Extracellular Vesicles from Different Mesenchymal Stem Cell Types Exhibit Distinctive Surface Protein Profiling and Molecular Characteristics: A Comparative Analysis
Abstract
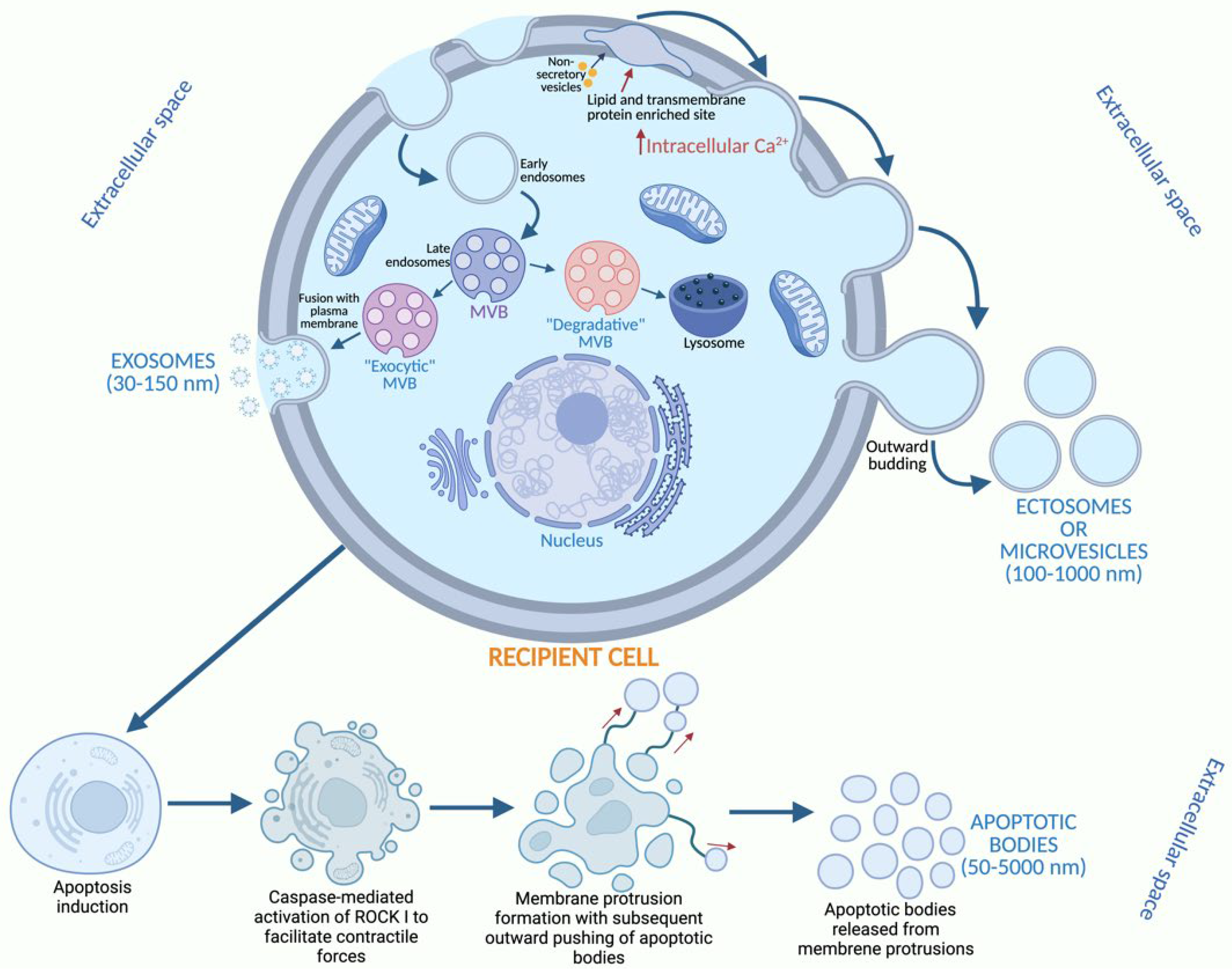
1. Introduction
2. Results
2.1. Characterization of MSC-EVs
2.2. MACSPlex Surface Protein Profiling
2.3. Fourier-Transform Infrared (FTIR)
2.4. Raman Spectroscopy
3. Discussion
4. Materials and Methods
4.1. MSC-EV Suspensions
4.2. Characterization of hP-MSC-EVs, hE-MSC-EVs and hDP-MSC-EVs
4.2.1. Dynamic Light Scattering
4.2.2. Transmission Electron Microscopy
4.2.3. Scanning Electron Microscopy
4.2.4. MACSPlex Surface Protein Profiling
4.2.5. Fourier-Transform Infrared
4.2.6. Raman Spectroscopy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EVs | Extracellular vesicles |
| MSCs | Mesenchymal stem cells |
| MSC-EVs | Extracellular vesicles derived from mesenchymal stem cells |
| MVs | Microvesicles |
| MVBs | Multivesicular bodies |
| hP-MSCs | Human placenta-derived mesenchymal stem cells |
| hE-MSCs | Human endometrium-derived mesenchymal stem cells |
| hDP-MSCs | Human dental pulp-derived mesenchymal stem cells |
| hP-MSC-EVs | EVs of human placenta-derived mesenchymal stem cells |
| hE-MSC-EVs | EVs of human endometrium-derived mesenchymal stem cells |
| hDP-MSC-EVs | EVs of human dental pulp-derived mesenchymal stem cells |
| DLS | Dynamic light scattering |
| CM | Conditional media |
| SMAs | Surface marker antibodies |
| PDI | Polydispersity index |
| TEM | Transmission electron microscopy |
| SEM | Scanning electron microscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| ddH2O | Filtered double-distilled water |
References
- Kim, J.; Xu, S.; Jung, S.R.; Nguyen, A.; Cheng, Y.; Zhao, M.; Fujimoto, B.S.; Nelson, W.; Schiro, P.; Franklinet, J.L.; et al. Comparison of EV characterization by commercial high-sensitivity flow cytometers and a custom single-molecule flow cytometer. J. Extracell. Vesicles 2024, 13, e12498. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Abyadeh, M.; Mirshahvaladi, S.; Kashani, S.A.; Paulo, J.A.; Amirkhani, A.; Mehryab, F.; Seydi, H.; Moradpour, N.; Jodeiryjabarzade, S.; Mirzaei, M.; et al. Proteomic profiling of mesenchymal stem cell-derived extracellular vesicles: Impact of isolation methods on protein cargo. J. Extracell. Biol. 2024, 3, e159. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, L.; Li, Y.; Holmes, C. Mesenchymal stromal/stem cell tissue source and in vitro expansion impact extracellular vesicle protein and miRNA compositions as well as angiogenic and immunomodulatory capacities. J. Extracell. Vesicles 2024, 13, e12472. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, T.; Cai, Y.; Liu, J.; Yu, B.; Fan, Y.; Su, J.; Zeng, Y.; Xiao, X.; Ren, L.; et al. Surface protein profiling and subtyping of extracellular vesicles in body fluids reveals non-CSF biomarkers of Alzheimer’s disease. J. Extracell. Vesicles 2024, 13, e12432. [Google Scholar] [CrossRef]
- Witwer, K.W.; Théry, C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles 2019, 8, 1648167. [Google Scholar] [CrossRef]
