Multi-Target Protective Effects of Sanghuangporus sanghuang Against 5-Fluorouracil-Induced Intestinal Injury Through Suppression of Inflammation, Oxidative Stress, Epitheli-Al-Mesenchymal Transition, and Tight Junction
Abstract
1. Introduction
2. Results
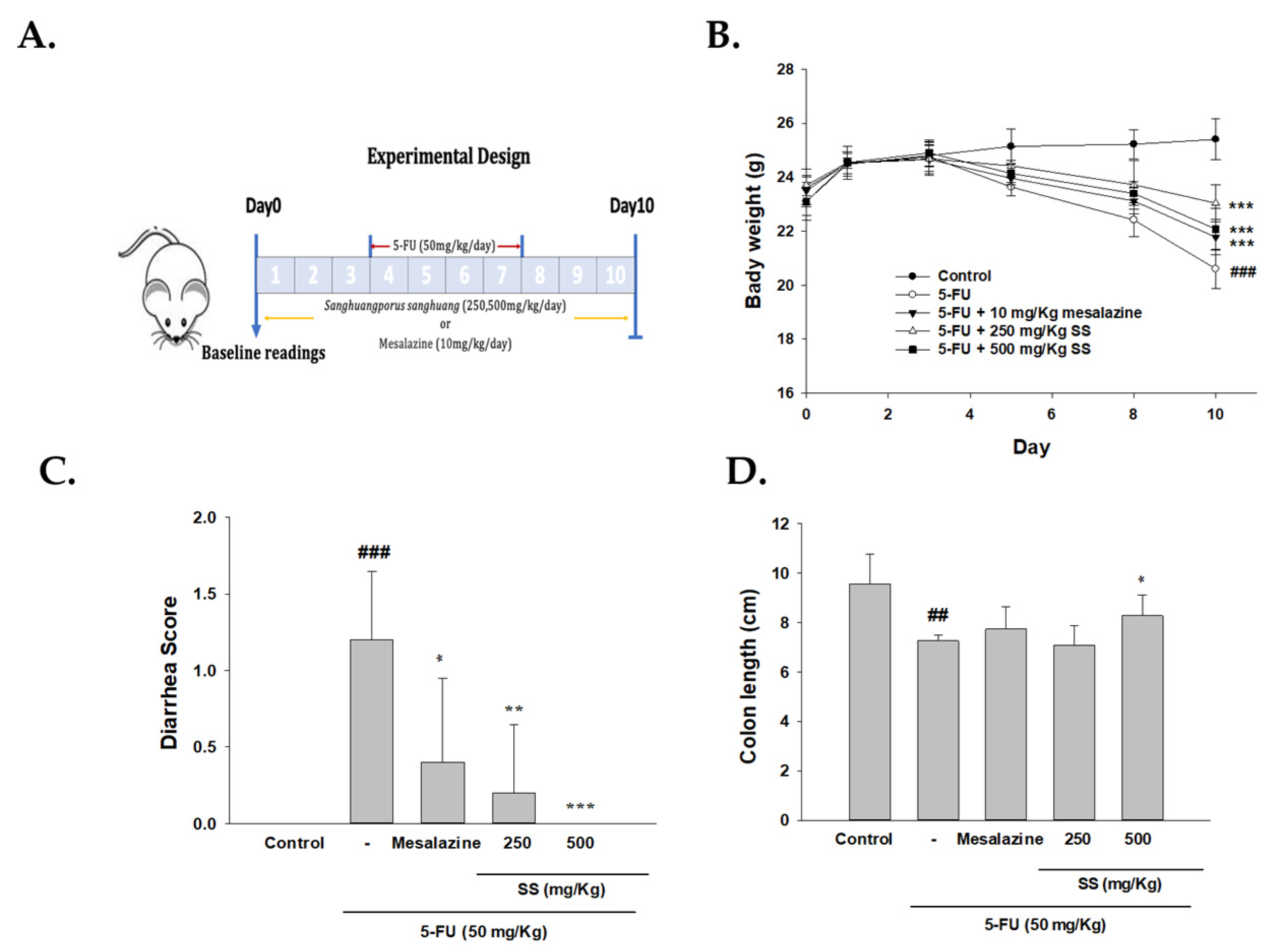
2.1. The Role of SS in Modulating Physical Symptoms, Including Weight Loss, Diarrhea, and Intestinal Length, Was Assessed in the 5-FU-Induced Intestinal Mucositis Model
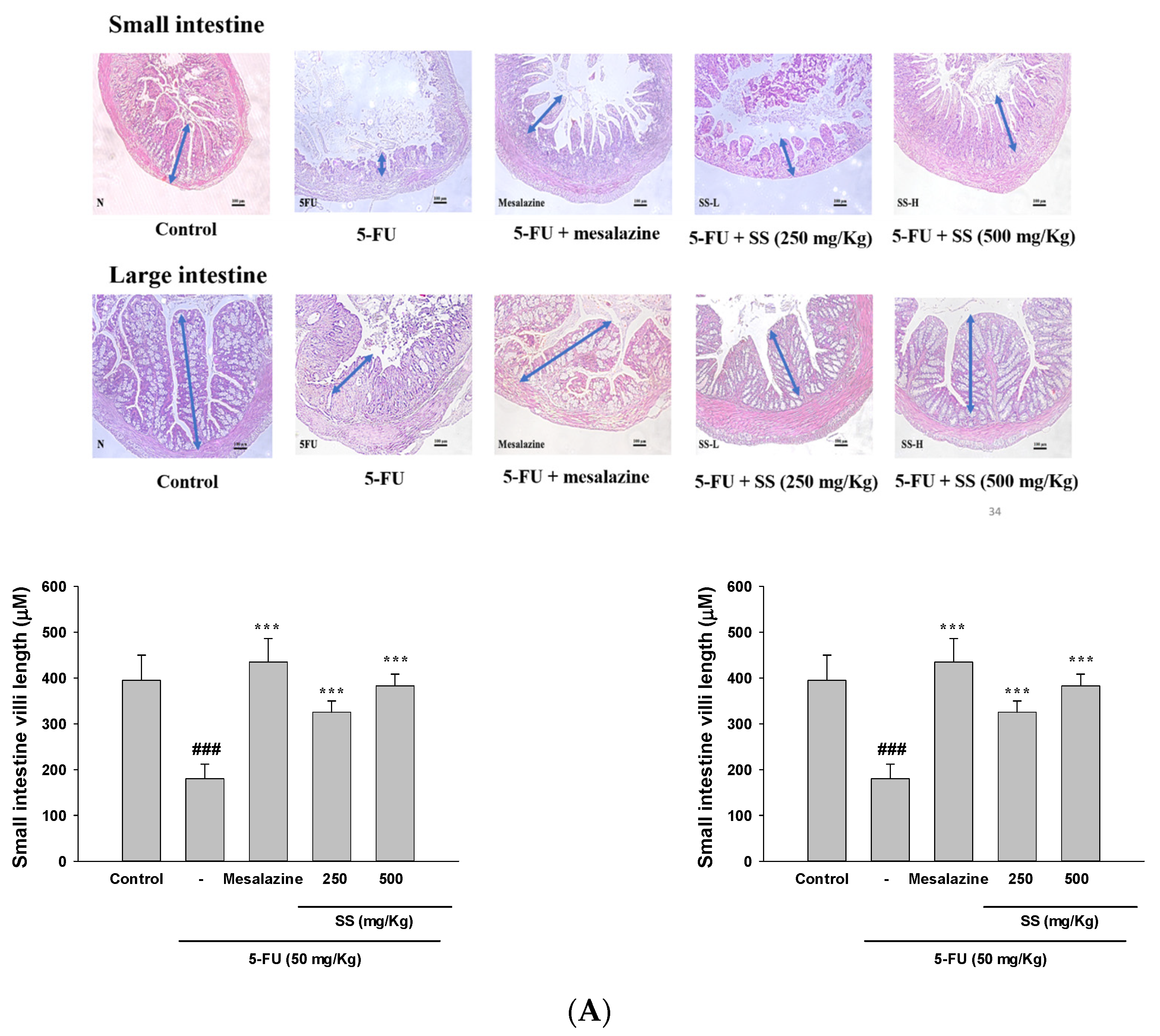
2.2. Effect of SS on 5-Fu-Induced Intestinal Histopathological Changes
2.3. Elevated Ki-67 Levels in the SS-Treated Group Indicated Improved Cellular Proliferation in the Mucositis Model
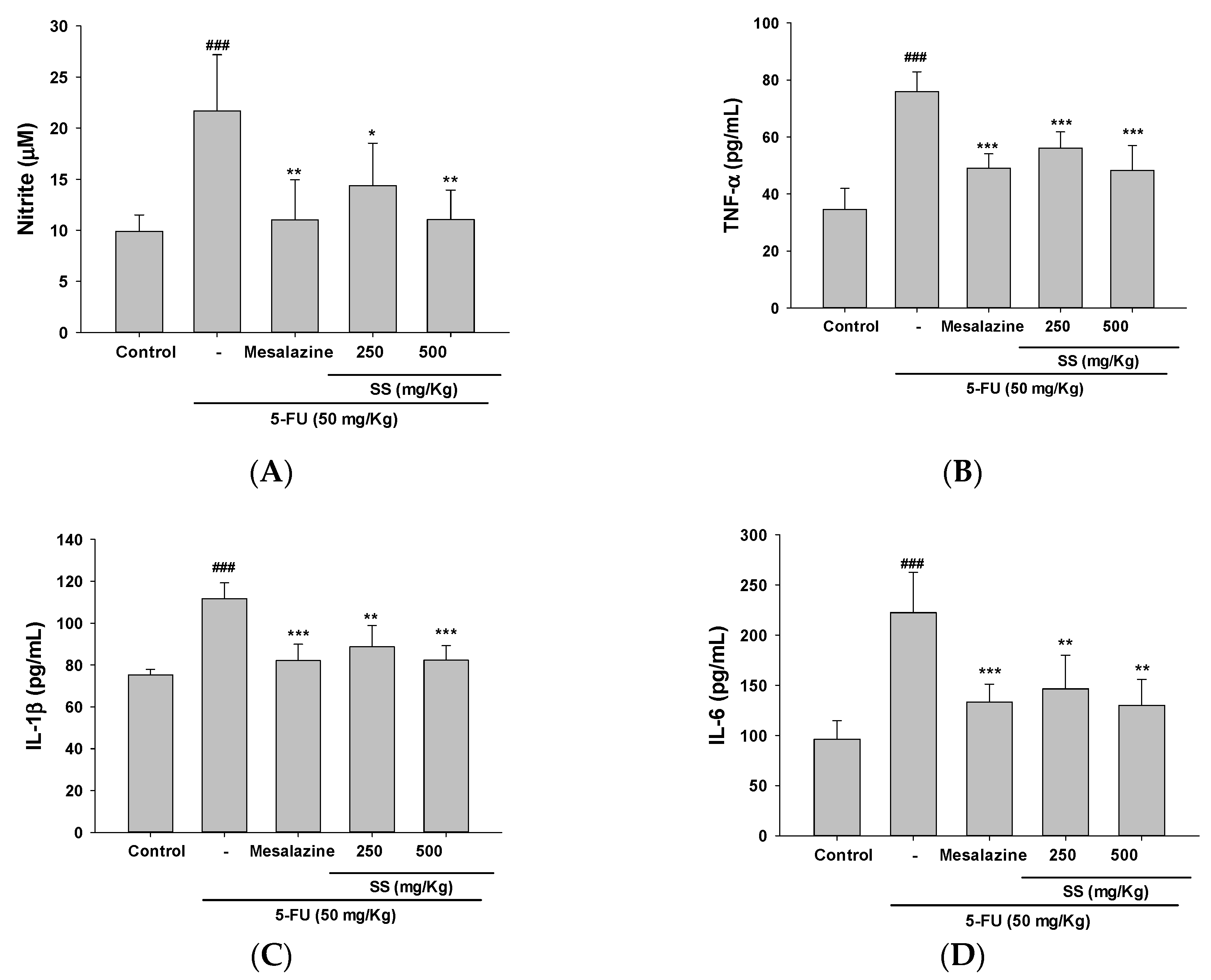
2.4. The Administration of SS Inhibited the Expression of Pro-Inflammatory Cytokines Triggered by 5-FU
2.5. SS Alleviates Oxidative Stress in the 5-FU-Induced Intestinal Mucositis
2.6. SS Mitigated Inflammation, NF-κB, and the MAPK Signaling Pathway in the 5-FU-Induced Intestinal Mucositis
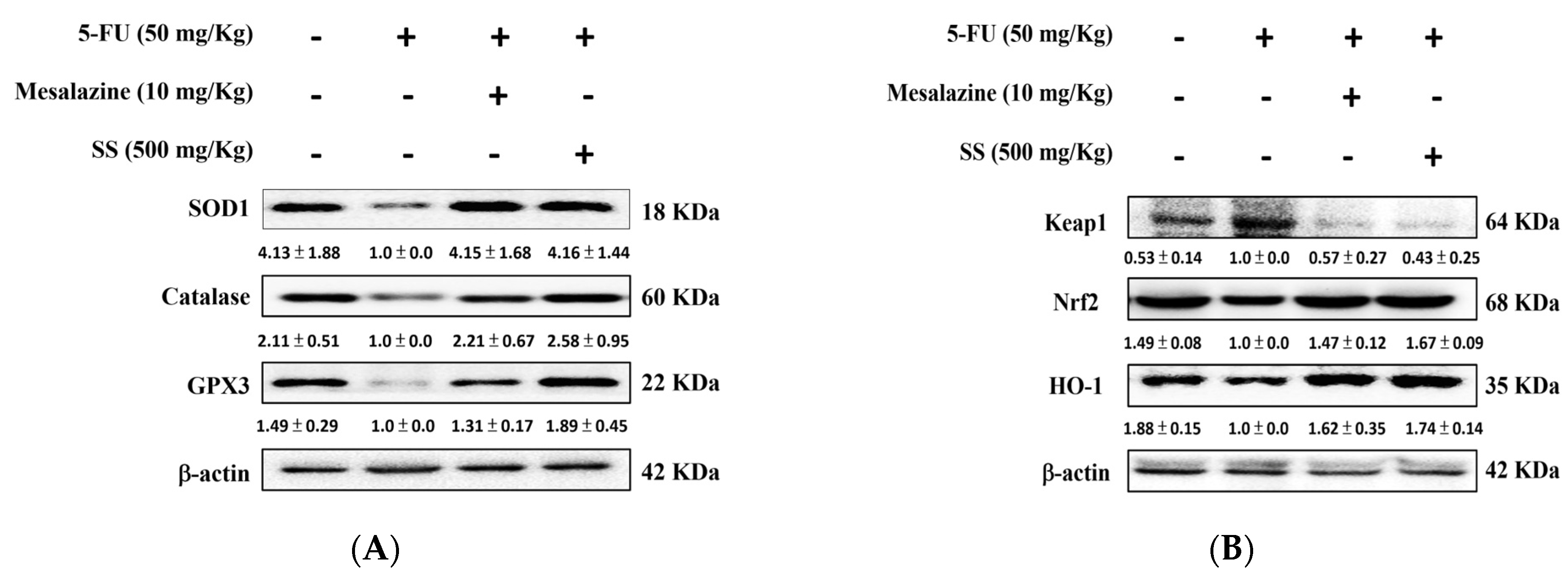
2.7. SS Administration in the 5-FU-Induced Mucositis Model Improves Intestinal Antioxidant Defenses and Promotes the Activation of the HO-1/Nrf2 Signaling Pathway
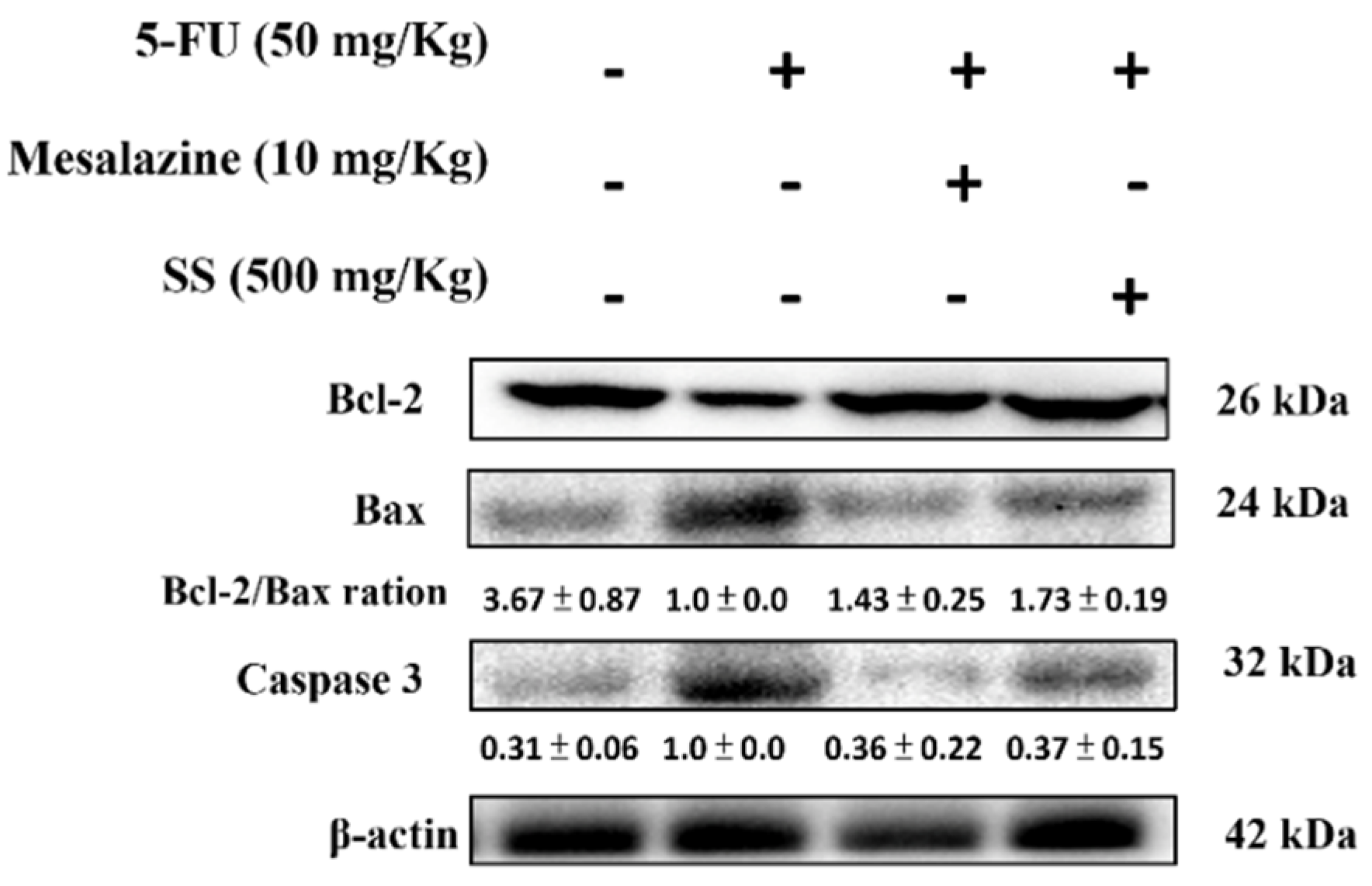
2.8. SS Mitigates the Apoptosis Signaling Pathway Induced by 5-FU
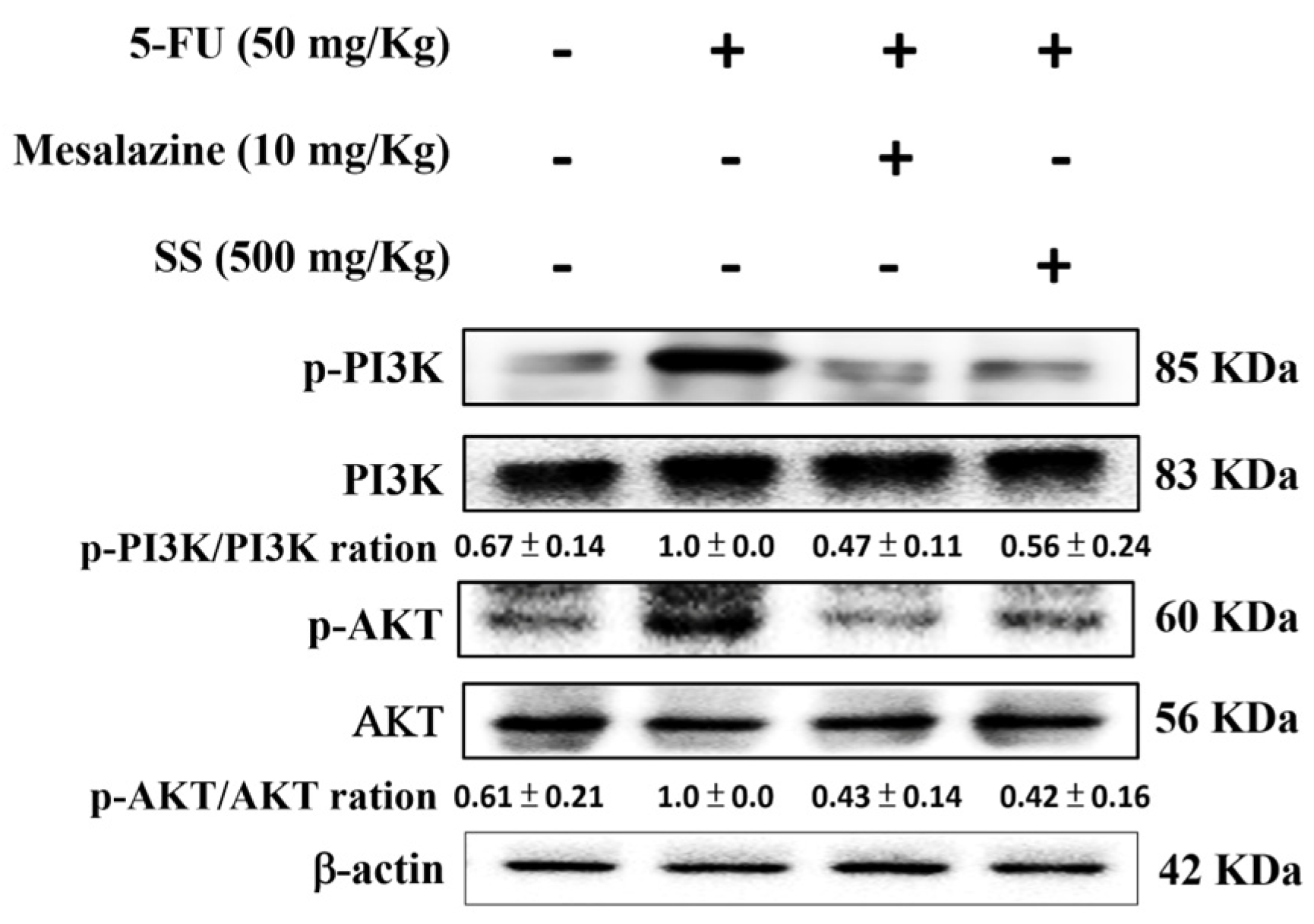
2.9. SS Attenuates the 5-FU-Induced Regulation of the PI3K-AKT Signaling Pathway
2.10. SS Alleviates the 5FU-Induced Epithelial–Mesenchymal Transition (EMT) Pathways
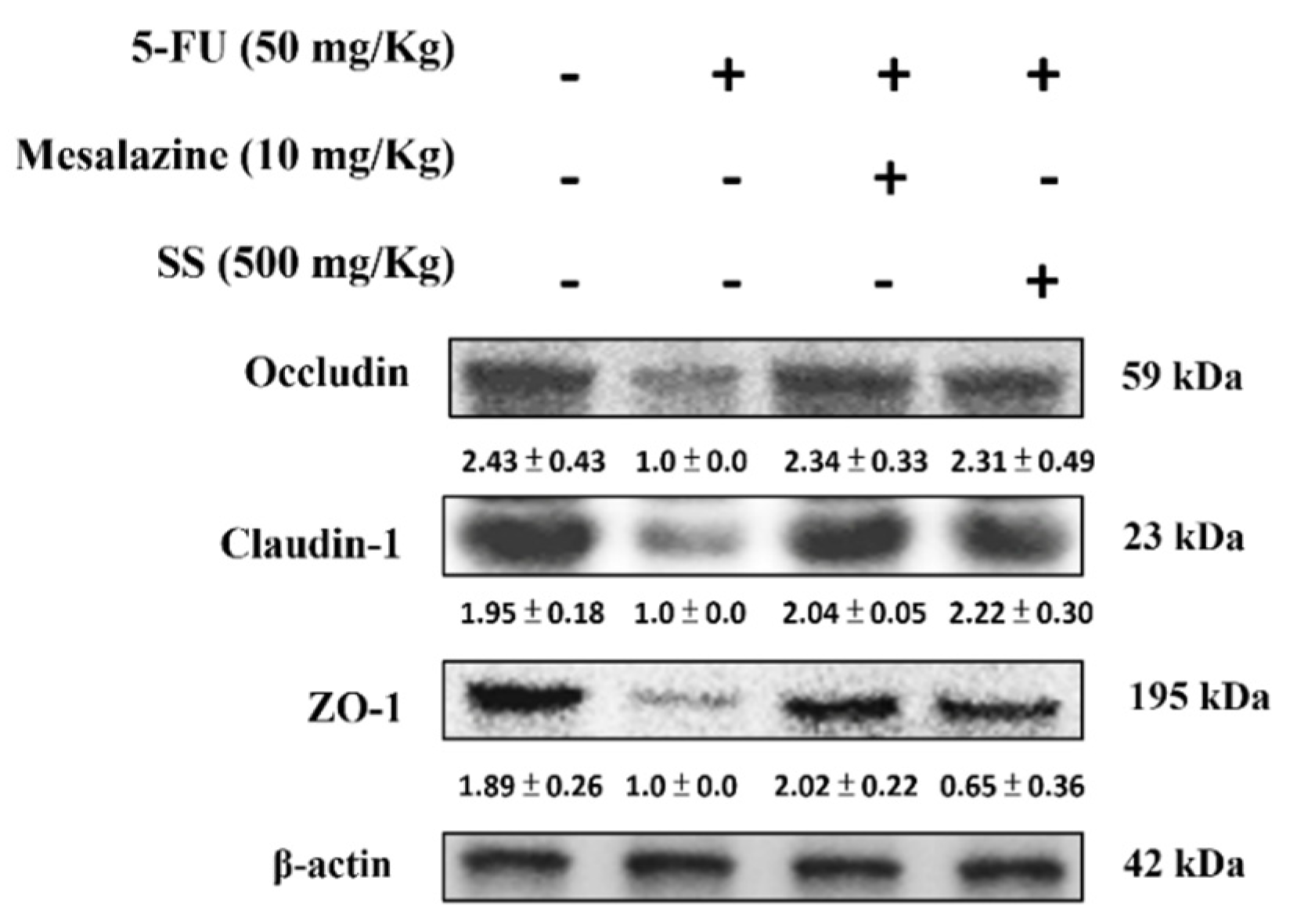
2.11. SS Alleviates the 5-FU-Induced Tight Junction Protein Expressions
3. Discussion
4. Materials and Methods
4.1. Preparation of Samples
4.2. Reagents
4.3. Animals
4.4. Research Design
4.5. Daily Weight and Diarrhea Monitoring
4.6. Histological Examination
4.7. Cytokine Assay
4.8. Nitrite Assay
4.9. The TBARS (Thiobarbituric Acid Reactive Substance) Assay
4.10. Glutathione (GSH) Assay
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef]
