Ovarian Endometriosis Accelerates Premature Ovarian Failure and Contributes to Osteoporosis and Cognitive Decline in Aging Mice
Abstract
1. Introduction
2. Results
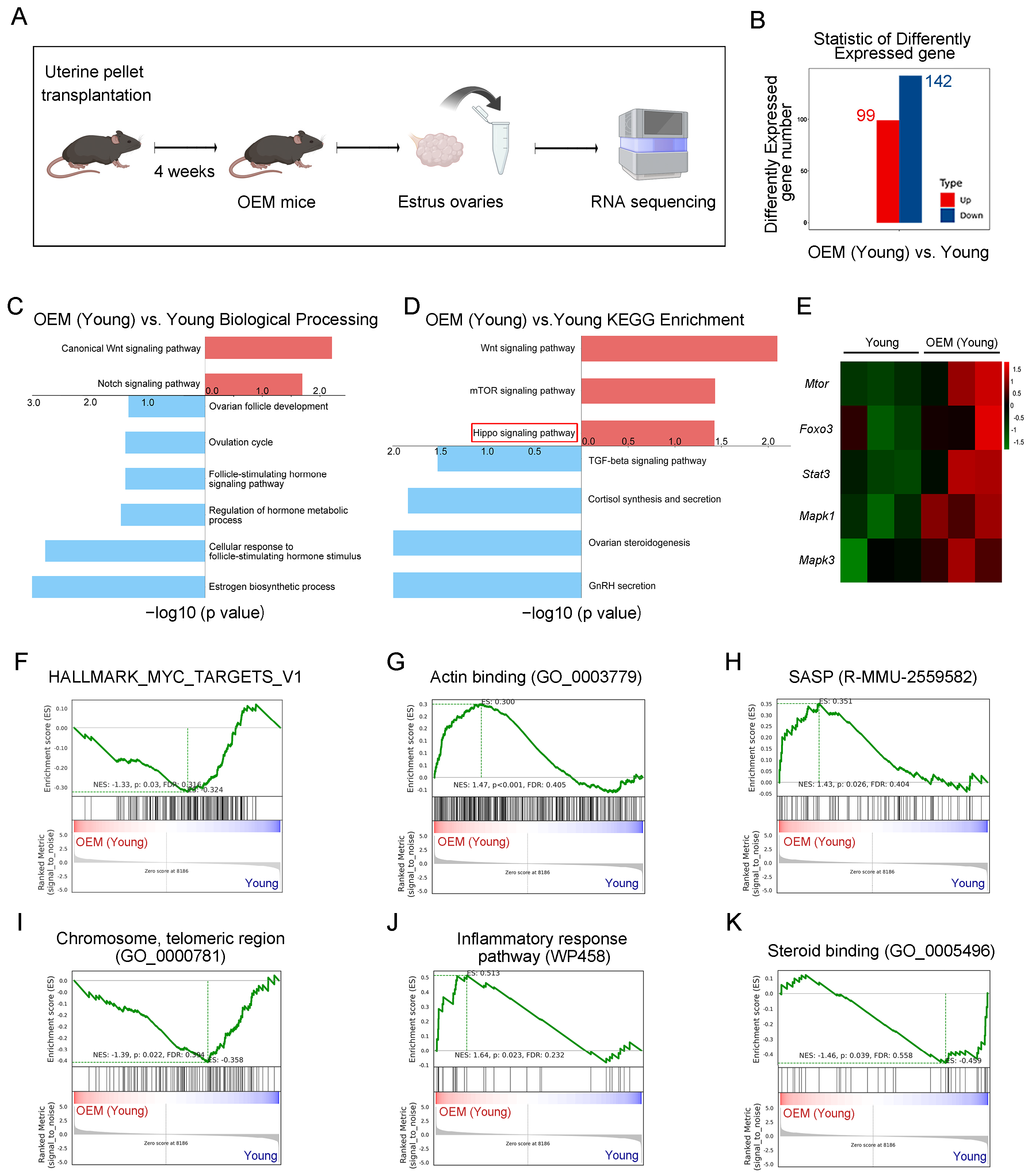
2.1. Abnormal Activation of Primordial Follicles in OEM