- Sonbhadra, S.; Mehak; Pandey, L.M. Biogenesis, Isolation, and Detection of Exosomes and Their Potential in Therapeutics and Diagnostics. Biosensors 2023, 13, 802. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Khan, K.; Kim, J.H. Biogenesis, Membrane Trafficking, Functions, and Next Generation Nanotherapeutics Medicine of Extracellular Vesicles. Int. J. Nanomed. 2021, 16, 3357. [Google Scholar] [CrossRef]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Keerthikumar, S.; Gangoda, L.; Liem, M.; Fonseka, P.; Atukorala, I.; Ozcitti, C.; Mechler, A.; Adda, C.G.; Ang, C.S.; Mathivanan, S. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget 2015, 6, 15375. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular Vesicles and Nanoparticles: Emerging Complexities. Trends Cell Biol. 2023, 33, 667. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428. [Google Scholar] [CrossRef]
- Bhat, A.; Malik, A.; Yadav, P.; Ware, W.-P.J.; Kakalij, P.; Chand, S. Mesenchymal stem cell-derived extracellular vesicles: Recent therapeutics and targeted drug delivery advances. J. Extracell. Biol. 2024, 3, e156. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; You, S.; Spinelli, C.; Morley, S.; Zandian, M.; Aspuria, P.J.; Cavallini, L.; Ciardiello, C.; Sobreiro, M.R.; Morello, M.; et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 2015, 6, 11327. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Park, Y.-B.; Ha, C.-W.; Lee, C.-H.; Yoon, Y.C.; Park, Y.-G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef]
- Tan, L.; Huang, Y.; Pan, X.; Quan, S.; Xu, S.; Li, D.; Song, L.; Zhang, X.; Chen, W.; Pan, J. Administration of bone marrow stromal cells in sepsis attenuates sepsis-related coagulopathy. Ann. Med. 2016, 48, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Perlee, D.; van Vught, L.A.; Scicluna, B.P.; Maag, A.; Lutter, R.; Kemper, E.M.; van ‘t Veer, C.; Punchard, M.A.; González, J.; Richard, M.P.; et al. Intravenous Infusion of Human Adipose Mesenchymal Stem Cells Modifies the Host Response to Lipopolysaccharide in Humans: A Randomized, Single-Blind, Parallel Group, Placebo Controlled Trial. Stem Cells 2018, 36, 1778–1788. [Google Scholar] [CrossRef]
- Harrell, C.R.; Miloradovic, D.; Sadikot, R.; Fellabaum, C.; Markovic, B.S.; Miloradovic, D.; Acovic, A.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular and Cellular Mechanisms Responsible for Beneficial Effects of Mesenchymal Stem Cell-Derived Product “exo-d-MAPPS” in Attenuation of Chronic Airway Inflammation. Anal. Cell. Pathol. 2020, 2020, 3153891. [Google Scholar] [CrossRef]
- Bi, B.; Schmitt, R.; Israilova, M.; Nishio, H.; Cantley, L.G. Stromal cells protect against acute tubular injury via an endocrine effect. J. Am. Soc. Nephrol. 2007, 18, 2486–2496. [Google Scholar] [CrossRef]
- de Pedro, M.Á.; López, E.; González-Nuño, F.M.; Pulido, M.; Álvarez, V.; Marchena, A.M.; Preußer, C.; Szymański, W.; Pogge von Strandmann, E.; Graumann, J.; et al. Menstrual blood-derived mesenchymal stromal cells: Impact of preconditioning on the cargo of extracellular vesicles as potential therapeutics. Stem Cell Res. Ther. 2023, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Evaluation of Safety and Exploratory Efficacy of CARTISTEM®, a Cell Therapy Product for Articular Cartilage Defects: A Phase I/IIa Clinical Trial in Patients with Focal, Full-thickness Grade 3–4 Articular Cartilage Defects of the Knee. Available online: https://clinicaltrials.gov/ct2/show/NCT01733186 (accessed on 17 March 2025).
