Aldosterone-Induced Transformation of Vascular Smooth Muscle Cells into Macrophage-like Cells Participates in Inflammatory Vascular Lesions
Abstract
1. Introduction
2. Results
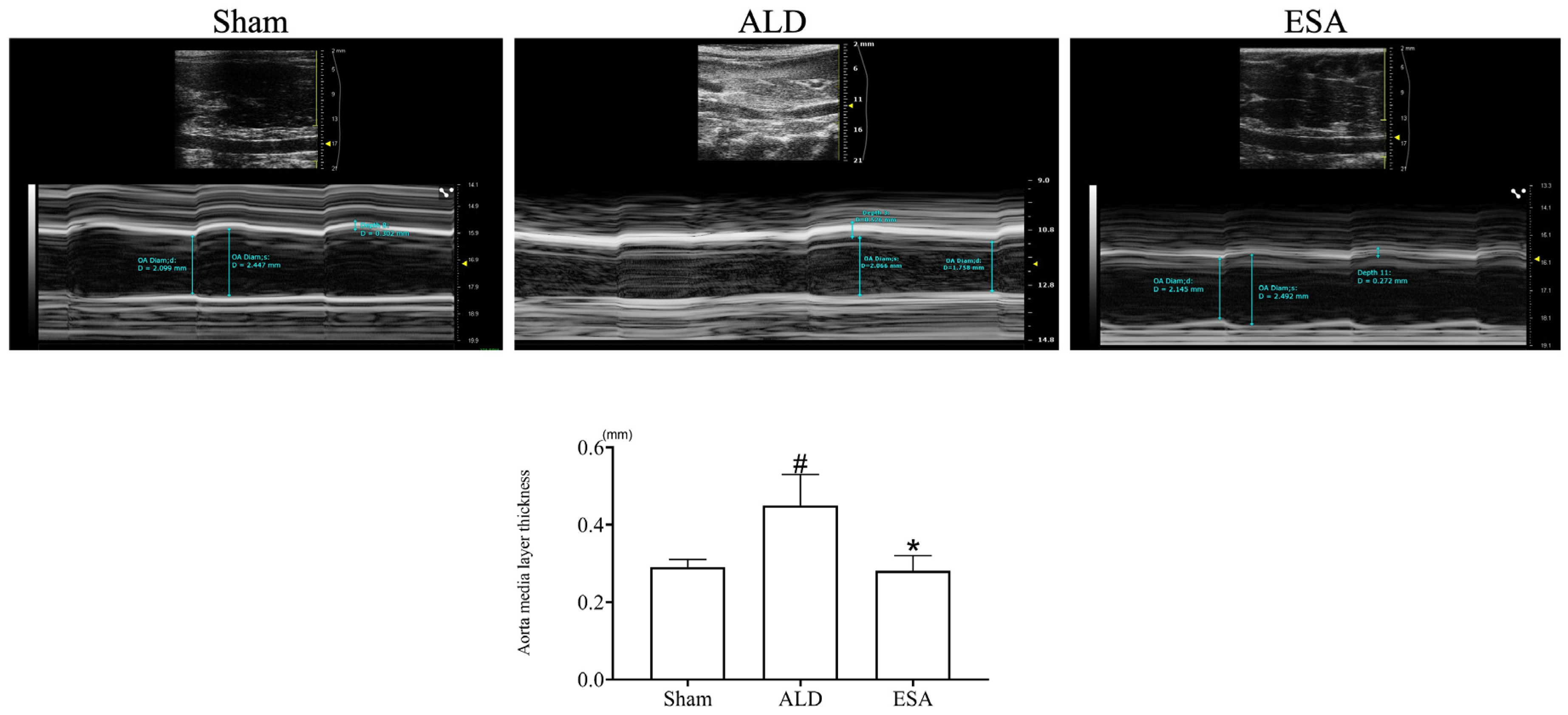
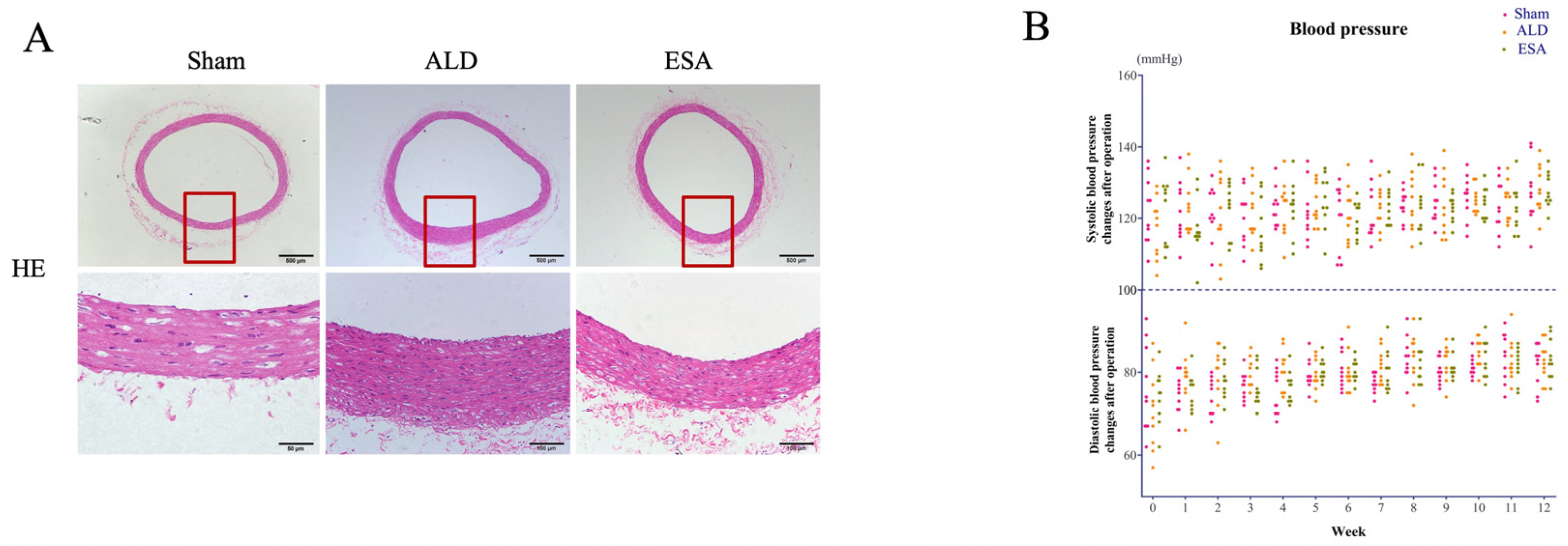
2.1. Aldosterone Induced Inflammatory Vascular Lesions
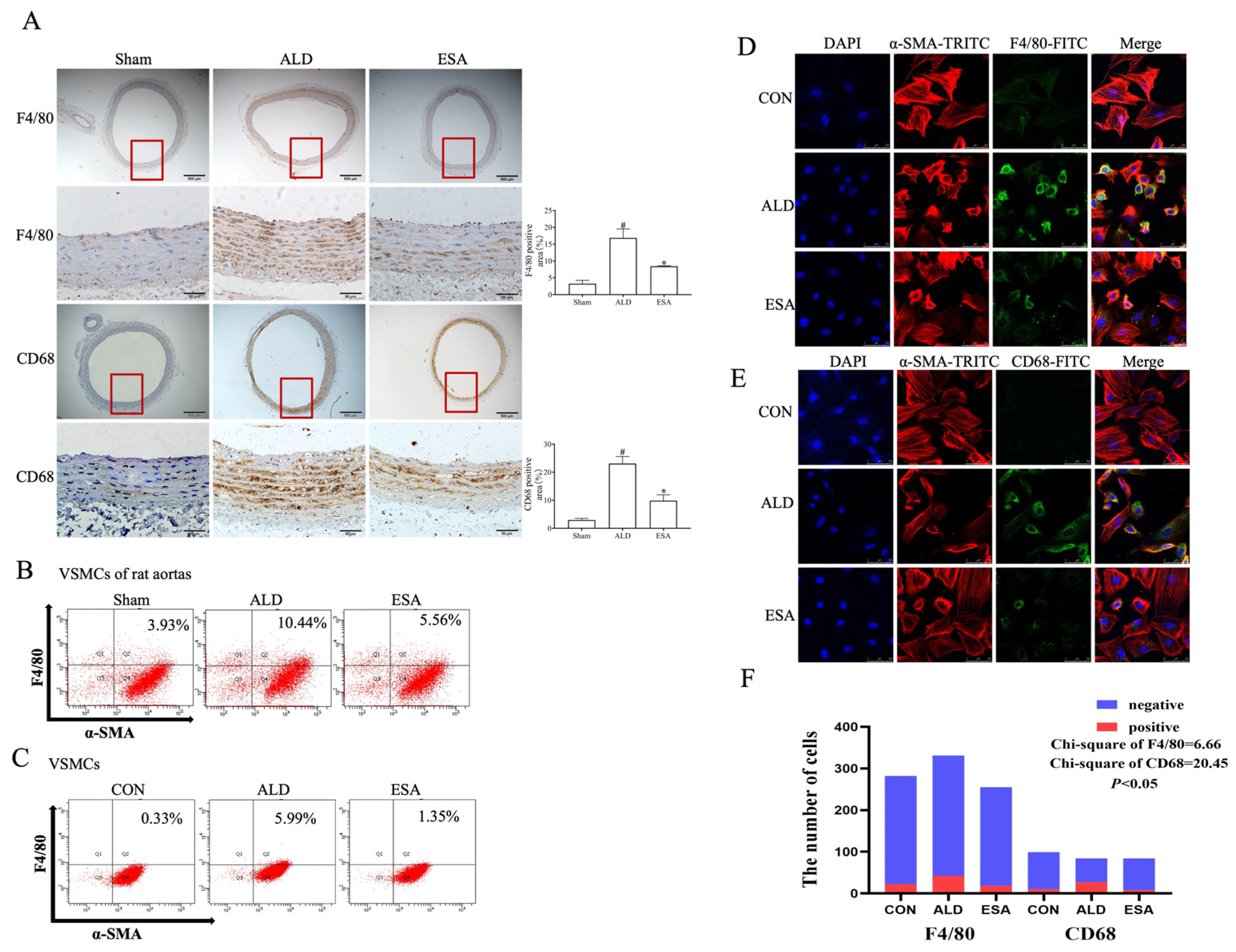
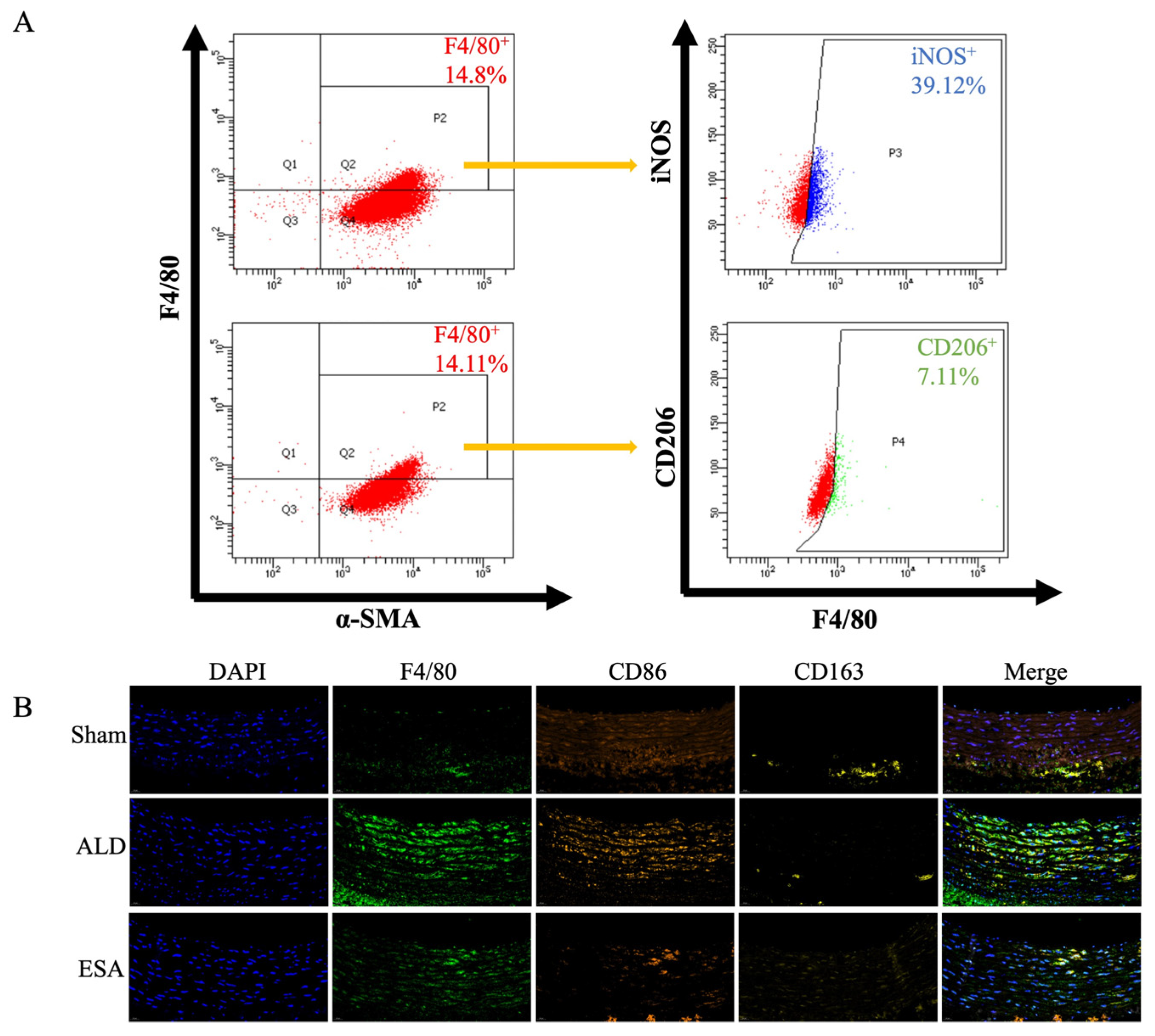
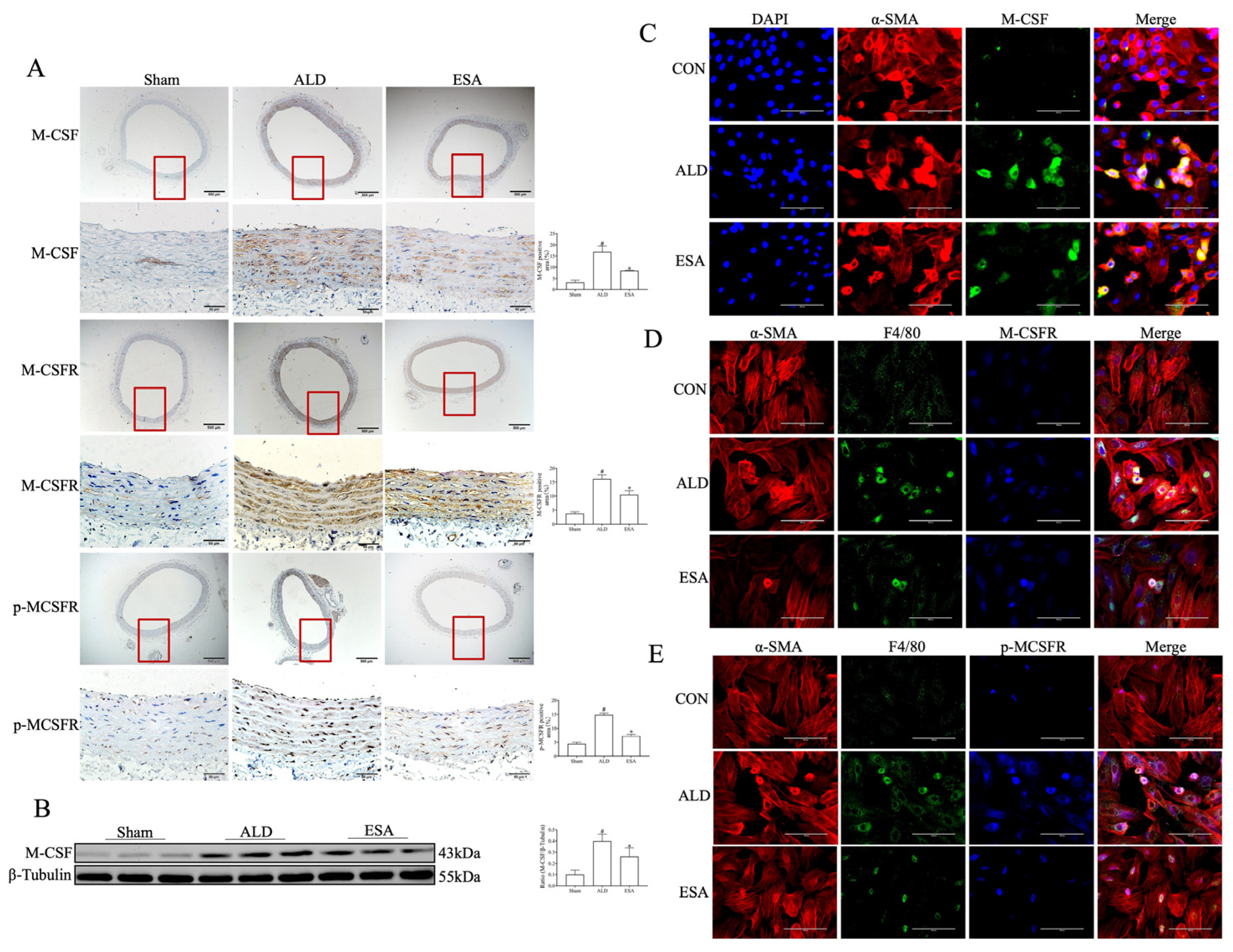
2.2. Transformation of VSMCs and Detection of the Macrophage-like Cell Type
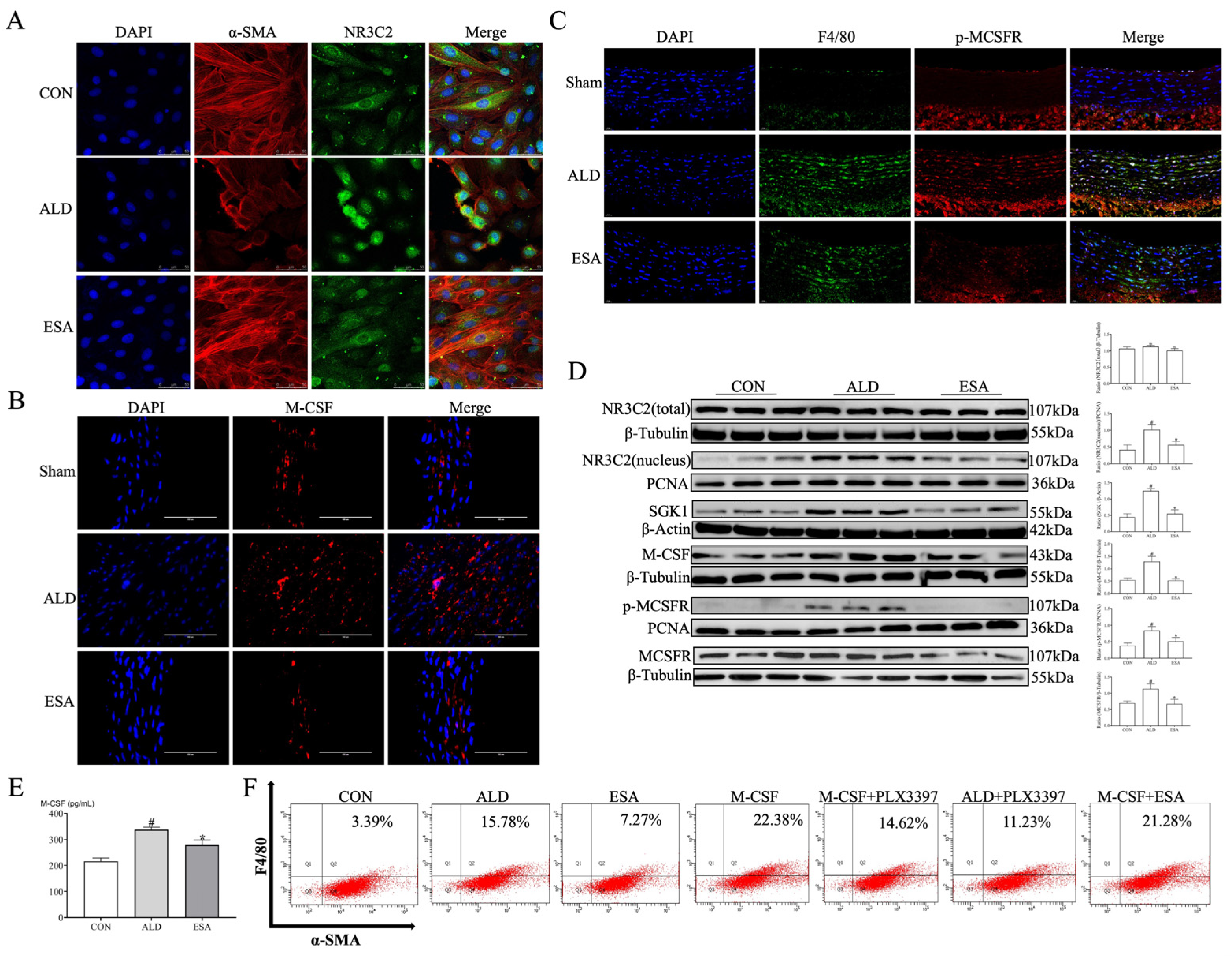
2.3. The MR/M-CSF Pathway-Mediated Transformation of VSMCs into Macrophage-like Cells Is Induced by Aldosterone
3. Discussion
4. Materials and Methods
4.1. Animals and Study Design
4.2. Blood Pressure
4.3. Intravascular Ultrasound Imaging of the Aorta
4.4. In Vitro Cell Culture Assays
4.5. Histopathological and Immunohistochemical Analyses
4.6. Immunofluorescence Staining
4.7. Cellular Immunofluorescence Analysis
4.8. Protein Extraction and Western Blot Analysis
4.9. Enzyme-Linked Immunosorbent Assays (ELISAs)
4.10. Flow Cytometry
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VSMCs | vascular smooth muscle cells |
| M-CSF | macrophage colony-stimulating factor |
| MR | mineralocorticoid receptor |
| RAAS | renin–angiotensin–aldosterone system |
| TNF-α | tumor necrosis factor-α |
| MCP-1 | monocyte chemoattractant protein-1 |
| IL-1β | interleukin-1β |
| M-CSFR | macrophage colony-stimulating factor receptor |
| CVD | cardiovascular disease |
| CHD | coronary heart disease |
| MRs | mineralocorticoid receptors |
| MRAs | mineralocorticoid receptor antagonists |
