Dppa2 Promotes Early Embryo Development Through Regulating PDH Expression Pattern During Zygotic Genome Activation
Abstract
1. Introduction
2. Results
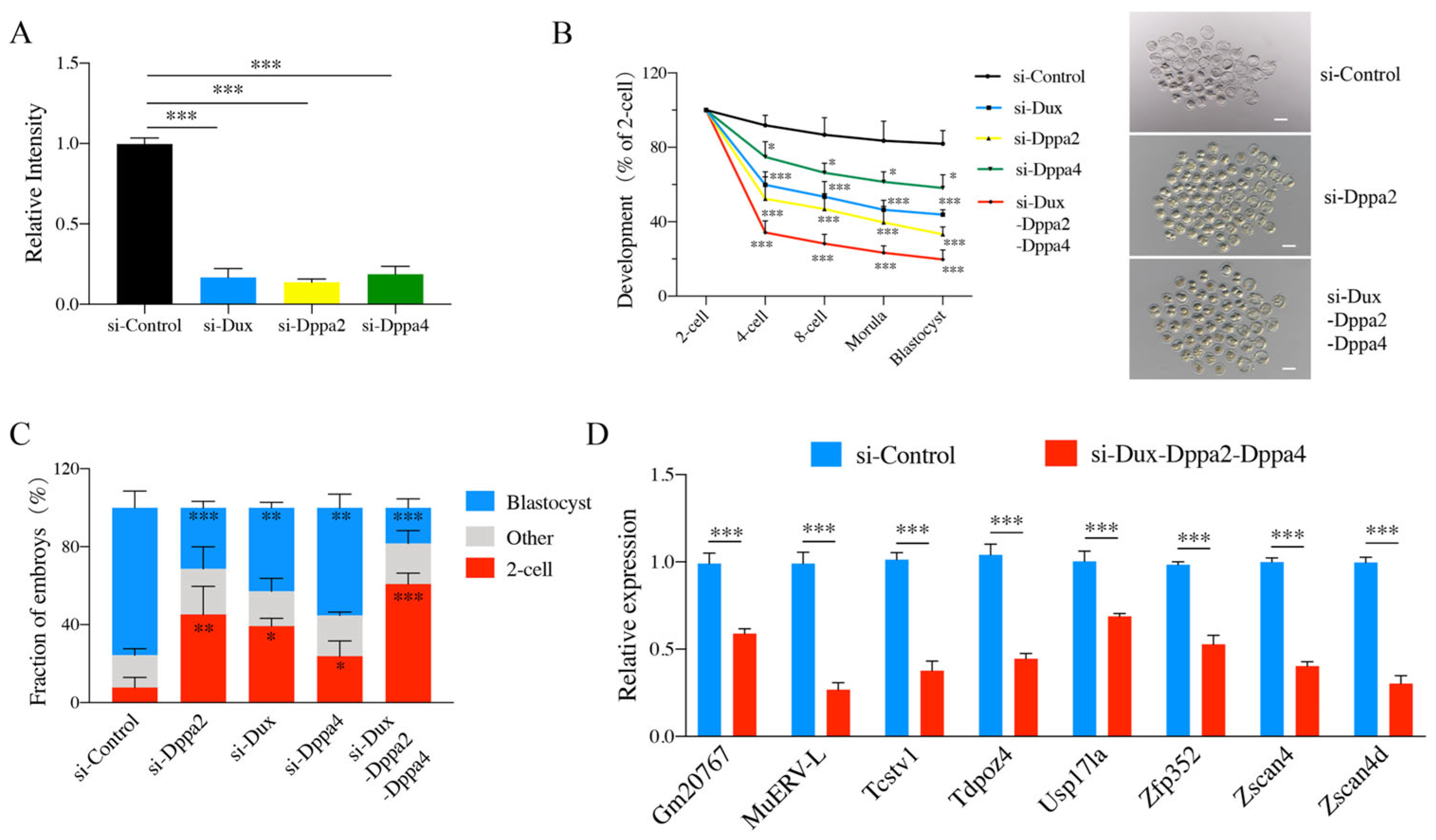
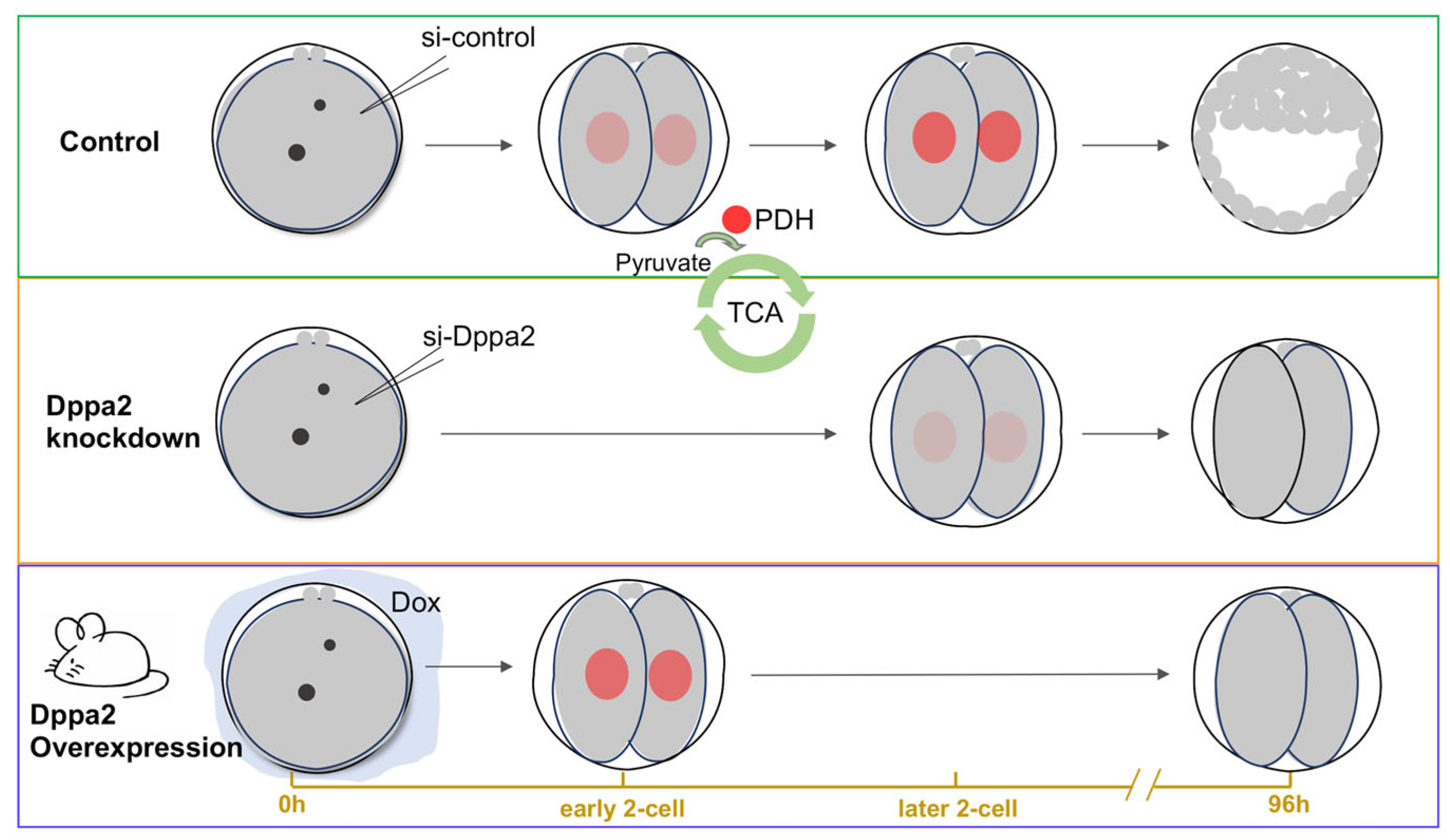
2.1. Effects of Dux, Dppa2, and Dppa4 Gene Knockdown on Embryonic Development
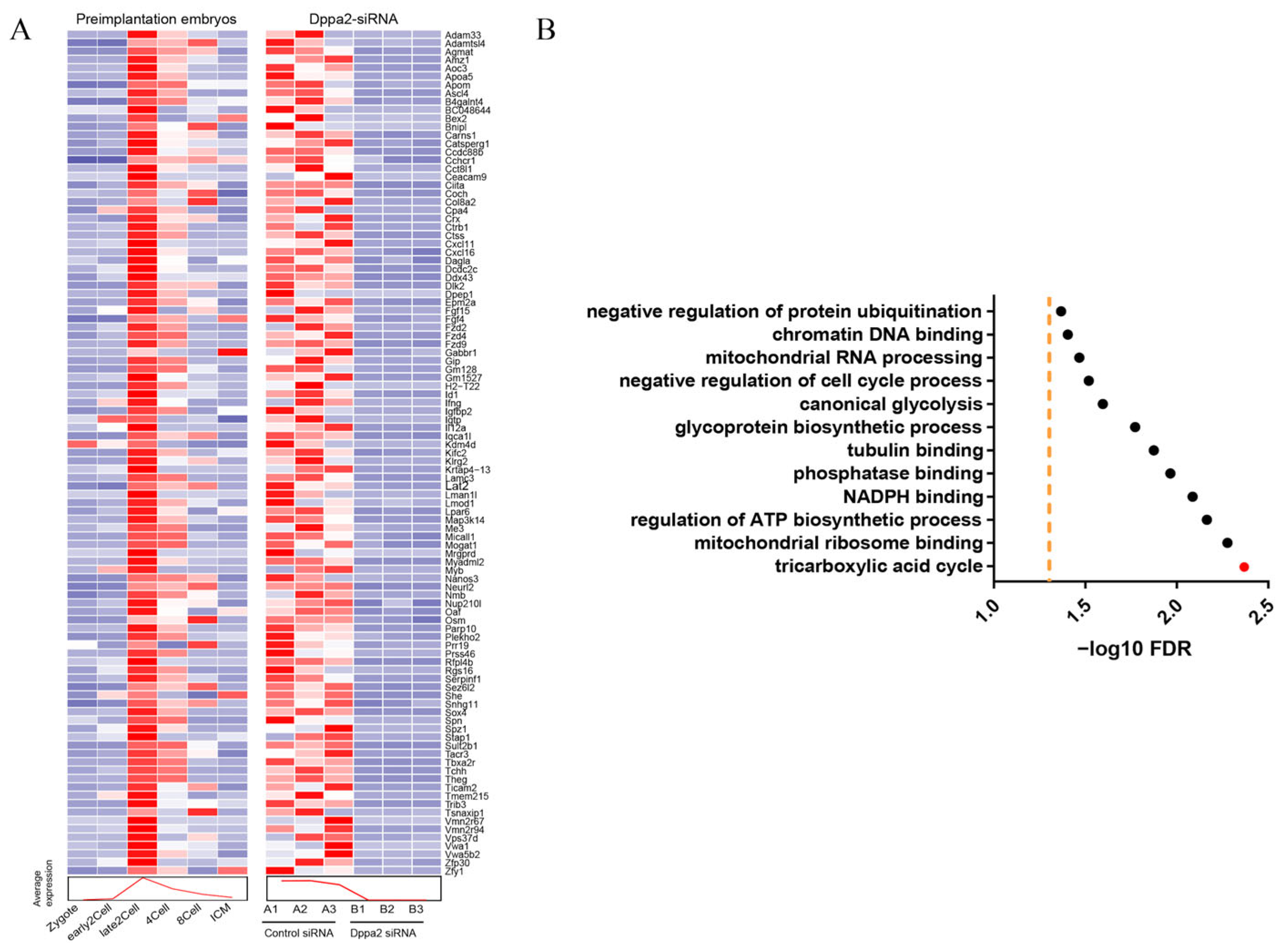
2.2. ScRNA-Seq Analysis of Dppa2 Knockdown Embryos
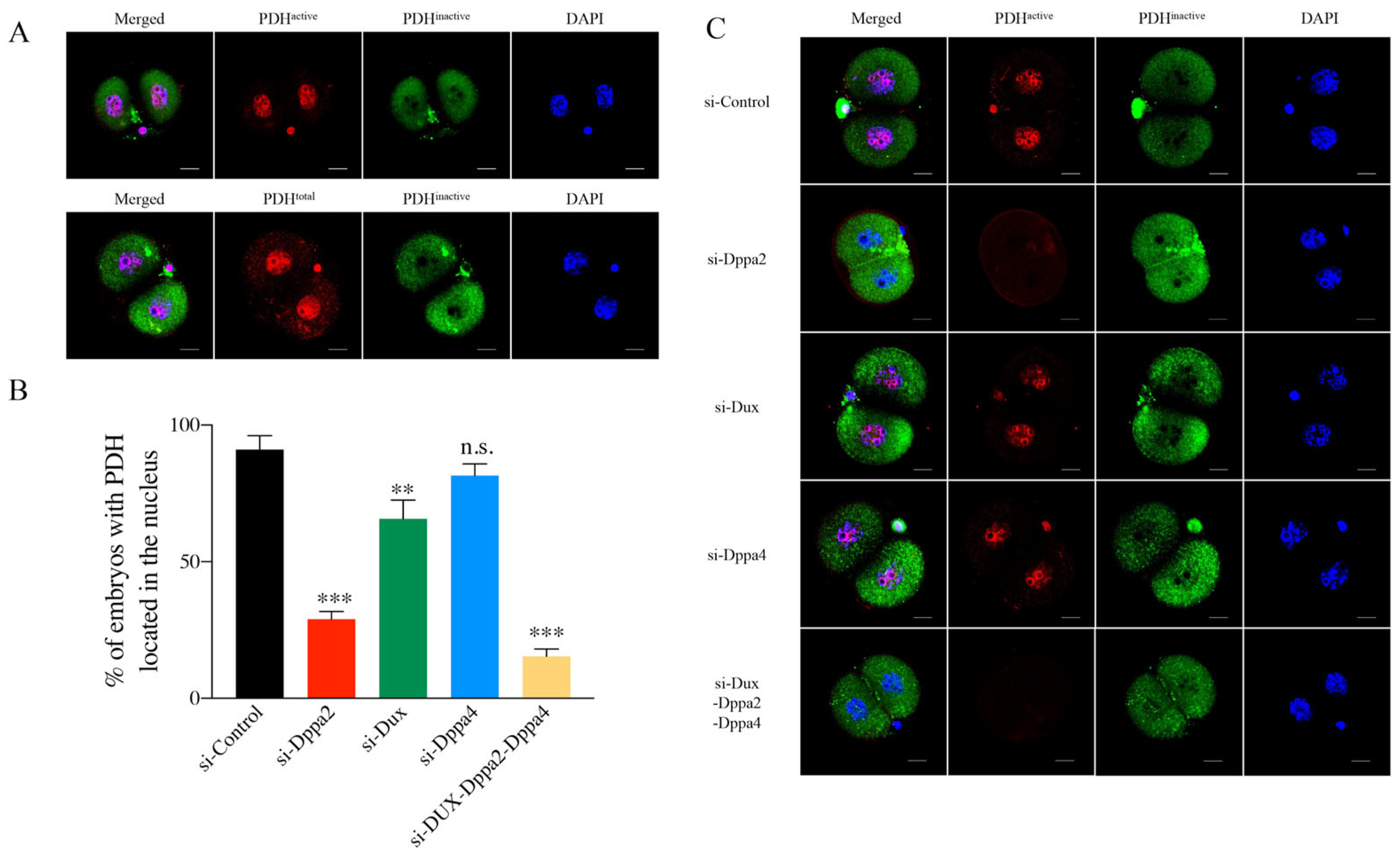
2.3. Relationship Between Dppa2 and PDH Position During Embryonic ZGA
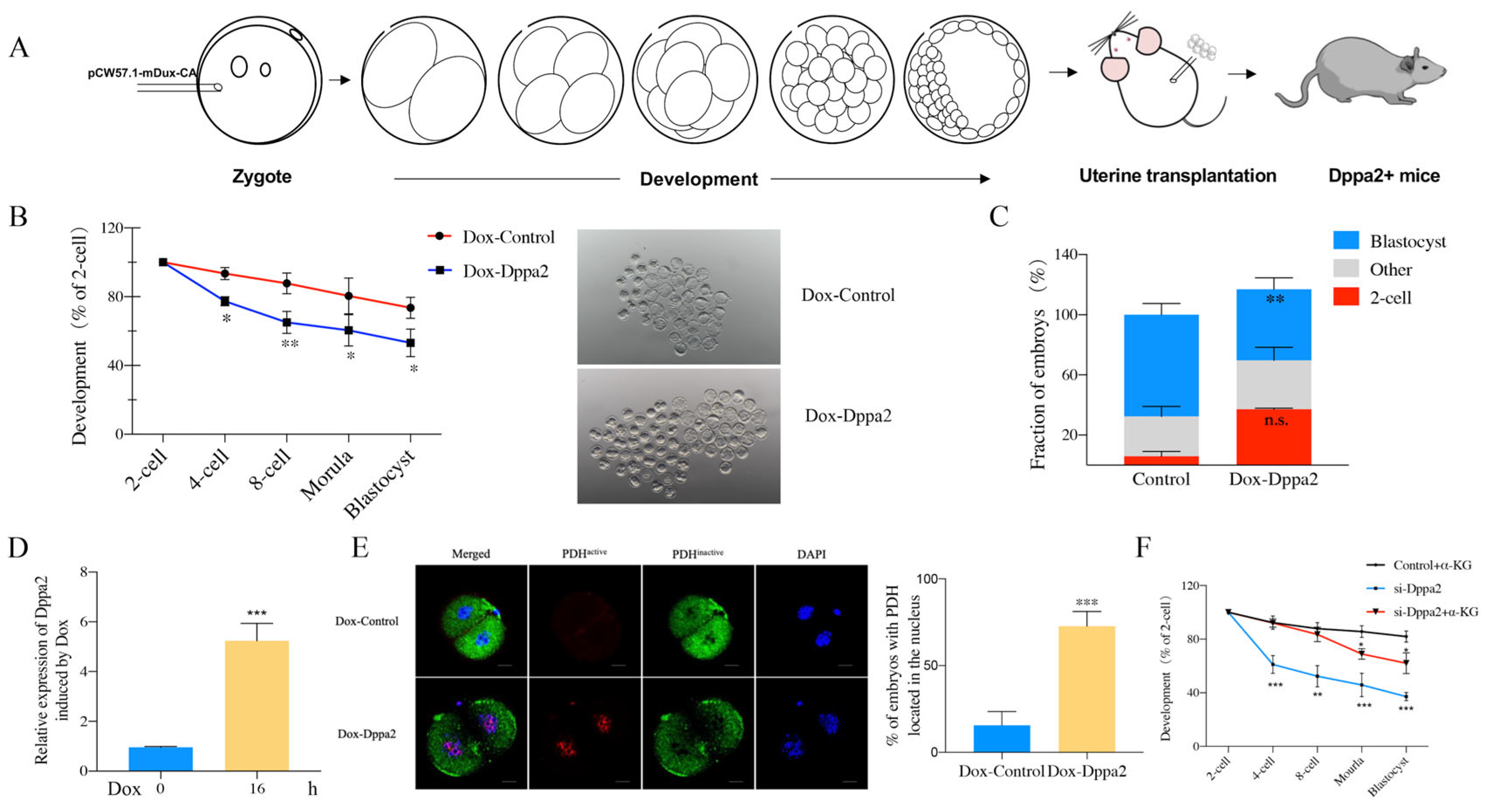
2.4. Effect of Dppa2 Overexpression on PDH Localization in Embryos
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Superovulation and Embryo Culture
4.3. siRNA Construction and Microinjection in Oocytes
4.4. RNA Extraction and RT–qPCR
4.5. Immunofluorescence Staining
4.6. Generation of Transgenic Mice
4.7. Dppa2-Overexpressing Embryo Collection
4.8. Low-Input ScRNA-Seq
4.9. RNA-Seq Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schulz, K.N.; Harrison, M.M. Mechanisms regulating zygotic genome activation. Nat. Rev. Genet. 2018, 20, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.-i.; Funaya, S.; Tsukioka, D.; Kawamura, M.; Suzuki, Y.; Suzuki, M.G.; Schultz, R.M.; Aoki, F. Minor zygotic gene activation is essential for mouse preimplantation development. Proc. Natl. Acad. Sci. USA 2018, 115, E6780–E6788. [Google Scholar] [CrossRef] [PubMed]