- Alcorta, A.; López-Gómez, L.; Capasso, R.; Abalo, R. Vitamins and fatty acids against chemotherapy-induced intestinal mucositis. Pharmacol. Ther. 2024, 261, 108689. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A.; Kokkali, S.; Digklia, A. Treatment of metastatic biliary cancers with irinotecan and 5-Fluorouracil based chemotherapy after platinum/gemcitabine progression: A systematic review and meta-analysis. Clin. Color. Cancer 2024, 23, 318–325.e1. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.M.; Ballalai, P.L.; de Lima, P.P.; Santo, R.M. Efficacy and safety of topical 0.5% 5-Fluorouracil as primary treatment of ocular surface squamous neoplasia. Can. J. Ophthalmol. 2024, 59, e510–e514. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, L.; Alcorta, A.; Abalo, R. Probiotics and probiotic-like agents against chemotherapy-induced intestinal mucositis: A narrative review. J. Pers. Med. 2023, 13, 1487. [Google Scholar] [CrossRef]
- Wei, L.; Wen, X.S.; Xian, C.J. Chemotherapy-induced intestinal microbiota dysbiosis impairs mucosal homeostasis by modulating toll-like receptor signaling pathways. Int. J. Mol. Sci. 2021, 22, 9474. [Google Scholar] [CrossRef]
- Chukwunyere, U.; Mercan, M.; Sehirli, A.O.; Abacioglu, N. Possible cytoprotective mechanisms of oxytocin against 5-fluorouracil-induced gastrointestinal mucositis. Mol. Biol. Rep. 2022, 49, 4055–4059. [Google Scholar] [CrossRef]
- Alan, N.; Oran, N.T.; Yılmaz, P.A.; Çelik, A.; Yılmaz, O. Fig seed oil improves intestinal damage caused by 5-FU-induced mucositis in rats. Food Sci. Nutr. 2024, 12, 6461–6471. [Google Scholar] [CrossRef]
- Teng, Y.; Li, J.; Guo, J.; Yan, C.; Wang, A.; Xia, X. Alginate oligosaccharide improves 5-fluorouracil-induced intestinal mucositis by enhancing intestinal barrier and modulating intestinal levels of butyrate and isovalerate. Int. J. Biol. Macromol. 2024, 276 Pt 1, 133699. [Google Scholar] [CrossRef]
- Jonan, S.; Haneda, M.; Amagase, K. Orally administrated glutamate restored EAAT1 and 3 expression levels suppressed in 5-fluorouracil-damaged intestinal epithelial cells. Anticancer Res. 2024, 44, 1143–1147. [Google Scholar] [CrossRef]
- Ivanov, S.M.; Zgoda, V.G.; Isakova, V.A.; Trukhanova, L.S.; Poroikov, V.V.; Shtil, A.A. Proteomic analysis identifies multiple mechanisms of 5-fluorouracil-induced gut mucositis in mice. Cancers 2024, 16, 4025. [Google Scholar] [CrossRef] [PubMed]