| Sham | Sham operation group |
| ALD | aldosterone group |
| ESA | esaxerenone group |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | fetal bovine serum |
| DMSO | dimethyl sulfoxide |
| PFA | paraformaldehyde |
| DAPI | 4′,6-diamino-2-phenylindole |
| RIPA | radioimmunoprecipitation assay |
| PVDF | polyvinylidene fluoride |
| HRP | horseradish peroxidase |
| PBS | phosphate-buffered saline |
| ANOVA | analysis of variance |
| α-SMA | α-smooth muscle actin |
| SM-MHC | smooth muscle myosin heavy chain |
| SM22α | smooth muscle 22α |
| ICAM-1 | intercellular cell adhesion molecule-1 |
| TNF | tumor necrosis factor |
| PDGF | platelet-derived growth factor |
| PIGF | placental growth factor |
| Gal-3 | galectin 3 |
| PIT-1 | sodium-dependent phosphate transporter |
| NR3C2 | nuclear receptor subfamily 3, group C, member 2 |
| HSP90 | heat shock protein 90 |
| MRA | aldosterone receptor antagonist |
| SGK1 | serum- and glucocorticoid-inducible kinase 1 |
References
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front. Immunol. 2022, 13, 1098725. [Google Scholar]
- Shi, J.; Yang, Y.; Cheng, A.; Xu, G.; He, F. Metabolism of vascular smooth muscle cells in vascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H613–H631. [Google Scholar]
- Elmarasi, M.; Elmakaty, I.; Elsayed, B.; Elsayed, A.; Zein, J.A.; Boudaka, A.; Eid, A.H. Phenotypic switching of vascular smooth muscle cells in atherosclerosis, hypertension, and aortic dissection. J. Cell. Physiol. 2024, 239, e31200. [Google Scholar]
