Intracranial Aneurysm Biomarkers: A Convergence of Genetics, Inflammation, Oxidative Stress, and the Extracellular Matrix
Abstract
1. Background
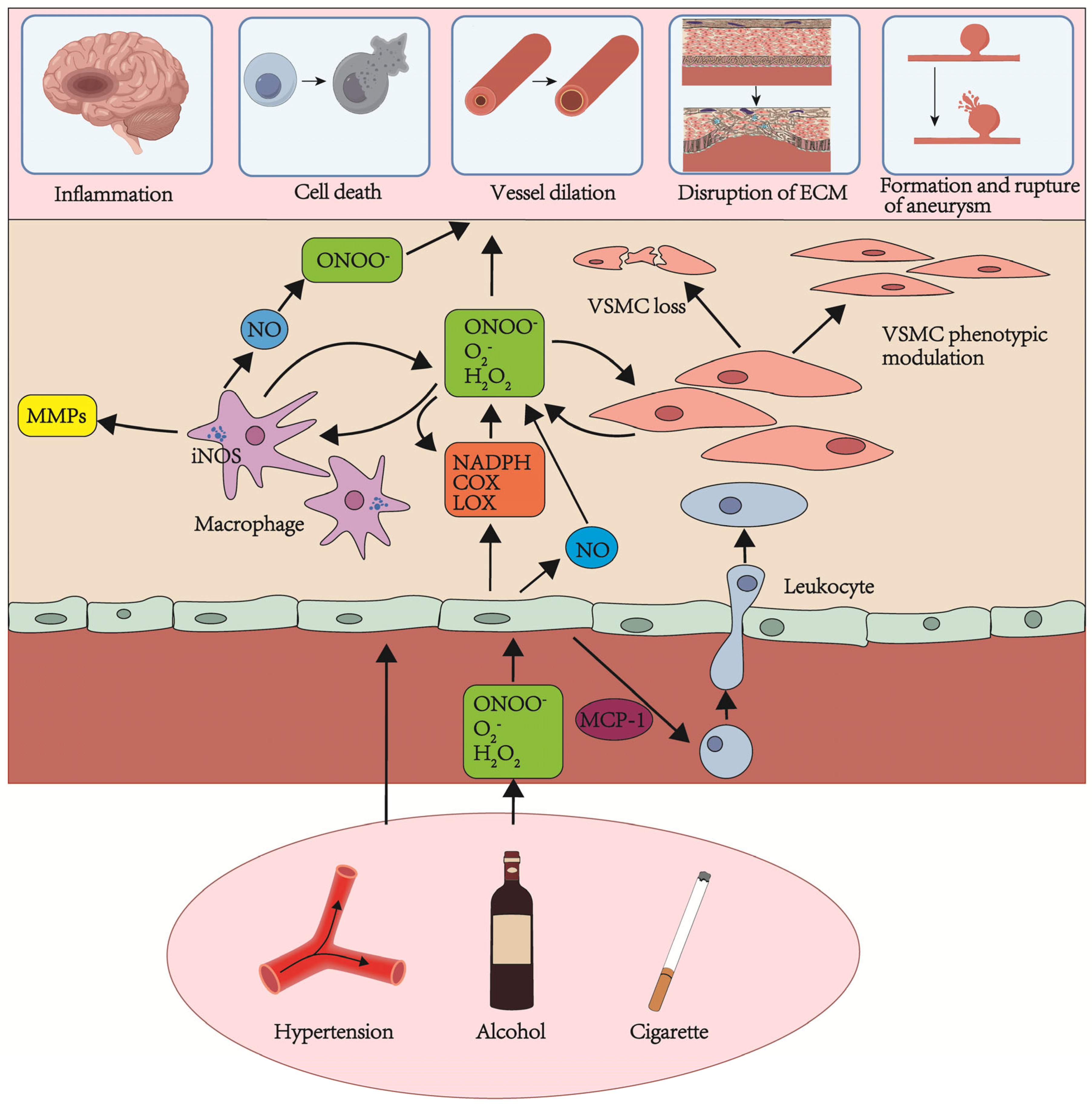
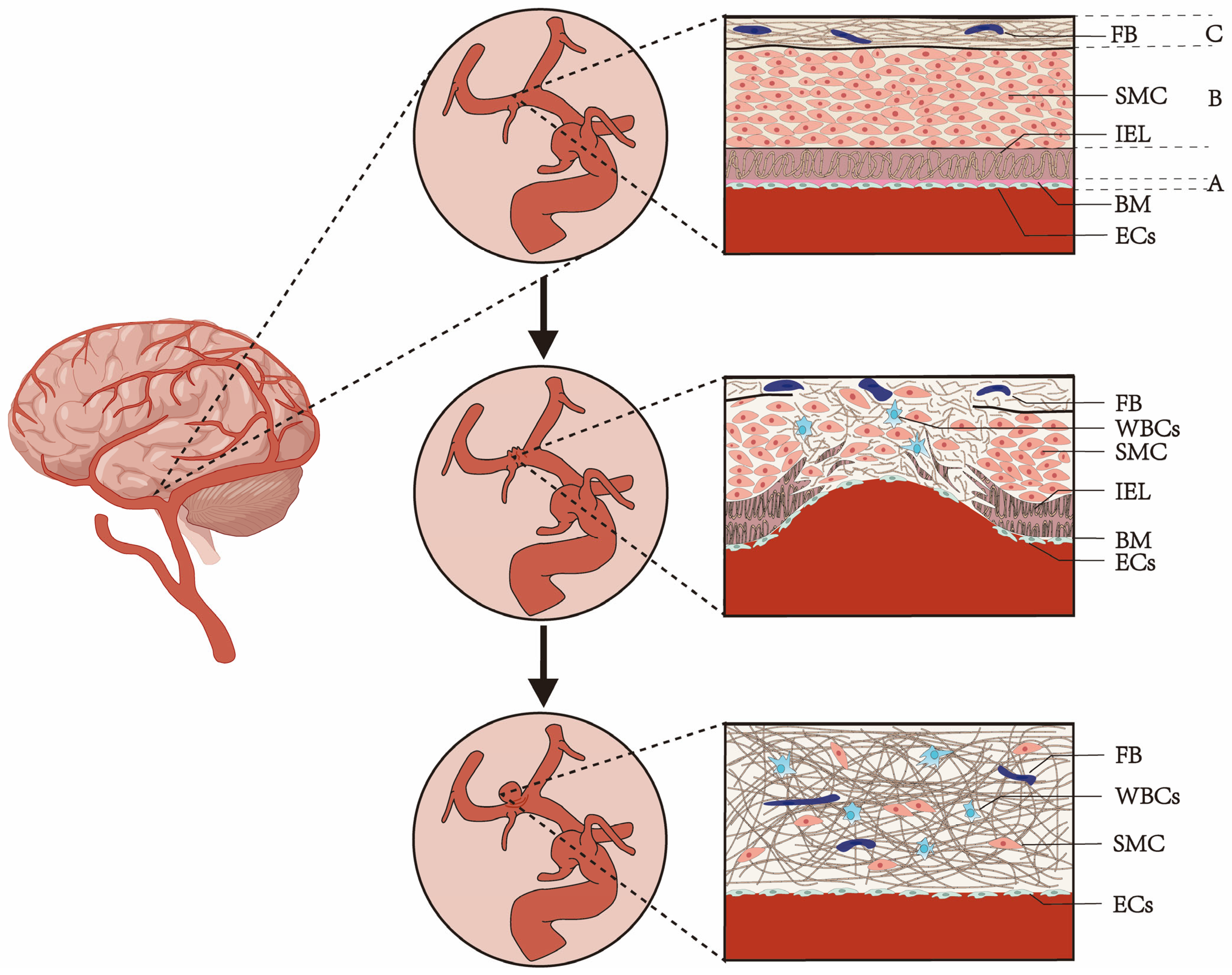
2. The Risk Factors and Causes of Aneurysm Formation and Rupture
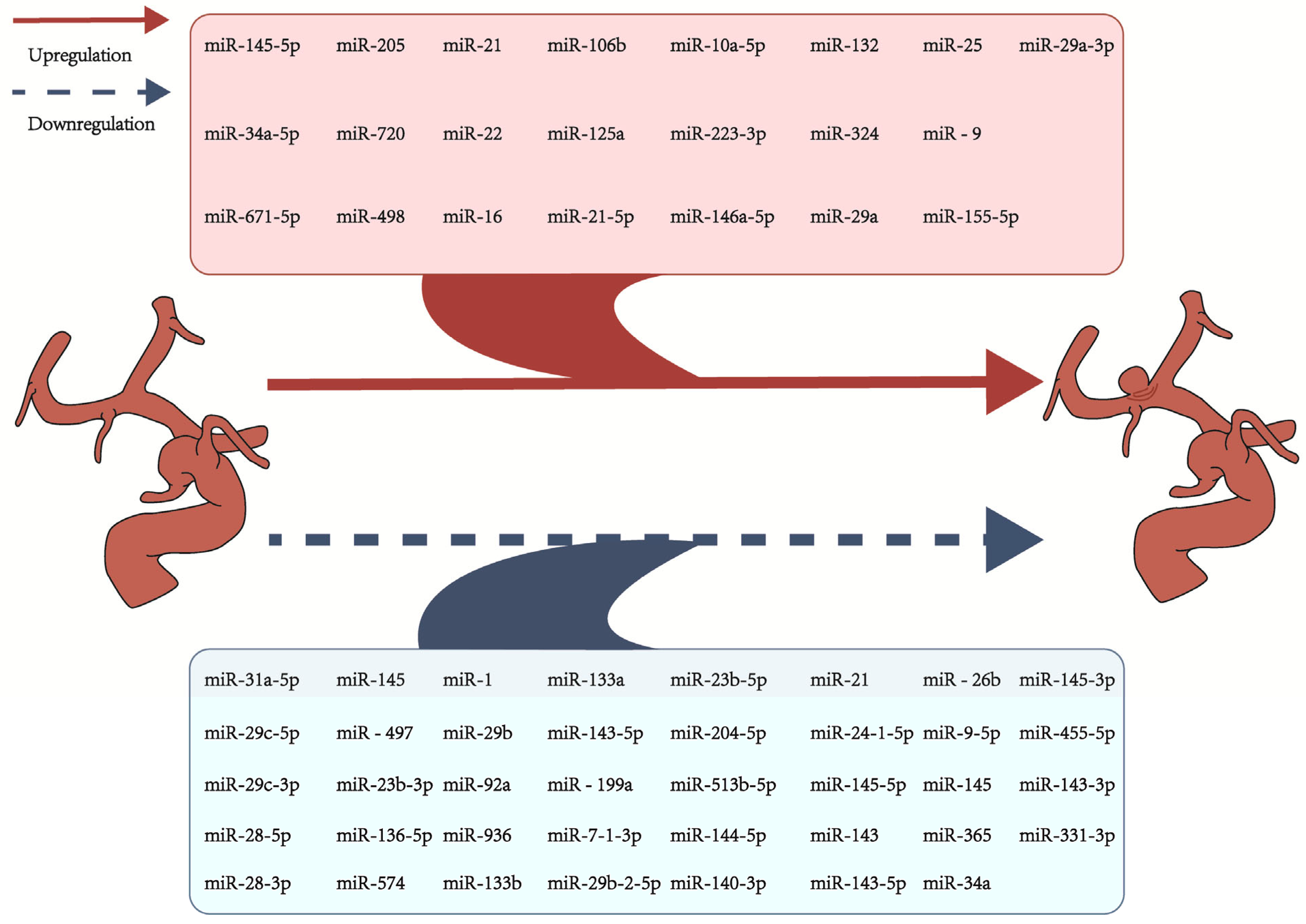
3. Research Progress on Biomarkers for Intracranial Aneurysms
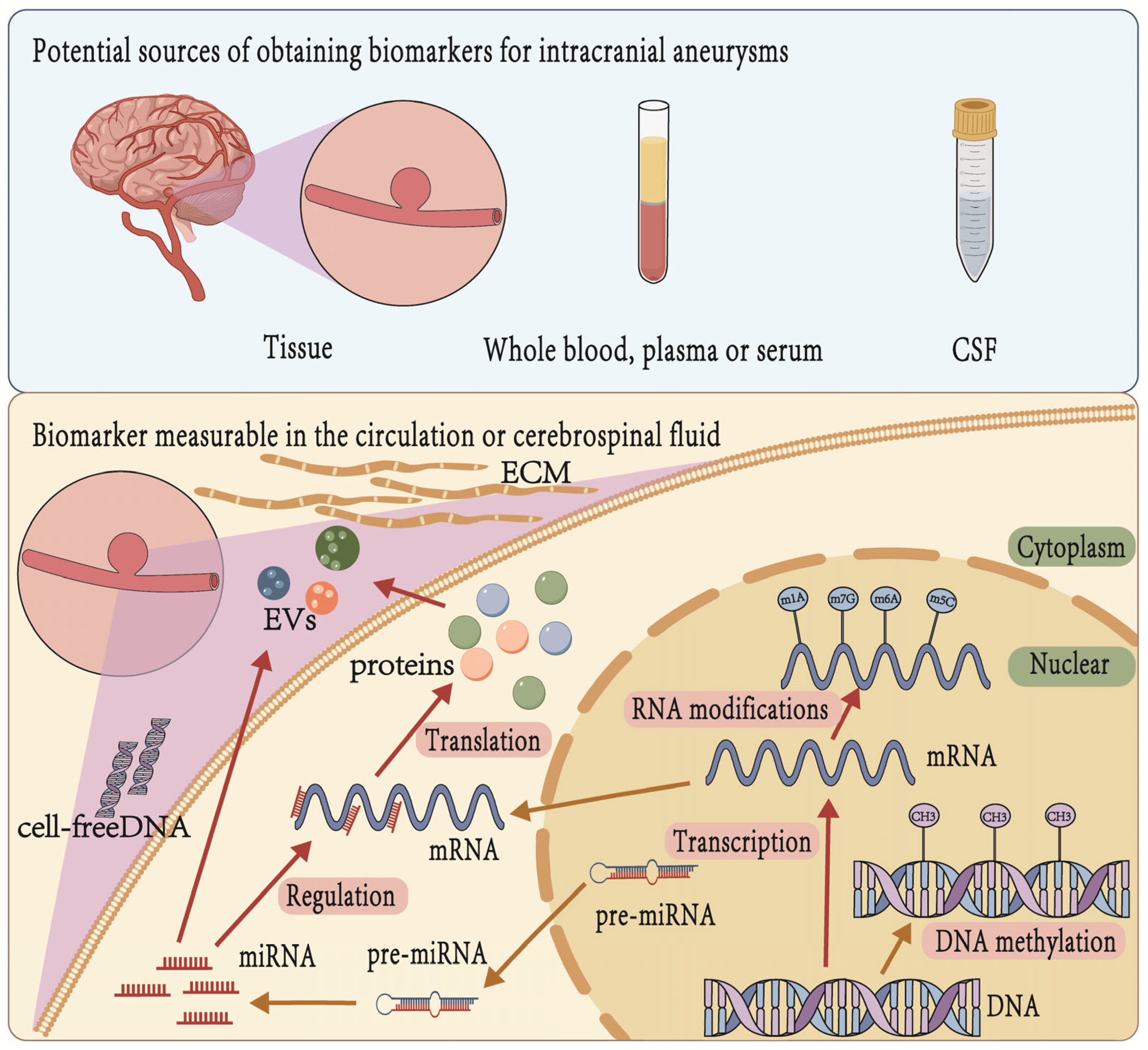
4. Sources of Biomarkers for IA
5. DNA
| Reference | Biomarker | Sample Source | n | Time of Take | Methodology | IA vs. Control | RIA vs. UIA |
|---|---|---|---|---|---|---|---|
| Zhong et al. [49] | miR-205 | human blood | 91 | pretreatment | qRT-PCR | ↑ | |
| Feng et al. [50] | miR-155-5p | rats | NA | pre/posttreatment | qRT-PCR | ↑ | |
| Yu et al. [51] | miR-31a-5p | rats | NA | pre/posttreatment | qRT-PCR | ↓ | |
| Xiong et al. [52] | miR-125a | human blood | 50 | pretreatment | qRT-PCR | ↑ | |
| Holcomb et al. [53] | miR-1 | rabbit | 6 | posttreatment | Sequencing | ↓ | |
| miR-9-5p | rabbit | 6 | posttreatment | Sequencing | ↓ | ||
| miR-204-5p | rabbit | 6 | posttreatment | Sequencing | ↓ | ||
| miR-10a-5p | rabbit | 6 | posttreatment | Sequencing | ↑ | ||
| miR-21-5p | rabbit | 6 | posttreatment | Sequencing | ↑ | ||
| miR-34a-5p | rabbit | 6 | posttreatment | Sequencing | ↑ | ||
| miR-146a-5p | rabbit | 6 | posttreatment | Sequencing | ↑ | ||
| miR-223-3p | rabbit | 6 | posttreatment | Sequencing | ↑ | ||
| Jiang et al. [54] | miR-1 | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | |
| miR-7-1-3p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-23b-5p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-23b-3p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-24-1-5p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-28-5p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-28-3p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-29b-2-5p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-29c-5p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-29c-3p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-133a | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-133b | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-140-3p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-143-5p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-143-3p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-145-5p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-145-3p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| miR-455-5p | IA tissues | 14 | intraoperative | qRT-PCR | ↓ | ||
| Zhao et al. [55] | miR-29a | human blood | 24 | pre/posttreatment | qRT-PCR | ↑ | |
| Zheng et al. [56] | miR-513b-5p | human serum | 100 | pretreatment | qRT-PCR | ↓ | ↓ |
| Fan et al. [57] | miR-331-3p | IA tissues | 96 | intraoperative | RT-qPCR | ↓ | |
| Wang et al. [25] | miR-29a | human plasma | 165 | pretreatment | qRT-PCR | ↑ | |
| Bekelis et al. [58] | miR-21 | IA tissues | 7 | intraoperative | sequencing | ↑ | |
| miR-143-5p | IA tissues | 7 | intraoperative | sequencing | ↓ | ||
| miR-145 | IA tissues | 7 | intraoperative | sequencing | ↓ | ||
| Lv et al. [59] | miR-136-5p | IA tissues | 82 | intraoperative | qRT-PCR | ↓ | |
| Cai et al. [60] | miR-92a | IA tissues | 90 | intraoperative | qRT-PCR | ↓ | |
| Liu et al. [61] | miR-29b | IA tissues | 6 | intraoperative | qRT-PCR | ↓ | |
| Guo et al. [62] | miR-23b-3p | IA tissues | 32 | intraoperative | qRT-PCR | ↓ | |
| Jin et al. [63] | miR-22 | human plasma | 24 | pretreatment | PCA | ↑ | |
| miR-671-5p | human plasma | 24 | pretreatment | PCA | ↑ | ||
| miR-720 | human plasma | 24 | pretreatment | PCA | ↑ | ||
| miR-365 | human plasma | 24 | pretreatment | PCA | ↓ | ||
| miR-498 | human plasma | 24 | pretreatment | PCA | ↑ | ||
| miR-574 | human plasma | 24 | pretreatment | PCA | ↓ | ||
| miR-106b | human plasma | 24 | pretreatment | PCA | ↑ | ||
| miR-21 | human plasma | 24 | pretreatment | PCA | ↑ | ||
| miR-936 | human plasma | 24 | pretreatment | PCA | ↓ | ||
| Su et al. [64] | miR-132 | human plasma | 58 | pretreatment | qRT-PCR | ↑ | |
| miR-324 | human plasma | 58 | pretreatment | qRT-PCR | ↑ | ||
| Supriya et al. [65] | miR-26b | IA tissues | 29 | intraoperative | qRT-PCR | ↓ | |
| miR-199a | IA tissues | 29 | intraoperative | qRT-PCR | ↓ | ||
| miR-497 | IA tissues | 29 | intraoperative | qRT-PCR | ↓ | ||
| miR-365 | IA tissues | 29 | intraoperative | qRT-PCR | ↓ | ||
| Luo et al. [66] | miR-9 | IA tissues | 13 | intraoperative | qRT-PCR | ↑ | |
| Yang et al. [67] | miR-144-5p | human plasma | 84 | pretreatment | qRT-PCR | ↓ | |
| Liao et al. [68] | miR-145-5p | human plasma | 12 | pretreatment | qRT-PCR | ↑ | |
| miR-29a-3p | human plasma | 12 | pretreatment | qRT-PCR | ↑ | ||
| Li et al. [69] | miR-16 | human plasma | 40 | pretreatment | qRT-PCR | ↑ | |
| miR-25 | human plasma | 40 | pretreatment | qRT-PCR | ↑ | ||
| Zou et al. [70] | miR-34a | human blood | 20 | intraoperative | qRT-PCR | ↓ | |
| Xu et al. [71] | miR-143 | human serum | 30 | intraoperative | qRT-PCR | ↓ | |
| miR-145 | human serum | 30 | intraoperative | qRT-PCR | ↓ | ||
| Yuan et al. [72] | miR-34a | human serum | 20 | intraoperative | qRT-PCR | ↓ | |
| Zhao et al. [45] | Methyl MAP3K10 | human plasma | 96 | pretreatment | MIRA-seq | ↓ | |
| Xu et al. [46] | Methyl GSTA4 | human blood | 44 | pretreatment | MIRA-seq | ↓ | |
| Zhou et al. [47] | Methyl PNPLA6 | human plasma | 96 | pretreatment | MIRA-seq | ↑ | |
| Roder et al. [37] | BCL2 | / | 30 | pretreatment | MIRA-seq | ↓ | |
| COL1A2 | / | 30 | pretreatment | MIRA-seq | ↑ | ||
| COL3A1 | / | 30 | pretreatment | MIRA-seq | ↑ | ||
| COL5A2 | / | 30 | pretreatment | MIRA-seq | ↑ | ||
| CXCL12 | / | 30 | pretreatment | MIRA-seq | ↓ | ||
| TIMP4 | / | 30 | pretreatment | MIRA-seq | ↓ | ||
| TNC | / | 30 | pretreatment | MIRA-seq | ↓ | ||
| Maimaiti et al. [48] | DNMT3A | / | 156 | pretreatment | MIRA-seq | ↓ | |
| MBD2 | / | 156 | pretreatment | MIRA-seq | ↑ |
6. RNA
7. Proteins
7.1. Inflammation-Related Proteins
| Reference | Biomarker | Sample Source | n | Time of Take | Methodology | IA vs. Control | RIA vs. UIA |
|---|---|---|---|---|---|---|---|
| Xiong et al. [52] | ET-1 | blood | 50 | pretreatment | WB | ↑ | |
| Lv et al. [59] | KDM1A | IA tissues | 82 | intraoperative | WB | ↑ | |
| Cai et al. [60] | HDAC9 | IA tissues | 90 | intraoperative | WB | ↑ | |