- Genet, M.; Torres-Padilla, M.-E. The molecular and cellular features of 2-cell-like cells: A reference guide. Development 2020, 147, dev189688. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, C.; Zhang, Y. Epigenetic regulation of mouse preimplantation embryo development. Curr. Opin. Genet. Dev. 2020, 64, 13–20. [Google Scholar] [CrossRef]
- Hendrickson, P.G.; Doráis, J.A.; Grow, E.J.; Whiddon, J.L.; Lim, J.-W.; Wike, C.L.; Weaver, B.D.; Pflueger, C.; Emery, B.R.; Wilcox, A.L.; et al. Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat. Genet. 2017, 49, 925–934. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.; Zhou, J.; Bi, Y.; Xu, J.; Xu, C.; Kou, X.; Zhao, Y.; Li, Y.; Tu, Z.; et al. Precise temporal regulation of Dux is important for embryo development. Cell Res. 2019, 29, 956–959. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y. Loss of DUX causes minor defects in zygotic genome activation and is compatible with mouse development. Nat. Genet. 2019, 51, 947–951. [Google Scholar] [PubMed]
- Chen, Z.; Xie, Z.; Zhang, Y. DPPA2 and DPPA4 are dispensable for mouse zygotic genome activation and pre-implantation development. Development 2021, 148, dev200178. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Y.-L.; Zhang, C.; Hao, J.; Wang, X.-L.; Ming, J.; Mi, L.; Na, J.; Hu, X.; Wang, Y. DPPA2/4 and SUMO E3 ligase PIAS4 opposingly regulate zygotic transcriptional program. PLoS Biol. 2019, 17, e3000324. [Google Scholar]
- De Iaco, A.; Coudray, A.; Duc, J.; Trono, D. DPPA2 and DPPA4 are necessary to establish a 2C—Like state in mouse embryonic stem cells. EMBO Rep. 2019, 20, e47382. [Google Scholar] [CrossRef]
- Masaki, H.; Nishida, T.; Kitajima, S.; Asahina, K.; Teraoka, H. Developmental Pluripotency-associated 4 (DPPA4) Localized in Active Chromatin Inhibits Mouse Embryonic Stem Cell Differentiation into a Primitive Ectoderm Lineage. J. Biol. Chem. 2007, 282, 33034–33042. [Google Scholar]
- Hardivillé, S.; Hart, G.W. Nutrient Regulation of Signaling, Transcription, and Cell Physiology by O-GlcNAcylation. Cell Metab. 2014, 20, 208–213. [Google Scholar] [PubMed]
- Martinez-Pastor, B.; Cosentino, C.; Mostoslavsky, R. A Tale of Metabolites: The Cross-Talk between Chromatin and Energy Metabolism. Cancer Discov. 2013, 3, 497–501. [Google Scholar] [PubMed]
- Flach, G.; Johnson, M.H.; Braude, P.R.; Taylor, R.A.; Bolton, V.N. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1982, 1, 681–686. [Google Scholar] [PubMed]