- Miano, J.M.; Fisher, E.A.; Majesky, M.W. Fate and State of Vascular Smooth Muscle Cells in Atherosclerosis. Circulation 2021, 143, 2110–2116. [Google Scholar]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam Cells in Atherosclerosis: Novel Insights into Its Origins, Consequences, and Molecular Mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar]
- Liu, Y.X.; Yuan, P.Z.; Wu, J.H.; Hu, B. Lipid accumulation and novel insight into vascular smooth muscle cells in atherosclerosis. J. Mol. Med. 2021, 99, 1511–1526. [Google Scholar] [PubMed]
- Biros, E.; Reznik, J.E.; Moran, C.S. Role of inflammatory cytokines in genesis and treatment of atherosclerosis. Trends Cardiovasc. Med. 2022, 32, 138–142. [Google Scholar]
- Xu, X.; Xu, X.D.; Ma, M.Q.; Liang, Y.; Cai, Y.B.; Zhu, Z.X.; Xu, T.; Zhu, L.; Ren, K. The mechanisms of ferroptosis and its role in atherosclerosis. Biomed. Pharmacother. 2024, 171, 116112. [Google Scholar]
- Madrigal-Matute, J.; Rotllan, N.; Aranda, J.F.; Fernández-Hernando, C. MicroRNAs and atherosclerosis. Curr. Atheroscler. Rep. 2013, 15, 322. [Google Scholar] [CrossRef]
- Pu, Y.; Cheng, C.K.; Zhang, H.; Luo, J.Y.; Wang, L.; Tomlinson, B.; Huang, Y. Molecular mechanisms and therapeutic perspectives of peroxisome proliferator-activated receptor α agonists in cardiovascular health and disease. Med. Res. Rev. 2023, 43, 2086–2114. [Google Scholar] [PubMed]
- Wu, B.; Mottola, G.; Schaller, M.; Upchurch, G.R., Jr.; Conte, M.S. Resolution of vascular injury: Specialized lipid mediators and their evolving therapeutic implications. Mol. Asp. Med. 2017, 58, 72–82. [Google Scholar]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar]