| BCL2L11 | IA tissues | 90 | intraoperative | WB | ↑ | ||
| Fan et al. [57] | TNF-α | IA tissues | 96 | intraoperative | WB | ↑ | |
| CD14 | IA tissues | 96 | intraoperative | WB | ↑ | ||
| Zheng et al. [56] | COL1A1 | serum | 100 | pretreatment | WB | ↑ | ↑ |
| COL1A2 | serum | 100 | pretreatment | WB | ↑ | ↑ | |
| TNF-α | serum | 100 | pretreatment | WB | ↑ | ↑ | |
| IL-1β | serum | 100 | pretreatment | WB | ↑ | ↑ | |
| MMP-2 | serum | 100 | pretreatment | WB | ↑ | ↑ | |
| MMP-3 | serum | 100 | pretreatment | WB | ↑ | ↑ | |
| MMP-9 | serum | 100 | pretreatment | WB | ↑ | ↑ | |
| Guo et al. [62] | PTEN | IA tissues | 32 | intraoperative | WB | ↑ | |
| Xu et al. [46] | GSTA4 | serum | 44 | pretreatment | WB | ↑ | |
| Zhou et al. [47] | PNPLA6 | serum | 96 | pretreatment | WB | ↓ | |
| Zhang et al. [82] | IL-1β | blood | 66 | pretreatment | Bio-Plex protein array systems | ↑ | |
| MCP-1 | blood | 66 | pretreatment | Bio-Plex protein array systems | ↑ | ||
| TNF-α | blood | 66 | pretreatment | Bio-Plex protein array systems | ↑ | ||
| Yamaguchi et al. [93] | MMP-3 | IA tissues | 24 | pre/intraoperative | WB | ↑ | |
| IL-1β | IA tissues | 24 | pre/intraoperative | WB | ↑ | ||
| Zou et al. [70] | MMP-2 | IA tissues | 20 | pre/intraoperative | WB | ↑ | |
| Kamińska et al. [94] | MCP-1 | CSF | 25 | pretreatment | ELISA | ↑ | |
| IL-8 | CSF | 25 | pretreatment | ELISA | ↑ | ||
| Lai et al. [85] | TNF-α | serum | 108 | pretreatment | ELISA | ↑ | ↑ |
| MCP-1 | serum | 108 | pretreatment | ELISA | ↑ | ↑ | |
| IL-1β | serum | 108 | pretreatment | ELISA | ↑ | ↑ | |
| IL-6 | serum | 108 | pretreatment | ELISA | ↑ | ↑ | |
| NF-κB p65 | serum | 108 | pretreatment | ELISA | ↑ | ||
| Kamińska et al. [95] | NF-κB p65 | CSF, serum | 25 | pretreatment | ELISA | ↓ | |
| CXCL1 | CSF, serum | 25 | pretreatment | ELISA | ↑ | ||
| CXCR2 | CSF, serum | 25 | pretreatment | ELISA | ↑ | ||
| Aoki et al. [96] | COX-2 | mice IA tissues | 5 | pretreatment | WB | ↑ | |
| mPGES1 | mice IA tissues | 5 | pretreatment | WB | ↑ | ||
| Sun et al. [97] | NOX4 | IA tissues | 27 | pretreatment | WB | ↑ | |
| p22phox | IA tissues | 27 | pretreatment | WB | ↑ | ||
| p47phox | IA tissues | 27 | pretreatment | WB | ↑ | ||
| TRPC6 | IA tissues | 27 | pretreatment | WB | ↑ | ||
| CN | IA tissues | 27 | pretreatment | WB | ↑ | ||
| NFATC1 | IA tissues | 27 | pretreatment | WB | ↑ | ||
| MMP-2 | IA tissues | 27 | pretreatment | WB | ↑ | ||
| MCP-1 | IA tissues | 27 | pretreatment | WB | ↑ | ||
| Aoki et al. [98] | procollagen type I | IA tissues | 6 | pretreatment | WB | ↓ | |
| procollagen type III | IA tissues | 6 | pretreatment | WB | ↓ |
7.2. Oxidative Stress-Related Proteins
7.3. Extracellular Matrix-Associated Proteins
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Abdominal aortic aneurysm |
| BCL2 | B-cell lymphoma-2 |
| CASP1/ICE | Caspase 1 |
| CD14 | Cluster of differentiation 14 |
| CNS | Central nervous system |
| COL1A1 | Collagen type I alpha 1 chain |
| COL1A2 | Collagen type I alpha 2 chain |
| COL3A1 | Collagen type III alpha 1 chain |
| COL5A1 | Collagen type V alpha 1 chain |
| COL5A2 | Collagen type V alpha 2 chain |
| COX-1 | Cyclooxygenase-1 |
| COX-2 | Cyclooxygenase-2 |
| CSF | Cerebrospinal fluid |
| CTA | Computed tomography angiography |
| CXCL12 | Chemokine (C-X-C motif) ligand 12 |
| CXCR3 | CXC chemokine receptor 3 |
| DNMT3A | DNA methyltransferase 3 alpha |
| DPP-4 | Dipeptidyl peptidase-4 |
| DSA | Digital subtraction angiography |
| ECM | Extracellular matrix |
| ECs | Endothelial cells |
| EDNRA | Endothelin receptor type A |
| ELISA | Enzyme-linked immunosorbent assay |
| eNOS | Endothelial nitric oxide synthase |
| ET-1 | Endothelin-1 |
| EVs | Extracellular vesicles |
| FAS | Fatty acid synthase |
| FTO | Fat mass and obesity-associated protein |
| GEO | Gene expression omnibus |
| gp130 | Glycoprotein 130 |
| GREM1 | Gremlin 1 |
| GSTA4 | Glutathione S-transferase alpha 4 |
| GWAS | Genome-wide association study |
| H2O2 | Hydrogen peroxide |
| HBVSMCs | Human brain vascular smooth muscle cells |
| HDAC9 | Histone deacetylase 9 |
| IA | Intracranial aneurysm |
| IAs | Intracranial aneurysms |
| IEL | Internal elastic lamina |
| IFN-γ | Interferon-gamma |
| IGF2BP1 | Insulin-like growth factor 2 mRNA binding protein 1 |
| IGF2BP3 | Insulin-like growth factor 2 mRNA binding protein 3 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| iNOS | Inducible NOS |
| KLF5 | Kruppel-like factor 5 |
| LOX | Lipoxygenase |
| KDM1A | Lysine-specific demethylase 1 |
| LRPPRC | Leucine-rich pentatricopeptide repeat containing |
| m1A | N1-methyladenosine |
| m5C | 2′-O-methylation, 5-methylcytosine |
| m6A | N6-methyladenosine |
| MAP3K10 | Mitogen-activated protein kinase 10 |
| MBD2 | Methyl-CpG-binding domain protein 2 |
| MCL-1 | Myeloid leukemia 1 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| Methyl | Methylation |
| MIRA-seq | Methylated-pyrosequencing |
| MMP-2 | Matrix metalloproteinase-2 |
| MMP-3 | Matrix metalloproteinase-3 |
| MMP-9 | Matrix metalloproteinase-9 |
| MMP-13 | Matrix metalloproteinase-13 |
| MMPs | Matrix metalloproteinases |
| MRA | Magnetic resonance angiography |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NFATC1 | Nuclear factor of activated T cell |
| NF-κB | Nuclear factor kappa-B |
| NLRP3 | The NOD-, LRR-, and pyrin domain-containing protein 3 |
| nNOS | Neuronal NOS |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NOX | Nicotinamide Adenine Dinucleotide Phosphate Oxidase |
| O2•- | Superoxide |
| ONOO•- | Peroxynitrite |
| PCA | Principal component analyzed |
| PGE2 | Prostaglandin E2 |
| PGG2 | Prostaglandin G2 |
| PMN | Polymorphonuclear neutrophils |
| PNPLA6 | Patatin-like phospholipase domain-containing protein 6 |
| PTEN | Phosphatase and tensin homolog |
| PTGS2/COX2 | Cyclooxygenase-2 |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RBM15 | RNA-binding motif protein 15 |
| RIA | Ruptured intracranial aneurysm |
| RNA-seq | RNA sequencing |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SAH | Subarachnoid hemorrhage |
| SERPINA3 | Serine protease inhibitor clade A member 3 |
| SMC | Smooth muscle cell |
| SMCs | Smooth muscle cells |
| SNPs | Single nucleotide polymorphisms |
| SOCS3 | Cytokine signaling suppressor 3 |
| TAM | Tumor-associated macrophage |
| TIMP4 | Tissue inhibitor of metalloproteinase 4 |