- De Iaco, A.; Verp, S.; Offner, S.; Grun, D.; Trono, D. DUX is a non-essential synchronizer of zygotic genome activation. Development 2020, 147, dev177725. [Google Scholar] [CrossRef]
- Yoshizawa-Sugata, N.; Yamazaki, S.; Mita-Yoshida, K.; Ono, T.; Nishito, Y.; Masai, H. Loss of full-length DNA replication regulator Rif1 in two-cell embryos is associated with zygotic transcriptional activation. J. Biol. Chem. 2021, 297, 101367. [Google Scholar]
- Nagaraj, R.; Sharpley, M.S.; Chi, F.; Braas, D.; Zhou, Y.; Kim, R.; Clark, A.T.; Banerjee, U. Nuclear Localization of Mitochondrial TCA Cycle Enzymes as a Critical Step in Mammalian Zygotic Genome Activation. Cell 2017, 168, 210–223.e11. [Google Scholar]
- Ji, S.; Chen, F.; Stein, P.; Wang, J.; Zhou, Z.; Wang, L.; Zhao, Q.; Lin, Z.; Liu, B.; Xu, K.; et al. OBOX regulates mouse zygotic genome activation and early development. Nature 2023, 620, 1047–1053. [Google Scholar]
- Jukam, D.; Shariati, S.A.M.; Skotheim, J.M. Zygotic Genome Activation in Vertebrates. Dev. Cell 2017, 42, 316–332. [Google Scholar] [CrossRef]
- Li, L.; Lu, X.; Dean, J. The maternal to zygotic transition in mammals. Mol. Asp. Med. 2013, 34, 919–938. [Google Scholar]
- Lee, M.T.; Bonneau, A.R.; Giraldez, A.J. Zygotic Genome Activation During the Maternal-to-Zygotic Transition. Annu. Rev. Cell Dev. Biol. 2014, 30, 581–613. [Google Scholar]