- Andreeva, E.R.; Pugach, I.M.; Orekhov, A.N. Subendothelial smooth muscle cells of human aorta express macrophage antigen in situ and in vitro. Atherosclerosis 1997, 135, 19–27. [Google Scholar] [CrossRef]
- Buffolo, F.; Tetti, M.; Mulatero, P.; Monticone, S. Aldosterone as a Mediator of Cardiovascular Damage. Hypertension 2022, 79, 1899–1911. [Google Scholar]
- Bauersachs, J.; López-Andrés, N. Mineralocorticoid receptor in cardiovascular diseases-Clinical trials and mechanistic insights. Br. J. Pharmacol. 2022, 179, 3119–3134. [Google Scholar]
- Young, M.J.; Clyne, C.D. Mineralocorticoid receptor actions in cardiovascular development and disease. Essays Biochem. 2021, 65, 901–911. [Google Scholar] [CrossRef]
- Campbell, J.H.; Campbell, G.R. Smooth muscle phenotypic modulation—A personal experience. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1784–1789. [Google Scholar] [PubMed]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [PubMed]
- Liu, M.; Gomez, D. Smooth Muscle Cell Phenotypic Diversity. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Wirka, R.C.; Wagh, D.; Paik, D.T.; Pjanic, M.; Nguyen, T.; Miller, C.L.; Kundu, R.; Nagao, M.; Coller, J.; Koyano, T.K.; et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat. Med. 2019, 25, 1280–1289. [Google Scholar]
- Pan, H.; Xue, C.; Auerbach, B.J.; Fan, J.; Bashore, A.C.; Cui, J.; Yang, D.Y.; Trignano, S.B.; Liu, W.; Shi, J.; et al. Single-Cell Genomics Reveals a Novel Cell State During Smooth Muscle Cell Phenotypic Switching and Potential Therapeutic Targets for Atherosclerosis in Mouse and Human. Circulation 2020, 142, 2060–2075. [Google Scholar]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar]