- Chien, L.H.; Deng, J.S.; Jiang, W.P.; Chou, Y.N.; Lin, J.G.; Huang, G.J. Evaluation of lung protection of Sanghuangporus sanghuang through TLR4/NF-kappaB/MAPK, keap1/Nrf2/HO-1, CaMKK/AMPK/Sirt1, and TGF-beta/SMAD3 signaling pathways mediating apoptosis and autophagy. Biomed. Pharmacother. 2023, 165, 115080. [Google Scholar]
- Jiang, W.P.; Deng, J.S.; Huang, S.S.; Wu, S.H.; Chen, C.C.; Liao, J.C.; Chen, H.Y.; Lin, H.Y.; Huang, G.J. Sanghuangporus sanghuang mycelium prevents paracetamol-induced hepatotoxicity through Regulating the MAPK/NF-kappaB, Keap1/Nrf2/HO-1, TLR4/PI3K/Akt, and CaMKKbeta/LKB1/AMPK pathways and suppressing oxidative stress and inflammation. Antioxidants 2021, 10, 897. [Google Scholar]
- Lin, W.C.; Deng, J.S.; Huang, S.S.; Wu, S.H.; Chen, C.C.; Lin, W.R.; Lin, H.Y.; Huang, G.J. Anti-inflammatory activity of Sanghuangporus sanghuang mycelium. Int. J. Mol. Sci. 2017, 18, 347. [Google Scholar] [CrossRef]
- Chien, L.H.; Deng, J.S.; Jiang, W.P.; Chen, C.C.; Chou, Y.N.; Lin, J.G.; Huang, G.J. Study on the potential of Sanghuangporus sanghuang and its components as COVID-19 spike protein receptor binding domain inhibitors. Biomed. Pharmacother. 2022, 153, 113434. [Google Scholar] [CrossRef] [PubMed]
- Atiq, A.; Shal, B.; Naveed, M.; Khan, A.; Ali, J.; Zeeshan, S.; Al-Sharari, S.D.; Kim, Y.S.; Khan, S. Diadzein ameliorates 5-fluorouracil-induced intestinal mucositis by suppressing oxidative stress and inflammatory mediators in rodents. Eur. J. Pharmacol. 2019, 843, 292–306. [Google Scholar]
- Chien, L.H.; Wu, C.T.; Deng, J.S.; Jiang, W.P.; Huang, W.C.; Huang, G.J. Salvianolic Acid C protects against cisplatin-induced acute kidney injury through attenuation of inflammation, oxidative stress and apoptotic effects and activation of the CaMKK-AMPK-Sirt1-associated signaling pathway in mouse models. Antioxidants 2021, 10, 1620. [Google Scholar] [CrossRef]
- Spencer, N.J. Motility patterns in mouse colon: Gastrointestinal dysfunction induced by anticancer chemotherapy. Neurogastroenterol. Motil. 2016, 28, 1759–1764. [Google Scholar] [PubMed]
- Cheng, M.R.; Li, Q.; Wan, T.; He, B.; Han, J.; Chen, H.X.; Yang, F.X.; Wang, W.; Xu, H.Z.; Ye, T.; et al. Galactosylated chitosan/5-fluorouracil nanoparticles inhibit mouse hepatic cancer growth and its side effects. World J. Gastroenterol. 2012, 18, 6076–6087. [Google Scholar] [CrossRef]
