CrossRef]
- Dathe, M.; Nikolenko, H.; Meyer, J.; Beyermann, M.; Bienert, M. Optimization of the antimicrobial activity of magainin peptides by modification of charge. FEBS Lett. 2001, 501, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Nannette, Y.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Yin, L.M.; Edwards, M.A.; Li, J.; Yip, C.M.; Deber, C.M. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. J. Biol. Chem. 2012, 287, 7738–7745. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.W.; Wei, S.Y.; Wang, S.H.; Wei, H.M.; Wang, Y.J.; Wang, C.-F.; Chen, C.; Liao, Y.-D. Hydrophobic residues are critical for the helix-forming, hemolytic and bactericidal activities of amphipathic antimicrobial peptide TP4. PLoS ONE 2017, 12, e0186442. [Google Scholar] [CrossRef] [PubMed]
- Wolkenstein, P.; Machovcová, A.; Szepietowski, J.C.; Tennstedt, D.; Veraldi, S.; Delarue, A. Acne prevalence and associations with lifestyle: A cross-sectional online survey of adolescents/young adults in 7 European countries. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 298–306. [Google Scholar] [CrossRef]
- Mazurkiewicz-Pisarek, A.; Baran, J.; Ciach, T. Antimicrobial Peptides: Challenging Journey to the Pharmaceutical, Biomedical, and Cosmeceutical Use. Int. J. Mol. Sci. 2023, 24, 9031. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Quan, Z.; Xiao, P.; Duan, J.A. New Insights into Antioxidant Peptides: An Overview of Efficient Screening, Evaluation Models, Molecular Mechanisms, and Applications. Antioxidants 2024, 13, 203. [Google Scholar] [CrossRef]
- Golonka, I.; Greber, K.E.; Oleksy-Wawrzyniak, M.; Paleczny, J.; Dryś, A.; Junka, A.; Sawicki, W.; Musiał, W. Antimicrobial and Antioxidative Activity of Newly Synthesized Peptides Absorbed into Bacterial Cellulose Carrier against Acne vulgaris. Int. J. Mol. Sci. 2021, 22, 7466. [Google Scholar] [CrossRef]
- Kramer, A.; Eberlein, T.; Müller, G.; Dissemond, J.; Assadian, O. Re-evaluation of polihexanide use in wound antisepsis in order to clarify ambiguities of two animal studies. J. Wound Care 2019, 28, 246–255. [Google Scholar] [PubMed]
- Hübner, N.-O.; Siebert, J.; Kramer, A. Octenidine Dihydrochloride, a Modern Antiseptic for Skin, Mucous Membranes and Wounds. Ski. Pharmacol. Physiol. 2010, 23, 244–258. [Google Scholar]
- Boraldi, F.; Lofaro, F.D.; Bonacorsi, S.; Mazzilli, A.; Garcia-Fernandez, M.; Quaglino, D. The Role of Fibroblasts in Skin Homeostasis and Repair. Biomedicines 2024, 12, 1586. [Google Scholar] [CrossRef]
- Golonka, I.; Pucułek, J.E.; Greber, K.E.; Dryś, A.; Sawicki, W.; Musiał, W. Evaluation of the Effect of Antibacterial Peptides on Model Monolayers. Int. J. Mol. Sci. 2023, 24, 14861. [Google Scholar] [CrossRef] [PubMed]
- Metreveli, N.O.; Jariashvili, K.K.; Namicheishvili, L.O.; Svintradze, D.V.; Chikvaidze, E.N.; Sionkowska, A.; Skopinska, J. UV-vis and FT-IR spectra of ultraviolet irradiated collagen in the presence of antioxidant ascorbic acid. Ecotoxicol. Environ. Saf. 2010, 73, 448–455. [Google Scholar] [CrossRef]
- Jeon, J.; Park, S.C.; Her, J.; Lee, J.W.; Han, J.-K.; Kim, Y.-K.; Kim, K.P.; Ban, C. Comparative Lipidomic Profiling of the HumanCommensal Bacterium Propionibacterium acnes and Its Extracellular Vesicles. RSC Adv. 2018, 8, 15241–15247. [Google Scholar]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic Acid in Skin Health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef]