- Lechartier, B.; Berrebeh, N.; Huertas, A.; Humbert, M.; Guignabert, C.; Tu, L. Phenotypic Diversity of Vascular Smooth Muscle Cells in Pulmonary Arterial Hypertension: Implications for Therapy. Chest 2022, 161, 219–231. [Google Scholar]
- Vengrenyuk, Y.; Nishi, H.; Long, X.; Ouimet, M.; Savji, N.; Martinez, F.O.; Cassella, C.P.; Moore, K.J.; Ramsey, S.A.; Miano, J.M.; et al. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 535–546. [Google Scholar] [CrossRef]
- Wu, J.H.; Zhang, L.; Nepliouev, I.; Brian, L.; Huang, T.; Snow, K.P.; Schickling, B.M.; Hauser, E.R.; Miller, F.J.; Freedman, N.J.; et al. Drebrin attenuates atherosclerosis by limiting smooth muscle cell transdifferentiation. Cardiovasc. Res. 2022, 118, 772–784. [Google Scholar]
- Gong, X.; Liu, Y.; Liu, H.; Cao, N.; Zeng, L.; Tian, M.; Zeng, C.; Hu, Y.; Zhang, R.; Chen, Y.; et al. Re-analysis of single-cell transcriptomics reveals a critical role of macrophage-like smooth muscle cells in advanced atherosclerotic plaque. Theranostics 2024, 14, 1450–1463. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; de Winther, M.P.; Weber, C.; Daemen, M.J.; Lutgens, E.; Soehnlein, O. Atherosclerotic plaque destabilization: Mechanisms, models, and therapeutic strategies. Circ. Res. 2014, 114, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, Hypertension, and Cardiac Dysfunction: Novel Roles of Immunometabolism in Macrophage Activation and Inflammation. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, L.; Wang, K.; Zhou, X.; Duan, M.; Wang, X.; Zhang, Z.; Liu, X. Ubiquitin Carboxyl Terminal Hydrolase L1 Attenuates TNF-α-Mediated Vascular Smooth Muscle Cell Migration Through Suppression of NF-κB Activation. Int. Heart J. 2018, 59, 1409–1415. [Google Scholar] [CrossRef]