- Prochymal® (Human Adult Stem Cells) Intravenous Infusion Following Acute Myocardial Infarction (AMI). Available online: https://clinicaltrials.gov/study/NCT00877903?tab=table (accessed on 17 March 2025).
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: A phase 1/2 randomized controlled trial (RIMECARD trial [Randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef]
- Tsang, K.S.; Ng, C.P.S.; Zhu, X.L.; Wong, G.K.C.; Lu, G.; Ahuja, A.T.; Wong, K.S.L.; Ng, H.K.; Poon, W.S. Phase I/II randomized controlled trial of autologous bone marrow-derived mesenchymal stem cell therapy for chronic stroke. World J. Stem Cells 2017, 9, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2019, 7, 154–162. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Hu, B.; Liu, J.; Kong, P.; Lou, S.; Su, Y.; Yang, T.; Li, H.; Liu, Y.; et al. Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord-derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-haploidentical stem-cell transplantation. J. Clin. Oncol. 2016, 34, 2843–2850. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, S.; Liu, X.; Song, B.; Shi, L. Umbilical cord mesenchymal stem cell treatment for Crohn’s disease: A randomized controlled clinical trial. Gut Liver 2018, 12, 73–78. [Google Scholar] [CrossRef]
- Melmed, G.Y.; Pandak, W.M.; Casey, K.; Abraham, B.; Valentine, J.; Schwartz, D.; Awais, D.; Bassan, I.; Lichtiger, S.; Sands, B.; et al. Human placenta-derived cells (PDA-001) for the treatment of moderate-to-severe Crohn’s disease: A phase 1b/2a study. Inflamm. Bowel Dis. 2015, 21, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Y.; Gong, H.; Yu, C.; Guo, C.; Wang, F.; Yan, S.; Xu, H. Long term effect and safety of wharton’s jelly-derived mesenchymal stem cells on type 2 diabetes. Exp. Ther. Med. 2016, 12, 1857–1866. [Google Scholar] [CrossRef]
- Yu, B.H.; Zhou, Q.; Wang, Z.L. Periodontal ligament versus bone marrow mesenchymal stem cells in combination with Bio-Oss scaffolds for ectopic and in situ bone formation: A comparative study in the rat. J. Biomater. Appl. 2014, 29, 243–253. [Google Scholar] [CrossRef]
- Čamernik, K.; Marc, J.; Zupan, J. Human Skeletal Muscle-Derived Mesenchymal Stem/Stromal Cell Isolation and Growth Kinetics Analysis. Methods Mol. Biol. 2019, 119–129. [Google Scholar] [CrossRef]
- Castro-Manrreza, M.E.; Bonifaz, L.; Castro-Escamilla, O.; Monroy-García, A.; Cortés-Morales, A.; Hernández-Estévez, E.; Hernández-Cristino, J.; Mayani, H.; Montesinos, J.J. Mesenchymal Stromal Cells from the Epidermis and Dermis of Psoriasis Patients: Morphology, Immunophenotype, Differentiation Patterns, and Regulation of T Cell Proliferation. Stem Cells Int. 2019, 2019, 4541797. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Altarejos, P.; Cabrera-Pastor, A.; Martínez-García, M.; Sánchez-Huertas, C.; Hernández, A.; Moreno-Manzano, V.; Felipo, V. Extracellular vesicles from mesenchymal stem cells reduce neuroinflammation in hippocampus and restore cognitive function in hyperammonemic rats. J. Neuroinflammation 2023, 20, 1. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, C.L.; Xu, Y.; Li, C.; Cao, Y.; Li, P. Exosomes derived from human placenta-derived mesenchymal stem cells improve neurologic function by promoting angiogenesis after spinal cord injury. Neurosci. Lett. 2020, 739, 135399. [Google Scholar] [CrossRef]
- Zheng, W.; Bian, S.; Qiu, S.; Bishop, C.E.; Wan, M.; Xu, N.; Sun, X.; Sequeira, R.C.; Atala, A.; Gu, Z.; et al. Placenta mesenchymal stem cell-derived extracellular vesicles alleviate liver fibrosis by inactivating hepatic stellate cells through a miR-378c/SKP2 axis. Inflamm. Regen. 2023, 43, 47. [Google Scholar] [CrossRef]
- Amaro-Prellezo, E.; Gómez-Ferrer, M.; Hakobyan, L.; Ontoria-Oviedo, I.; Peiró-Molina, E.; Tarazona, S.; Salguero, P.; Ruiz-Saurí, A.; Selva-Roldán, M.; Vives-Sanchez, R.; et al. Extracellular vesicles from dental pulp mesenchymal stem cells modulate macrophage phenotype during acute and chronic cardiac inflammation in athymic nude rats with myocardial infarction. Inflamm. Regen. 2024, 44, 25. [Google Scholar] [CrossRef]