- Al-Hoshary, D.M.; Zalzala, M.H. Mucoprotective effect of ellagic acid in 5 fluorouracil-induced intestinal mucositis model. J. Med. Life 2023, 16, 712–718. [Google Scholar]
- Li, T.; Si, W.; Zhu, J.; Yin, L.; Zhong, C. Emodin reverses 5-Fu resistance in human colorectal cancer via downregulation of PI3K/Akt signaling pathway. Am. J. Transl. Res. 2020, 12, 1851–1861. [Google Scholar] [PubMed]
- Sotos, G.A.; Grogan, L.; Allegra, C.J. Preclinical and clinical aspects of biomodulation of 5-fluorouracil. Cancer Treat. Rev. 1994, 20, 11–49. [Google Scholar] [CrossRef]
- Kreuser, E.D.; Wadler, S.; Thiel, E. Biochemical modulation of cytotoxic drugs by cytokines: Molecular mechanisms in experimental oncology. Recent Results Cancer Res. 1995, 139, 371–382. [Google Scholar] [PubMed]
- Huang, J.; Hwang, A.Y.M.; Jia, Y.; Kim, B.; Iskandar, M.; Mohammed, A.I.; Cirillo, N. Experimental chemotherapy-induced mucositis: A scoping review guiding the design of suitable preclinical models. Int. J. Mol. Sci. 2022, 23, 15434. [Google Scholar] [CrossRef]
- Wang, L.; Song, B.; Hu, Y.; Chen, J.; Zhang, S.; Chen, D.; Wang, J. Puerarin ameliorates 5-Fluorouracil-induced intestinal mucositis in mice by inhibiting JAKs. J. Pharmacol. Exp. Ther. 2021, 379, 147–155. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.H.; Moon, W.; Park, J.; Park, S.J.; Song, G.A.; Han, S.H.; Lee, J.H. Rebamipide attenuates 5-Fluorouracil-induced small intestinal mucositis in a mouse model. Biol. Pharm. Bull. 2015, 38, 179–183. [Google Scholar] [CrossRef]
- Sougiannis, A.T.; VanderVeen, B.N.; Davis, J.M.; Fan, D.; Murphy, E.A. Understanding chemotherapy-induced intestinal mucositis and strategies to improve gut resilience. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G712–G719. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Wardill, H.R.; Bowen, J.M. Role of toll-like receptor 4 (TLR4)-mediated interleukin-6 (IL-6) production in chemotherapy-induced mucositis. Cancer Chemother. Pharmacol. 2018, 82, 31–37. [Google Scholar] [CrossRef]