- Simpson, S.A.; Tait, J.F.; Wettstein, A.; Neher, R.; Von Euw, J.; Reichstein, T. Isolation from the adrenals of a new crystalline hormone with especially high effectiveness on mineral metabolism. Experientia 1953, 9, 333–335. [Google Scholar] [CrossRef]
- Treesaranuwattana, T.; Wong, K.Y.H.; Brooks, D.L.; Tay, C.S.; Williams, G.H.; Williams, J.S.; Pojoga, L.H. Lysine-Specific Demethylase-1 Deficiency Increases Agonist Signaling via the Mineralocorticoid Receptor. Hypertension 2020, 75, 1045–1053. [Google Scholar] [CrossRef]
- Gromotowicz-Poplawska, A.; Flaumenhaft, R.; Gholami, S.K.; Merrill-Skoloff, G.; Chabielska, E.; Williams, G.H.; Romero, J.R. Enhanced Thrombotic Responses Are Associated with Striatin Deficiency and Aldosterone. J. Am. Heart Assoc. 2021, 10, e022975. [Google Scholar] [CrossRef]
- DuPont, J.J.; Jaffe, I.Z. 30 Years of The Mineralocorticoid Receptor: The role of the mineralocorticoid receptor in the vasculature. J. Endocrinol. 2017, 234, T67–T82. [Google Scholar] [CrossRef]
- McGraw, A.P.; Bagley, J.; Chen, W.S.; Galayda, C.; Nickerson, H.; Armani, A.; Caprio, M.; Carmeliet, P.; Jaffe, I.Z. Aldosterone increases early atherosclerosis and promotes plaque inflammation through a placental growth factor-dependent mechanism. J. Am. Heart Assoc. 2013, 2, e000018. [Google Scholar] [CrossRef]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; de Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef]