- Telang, P.S. Vitamin C in dermatology. Indian Dermatol. Online J. 2013, 4, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Lee, Y.I.; Jang, S.; Song, S.Y.; Lee, W.J.; Lee, J.H. Particulate matter-induced atmospheric skin aging is aggravated by UVA and inhibited by a topical L-ascorbic acid compound. Photodermatol. Photoimmunol. Photomed. 2022, 38, 123–131. [Google Scholar] [PubMed]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013, 2013, 930164. [Google Scholar]
- Pinnell, S.R.; Yang, H.; Omar, M.; Monteiro-Riviere, N.; DeBuys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical L-ascorbic acid: Percutaneous absorption studies. Dermatol. Surg. 2001, 27, 137–142. [Google Scholar] [PubMed]
- Iliopoulos, F.; Sil, B.C.; Moore Robert, D.J.; Lucas, A.; Lane, M.E. 3-O-ethyl-l-ascorbic acid: Characterisation and investigation of single solvent systems for delivery to the skin. Int. J. Pharm. X 2019, 1, 100025. [Google Scholar] [PubMed]
- Liu, Y.; Wang, L.; Liu, J.; Di, Y. A study of human skin and surface degrees in stable and unstable thermal environments. J. Therm. Biol. 2013, 38, 440–448. [Google Scholar]
- Lee, C.M.; Jin, S.P.; Doh, E.J.; Lee, D.H.; Chung, J.H. Regional Variation of Human Skin Surface Temperature. Ann. Dermatol. 2019, 31, 349–352. [Google Scholar]
- Tian, X.; Yu, J.; Liu, W. Facial skin temperature and its relationship with overall thermal sensation in winter in Changsha, China. Indoor Air 2022, 32, e13138. [Google Scholar]
- Golonka, I.; Łukasiewicz, I.W.; Sebastiańczyk, A.; Greber, K.E.; Sawicki, W.; Musiał, W. The Influence of the Amphiphilic Properties of Peptides on the Phosphatidylinositol Monolayer in the Presence of Ascorbic Acid. Int. J. Mol. Sci. 2024, 25, 12484. [Google Scholar] [CrossRef]
- Davies, J.T.; Rideal, E.K. Interfacial Phenomena; Academic Press: New York, NY, USA, 1963. [Google Scholar]
- Alkhawaja, E.; Hammadi, S.; Abdelmalek, M.; Mahasneh, N.; Alkhawaja, B.; Abdelmalek, S.M. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: A cross sectional study. BMC Dermatol. 2020, 20, 17. [Google Scholar]
- McLaughlin, J.; Watterson, S.; Layton, A.M.; Bjourson, A.J.; Barnard, E.; McDowell, A. Propionibacterium acnes and Acne Vulgaris: New Insights from the Integration of Population Genetic, Multi-Omic, Biochemical and Host-Microbe Studies. Microorganisms 2019, 7, 128. [Google Scholar] [CrossRef]
- Martinez-Seara, H.; Róg, T.; Pasenkiewicz-Gierula, M.; Vattulainen, I.; Karttunen, M.; Reigada, R. Effect of double bond position on lipid bilayer properties: Insight through atomistic simulations. J. Phys. Chem. B 2007, 111, 11162–11168. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, S.; Kurisu, S.; Takenawa, T. Dynamic shaping of cellular membranes by phospholipids and membrane-deforming proteins. Physiol. Rev. 2014, 94, 1219–1248. [Google Scholar] [CrossRef]
- Golonka, I.; Greber, K.E.; Szyja, B.M.; Petrus, P.P.; Pucułek, J.E.; Musiał, W. Effect of Newly Synthesized Structures of Peptides on the Stability of the Monolayers Formed. Int. J. Mol. Sci. 2023, 24, 4318. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.E.; Caseli, L. Understanding the collapse mechanism in Langmuir monolayers through polarization modulation-infrared reflection absorption spectroscopy. Langmuir 2013, 29, 9063–9071. [Google Scholar] [CrossRef]