- He, X.; Chu, X.Y.; Chen, X.; Xiang, Y.L.; Li, Z.L.; Gao, C.Y.; Luan, Y.Y.; Yang, K.; Zhang, D.L. Dental pulp stem cell-derived extracellular vesicles loaded with hydrogels promote osteogenesis in rats with alveolar bone defects. Mol. Med. Rep. 2025, 31, 29. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, Z.; Webster, K.A.; Chen, J.; Hu, H.; Zhou, Y.; Zhao, J.; Wang, L.; Wang, Y.; Zhong, Z.; et al. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem Cells Transl. Med. 2017, 6, 209–222. [Google Scholar] [CrossRef]
- Marinaro, F.; Pericuesta, E.; Sánchez-Margallo, F.M.; Casado, J.G.; Álvarez, V.; Matilla, E.; Hernández, N.; Blázquez, R.; González-Fernández, L.; Gutiérrez-Adán, A.; et al. Extracellular vesicles derived from endometrial human mesenchymal stem cells improve IVF outcome in an aged murine model. Reprod. Domest. Anim. 2018, 53, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Vonk, L.A.; van Dooremalen, S.F.J.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.F.; Lorenowicz, M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics 2018, 8, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tao, R.; Chen, L.; Xiong, Y.; Xue, H.; Hu, L.; Yan, C.; Xie, X.; Lin, Z.; Panayi, A.C.; et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J. Nanobiotechnology 2021, 19, 150. [Google Scholar] [CrossRef]
- Görgens, A.; Wiklander, O.; Bostancıoglu, R.B.; Zickler, A.; Murke, F.; Heider, U.; Wild, S.; Giebel, B.; Andaloussi, S. A robust and semi-quantitative method for analyzing exosomes by flow cytometry. Trillium Extracell. Vesicles 2021, 3, 41–45. [Google Scholar]
- Ekström, K.; Crescitelli, R.; Pétursson, H.I.; Johansson, J.; Lässer, C.; Bagge, R.O. Characterization of surface markers on extracellular vesicles isolated from lymphatic exudate from patients with breast cancer. BMC Cancer 2022, 22, 50. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lässer, C.; Szabó, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzás, E.I.; Lötvall, J. Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles 2013, 2, 20677. [Google Scholar] [CrossRef]
- Fan, Y.; Pionneau, C.; Cocozza, F.; Boëlle, P.Y.; Chardonnet, S.; Charrin, S.; Théry, C.; Zimmermann, P.; Rubinstein, E. Differential proteomics argues against a general role for CD9, CD81 or CD63 in the sorting of proteins into extracellular vesicles. J. Extracell. Vesicles 2023, 12, 12352. [Google Scholar] [CrossRef]
- Li, L.; Görgens, A.; Mussack, V.; Pepeldjiyska, E.; Hartz, A.S.; Rank, A.; Schmohl, J.; Krämer, D.; El-Andaloussi, S.; Pfaffl, M.W.; et al. Description and optimization of a multiplex bead-based flow cytometry method (MBFCM) to characterize extracellular vesicles in serum samples from patients with hematological malignancies. Cancer Gene Ther. 2022, 29, 1600–1615. [Google Scholar] [CrossRef]
- Hernando, S.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Extracellular vesicles released by hair follicle and adipose mesenchymal stromal cells induce comparable neuroprotective and anti-inflammatory effects in primary neuronal and microglial cultures. Cytotherapy 2023, 25, 1027–1032. [Google Scholar] [CrossRef]
- Balbi, C.; Burrello, J.; Bolis, S.; Lazzarini, E.; Biemmi, V.; Pianezzi, E.; Burrello, A.; Caporali, E.; Grazioli, L.G.; Martinetti, G.; et al. Circulating extracellular vesicles are endowed with enhanced procoagulant activity in SARS-CoV-2 infection. EBioMedicine 2021, 67, 103369. [Google Scholar] [CrossRef]
- Weber, B.; Sturm, R.; Henrich, D.; Marzi, I.; Leppik, L. CD44+ and CD31+ extracellular vesicles (EVs) are significantly reduced in polytraumatized patients with hemorrhagic shock-evaluation of their diagnostic and prognostic potential. Front. Immunol. 2023, 14, 1196241. [Google Scholar] [CrossRef]
- de Paula Silva, E.; Marti, L.C.; Andreghetto, F.M.; de Sales, R.O.; Hoberman, M.; dos Santos Dias, B.; Diniz, L.F.A.; dos Santos, A.M.; Moyses, R.A.; Curioni, O.A.; et al. Extracellular vesicles cargo from head and neck cancer cell lines disrupt dendritic cells function and match plasma microRNAs. Sci. Rep. 2021, 11, 18534. [Google Scholar] [CrossRef] [PubMed]
- Giovanazzi, A.; van Herwijnen, M.J.C.; Kleinjan, M.; van der Meulen, G.N.; Wauben, M.H.M. Surface protein profiling of milk and serum extracellular vesicles unveils body fluid-specific signatures. Sci. Rep. 2023, 13, 8758. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhang, J.; Yarden, Y.; Fu, L. The key roles of cancer stem cell-derived extracellular vesicles. Signal Transduct. Target. Ther. 2021, 6, 109. [Google Scholar] [CrossRef]