- Voelkl, J.; Alesutan, I.; Leibrock, C.B.; Quintanilla-Martinez, L.; Kuhn, V.; Feger, M.; Mia, S.; Ahmed, M.S.; Rosenblatt, K.P.; Kuro, O.M.; et al. Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice. J. Clin. Investig. 2013, 123, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K.; Miikeda, A.; Fouladian, Z.; Mehrabian, M.; Edillor, C.; Shih, D.; Zhou, Z.; Paul, M.K.; Charugundla, S.; Davis, R.C.; et al. Local M-CSF (Macrophage Colony-Stimulating Factor) Expression Regulates Macrophage Proliferation and Apoptosis in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 220–233. [Google Scholar]
- Xiang, C.; Li, H.; Tang, W. Targeting CSF-1R represents an effective strategy in modulating inflammatory diseases. Pharmacol. Res. 2023, 187, 106566. [Google Scholar]
- Sjaarda, J.; Gerstein, H.; Chong, M.; Yusuf, S.; Meyre, D.; Anand, S.S.; Hess, S.; Paré, G. Blood CSF1 and CXCL12 as Causal Mediators of Coronary Artery Disease. J. Am. Coll. Cardiol. 2018, 72, 300–310. [Google Scholar]
- Shaposhnik, Z.; Wang, X.; Lusis, A.J. Arterial colony stimulating factor-1 influences atherosclerotic lesions by regulating monocyte migration and apoptosis. J. Lipid Res. 2010, 51, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, Z.; Daugherty, A.; Cassis, L.A.; Pearson, K.J.; Gong, M.C.; Guo, Z. Mineralocorticoid receptor agonists induce mouse aortic aneurysm formation and rupture in the presence of high salt. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1568–1579. [Google Scholar] [PubMed]
- Fuller, P.J.; Yang, J.; Young, M.J. 30 Years of The Mineralocorticoid Receptor: Coregulators as mediators of mineralocorticoid receptor signalling diversity. J. Endocrinol. 2017, 234, T23–T34. [Google Scholar]