| TNC | Tenascin C |
| TNF-α | Tumor necrosis factor-alpha |
| TRPC6 | Transient receptor potential-6 |
| UIA | Unruptured intracranial aneurysm |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| VSMC | Vascular smooth muscle cell |
| WB | Western blotting |
| WSS | Wall shear stress |
| YTHDC1 | YT521-B homology domain-containing protein 1 |
| YTHDF2 | YT521-B homology domain family member 2 |
| YTHDF3 | YT521-B homology domain family member 2 |
| ZNF217 | Zinc finger protein 217 |
References
- Brown, R.D., Jr.; Broderick, J.P. Unruptured intracranial aneurysms: Epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014, 13, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Chalouhi, N.; Hoh, B.L.; Hasan, D. Review of cerebral aneurysm formation, growth, and rupture. Stroke 2013, 44, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Chen, S.W.; Li, Y.D.; Chen, Y.C.; Cheng, Y.S.; Hu, D.J.; Tan, H.Q.; Wu, Q.; Wang, W.; Sun, Z.K.; et al. Prevalence of unruptured cerebral aneurysms in Chinese adults aged 35 to 75 years: A cross-sectional study. Ann. Intern. Med. 2013, 159, 514–521. [Google Scholar] [CrossRef]
- Vernooij, M.W.; Ikram, M.A.; Tanghe, H.L.; Vincent, A.J.; Hofman, A.; Krestin, G.P.; Niessen, W.J.; Breteler, M.M.; van der Lugt, A. Incidental findings on brain MRI in the general population. N. Engl. J. Med. 2007, 357, 1821–1828. [Google Scholar] [CrossRef]
- Chason, J.L.; Hindman, W.M. Berry aneurysms of the circle of Willis; results of a planned autopsy study. Neurology 1958, 8, 41–44. [Google Scholar] [CrossRef]
- Inagawa, T.; Hirano, A. Autopsy study of unruptured incidental intracranial aneurysms. Surg. Neurol. 1990, 34, 361–365. [Google Scholar] [CrossRef]
- Rackauskaite, D.; Svanborg, E.; Andersson, E.; Löwhagen, K.; Csajbok, L.; Nellgård, B. Prospective study: Long-term outcome at 12–15 years after aneurysmal subarachnoid hemorrhage. Acta Neurol. Scand. 2018, 138, 400–407. [Google Scholar] [CrossRef]
- Etminan, N.; Rinkel, G.J. Unruptured intracranial aneurysms: Development, rupture and preventive management. Nat. Rev. Neurol. 2016, 12, 699–713. [Google Scholar] [CrossRef]
- Etminan, N.; Dreier, R.; Buchholz, B.A.; Beseoglu, K.; Bruckner, P.; Matzenauer, C.; Torner, J.C.; Brown, R.D., Jr.; Steiger, H.J.; Hänggi, D.; et al. Age of collagen in intracranial saccular aneurysms. Stroke 2014, 45, 1757–1763. [Google Scholar] [CrossRef]
- Sluijter, J.P.; Smeets, M.B.; Velema, E.; Pasterkamp, G.; de Kleijn, D.P. Increased collagen turnover is only partly associated with collagen fiber deposition in the arterial response to injury. Cardiovasc. Res. 2004, 61, 186–195. [Google Scholar] [CrossRef]
- Rowe, A.J.; Finlay, H.M.; Canham, P.B. Collagen biomechanics in cerebral arteries and bifurcations assessed by polarizing microscopy. J. Vasc. Res. 2003, 40, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Tutino, V.M.; Xiang, J.; Siddiqui, A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: Toward a unifying hypothesis. AJNR Am. J. Neuroradiol. 2014, 35, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Frösen, J.; Tulamo, R.; Paetau, A.; Laaksamo, E.; Korja, M.; Laakso, A.; Niemelä, M.; Hernesniemi, J. Saccular intracranial aneurysm: Pathology and mechanisms. Acta Neuropathol. 2012, 123, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Pradilla, G.; Wicks, R.T.; Hadelsberg, U.; Gailloud, P.; Coon, A.L.; Huang, J.; Tamargo, R.J. Accuracy of computed tomography angiography in the diagnosis of intracranial aneurysms. World Neurosurg. 2013, 80, 845–852. [Google Scholar] [CrossRef]
- Sailer, A.M.; Wagemans, B.A.; Nelemans, P.J.; de Graaf, R.; van Zwam, W.H. Diagnosing intracranial aneurysms with MR angiography: Systematic review and meta-analysis. Stroke 2014, 45, 119–126. [Google Scholar] [CrossRef]
- Etminan, N.; Buchholz, B.A.; Dreier, R.; Bruckner, P.; Torner, J.C.; Steiger, H.J.; Hänggi, D.; Macdonald, R.L. Cerebral aneurysms: Formation, progression, and developmental chronology. Transl. Stroke Res. 2014, 5, 167–173. [Google Scholar] [CrossRef]
- Alg, V.S.; Sofat, R.; Houlden, H.; Werring, D.J. Genetic risk factors for intracranial aneurysms: A meta-analysis in more than 116,000 individuals. Neurology 2013, 80, 2154–2165. [Google Scholar] [CrossRef]
- Korja, M.; Silventoinen, K.; McCarron, P.; Zdravkovic, S.; Skytthe, A.; Haapanen, A.; de Faire, U.; Pedersen, N.L.; Christensen, K.; Koskenvuo, M.; et al. Genetic epidemiology of spontaneous subarachnoid hemorrhage: Nordic Twin Study. Stroke 2010, 41, 2458–2462. [Google Scholar] [CrossRef]
- Vlak, M.H.; Rinkel, G.J.; Greebe, P.; Algra, A. Independent risk factors for intracranial aneurysms and their joint effect: A case-control study. Stroke 2013, 44, 984–987. [Google Scholar] [CrossRef]
- Meng, H.; Wang, Z.; Hoi, Y.; Gao, L.; Metaxa, E.; Swartz, D.D.; Kolega, J. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke 2007, 38, 1924–1931. [Google Scholar] [CrossRef]
- Miura, Y.; Ishida, F.; Umeda, Y.; Tanemura, H.; Suzuki, H.; Matsushima, S.; Shimosaka, S.; Taki, W. Low wall shear stress is independently associated with the rupture status of middle cerebral artery aneurysms. Stroke 2013, 44, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Hasan, D.; Chalouhi, N.; Jabbour, P.; Hashimoto, T. Macrophage imbalance (M1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: Preliminary results. J. Neuroinflammation 2012, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Sibon, I.; Mercier, N.; Darret, D.; Lacolley, P.; Lamazière, J.M. Association between semicarbazide-sensitive amine oxidase, a regulator of the glucose transporter, and elastic lamellae thinning during experimental cerebral aneurysm development: Laboratory investigation. J. Neurosurg. 2008, 108, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Wang, W.H.; Wang, Y.H.; Zheng, L.L.; Li, X.W.; Hao, F.; Guo, D. MicroRNA-29a: A potential biomarker in the development of intracranial aneurysm. J. Neurol. Sci. 2016, 364, 84–89. [Google Scholar] [CrossRef]
- Kao, H.W.; Lee, K.W.; Kuo, C.L.; Huang, C.S.; Tseng, W.M.; Liu, C.S.; Lin, C.P. Interleukin-6 as a Prognostic Biomarker in Ruptured Intracranial Aneurysms. PLoS ONE 2015, 10, e0132115. [Google Scholar] [CrossRef]
- Richards, E.J. Inherited epigenetic variation--revisiting soft inheritance. Nat. Rev. Genet. 2006, 7, 395–401. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef]