- Ji, L.; Hao, S.; Wang, J.; Zou, J.; Wang, Y. Roles of toll-like receptors in radiotherapy- and chemotherapy-induced oral mucositis: A concise review. Front. Cell. Infect. Microbiol. 2022, 12, 831387. [Google Scholar] [CrossRef]
- Cario, E. Toll-like receptors in the pathogenesis of chemotherapy-induced gastrointestinal toxicity. Curr. Opin. Support. Palliat. Care 2016, 10, 157–164. [Google Scholar] [CrossRef]
- Sukhotnik, I.; Pollak, Y.; Coran, A.G.; Pilatov, J.; Bejar, J.; Mogilner, J.G.; Berkowitz, D. Glutamine attenuates the inhibitory effect of methotrexate on TLR signaling during intestinal chemotherapy-induced mucositis in a rat. Nutr. Metab. 2014, 11, 17. [Google Scholar]
- Liao, P.L.; Huang, S.H.; Hung, C.H.; Huang, W.K.; Tsai, C.H.; Kang, J.J.; Wang, H.P.; Cheng, Y.W. Efficacy of azatyrosine-phenylbutyric hydroxamides, a histone deacetylase inhibitor, on chemotherapy-induced gastrointestinal mucositis. Int. J. Mol. Sci. 2019, 20, 249. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.F.; Luo, F.L.; Tang, S.S.; Huang, J.W.; Yang, Y.; Wang, S.; Jiang, T.Y.; Man, Q.; Liu, S.; Wu, Y.Y. Network analysis and experimental pharmacology study explore the protective effects of Isoliquiritigenin on 5-fluorouracil-induced intestinal mucositis. Front. Pharmacol. 2022, 3, 1014160. [Google Scholar]
- Lin, C.H.; Jiang, W.P.; Itokazu, N.; Huang, G.J. Chlorogenic acid attenuates 5-fluorouracil-induced intestinal mucositis in mice through SIRT1 signaling-mediated oxidative stress and inflammatory pathways. Biomed. Pharmacother. 2025, 161, 114481. [Google Scholar] [CrossRef]
- Kuo, Y.R.; Lin, C.H.; Lin, W.S.; Pan, M.H. L-Glutamine substantially improves 5-fluorouracil-induced intestinal mucositis by modulating gut microbiota and maintaining the integrity of the gut barrier in mice. Mol. Nutr. Food Res. 2024, 68, e2300704. [Google Scholar]
- Ali, J.; Khan, A.U.; Shah, F.A.; Ali, H.; Islam, S.U.; Kim, Y.S.; Khan, S. Mucoprotective effects of Saikosaponin-A in 5-fluorouracil-induced intestinal mucositis in mice model. Life Sci. 2019, 239, 116888. [Google Scholar]
- Chen, S.; Tamaki, N.; Kudo, Y.; Tsunematsu, T.; Miki, K.; Ishimaru, N.; Ito, H.O. Protective effects of resveratrol against 5-fluorouracil-induced oxidative stress and inflammatory responses in human keratinocytes. J. Clin. Biochem. Nutr. 2021, 69, 238–246. [Google Scholar]