- Partridge, M.; Wong, R.; Collins, M.; James, S.W.; Davis, F.; Tatam, R.P.; Séamus, P.J. Higson, Modifying monolayer behaviour by incorporating subphase additives and improving Langmuir–Blodgett thin film deposition on optical fibres. Mater. Chem. Phys. 2014, 144, 179–185. [Google Scholar] [CrossRef][Green Version]
- Wydro, P.; Krajewska, B.; Hac-Wydro, K. Chitosan as a Lipid Binder: A Langmuir Monolayer Study of Chitosan—Lipid Interactions. Biomacromolecules 2007, 8, 2611–2617. [Google Scholar] [CrossRef]
- Ahmed, I.; Dildar, L.; Haque, A.; Patra, P.; Mukhopadhyay, M.; Hazra, S.; Kulkarni, M.; Thomas, S.; Plaisier, J.R.; Dutta, S.B.; et al. Chitosan-Fatty Acid Interaction Mediated Growth of Langmuir Monolayer and Langmuir-Blodgett Films. J. Colloid Interface Sci. 2018, 514, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Shalmashi, A.; Eliassi, A. Solubility of L-(+)-Ascorbic Acid in Water, Ethanol, Methanol, Propan-2-Ol, Acetone, Acetonitrile, Ethyl Acetate, and Tetrahydrofuran from (293 to 323) K. J. Chem. Eng. Data 2008, 53, 1332–1334. [Google Scholar] [CrossRef]
- Nath, J.; Nath, R.K.; Chakraborty, A.; Husain, S.A. Monolayer characteristics of chitosan assembled in Langmuir films mixed with arachidic acid. Surf. Rev. Lett. 2014, 21, 1450049. [Google Scholar] [CrossRef]
- Banerjee, R.; Sanyal, M.K.; Bera, M.K.; Gibaud, A.; Lin, B.; Meron, M. Reversible monolayer-to-crystalline phase transition in amphiphilic silsesquioxane at the air-water interface. Sci. Rep. 2015, 5, 8497. [Google Scholar] [CrossRef]
- Sudheesh, S.; Ahmad, J. Effect of Wilhelmy Plate Material on Hysteresis of Langmuir Film Isotherms. Asian J. Chem. 2013, 25, 3535–3538. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Yokoi, N.; Ivanova, S.; Krastev, R.; Lalchev, Z. Surface chemistry study of the interactions of pharmaceutical ingredients with human meibum films. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4605–4615. [Google Scholar] [CrossRef] [PubMed]
| Evaluated Systems | Monolayer | Subphase | ||
|---|---|---|---|---|
| PI | AA | EAA | P5 | |
| PI * | 1.20 × 1016 | − | − | − |
| PI + AA * | 2.30 × 1016 | − | − | |
| PI + EAA * | − | 2.30 × 1016 | ||
| PI + P5 | − | − | 2.30 × 1016 | |
| PI + P5 + AA | 2.30 × 1016 | − | 2.30 × 1016 | |
| PI + P5 + EAA | − | 2.30 × 1016 | 2.30 × 1016 | |
| Evaluated System | Temperature (°C) | Alift-off (Å2/molec.) | Acollapse (Å2/molec.) | πcollapse (mN/m) | χ | aLE/LC |
|---|---|---|---|---|---|---|
| PI | 20 | 101.35 | 13.50 | 38.43 | 0.13 | −0.571 |
| 25 | 101.27 | 17.09 | 38.89 | 0.17 | −0.594 | |
| 30 | 101.30 | 21.56 | 35.06 | 0.21 | −0.573 | |
| 35 | 101.26 | 21.38 | 35.08 | 0.21 | −0.572 | |
| PI + AA | 20 | 101.53 | 24.11 | 37.96 | 0.24 | −0.588 |
| 25 | 102.80 | 33.73 | 21.23 | 0.33 | −0.552 | |
| 30 | 101.54 | 18.23 | 27.67 | 0.18 | −0.529 | |
| 35 | 101.54 | 34.00 | 32.41 | 0.33 | −0.547 | |
| PI + EAA | 20 | 100.72 | 13.50 | 41.74 | 0.13 | −0.581 |
| 25 | 101.10 | 22.05 | 38.46 | 0.22 | −0.586 | |
| 30 | 101.86 | 33.27 | 35.11 | 0.33 | −0.558 | |
| 35 | 101.60 | 13.68 | 29.97 | 0.13 | −0.547 | |