- Tholen, S.; Schilling, O.; Gießl, A.; Kistenmacher, S.; Schwämmle, M.; Martin, G.; Ulrich, E.; Schlötzer-Schrehardt, U.; Bucher, F.; Schlunck, G.; et al. Enrichment, Characterization, and Proteomic Profiling of Small Extracellular Vesicles Derived from Human Limbal Mesenchymal Stromal Cells and Melanocytes. Cells 2024, 13, 623. [Google Scholar] [CrossRef]
- Nguyen, V.V.T.; Welsh, J.A.; Tertel, T.; Choo, A.; van de Wakker, S.I.; Defourny, K.A.Y.; Giebel, B.; Vader, P.; Padmanabhan, J.; Lim, S.K.; et al. Inter-laboratory multiplex bead-based surface protein profiling of MSC-derived EV preparations identifies MSC-EV surface marker signatures. J. Extracell. Vesicles 2024, 13, e12463. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- Stępień, E.; Kamińska, A.; Surman, M.; Karbowska, D.; Wróbel, A.; Przybyło, M. Fourier-Transform InfraRed (FT-IR) spectroscopy to show alterations in molecular composition of EV subpopulations from melanoma cell lines in different malignancy. Biochem. Biophys. Rep. 2021, 25, 100888. [Google Scholar] [CrossRef]
- Mereghetti, P.; Corsetto, P.A.; Cremona, A.; Rizzo, A.M.; Doglia, S.M.; Ami, D. A Fourier transform infrared spectroscopy study of cell membrane domain modifications induced by docosahexaenoic acid. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 3115–3122. [Google Scholar] [CrossRef]
- Szentirmai, V.; Wacha, A.; Németh, C.; Kitka, D.; Rácz, A.; Héberger, K.; Mihály, J.; Varga, Z. Reagent-free total protein quantification of intact extracellular vesicles by attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy. Anal. Bioanal. Chem. 2020, 412, 4619–4628. [Google Scholar] [CrossRef] [PubMed]
- Mihály, J.; Deák, R.; Szigyártó, I.C.; Bóta, A.; Beke-Somfai, T.; Varga, Z. Characterization of extracellular vesicles by IR spectroscopy: Fast and simple classification based on amide and CH stretching vibrations. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 459–466. [Google Scholar] [CrossRef]
- Wong, L.W.; Mak, S.H.; Goh, B.H.; Lee, W.L. The Convergence of FTIR and EVs: Emergence Strategy for Non-Invasive Cancer Markers Discovery. Diagnostics 2023, 13, 22. [Google Scholar] [CrossRef]
- Kruglik, S.G.; Royo, F.; Guigner, J.M.; Palomo, L.; Seksek, O.; Turpin, P.Y.; Tatischeff, I.; Falcón-Pérez, J.M. Raman tweezers microspectroscopy of: Circa 100 nm extracellular vesicles. Nanoscale 2019, 11, 1661–1679. [Google Scholar] [CrossRef]
- Gualerzi, A.; Niada, S.; Giannasi, C.; Picciolini, S.; Morasso, C.; Vanna, R.; Rossella, V.; Masserini, M.; Bedoni, M.; Ciceri, F.; et al. Raman spectroscopy uncovers biochemical tissue-related features of extracellular vesicles from mesenchymal stromal cells. Sci. Rep. 2017, 7, 9820. [Google Scholar] [CrossRef]
- Szekeres, G.P.; Kneipp, J. SERS probing of proteins in gold nanoparticle agglomerates. Front. Chem. 2019, 7, 30. [Google Scholar] [CrossRef]
- Liangsupree, T.; Multia, E.; Saarinen, J.; Ruiz-Jimenez, J.; Kemell, M.; Riekkola, M.L. Raman spectroscopy combined with comprehensive gas chromatography for label-free characterization of plasma-derived extracellular vesicle subpopulations. Anal. Biochem. 2022, 647, 114672. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. Imaging of polysaccharides in the tomato cell wall with Raman microspectroscopy. Plant Methods 2014, 10, 14. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A.; Baez, C.; Reiner, R.S. Detection and quantitation of cellulose II by Raman spectroscopy. Cellulose 2021, 28, 9069–9079. [Google Scholar] [CrossRef]
- Zhang, H.; Silva, A.C.; Zhang, W.; Rutigliano, H.; Zhou, A. Raman Spectroscopy characterization extracellular vesicles from bovine placenta and peripheral blood mononuclear cells. PLoS ONE 2020, 15, e0235214. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101. [Google Scholar] [CrossRef]
- Shekari, F.; Nazari, A.; Assar Kashani, S.; Hajizadeh-Saffar, E.; Lim, R.; Baharvand, H. Pre-clinical investigation of mesenchymal stromal cell-derived extracellular vesicles: A systematic review. Cytotherapy 2021, 23, 277–284. [Google Scholar] [CrossRef]