- Hosaka, K.; Hoh, B.L. Inflammation and cerebral aneurysms. Transl. Stroke Res. 2014, 5, 190–198. [Google Scholar] [CrossRef]
- Liu, Q.; Li, K.; He, H.; Miao, Z.; Cui, H.; Wu, J.; Ding, S.; Wen, Z.; Chen, J.; Lu, X.; et al. The markers and risk stratification model of intracranial aneurysm instability in a large Chinese cohort. Sci. Bull. 2023, 68, 1162–1175. [Google Scholar] [CrossRef]
- Tutino, V.M.; Poppenberg, K.E.; Li, L.; Shallwani, H.; Jiang, K.; Jarvis, J.N.; Sun, Y.; Snyder, K.V.; Levy, E.I.; Siddiqui, A.H.; et al. Biomarkers from circulating neutrophil transcriptomes have potential to detect unruptured intracranial aneurysms. J. Transl. Med. 2018, 16, 373. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, J.; Maciejczyk, M.; Ćwiklińska, A.; Matowicka-Karna, J.; Koper-Lenkiewicz, O.M. Pro-Inflammatory and Anti-Inflammatory Cytokines Levels are Significantly Altered in Cerebrospinal Fluid of Unruptured Intracranial Aneurysm (UIA) Patients. J. Inflamm. Res. 2022, 15, 6245–6261. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ma, X.; Kong, D.; Dai, Y.; Li, T.; Yu, H.; Liu, J.; Li, M.; Xu, Y.; Xiang, G.; et al. Multiomics integrated analysis and experimental validation identify TLR4 and ALOX5 as oxidative stress-related biomarkers in intracranial aneurysms. J. Neuroinflammation 2024, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Gaetani, L.; Blennow, K.; Calabresi, P.; Di Filippo, M.; Parnetti, L.; Zetterberg, H. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 2019, 90, 870–881. [Google Scholar] [CrossRef]
- Wiśniewski, K.; Zaczkowski, K.; Szmyd, B.M.; Popęda, M.; Bieńkowski, M.; Posmyk, B.; Bobeff, E.J.; Jaskólski, D.J. Evaluation of CSF 8-iso-prostaglandin F2α and erythrocyte anisocytosis as prognostic biomarkers for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Sci. Rep. 2024, 14, 11302. [Google Scholar] [CrossRef]
- Roder, C.; Kasuya, H.; Harati, A.; Tatagiba, M.; Inoue, I.; Krischek, B. Meta-analysis of microarray gene expression studies on intracranial aneurysms. Neuroscience 2012, 201, 105–113. [Google Scholar] [CrossRef]
- McColgan, P.; Thant, K.Z.; Sharma, P. The genetics of sporadic ruptured and unruptured intracranial aneurysms: A genetic meta-analysis of 8 genes and 13 polymorphisms in approximately 20,000 individuals. J. Neurosurg. 2010, 112, 714–721. [Google Scholar] [CrossRef]
- Slowik, A.; Borratynska, A.; Turaj, W.; Pera, J.; Dziedzic, T.; Figlewicz, D.A.; Betlej, M.; Krzyszkowski, T.; Czepko, R.; Szczudlik, A. Alpha1-antichymotrypsin gene (SERPINA3) A/T polymorphism as a risk factor for aneurysmal subarachnoid hemorrhage. Stroke 2005, 36, 737–740. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, Y.; Ge, M.; Pang, Q.; Yu, Y. Polymorphism rs4934 of SERPINA3 and sporadic intracranial aneurysms in the Chinese population. Cerebrovasc. Dis. 2010, 29, 68–72. [Google Scholar] [CrossRef]
- Bakker, M.K.; Ruigrok, Y.M. Genetics of Intracranial Aneurysms. Stroke 2021, 52, 3004–3012. [Google Scholar] [CrossRef]
- Yasuno, K.; Bakırcıoğlu, M.; Low, S.K.; Bilgüvar, K.; Gaál, E.; Ruigrok, Y.M.; Niemelä, M.; Hata, A.; Bijlenga, P.; Kasuya, H.; et al. Common variant near the endothelin receptor type A (EDNRA) gene is associated with intracranial aneurysm risk. Proc. Natl. Acad. Sci. USA 2011, 108, 19707–19712. [Google Scholar] [CrossRef]
- Yu, L.; Wang, J.; Wang, S.; Zhang, D.; Zhao, Y.; Wang, R.; Zhao, J. DNA Methylation Regulates Gene Expression in Intracranial Aneurysms. World Neurosurg. 2017, 105, 28–36. [Google Scholar] [CrossRef]
- Ma, J.; Joehanes, R.; Liu, C.; Keshawarz, A.; Hwang, S.J.; Bui, H.; Tejada, B.; Sooda, M.; Munson, P.J.; Demirkale, C.Y.; et al. Elucidating the genetic architecture of DNA methylation to identify promising molecular mechanisms of disease. Sci. Rep. 2022, 12, 19564. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, T.; Zhou, S.; Zhang, J.; Wu, Y.; Zhou, C.; Sun, J.; Gao, X.; Huang, Y. DNA methylation of the MAP3K10 gene may participate in the development of intracranial aneurysm. Gene 2023, 851, 147024. [Google Scholar] [CrossRef]
- Xu, T.; Yu, X.; Zhou, S.; Wu, Y.; Deng, X.; Wu, Y.; Wang, S.; Gao, X.; Nie, S.; Zhou, C.; et al. DNA methylation and mRNA expression of glutathione S-transferase alpha 4 are associated with intracranial aneurysms in a gender-dependent manner. Front. Genet. 2022, 13, 1079455. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, J.; Zhou, C.; Gong, F.; Zhu, X.; Pan, X.; Sun, J.; Gao, X.; Huang, Y. DNA Methylation of Patatin-Like Phospholipase Domain-Containing Protein 6 Gene Contributes to the Risk of Intracranial Aneurysm in Males. Front. Aging Neurosci. 2022, 14, 885680. [Google Scholar] [CrossRef]
- Maimaiti, A.; Turhon, M.; Abulaiti, A.; Dilixiati, Y.; Zhang, F.; Axieer, A.; Kadeer, K.; Zhang, Y.; Maimaitili, A.; Yang, X. DNA methylation regulator-mediated modification patterns and risk of intracranial aneurysm: A multi-omics and epigenome-wide association study integrating machine learning, Mendelian randomization, eQTL and mQTL data. J. Transl. Med. 2023, 21, 660. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, J.; Yuan, K.; Song, Z.; Ma, Z.; Zhong, Y.; Fang, X.; Zhang, W. Upregulation of microRNA-205 is a potential biomarker for intracranial aneurysms. Neuroreport 2019, 30, 812–816. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, X.; Li, L.; Wang, C.; Feng, M.; Zhao, K.; Zhao, R.; Liu, J.; Fang, Y. Tumor-associated macrophage-derived exosomal microRNA-155-5p stimulates intracranial aneurysm formation and macrophage infiltration. Clin. Sci. 2019, 133, 2265–2282. [Google Scholar] [CrossRef]
- Yu, G.; Liu, P.; Shi, Y.; Li, S.; Liu, Y.; Fan, Z.; Zhu, W. Stimulation of endothelial progenitor cells by microRNA-31a-5p to induce endothelialization in an aneurysm neck after coil embolization by modulating the Axin1-mediated β-catenin/vascular endothelial growth factor pathway. J. Neurosurg. 2019, 133, 918–926. [Google Scholar] [CrossRef]
- Xiong, W.; Yao, W.; Gao, Z.; Liu, K. Rs12976445 polymorphism is associated with the risk of post-SAH re-bleeding by modulating the expression of microRNA-125 and ET-1. Sci. Rep. 2022, 12, 2062. [Google Scholar] [CrossRef]