| PI + P5 | 20 | 100.72 | 10.55 | 44.41 | 0.10 | −0.588 |
| 25 | 98.34 | 9.41 | 36.46 | 0.10 | −0.549 | |
| 30 | 98.13 | 5.00 | 39.01 | 0.05 | −0.559 | |
| 35 | 97.92 | 9.06 | 37.15 | 0.09 | −0.553 | |
| PI + P5 + AA | 20 | 101.70 | 33.58 | 25.85 | 0.33 | −0.545 |
| 25 | 100.53 | 12.70 | 26.19 | 0.13 | −0.403 | |
| 30 | 102.36 | 10.79 | 25.01 | 0.11 | −0.569 | |
| 35 | 101.83 | 11.21 | 29.21 | 0.11 | −0.540 | |
| PI + P5 + EAA | 20 | 101.76 | 11.21 | 39.45 | 0.11 | −0.559 |
| 25 | 102.06 | 12.51 | 34.45 | 0.12 | −0.546 | |
| 30 | 102.12 | 14.31 | 29.09 | 0.14 | −0.578 | |
| 35 | 102.40 | 10.02 | 24.29 | 0.10 | −0.575 |
| Evaluated Systems | 20 °C | 25 °C | 30 °C | 35 °C |
|---|---|---|---|---|
| PI * | 46.23 | 48.18 | 48.22 | 49.95 |
| PI + AA * | 49.39 | 51.58 | 47.53 | 44.60 |
| PI + EAA * | 45.47 | 49.01 | 46.77 | 47.21 |
| PI + P5 | 45.39 | 43.85 | 45.87 | 45.47 |
| PI + P5 + AA | 48.68 | 35.28 | 54.32 | 51.50 |
| PI + P5 + EAA | 45.97 | 49.53 | 52.28 | 57.49 |
| Temperature | Rv (%) Parameter for the Evaluated Systems | ||||||
|---|---|---|---|---|---|---|---|
| PI | PI + AA | PI + EAA | PI + P5 | PI + P5 + AA | PI + P5 + EAA | ||
| 20 °C | loop 1 | 70.77 | 70.13 | 72.84 | 80.59 | 68.75 | 77.24 |
| loop 2 | 73.06 | 81.89 | 80.04 | 88.67 | 78.96 | 86.20 | |
| loop 3 | 72.66 | 83.64 | 82.32 | 96.43 | 80.76 | 85.04 | |
| 25 °C | loop 1 | 74.54 | 73.30 | 72.63 | 87.34 | 71.75 | 73.51 |
| loop 2 | 77.57 | 77.64 | 80.30 | 90.32 | 78.46 | 79.72 | |
| loop 3 | 79.03 | 79.60 | 81.95 | 87.11 | 77.11 | 81.36 | |
| 30 °C | loop 1 | 86.81 | 59.59 | 67.03 | 83.47 | 72.62 | 73.35 |
| loop 2 | 77.23 | 73.46 | 76.80 | 97.06 | 71.13 | 77.70 | |
| loop 3 | 80.80 | 79.49 | 80.61 | 95.94 | 73.20 | 86.87 | |
| 35 °C | loop 1 | 66.43 | 51.74 | 60.53 | 87.34 | 69.49 | 77.60 |
| loop 2 | 79.77 | 69.83 | 74.97 | 93.87 | 68.66 | 75.34 | |
| loop 3 | 86.83 | 74.38 | 78.56 | 97.77 | 68.60 | 70.59 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golonka, I.; Łukasiewicz, I.W.; Sebastiańczyk, A.; Greber, K.E.; Sawicki, W.; Musiał, W. The Role of Temperature and Subphase Components in Shaping Selected Physicochemical Properties of the Phosphatidylinositol Monolayer. Int. J. Mol. Sci. 2025, 26, 3472. https://doi.org/10.3390/ijms26083472
Golonka I, Łukasiewicz IW, Sebastiańczyk A, Greber KE, Sawicki W, Musiał W. The Role of Temperature and Subphase Components in Shaping Selected Physicochemical Properties of the Phosphatidylinositol Monolayer. International Journal of Molecular Sciences. 2025; 26(8):3472. https://doi.org/10.3390/ijms26083472
Chicago/Turabian StyleGolonka, Iwona, Izabela W. Łukasiewicz, Aleksandra Sebastiańczyk, Katarzyna E. Greber, Wiesław Sawicki, and Witold Musiał. 2025. "The Role of Temperature and Subphase Components in Shaping Selected Physicochemical Properties of the Phosphatidylinositol Monolayer" International Journal of Molecular Sciences 26, no. 8: 3472. https://doi.org/10.3390/ijms26083472
APA StyleGolonka, I., Łukasiewicz, I. W., Sebastiańczyk, A., Greber, K. E., Sawicki, W., & Musiał, W. (2025). The Role of Temperature and Subphase Components in Shaping Selected Physicochemical Properties of the Phosphatidylinositol Monolayer. International Journal of Molecular Sciences, 26(8), 3472. https://doi.org/10.3390/ijms26083472