- Seyedrazizadeh, S.Z.; Poosti, S.; Nazari, A.; Alikhani, M.; Shekari, F.; Pakdel, F.; Shahpasand, K.; Satarian, L.; Baharvand, H. Extracellular vesicles derived from human ES-MSCs protect retinal ganglion cells and preserve retinal function in a rodent model of optic nerve injury. Stem Cell Res. Ther. 2020, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Mardpour, S.; Ghanian, M.H.; Sadeghi-Abandansari, H.; Mardpour, S.; Nazari, A.; Shekari, F.; Baharvand, H. Hydrogel-Mediated Sustained Systemic Delivery of Mesenchymal Stem Cell-Derived Extracellular Vesicles Improves Hepatic Regeneration in Chronic Liver Failure. ACS Appl. Mater. Interfaces 2019, 11, 37421–37433. [Google Scholar] [CrossRef]
- Kalimuthu, S.; Gangadaran, P.; Rajendran, R.L.; Zhu, L.; Oh, J.M.; Lee, H.W.; Gopal, A.; Baek, S.H.; Jeong, S.Y.; Lee, S.W.; et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front. Pharmacol. 2018, 9, 395447. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Vs, M.; Gerber, P.; Sv, V.; Jm, P.; Pasquini, J.M. Extracellular vesicles containing the transferrin receptor as nanocarriers of apotransferrin. J. Neurochem. 2020, 155, 327–338. [Google Scholar] [CrossRef]
- Jakubec, M.; Maple-Grødem, J.; Akbari, S.; Nesse, S.; Halskau, Ø.; Mork-Jansson, A.E. Plasma-derived exosome-like vesicles are enriched in lyso-phospholipids and pass the blood-brain barrier. PLoS ONE 2020, 15, e0232442. [Google Scholar] [CrossRef]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Kaddour, H.; Panzner, T.D.; Welch, J.L.; Shouman, N.; Mohan, M.; Stapleton, J.T.; Okeoma, C.M. Electrostatic Surface Properties of Blood and Semen Extracellular Vesicles: Implications of Sialylation and HIV-Induced Changes on EV Internalization. Viruses 2020, 12, 1117. [Google Scholar] [CrossRef]
- Rupert, D.L.M.; Claudio, V.; Lässer, C.; Bally, M. Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 3164–3179. [Google Scholar] [CrossRef]
- Mendivil-Alvarado, H.; Limon-Miro, A.T.; Carvajal-Millan, E.; Lizardi-Mendoza, J.; Mercado-Lara, A.; Coronado-Alvarado, C.D.; Rascón-Durán, M.L.; Anduro-Corona, I.; Talamás-Lara, D.; Rascón-Careaga, A.; et al. Extracellular Vesicles and Their Zeta Potential as Future Markers Associated with Nutrition and Molecular Biomarkers in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6810. [Google Scholar] [CrossRef]
- Schöler, D.; Loosen, S.H.; Wirtz, T.H.; Brozat, J.F.; dos Santos Ferreira Grani, L.A.; Luedde, T.; Heinrichs, L.; Frank, D.; Koch, A.; Roderburg, C.; et al. Low extracellular vesicle concentrations predict survival in patients with heart failure. Front. Cardiovasc. Med. 2023, 10, 1163525. [Google Scholar] [CrossRef] [PubMed]
- Kooijmans, S.A.A.; Fliervoet, L.A.L.; Van Der Meel, R.; Fens, M.H.A.M.; Heijnen, H.F.G.; Van Bergen En Henegouwen, P.M.P.; Vader, P.; Schiffelers, R.M. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Control Release 2016, 224, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Futaki, S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci. Rep. 2015, 5, 10112. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, L.; Zhu, C.; Zheng, Q.; Wang, G.; Tong, J.; Fang, Y.; Xia, Y.; Cheng, G.; He, X.; et al. Aptamer-conjugated extracellular nanovesicles for targeted drug delivery. Cancer Res. 2017, 78, 798. [Google Scholar] [CrossRef]
- Ghadami, S.; Dellinger, K. The lipid composition of extracellular vesicles: Applications in diagnostics and therapeutic delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef]
- Carlomagno, C.; Giannasi, C.; Niada, S.; Bedoni, M.; Gualerzi, A.; Brini, A.T. Raman Fingerprint of Extracellular Vesicles and Conditioned Media for the Reproducibility Assessment of Cell-Free Therapeutics. Front. Bioeng. Biotechnol. 2021, 9, 640617. [Google Scholar] [CrossRef]
- Yin, N.; Zhao, W.; Luo, Q.; Yuan, W.; Luan, X.; Zhang, H. Restoring ovarian function with human placenta-derived mesenchymal stem cells in autoimmune-induced premature ovarian failure mice mediated by Treg cells and associated cytokines. Reprod. Sci. 2018, 25, 1073–1082. [Google Scholar] [CrossRef]
- Blázquez, R.; Sánchez-Margallo, F.M.; Álvarez, V.; Matilla, E.; Hernández, N.; Marinaro, F.; Gómez-Serrano, M.; Jorge, I.; Casado, J.G.; Macías-García, B. Murine embryos exposed to human endometrial MSCs-derived extracellular vesicles exhibit higher VEGF/PDGF AA release, increased blastomere count and hatching rates. PLoS ONE 2018, 13, e0196080. [Google Scholar] [CrossRef] [PubMed]