- Koizumi, R.; Azuma, K.; Izawa, H.; Morimoto, M.; Ochi, K.; Tsuka, T.; Imagawa, T.; Osaki, T.; Ito, N.; Okamoto, Y.; et al. Oral administration of surface-deacetylated chitin nanofibers and chitosan inhibit 5-fluorouracil-induced intestinal mucositis in mice. Int. J. Mol. Sci. 2017, 18, 279. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Y.; Peng, J.; Chen, W.; Lisha, L.; Lin, J. Qingjie Fuzheng granule attenuates 5-fluorouracil-induced intestinal mucosal damage. Biomed. Pharmacother. 2019, 118, 109223. [Google Scholar]
- Xu, L.; Zhao, X.; Tang, F.; Zhang, J.; Peng, C.; Ao, H. Ameliorative effect of ginsenoside Rc on 5-Fluorouracil-induced chemotherapeutic intestinal mucositis via the PI3K-AKT/NF-kappaB signaling pathway: In vivo and in vitro evaluations. Int. J. Mol. Sci. 2024, 25, 13085. [Google Scholar]
- Manfioletti, G.; Fedele, M. Epithelial-Mesenchymal Transition (EMT). Int. J. Mol. Sci. 2023, 24, 11386. [Google Scholar] [PubMed]
- Meng, Z.; Yang, T.; Liu, D. Type-2 epithelial-mesenchymal transition in oral mucosal nonneoplastic diseases. Front. Immunol. 2022, 13, 1020768. [Google Scholar]
- Chattopadhyay, I.; Ambati, R.; Gundamaraju, R. Exploring the crosstalk between inflammation and epithelial-mesenchymal transition in cancer. Mediat. Inflamm. 2021, 2021, 9918379. [Google Scholar]
- Saikia, P.J.; Pathak, L.; Mitra, S.; Das, B. The emerging role of oral microbiota in oral cancer initiation, progression and stemness. Front. Immunol. 2023, 14, 1198269. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.W.; Al-Sadi, R.; Ma, T.Y. IL-1beta and the intestinal epithelial tight junction barrier. Front Immunol. 2021, 12, 767456. [Google Scholar]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky gut: Effect of dietary fiber and fats on microbiome and intestinal barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; Aherne, C.M. Adenosine in intestinal epithelial barrier function. Cells 2024, 13, 381. [Google Scholar] [CrossRef]
- Lin, W.C.; Deng, J.S.; Huang, S.S.; Wu, S.H.; Lin, H.Y.; Huang, G.J. Anti-inflammatory activity of Sanghuangporus sanghuang by suppressing TLR4-mediated PI3K/AKT/mTOR/IKKß signaling pathway. RSC Adv. 2017, 7, 21234–21251. [Google Scholar] [CrossRef]