- Holcomb, M.; Ding, Y.H.; Dai, D.; McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Kadirvel, R. RNA-Sequencing Analysis of Messenger RNA/MicroRNA in a Rabbit Aneurysm Model Identifies Pathways and Genes of Interest. AJNR Am. J. Neuroradiol. 2015, 36, 1710–1715. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; He, H.; Chen, J.; Zeng, H.; Li, J.; Duan, R. MicroRNA/mRNA profiling and regulatory network of intracranial aneurysm. BMC Med. Genom. 2013, 6, 36. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, H.; Su, J.Y. MicroRNA-29a contributes to intracranial aneurysm by regulating the mitochondrial apoptotic pathway. Mol. Med. Rep. 2018, 18, 2945–2954. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, Y.; Wang, Y.; Li, Y.; Cheng, Q. MicroRNA-513b-5p targets COL1A1 and COL1A2 associated with the formation and rupture of intracranial aneurysm. Sci. Rep. 2021, 11, 14897. [Google Scholar] [CrossRef]
- Fan, W.; Liu, Y.; Li, C.; Qu, X.; Zheng, G.; Zhang, Q.; Pan, Z.; Wang, Y.; Rong, J. microRNA-331-3p maintains the contractile type of vascular smooth muscle cells by regulating TNF-α and CD14 in intracranial aneurysm. Neuropharmacology 2020, 164, 107858. [Google Scholar] [CrossRef]
- Bekelis, K.; Kerley-Hamilton, J.S.; Teegarden, A.; Tomlinson, C.R.; Kuintzle, R.; Simmons, N.; Singer, R.J.; Roberts, D.W.; Kellis, M.; Hendrix, D.A. MicroRNA and gene expression changes in unruptured human cerebral aneurysms. J. Neurosurg. 2016, 125, 1390–1399. [Google Scholar] [CrossRef]
- Lv, C.; Wang, J.; Dai, S.; Chen, Y.; Jiang, X.; Li, X. Long non-coding RNA NORAD induces phenotypic regulation of vascular smooth muscle cells through regulating microRNA-136-5p-targeted KDM1A. Cell Cycle 2021, 20, 2137–2148. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, D.; Ma, W.; Wang, M.; Qin, Q.; Jiang, Z.; Liu, M. Histone deacetylase 9 inhibition upregulates microRNA-92a to repress the progression of intracranial aneurysm via silencing Bcl-2-like protein 11. J. Drug Target. 2021, 29, 761–770. [Google Scholar] [CrossRef]
- Liu, D.; Han, L.; Wu, X.; Yang, X.; Zhang, Q.; Jiang, F. Genome-wide microRNA changes in human intracranial aneurysms. BMC Neurol. 2014, 14, 188. [Google Scholar] [CrossRef]
- Guo, D.; Wang, Y.W.; Yan, L.; Ma, J.; Han, X.W.; Shui, S.F. Dysregulation of microRNA-23b-3p contributes to the development of intracranial aneurysms by targeting phosphatase and tensin homolog. Int. J. Mol. Med. 2018, 42, 1637–1643. [Google Scholar] [CrossRef]
- Jin, H.; Li, C.; Ge, H.; Jiang, Y.; Li, Y. Circulating microRNA: A novel potential biomarker for early diagnosis of intracranial aneurysm rupture a case control study. J. Transl. Med. 2013, 11, 296. [Google Scholar] [CrossRef]
- Su, X.W.; Chan, A.H.; Lu, G.; Lin, M.; Sze, J.; Zhou, J.Y.; Poon, W.S.; Liu, Q.; Zheng, V.Z.; Wong, G.K. Circulating microRNA 132-3p and 324-3p Profiles in Patients after Acute Aneurysmal Subarachnoid Hemorrhage. PLoS ONE 2015, 10, e0144724. [Google Scholar] [CrossRef][Green Version]
- Supriya, M.; Christopher, R.; Devi, B.I.; Bhat, D.I.; Shukla, D.; Kalpana, S.R. Altered MicroRNA Expression in Intracranial Aneurysmal Tissues: Possible Role in TGF-β Signaling Pathway. Cell Mol. Neurobiol. 2022, 42, 2393–2405. [Google Scholar] [CrossRef]
- Luo, J.; Jin, H.; Jiang, Y.; Ge, H.; Wang, J.; Li, Y. Aberrant Expression of microRNA-9 Contributes to Development of Intracranial Aneurysm by Suppressing Proliferation and Reducing Contractility of Smooth Muscle Cells. Med. Sci. Monit. 2016, 22, 4247–4253. [Google Scholar] [CrossRef]
- Yang, G.; Qin, H.; Liu, B.; Zhao, X.; Yin, H. Mesenchymal stem cells-derived exosomes modulate vascular endothelial injury via miR-144-5p/PTEN in intracranial aneurysm. Hum. Cell 2021, 34, 1346–1359. [Google Scholar] [CrossRef]
- Liao, B.; Zhou, M.X.; Zhou, F.K.; Luo, X.M.; Zhong, S.X.; Zhou, Y.F.; Qin, Y.S.; Li, P.P.; Qin, C. Exosome-Derived MiRNAs as Biomarkers of the Development and Progression of Intracranial Aneurysms. J. Atheroscler. Thromb. 2020, 27, 545–610. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Q.; Wu, X.; Yang, X.; Zhang, Y.; Li, Y.; Jiang, F. Circulating microRNAs serve as novel biological markers for intracranial aneurysms. J. Am. Heart Assoc. 2014, 3, e000972. [Google Scholar] [CrossRef]
- Zou, L.; Hou, Y.; Yu, B.; Li, S.; Du, Y. The effect of intravascular interventional embolization and craniotomy on MMP-2, MMP-9 and caspase3 in serum of intracranial aneurysm patients. Exp. Ther. Med. 2018, 16, 4511–4518. [Google Scholar] [CrossRef]
- Xu, J.; Yan, S.; Tan, H.; Ma, L.; Feng, H.; Han, H.; Pan, M.; Yu, L.; Fang, C. The miR-143/145 cluster reverses the regulation effect of KLF5 in smooth muscle cells with proliferation and contractility in intracranial aneurysm. Gene 2018, 679, 266–273. [Google Scholar] [CrossRef]
- Yuan, X.; Bian, X.; Wei, W.; Bao, Q.; Liu, P.; Jiang, W. miR-34a regulates phenotypic modulation of vascular smooth muscle cells in intracranial aneurysm by targeting CXCR3 and MMP-2. Genet. Mol. Biol. 2021, 44, e20200124. [Google Scholar] [CrossRef]
- Marks, L.B.; Yu, X.; Vujaskovic, Z.; Small, W., Jr.; Folz, R.; Anscher, M.S. Radiation-induced lung injury. Semin. Radiat. Oncol. 2003, 13, 333–345. [Google Scholar] [CrossRef]
- Skoczylas, J.Z.; Bentzen, S.M.; Overgaard, M.; Overgaard, J. Time course of radiological lung density changes after postmastectomy radiotherapy. Acta Oncol. 2000, 39, 181–187. [Google Scholar] [CrossRef]
- Ozsolak, F.; Milos, P.M. RNA sequencing: Advances, challenges and opportunities. Nat. Rev. Genet. 2011, 12, 87–98. [Google Scholar] [CrossRef]
- Lin, W.; Ye, H.; You, K.; Chen, L. Up-regulation of circ_LARP4 suppresses cell proliferation and migration in ovarian cancer by regulating miR-513b-5p/LARP4 axis. Cancer Cell Int. 2020, 20, 5. [Google Scholar] [CrossRef]
- Desrosiers, R.; Friderici, K.; Rottman, F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl. Acad. Sci. USA 1974, 71, 3971–3975. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Q.; Li, B.; Wang, D.; Wang, L.; Zhou, Y.L. m(6)A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol. Cancer 2020, 19, 53. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Li, S.; Wang, J.; Feng, C.; Li, T.; Du, X. N6-Methylandenosine-Related lncRNAs in Tumor Microenvironment Are Potential Prognostic Biomarkers in Colon Cancer. Front. Oncol. 2021, 11, 697949. [Google Scholar] [CrossRef]