- Brunello, G.; Zanotti, F.; Trentini, M.; Zanolla, I.; Pishavar, E.; Favero, V.; Favero, R.; Favero, L.; Bressan, E.; Bonora, M.; et al. Exosomes Derived from Dental Pulp Stem Cells Show Different Angiogenic and Osteogenic Properties in Relation to the Age of the Donor. Pharmaceutics 2022, 14, 908. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of extracellular vesicles: General methodologies and latest trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]







| Exosomes | Ectosomes | Apoptotic Bodies | |
|---|---|---|---|
| Size | 30–150 nm | 100–1000 nm | 50–5000 nm |
| Biogenesis | MVBs | Plasma membrane | Cellular disassembly/fragmentation of cell components |
| Density | ≈1.10–1.14 g/mL | ≈1.12–1.20 g/mL | 1.16–1.28 g/mL |
| Enriched proteins | Alix, TSG101, CD63, CD9, CD81, Syntenin-1 | MMP2, CK18, Annexin A1, Annexin A2, CD147, CD9 | Annexin V |
| Composition | Nucleic acids, proteins, lipids and metabolites. | Nucleic acids, proteins, lipids and metabolites. | Chromatin remnants, cytosol portions, DNA fragments and histone, degraded proteins. |
| MSC-EVs Source | Biomarker Characterization of MSC-EVs | Molecular Effect of MSC-EVs | References |
|---|---|---|---|
| Human adipocyte-derived MSCs | Alix, Hsp70, Flotillin-2 and CD9, as well as TGFβ | MSC-EVs reach the hippocampus, reduce glial activation and neuroinflammation, and restore cognitive function. | [38] |
| Human placenta-derived MSCs | CD9, CD63 | MSC-EVs promote the tube formation and migration of human umbilical vein endothelial cells. | [39] |
| Human placenta-derived MSCs | CD63, CD81, CD9, and TSG101 | MSC-EVs showed therapeutic effects on liver fibrosis. | [40] |
| Dental pulp-derived MSCs | HSP70, TSG101, CD9, CD63, CD81 | MSC-EVs facilitated the transformation of pro-inflammatory macrophages into a pro-resolving phenotype, as demonstrated by elevated expression of M2 markers and reduced secretion of pro-inflammatory cytokines. | [41] |
| Dental pulp-derived MSCs | CD9, CD63, TSG101 and CD81 | MSC-EVs significantly enhanced the expression of osteogenic transcription factors and enzymes, including runt-related transcription factor 2 and alkaline phosphatase, thereby increasing the osteogenic differentiation capacity of Hertwig’s epithelial root sheath cells. | [42] |
| Human endometrium-derived MSCs | CD63 | MSC-EV MicroRNA-21 is a potential mediator of human endometrium-derived MSCs in therapy by enhancing cell survival through the PTEN/Akt pathway. | [43] |
| Human endometrium-derived MSCs | CD9 and CD63 | MSC-EVs exert an antioxidant effect and increase the developmental competence of in vitro fertilization-derived embryos from older females. | [44] |
| Human bone marrow-derived MSCs | CD9 and CD63 | MSC-EVs promoted cartilage regeneration in vitro, inhibited the adverse effects of inflammatory mediators on cartilage homeostasis, and stimulated the production of proteoglycans and type II collagen by these cells. | [45] |
| Bone marrow-derived MSCs | CD9 and CD63 | MSC-EVs accelerated diabetic wound healing via enhanced angiogenesis. | [46] |
| Surface Marker Antibodies (SMAs) | Target | SMAs Present in hP-MSC-EVs | SMAs Present in hE-MSC-EVs | SMAs Present in hDP-MSC-EVs | Reference |
|---|---|---|---|---|---|
| CD9, CD63, CD81 | Tetraspanins, cell adhesion, migration, and signaling. | CD9, CD63 | CD9, CD63 | CD9, CD63 | [47,48,49,50,51] |
| CD31, CD40, CD44, CD69, CD146, CD142 | Cell origin (endothelial, epithelial and MSCs), and cell activation and EV functional status markers. | CD31, CD44, CD40, CD142 | CD31, CD44, CD40, CD146, CD31, CD142 | CD31, CD146, CD142 | [47,52,53,54,55] |
| CD29, CD49e | Integrins, cell activation and EV functional status markers. | CD29 | CD29, CD49e | CD29, CD49e | [52] |
| CD8, CD14, CD19, CD24, CD41b, CD42a, CD62P, CD86, R0R1, HLA-ABC | Lineage-associated markers present in healthy cells. | CD8 *, CD19 *, CD62P, R0R1 * | CD62P, CD86, CD41b *, HLA-ABC * | CD42a *, CD86 | [47,52,56] |
| CD326 | Epithelial cell adhesion molecule/EV functional status markers. | CD326 | CD326 | CD326 | [48,52] |