- Lin, W.C.; Deng, J.S.; Huang, S.S.; Wu, S.H.; Lin, H.Y.; Huang, G.J. Evaluation of antioxidant, anti-inflammatory and anti-proliferative activities of ethanol extracts from different varieties of Sanghuang species. RSC Adv. 2017, 7, 7780–7788. [Google Scholar]
- Motamedi, Z.; Amini, S.A.; Raeisi, E.; Lemoigne, Y.; Heidarian, E. Combined effects of protocatechuic acid and 5-Fluorouracil on p53 gene expression and apoptosis in gastric adenocarcinoma cells. Turk. J. Pharm. Sci. 2020, 17, 578–585. [Google Scholar]
- Cao, S.; Chen, S.; Qiao, X.; Guo, Y.; Liu, F.; Ding, Z.; Jin, B. Protocatechualdehyde rescues oxygen-glucose deprivation/reoxygenation-induced endothelial cells injury by inducing autophagy and inhibiting apoptosis via regulation of SIRT1. Front. Pharmacol. 2022, 13, 846513. [Google Scholar]
- Wang, P.; Zhang, Q. Protocatechuic aldehyde alleviates d -galactose-induced cardiomyocyte senescence by regulating the TCF3/ATG5 Axis. J. Cardiovasc. Pharmacol. 2023, 81, 221–231. [Google Scholar] [CrossRef]
- Xiang, C.; Liu, M.; Lu, Q.; Fan, C.; Lu, H.; Feng, C.; Yang, X.; Li, H.; Tang, W. Blockade of TLRs-triggered macrophage activation by caffeic acid exerted protective effects on experimental ulcerative colitis. Cell. Immunol. 2021, 365, 104364. [Google Scholar] [PubMed]
- Min, J.; Shen, H.; Xi, W.; Wang, Q.; Yin, L.; Zhang, Y.; Yu, Y.; Yang, Q.; Wang, Z.N. Synergistic anticancer activity of combined use of caffeic acid with paclitaxel enhances apoptosis of non-small-cell lung cancer H1299 cells in vivo and in vitro. Cell. Physiol. Biochem. 2018, 48, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.A. Syringic acid alleviates cisplatin-induced ovarian injury through modulating endoplasmic reticulum stress, inflammation and Nrf2 pathway. J. Trace Elem. Med. Biol. 2024, 82, 127356. [Google Scholar]
- Ikemoto, M.J.; Aihara, Y.; Ishii, N.; Shigemori, H. 3,4-dihydroxybenzalacetone inhibits the propagation of hydrogen peroxide-induced oxidative effect via secretory components from SH-SY5Y Cells. Biol. Pharm. Bull. 2023, 46, 599–607. [Google Scholar] [CrossRef]