- Maimaiti, A.; Turhon, M.; Cheng, X.; Su, R.; Kadeer, K.; Axier, A.; Ailaiti, D.; Aili, Y.; Abudusalamu, R.; Kuerban, A.; et al. m6A regulator-mediated RNA methylation modification patterns and immune microenvironment infiltration characterization in patients with intracranial aneurysms. Front. Neurol. 2022, 13, 889141. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Q.; Weng, L.; Han, Y.; Li, J. Novel insight into m6A regulator-mediated methylation modification patterns and immune characteristics in intracranial aneurysm. Front. Aging Neurosci. 2022, 14, 973258. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhao, M.G.; Liang, G.B.; Song, Z.Q.; Li, Z.Q. Expression of pro-inflammatory cytokines and the risk of intracranial aneurysm. Inflammation 2013, 36, 1195–1200. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Ishibashi, R.; Nozaki, K.; Egashira, K.; Hashimoto, N. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke 2009, 40, 942–951. [Google Scholar] [CrossRef]
- Chu, C.; Xu, G.; Li, X.; Duan, Z.; Tao, L.; Cai, H.; Yang, M.; Zhang, X.; Chen, B.; Zheng, Y.; et al. Sustained expression of MCP-1 induced low wall shear stress loading in conjunction with turbulent flow on endothelial cells of intracranial aneurysm. J. Cell Mol. Med. 2021, 25, 110–119. [Google Scholar] [CrossRef]
- Lai, X.L.; Deng, Z.F.; Zhu, X.G.; Chen, Z.H. Apc gene suppresses intracranial aneurysm formation and rupture through inhibiting the NF-κB signaling pathway mediated inflammatory response. Biosci. Rep. 2019, 39, BSR20181909. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Dai, D.; Xu, Z. Expression of NF-κB, MCP-1 and MMP-9 in a Cerebral Aneurysm Rabbit Model. Can. J. Neurol. Sci. 2014, 41, 200–205. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Taleb, S.; Mallat, Z.; Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 969–979. [Google Scholar] [CrossRef]
- Ali, M.S.; Starke, R.M.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Owens, G.K.; Koch, W.J.; Greig, N.H.; Dumont, A.S. TNF-α induces phenotypic modulation in cerebral vascular smooth muscle cells: Implications for cerebral aneurysm pathology. J. Cereb. Blood Flow. Metab. 2013, 33, 1564–1573. [Google Scholar] [CrossRef]
- Starke, R.M.; Raper, D.M.; Ding, D.; Chalouhi, N.; Owens, G.K.; Hasan, D.M.; Medel, R.; Dumont, A.S. Tumor necrosis factor-α modulates cerebral aneurysm formation and rupture. Transl. Stroke Res. 2014, 5, 269–277. [Google Scholar] [CrossRef]
- Kanematsu, Y.; Kanematsu, M.; Kurihara, C.; Tada, Y.; Tsou, T.L.; van Rooijen, N.; Lawton, M.T.; Young, W.L.; Liang, E.I.; Nuki, Y.; et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke 2011, 42, 173–178. [Google Scholar] [CrossRef]
- Starke, R.M.; Chalouhi, N.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Wada, K.; Shimada, K.; Hasan, D.M.; Greig, N.H.; et al. Critical role of TNF-α in cerebral aneurysm formation and progression to rupture. J. Neuroinflammation 2014, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, T.; Berenstein, V.; Li, X.; Mayer, J.; Silane, M.; Shin, Y.S.; Niimi, Y.; Kiliç, T.; Gunel, M.; Berenstein, A. Tumor necrosis factor alpha is a key modulator of inflammation in cerebral aneurysms. Neurosurgery 2005, 57, 558–564; discussion 558–564. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Miyamoto, T.; Shikata, E.; Yamaguchi, I.; Shimada, K.; Yagi, K.; Tada, Y.; Korai, M.; Kitazato, K.T.; Kanematsu, Y.; et al. Activation of the NLRP3/IL-1β/MMP-9 pathway and intracranial aneurysm rupture associated with the depletion of ERα and Sirt1 in oophorectomized rats. J. Neurosurg. 2023, 138, 191–198. [Google Scholar] [CrossRef]
- Kamińska, J.; Lyson, T.; Chrzanowski, R.; Sawicki, K.; Milewska, A.J.; Tylicka, M.; Zińczuk, J.; Matowicka-Karna, J.; Dymicka-Piekarska, V.; Mariak, Z.; et al. Ratio of IL-8 in CSF versus Serum Is Elevated in Patients with Unruptured Brain Aneurysm. J. Clin. Med. 2020, 9, 1761. [Google Scholar] [CrossRef]
- Kamińska, J.; Tylicka, M.; Dymicka-Piekarska, V.; Mariak, Z.; Matowicka-Karna, J.; Koper-Lenkiewicz, O.M. Canonical NF-κB signaling pathway and GRO-α/CXCR2 axis are activated in unruptured intracranial aneurysm patients. Sci. Rep. 2022, 12, 21375. [Google Scholar] [CrossRef]
- Aoki, T.; Nishimura, M.; Matsuoka, T.; Yamamoto, K.; Furuyashiki, T.; Kataoka, H.; Kitaoka, S.; Ishibashi, R.; Ishibazawa, A.; Miyamoto, S.; et al. PGE(2)-EP(2) signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-κB. Br. J. Pharmacol. 2011, 163, 1237–1249. [Google Scholar] [CrossRef]
- Sun, Z.H.; Liu, F.; Kong, L.L.; Ji, P.M.; Huang, L.; Zhou, H.M.; Sun, R.; Luo, J.; Li, W.Z. Interruption of TRPC6-NFATC1 signaling inhibits NADPH oxidase 4 and VSMCs phenotypic switch in intracranial aneurysm. Biomed. Pharmacother. 2023, 161, 114480. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Ishibashi, R.; Nozaki, K.; Morishita, R.; Hashimoto, N. Reduced collagen biosynthesis is the hallmark of cerebral aneurysm: Contribution of interleukin-1beta and nuclear factor-kappaB. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1080–1086. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Petrasek, J.; Bala, S.; Csak, T.; Lippai, D.; Kodys, K.; Menashy, V.; Barrieau, M.; Min, S.Y.; Kurt-Jones, E.A.; Szabo, G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig. 2012, 122, 3476–3489. [Google Scholar] [CrossRef]
- Johnston, W.F.; Salmon, M.; Su, G.; Lu, G.; Stone, M.L.; Zhao, Y.; Owens, G.K.; Upchurch, G.R., Jr.; Ailawadi, G. Genetic and pharmacologic disruption of interleukin-1β signaling inhibits experimental aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, T.; Takagi, Y.; Sadamasa, N.; Aoki, T.; Nozaki, K.; Hashimoto, N. Impaired progression of cerebral aneurysms in interleukin-1beta-deficient mice. Stroke 2006, 37, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Zhu, C.; Liu, W.; Ma, X.; Chen, J.; Mo, S.; Dong, L.; Wang, N.; Wu, J.; et al. Serum IL-1, Pyroptosis and Intracranial Aneurysm Wall Enhancement: Analysis Integrating Radiology, Serum Cytokines and Histology. Front. Cardiovasc. Med. 2022, 9, 818789. [Google Scholar] [CrossRef]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Shimamura, M.; Nakagami, H.; Wakayama, K.; Moriwaki, T.; Ishibashi, R.; Nozaki, K.; Morishita, R.; Hashimoto, N. NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation 2007, 116, 2830–2840. [Google Scholar] [CrossRef]
- Zhou, H.; Khan, D.; Hussain, S.M.; Gerdes, N.; Hagenbeck, C.; Rana, M.; Cornelius, J.F.; Muhammad, S. Colchicine prevents oxidative stress-induced endothelial cell senescence via blocking NF-κB and MAPKs: Implications in vascular diseases. J. Inflamm. 2023, 20, 41. [Google Scholar] [CrossRef]