- De Iaco, A.; Planet, E.; Coluccio, A.; Verp, S.; Duc, J.; Trono, D. DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat. Genet. 2017, 49, 941–945. [Google Scholar]
- Whiddon, J.L.; Langford, A.T.; Wong, C.-J.; Zhong, J.W.; Tapscott, S.J. Conservation and innovation in the DUX4-family gene network. Nat. Genet. 2017, 49, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Saldivia, J.; van den Bergen, J.; Krouskos, M.; Gilchrist, M.; Lee, C.; Li, R.; Sinclair, A.H.; Surani, M.A.; Western, P.S. Dppa2 and Dppa4 Are Closely Linked SAP Motif Genes Restricted to Pluripotent Cells and the Germ Line. Stem Cells 2007, 25, 19–28. [Google Scholar] [PubMed]
- Eckersley-Maslin, M.; Alda-Catalinas, C.; Blotenburg, M.; Kreibich, E.; Krueger, C.; Reik, W. Dppa2 and Dppa4 directly regulate the Dux-driven zygotic transcriptional program. Genes Dev. 2019, 33, 194–208. [Google Scholar] [PubMed]
- Boiani, M.; Schöler, H.R. Regulatory networks in embryo-derived pluripotent stem cells. Nat. Rev. Mol. Cell Biol. 2005, 6, 872–881. [Google Scholar] [PubMed]
- Leese, H.J.; Guerif, F.; Allgar, V.; Brison, D.R.; Lundin, K.; Sturmey, R.G. Biological optimization, the Goldilocks principle, and how much islagomin the preimplantation embryo. Mol. Reprod. Dev. 2016, 83, 748–754. [Google Scholar]