| CD4, CD20, CD209 | Markers associated with immune and antigen-presenting cells. | CD4 *, CD209 * | CD20 | CD20 | [48,56,57] |
| CD45 | Hematopoietic marker. | CD45 * | N/A | N/A | [48,56,57] |
| CD133/1 (prominin-1) | Transmembrane protein present in MSCs. | CD133/1 | CD133/1 | CD133/1 | [58,59] |
| HLA-DR | Negative cellular marker for MSCs. | N/A | N/A | N/A | [60] |
| MSCP | A type I transmembrane proteoglycan found on the cell surface that plays a crucial role in cell survival, migration, and the formation of new blood vessels. | MSCP | MSCP | MSCP | [61] |
| Wavenumber (cm−1) | Vibrational Mode | References |
|---|---|---|
| 1300−1000 cm−1 | Phosphates and carbohydrates | [62,63] |
| 1700−1500 cm−1 | Amide bands | [62,64] |
| 1600−1700 cm−1 | Amide I | [62,64] |
| 1550, 1546, 1540, 1537 cm−1 | Amide II | [62,64] |
| 1743, 1728 cm−1 | Interfacial region | [62] |
| 1740 cm−1 | Ester carbonyl | [63,65] |
| 3100, 3078 cm−1 | Amide B | [62,66] |
| 3300, 3298, 3290, 3285 cm−1 | Amide A | [62,66] |
| Raman Shift (cm−1) | Tentative Assignment | References |
|---|---|---|
| 700–704 | Lipids | [67] |
| 725–751 | Nucleic acids | [67] |
| 770–776 | Nucleic acids | [68] |
| 812–839 | Nucleic acids, phospholipids | [67,68] |
| 838 | Tyr | [69] |
| 852–859 | Pro: ν(C-C), Tyr: ring breathing (proteins), polysaccharide structure | [67,68,69] |
| 886 | Phospholipids | [67] |
| 900 | Phospholipids | [67] |
| 934–935 | C-C backbone/C-C stretching (collagen/protein assignment) | [67,68,70] |
| 928–960 | Polysaccharide structure, d(C-C-N) symm, a-helical skeletal, proteins | [67,68,69] |
| 971 | p(CH2) (lipids) | [71,72] |
| 1000–1008 | Proteins | [70] |
| 1003 | Phenylalanine | [68] |
| 1000–1008 | Phe: ring breathing (proteins) | [70] |
| 1090–1100 | Nucleic acids | [67] |
| 1050–1160 | Proteins | [67] |
| 1155–1160 | Carotenoids | [67] |
| 1169–1180 | Nucleic acids and proteins | [70] |
| 1552–1554 | Tryptophan (protein assignment)/porphyrin/amide II | [73] |
| 1200–1208 | Nucleic acids and proteins | [67,70] |
| 1200–1260 | Nucleic acids | [67] |
| 1295–1314 | Lipids | [67,70] |
| 1355–1365 | Nucleic acids and proteins | [70] |
| 1400–1430 | Proteins | [70] |
| 1420–1470 | Lipids | [70] |
| 1515–1540 | Carotenoids | [67,70] |
| 1570–1580 | Nucleic acids | [67] |
| 1602–1604 | Lipids | [67] |
| 1619–1622 | Proteins | [67,70] |
| 1640–1700 | Nucleic acids, lipids, and proteins | [67,70] |
| 1720–1750 | Lipids | [67] |
| 2928 | Lipids | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Pérez, A.G.; Herrera-González, A.; López-Naranjo, E.J.; Martínez-Álvarez, I.A.; Uribe-Rodríguez, D.; Ramírez-Arreola, D.E.; Sánchez-Peña, M.J.; Navarro-Partida, J. Extracellular Vesicles from Different Mesenchymal Stem Cell Types Exhibit Distinctive Surface Protein Profiling and Molecular Characteristics: A Comparative Analysis. Int. J. Mol. Sci. 2025, 26, 3393. https://doi.org/10.3390/ijms26073393
Fernández-Pérez AG, Herrera-González A, López-Naranjo EJ, Martínez-Álvarez IA, Uribe-Rodríguez D, Ramírez-Arreola DE, Sánchez-Peña MJ, Navarro-Partida J. Extracellular Vesicles from Different Mesenchymal Stem Cell Types Exhibit Distinctive Surface Protein Profiling and Molecular Characteristics: A Comparative Analysis. International Journal of Molecular Sciences. 2025; 26(7):3393. https://doi.org/10.3390/ijms26073393
Chicago/Turabian StyleFernández-Pérez, Atziri G., Azucena Herrera-González, Edgar J. López-Naranjo, Iliany Annel Martínez-Álvarez, David Uribe-Rodríguez, Daniel E. Ramírez-Arreola, María Judith Sánchez-Peña, and Jose Navarro-Partida. 2025. "Extracellular Vesicles from Different Mesenchymal Stem Cell Types Exhibit Distinctive Surface Protein Profiling and Molecular Characteristics: A Comparative Analysis" International Journal of Molecular Sciences 26, no. 7: 3393. https://doi.org/10.3390/ijms26073393
APA StyleFernández-Pérez, A. G., Herrera-González, A., López-Naranjo, E. J., Martínez-Álvarez, I. A., Uribe-Rodríguez, D., Ramírez-Arreola, D. E., Sánchez-Peña, M. J., & Navarro-Partida, J. (2025). Extracellular Vesicles from Different Mesenchymal Stem Cell Types Exhibit Distinctive Surface Protein Profiling and Molecular Characteristics: A Comparative Analysis. International Journal of Molecular Sciences, 26(7), 3393. https://doi.org/10.3390/ijms26073393