- Nakayama, K. cAMP-response element-binding protein (CREB) and NF-κB transcription factors are activated during prolonged hypoxia and cooperatively regulate the induction of matrix metalloproteinase MMP1. J. Biol. Chem. 2013, 288, 22584–22595. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, K.; Li, W.; Yang, N.; Liu, Y.; Chen, C.; Wei, T. The interactions between pristine graphene and macrophages and the production of cytokines/chemokines via TLR- and NF-κB-related signaling pathways. Biomaterials 2012, 33, 6933–6942. [Google Scholar] [CrossRef]
- Chucair-Elliott, A.J.; Conrady, C.; Zheng, M.; Kroll, C.M.; Lane, T.E.; Carr, D.J. Microglia-induced IL-6 protects against neuronal loss following HSV-1 infection of neural progenitor cells. Glia 2014, 62, 1418–1434. [Google Scholar] [CrossRef]
- Taga, T.; Hibi, M.; Hirata, Y.; Yamasaki, K.; Yasukawa, K.; Matsuda, T.; Hirano, T.; Kishimoto, T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell 1989, 58, 573–581. [Google Scholar] [CrossRef]
- Monsour, M.; Croci, D.M.; Grüter, B.E.; Taussky, P.; Marbacher, S.; Agazzi, S. Cerebral Aneurysm and Interleukin-6: A Key Player in Aneurysm Generation and Rupture or Just One of the Multiple Factors? Transl. Stroke Res. 2023, 14, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Hodes, G.E.; Ménard, C.; Russo, S.J. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol. Stress. 2016, 4, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zheng, S.G. Hall of Fame among Pro-inflammatory Cytokines: Interleukin-6 Gene and Its Transcriptional Regulation Mechanisms. Front. Immunol. 2016, 7, 604. [Google Scholar] [CrossRef]
- Ikedo, T.; Minami, M.; Kataoka, H.; Hayashi, K.; Nagata, M.; Fujikawa, R.; Higuchi, S.; Yasui, M.; Aoki, T.; Fukuda, M.; et al. Dipeptidyl Peptidase-4 Inhibitor Anagliptin Prevents Intracranial Aneurysm Growth by Suppressing Macrophage Infiltration and Activation. J. Am. Heart Assoc. 2017, 6, e004777. [Google Scholar] [CrossRef]
- Wajima, D.; Hourani, S.; Dodd, W.; Patel, D.; Jones, C.; Motwani, K.; Fazal, H.Z.; Hosaka, K.; Hoh, B.L. Interleukin-6 Promotes Murine Estrogen Deficiency-Associated Cerebral Aneurysm Rupture. Neurosurgery 2020, 86, 583–592. [Google Scholar] [CrossRef]
- Kamińska, J.; Dymicka-Piekarska, V.; Chrzanowski, R.; Sawicki, K.; Milewska, A.J.; Zińczuk, J.; Tylicka, M.; Jadeszko, M.; Mariak, Z.; Kratz, E.M.; et al. IL-6 Quotient (The Ratio of Cerebrospinal Fluid IL-6 to Serum IL-6) as a Biomarker of an Unruptured Intracranial Aneurysm. J. Inflamm. Res. 2021, 14, 6103–6114. [Google Scholar] [CrossRef]
- Croci, D.M.; Wanderer, S.; Strange, F.; Grüter, B.E.; Sivanrupan, S.; Andereggen, L.; Casoni, D.; von Gunten, M.; Widmer, H.R.; Di Santo, S.; et al. Tocilizumab Reduces Vasospasms, Neuronal Cell Death, and Microclot Formation in a Rabbit Model of Subarachnoid Hemorrhage. Transl. Stroke Res. 2021, 12, 894–904. [Google Scholar] [CrossRef]
- Griendling, K.K.; FitzGerald, G.A. Oxidative stress and cardiovascular injury: Part I: Basic mechanisms and in vivo monitoring of ROS. Circulation 2003, 108, 1912–1916. [Google Scholar] [CrossRef]
- Starke, R.M.; Chalouhi, N.; Ali, M.S.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Koch, W.J.; Dumont, A.S. The role of oxidative stress in cerebral aneurysm formation and rupture. Curr. Neurovasc. Res. 2013, 10, 247–255. [Google Scholar] [CrossRef]
- Chalouhi, N.; Chitale, R.; Jabbour, P.; Tjoumakaris, S.; Dumont, A.S.; Rosenwasser, R.; Gonzalez, L.F. The case for family screening for intracranial aneurysms. Neurosurg. Focus. 2011, 31, E8. [Google Scholar] [CrossRef]
- Talukder, M.A.; Johnson, W.M.; Varadharaj, S.; Lian, J.; Kearns, P.N.; El-Mahdy, M.A.; Liu, X.; Zweier, J.L. Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H388–H396. [Google Scholar] [CrossRef] [PubMed]
- Juvela, S. Prevalence of and risk factors for intracranial aneurysms. Lancet Neurol. 2011, 10, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Touyz, R.M. Molecular mechanisms of hypertension--reactive oxygen species and antioxidants: A basic science update for the clinician. Can. J. Cardiol. 2012, 28, 288–295. [Google Scholar] [CrossRef]
- Miller, A.A.; Drummond, G.R.; Sobey, C.G. Reactive oxygen species in the cerebral circulation: Are they all bad? Antioxid. Redox Signal 2006, 8, 1113–1120. [Google Scholar] [CrossRef]
- Förstermann, U.; Li, H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br. J. Pharmacol. 2011, 164, 213–223. [Google Scholar] [CrossRef]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Marnett, L.J. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem. Rev. 2003, 103, 2239–2304. [Google Scholar] [CrossRef]
- Hasan, D.; Hashimoto, T.; Kung, D.; Macdonald, R.L.; Winn, H.R.; Heistad, D. Upregulation of cyclooxygenase-2 (COX-2) and microsomal prostaglandin E2 synthase-1 (mPGES-1) in wall of ruptured human cerebral aneurysms: Preliminary results. Stroke 2012, 43, 1964–1967. [Google Scholar] [CrossRef]
- Vignais, P.V. The superoxide-generating NADPH oxidase: Structural aspects and activation mechanism. Cell Mol. Life Sci. 2002, 59, 1428–1459. [Google Scholar] [CrossRef]
- Liu, X.; Qin, Z.; Liu, C.; Song, M.; Luo, X.; Zhao, H.; Qian, D.; Chen, J.; Huang, L. Nox4 and soluble epoxide hydrolase synergistically mediate homocysteine-induced inflammation in vascular smooth muscle cells. Vascul. Pharmacol. 2019, 120, 106544. [Google Scholar] [CrossRef]
- Tamura, T.; Jamous, M.A.; Kitazato, K.T.; Yagi, K.; Tada, Y.; Uno, M.; Nagahiro, S. Endothelial damage due to impaired nitric oxide bioavailability triggers cerebral aneurysm formation in female rats. J. Hypertens. 2009, 27, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Kreuzer, J. Vascular NADPH oxidases: Molecular mechanisms of activation. Cardiovasc. Res. 2005, 65, 16–27. [Google Scholar] [CrossRef]
- Tan, X.; Li, T.; Zhu, S.; Zhong, W.; Li, F.; Wang, Y. Induction of SPARC on Oxidative Stress, Inflammatory Phenotype Transformation, and Apoptosis of Human Brain Smooth Muscle Cells Via TGF-β1-NOX4 Pathway. J. Mol. Neurosci. 2020, 70, 1728–1741. [Google Scholar] [CrossRef]