- Chao, W.; Deng, J.S.; Huang, S.S.; Li, P.Y.; Liang, Y.C.; Huang, G.J. 3,4-dihydroxybenzalacetone attenuates lipopolysaccharide-induced inflammation in acute lung injury via down-regulation of MMP-2 and MMP-9 activities through suppressing ROS-mediated MAPK and PI3K/AKT signaling pathways. Int. Immunopharmacol. 2017, 50, 77–86. [Google Scholar]
- Lee, S.W.; Song, J.G.; Hwang, B.S.; Kim, D.W.; Lee, Y.J.; Woo, E.E.; Kim, J.Y.; Lee, I.K.; Yun, B.S. Lipoxygenase inhibitory activity of korean indigenous mushroom extracts and isolation of an active compound from Phellinus baumii. Mycobiology 2014, 42, 185–188. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-G.; Sun, Y.-W.; Wu, W.-L.; Jiang, W.-P.; Zhung, F.-Y.; Huang, G.-J. Multi-Target Protective Effects of Sanghuangporus sanghuang Against 5-Fluorouracil-Induced Intestinal Injury Through Suppression of Inflammation, Oxidative Stress, Epitheli-Al-Mesenchymal Transition, and Tight Junction. Int. J. Mol. Sci. 2025, 26, 3444. https://doi.org/10.3390/ijms26073444
Lin J-G, Sun Y-W, Wu W-L, Jiang W-P, Zhung F-Y, Huang G-J. Multi-Target Protective Effects of Sanghuangporus sanghuang Against 5-Fluorouracil-Induced Intestinal Injury Through Suppression of Inflammation, Oxidative Stress, Epitheli-Al-Mesenchymal Transition, and Tight Junction. International Journal of Molecular Sciences. 2025; 26(7):3444. https://doi.org/10.3390/ijms26073444
Chicago/Turabian StyleLin, Jaung-Geng, Yu-Wen Sun, Wen-Liang Wu, Wen-Ping Jiang, Fang-Yu Zhung, and Guan-Jhong Huang. 2025. "Multi-Target Protective Effects of Sanghuangporus sanghuang Against 5-Fluorouracil-Induced Intestinal Injury Through Suppression of Inflammation, Oxidative Stress, Epitheli-Al-Mesenchymal Transition, and Tight Junction" International Journal of Molecular Sciences 26, no. 7: 3444. https://doi.org/10.3390/ijms26073444
APA StyleLin, J.-G., Sun, Y.-W., Wu, W.-L., Jiang, W.-P., Zhung, F.-Y., & Huang, G.-J. (2025). Multi-Target Protective Effects of Sanghuangporus sanghuang Against 5-Fluorouracil-Induced Intestinal Injury Through Suppression of Inflammation, Oxidative Stress, Epitheli-Al-Mesenchymal Transition, and Tight Junction. International Journal of Molecular Sciences, 26(7), 3444. https://doi.org/10.3390/ijms26073444