- Gardner, D.K.; Harvey, A.J. Blastocyst metabolism. Reprod. Fertil. Dev. 2015, 27, 638–654. [Google Scholar]
- Barbehenn, E.K.; Wales, R.G.; Lowry, O.H. Measurement of metabolites in single preimplantation embryos; a new means to study metabolic control in early embryos. J. Embryol. Exp. Morphol. 1978, 43, 29–46. [Google Scholar]
- Holness, M.J.; Sugden, M.C. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem. Soc. Trans. 2003, 31, 1143–1151. [Google Scholar] [CrossRef]
- Choi, E.S.; Kawano, K.; Hiraya, M.; Matsukawa, E.; Yamada, M. Effects of pyruvate and dimethyl-α-ketoglutarate, either alone or in combination, on pre- and post-implantation development of mouse zygotes cultured in vitro. Reprod. Med. Biol. 2019, 18, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, L.-S.; Liu, X.-F.; Xia, Q.; Bai, L.-G.; Gao, L.; Gao, G.-Q.; Wang, Y.; Wei, Z.-Y.; Bai, C.-L.; et al. The Maternal Effect Genes UTX and JMJD3 Play Contrasting Roles in Mus musculus Preimplantation Embryo Development. Sci. Rep. 2016, 6, 26711. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, L.; Liu, X.; Bai, L.; Li, G. KDM6A and KDM6B play contrasting roles in nuclear transfer embryos revealed by MERVLreporter system. EMBO Rep. 2018, 19, e46240. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, A.; Zhang, X.; Song, L.; Wang, S.; Liu, X.; Bai, C.; Su, G.; Li, G.; Yang, L. Dppa2 Promotes Early Embryo Development Through Regulating PDH Expression Pattern During Zygotic Genome Activation. Int. J. Mol. Sci. 2025, 26, 3436. https://doi.org/10.3390/ijms26073436
Di A, Zhang X, Song L, Wang S, Liu X, Bai C, Su G, Li G, Yang L. Dppa2 Promotes Early Embryo Development Through Regulating PDH Expression Pattern During Zygotic Genome Activation. International Journal of Molecular Sciences. 2025; 26(7):3436. https://doi.org/10.3390/ijms26073436
Chicago/Turabian StyleDi, Anqi, Xinyi Zhang, Lishuang Song, Song Wang, Xuefei Liu, Chunling Bai, Guanghua Su, Guangpeng Li, and Lei Yang. 2025. "Dppa2 Promotes Early Embryo Development Through Regulating PDH Expression Pattern During Zygotic Genome Activation" International Journal of Molecular Sciences 26, no. 7: 3436. https://doi.org/10.3390/ijms26073436
APA StyleDi, A., Zhang, X., Song, L., Wang, S., Liu, X., Bai, C., Su, G., Li, G., & Yang, L. (2025). Dppa2 Promotes Early Embryo Development Through Regulating PDH Expression Pattern During Zygotic Genome Activation. International Journal of Molecular Sciences, 26(7), 3436. https://doi.org/10.3390/ijms26073436