- Juvela, S.; Poussa, K.; Porras, M. Factors affecting formation and growth of intracranial aneurysms: A long-term follow-up study. Stroke 2001, 32, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.F.; Dinh-Xuan, A.T.; Pueyo, M.; Darblade, B.; Rami, J. Endothelium-derived nitric oxide and vascular physiology and pathology. Cell Mol. Life Sci. 1999, 55, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Hashimoto, N.; Naritomi, H.; Nagata, I.; Nozaki, K.; Kondo, S.; Kurino, M.; Kikuchi, H. Prevention of rat cerebral aneurysm formation by inhibition of nitric oxide synthase. Circulation 2000, 101, 2532–2538. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef]
- Sheinberg, D.L.; McCarthy, D.J.; Elwardany, O.; Bryant, J.P.; Luther, E.; Chen, S.H.; Thompson, J.W.; Starke, R.M. Endothelial dysfunction in cerebral aneurysms. Neurosurg. Focus. 2019, 47, E3. [Google Scholar] [CrossRef]
- Khurana, V.G.; Sohni, Y.R.; Mangrum, W.I.; McClelland, R.L.; O’Kane, D.J.; Meyer, F.B.; Meissner, I. Endothelial nitric oxide synthase gene polymorphisms predict susceptibility to aneurysmal subarachnoid hemorrhage and cerebral vasospasm. J. Cereb. Blood Flow. Metab. 2004, 24, 291–297. [Google Scholar] [CrossRef]
- Khurana, V.G.; Sohni, Y.R.; Mangrum, W.I.; McClelland, R.L.; O’Kane, D.J.; Meyer, F.B.; Meissner, I. Endothelial nitric oxide synthase T-786C single nucleotide polymorphism: A putative genetic marker differentiating small versus large ruptured intracranial aneurysms. Stroke 2003, 34, 2555–2559. [Google Scholar] [CrossRef]
- Sadamasa, N.; Nozaki, K.; Hashimoto, N. Disruption of gene for inducible nitric oxide synthase reduces progression of cerebral aneurysms. Stroke 2003, 34, 2980–2984. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.J.A.; Mostaço-Guidolin, L.B. Optical Microscopy and the Extracellular Matrix Structure: A Review. Cells 2021, 10, 1760. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Heino, J. The collagen family members as cell adhesion proteins. Bioessays 2007, 29, 1001–1010. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef]
- Chyatte, D.; Bruno, G.; Desai, S.; Todor, D.R. Inflammation and intracranial aneurysms. Neurosurgery 1999, 45, 1137–1146; discussion 1146–1137. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Ishibashi, R.; Nozaki, K.; Hashimoto, N. Cathepsin B, K, and S are expressed in cerebral aneurysms and promote the progression of cerebral aneurysms. Stroke 2008, 39, 2603–2610. [Google Scholar] [CrossRef]
- Lepucki, A.; Orlińska, K.; Mielczarek-Palacz, A.; Kabut, J.; Olczyk, P.; Komosińska-Vassev, K. The Role of Extracellular Matrix Proteins in Breast Cancer. J. Clin. Med. 2022, 11, 1250. [Google Scholar] [CrossRef]
- Watton, P.N.; Selimovic, A.; Raberger, N.B.; Huang, P.; Holzapfel, G.A.; Ventikos, Y. Modelling evolution and the evolving mechanical environment of saccular cerebral aneurysms. Biomech. Model. Mechanobiol. 2011, 10, 109–132. [Google Scholar] [CrossRef]
- Nakagawa, D.; Zanaty, M.; Hudson, J.; Teferi, N.; Ishii, D.; Allan, L.; Jabbour, P.; Ortega-Gutierrez, S.; Samaniego, E.A.; Hasan, D.M. Plasma Soluble Human Elastin Fragments as an Intra-Aneurysmal Localized Biomarker for Ruptured Intracranial Aneurysm. J. Am. Heart Assoc. 2018, 7, e010051. [Google Scholar] [CrossRef]
- Yang, S.; Wang, T.; You, C.; Liu, W.; Zhao, K.; Sun, H.; Mao, B.; Li, X.; Xiao, A.; Mao, X.; et al. Association of polymorphisms in the elastin gene with sporadic ruptured intracranial aneurysms and unruptured intracranial aneurysms in Chinese patients. Int. J. Neurosci. 2013, 123, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Onda, H.; Kasuya, H.; Yoneyama, T.; Takakura, K.; Hori, T.; Takeda, J.; Nakajima, T.; Inoue, I. Genomewide-linkage and haplotype-association studies map intracranial aneurysm to chromosome 7q11. Am. J. Hum. Genet. 2001, 69, 804–819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ares, W.J.; Taussky, P.; Ducruet, A.F.; Grandhi, R. Role of matrix metalloproteinases in the pathogenesis of intracranial aneurysms. Neurosurg. Focus. 2019, 47, E4. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef]
- Hu, Z.; Deng, X.; Zhou, S.; Zhou, C.; Shen, M.; Gao, X.; Huang, Y. Pathogenic mechanisms and therapeutic implications of extracellular matrix remodelling in cerebral vasospasm. Fluids Barriers CNS 2023, 20, 81. [Google Scholar] [CrossRef]
- Tao, Z.; Jie, Y.; Mingru, Z.; Changping, G.; Fan, Y.; Haifeng, W.; Yuelan, W. The Elk1/MMP-9 axis regulates E-cadherin and occludin in ventilator-induced lung injury. Respir. Res. 2021, 22, 233. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Sun, H.; Zhang, Y.; Zou, D. New insights into fibrosis from the ECM degradation perspective: The macrophage-MMP-ECM interaction. Cell Biosci. 2022, 12, 117. [Google Scholar] [CrossRef]
- Liu, P.; Shi, Y.; Fan, Z.; Zhou, Y.; Song, Y.; Liu, Y.; Yu, G.; An, Q.; Zhu, W. Inflammatory Smooth Muscle Cells Induce Endothelial Cell Alterations to Influence Cerebral Aneurysm Progression via Regulation of Integrin and VEGF Expression. Cell Transplant. 2019, 28, 713–722. [Google Scholar] [CrossRef]
- Kim, B.J.; Hong, E.P.; Youn, D.H.; Jeon, J.P. Genome-Wide Association Study of the Relationship Between Matrix Metalloproteinases and Intracranial Aneurysms. J. Clin. Neurol. 2022, 18, 163–170. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Morimoto, M.; Nozaki, K.; Hashimoto, N. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke 2007, 38, 162–169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, E.; Yan, X.; Shen, H.; Zhao, M.; Gao, X.; Huang, Y. Intracranial Aneurysm Biomarkers: A Convergence of Genetics, Inflammation, Oxidative Stress, and the Extracellular Matrix. Int. J. Mol. Sci. 2025, 26, 3316. https://doi.org/10.3390/ijms26073316
Zhang E, Yan X, Shen H, Zhao M, Gao X, Huang Y. Intracranial Aneurysm Biomarkers: A Convergence of Genetics, Inflammation, Oxidative Stress, and the Extracellular Matrix. International Journal of Molecular Sciences. 2025; 26(7):3316. https://doi.org/10.3390/ijms26073316
Chicago/Turabian StyleZhang, Enhao, Xu Yan, Hangyu Shen, Mingyue Zhao, Xiang Gao, and Yi Huang. 2025. "Intracranial Aneurysm Biomarkers: A Convergence of Genetics, Inflammation, Oxidative Stress, and the Extracellular Matrix" International Journal of Molecular Sciences 26, no. 7: 3316. https://doi.org/10.3390/ijms26073316
APA StyleZhang, E., Yan, X., Shen, H., Zhao, M., Gao, X., & Huang, Y. (2025). Intracranial Aneurysm Biomarkers: A Convergence of Genetics, Inflammation, Oxidative Stress, and the Extracellular Matrix. International Journal of Molecular Sciences, 26(7), 3316. https://doi.org/10.3390/ijms26073316