- Xian, Y.; Wang, X.; Chang, Y.; Qiang, P.; Han, Y.; Hao, J.; Gao, X.; Shimosawa, T.; Xu, Q.; Yang, F. Esaxerenone Attenuates Aldosterone-Induced Mitochondrial Damage-Mediated Pyroptosis in Mouse Aorta and Rat Vascular Smooth Muscle Cells. Life 2024, 14, 967. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Liu, Z.; Chang, Y.; Lv, R.; Guo, H.; Qiang, P.; Shimosawa, T.; Xu, Q.; Yang, F. Aldosterone-Induced Transformation of Vascular Smooth Muscle Cells into Macrophage-like Cells Participates in Inflammatory Vascular Lesions. Int. J. Mol. Sci. 2025, 26, 3345. https://doi.org/10.3390/ijms26073345
Zhang B, Liu Z, Chang Y, Lv R, Guo H, Qiang P, Shimosawa T, Xu Q, Yang F. Aldosterone-Induced Transformation of Vascular Smooth Muscle Cells into Macrophage-like Cells Participates in Inflammatory Vascular Lesions. International Journal of Molecular Sciences. 2025; 26(7):3345. https://doi.org/10.3390/ijms26073345
Chicago/Turabian StyleZhang, Boya, Ziqian Liu, Yi Chang, Ruyan Lv, Haixia Guo, Panpan Qiang, Tatsuo Shimosawa, Qingyou Xu, and Fan Yang. 2025. "Aldosterone-Induced Transformation of Vascular Smooth Muscle Cells into Macrophage-like Cells Participates in Inflammatory Vascular Lesions" International Journal of Molecular Sciences 26, no. 7: 3345. https://doi.org/10.3390/ijms26073345
APA StyleZhang, B., Liu, Z., Chang, Y., Lv, R., Guo, H., Qiang, P., Shimosawa, T., Xu, Q., & Yang, F. (2025). Aldosterone-Induced Transformation of Vascular Smooth Muscle Cells into Macrophage-like Cells Participates in Inflammatory Vascular Lesions. International Journal of Molecular Sciences, 26(7), 3345. https://doi.org/10.3390/ijms26073345