2.2. Estrous Cycle Disruption and Ovarian Reduction in Aging Mice with OEM
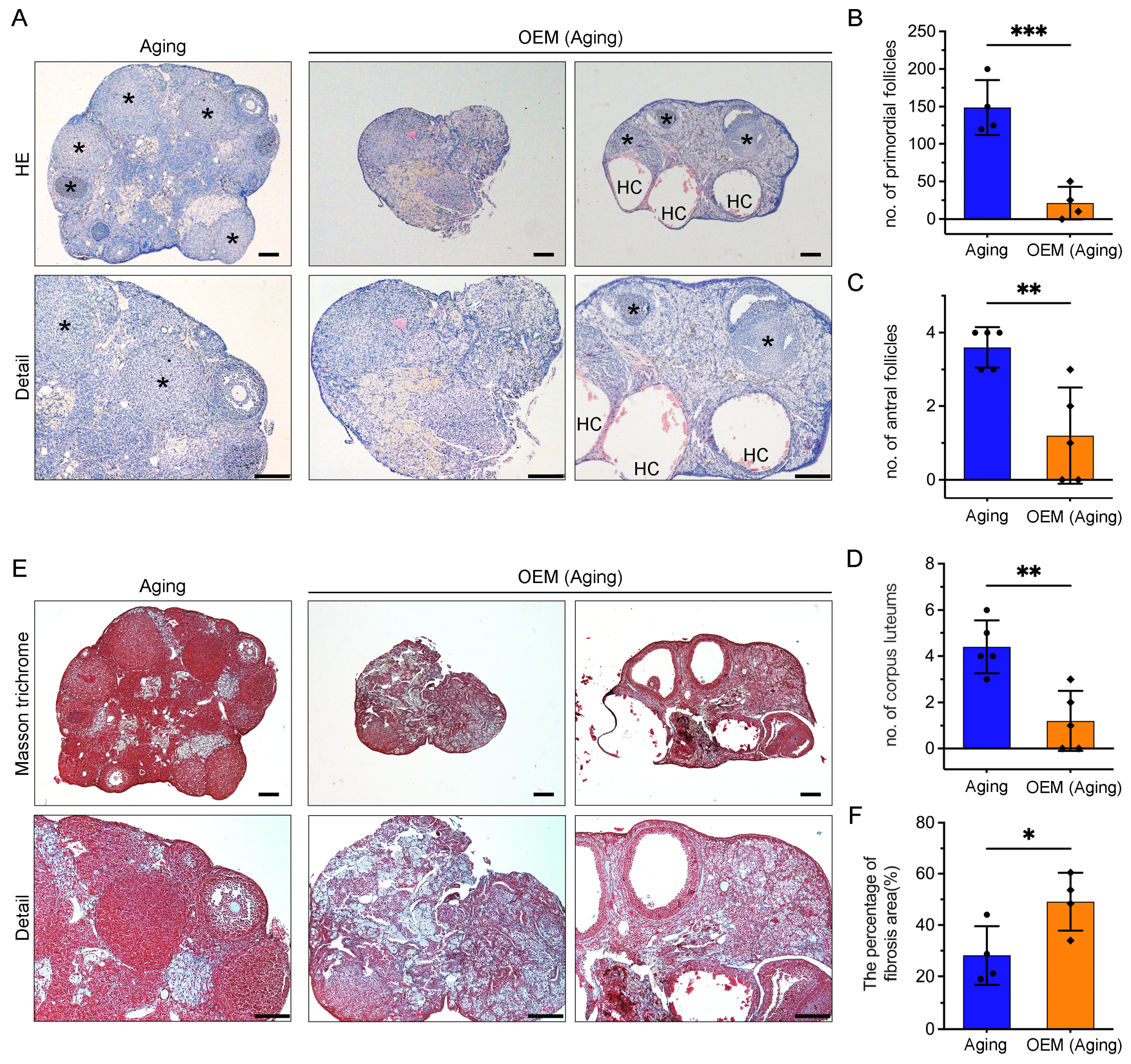
2.3. Premature Exhaustion of Primordial Follicles and Accumulation of Ovarian Fibrosis in OEM
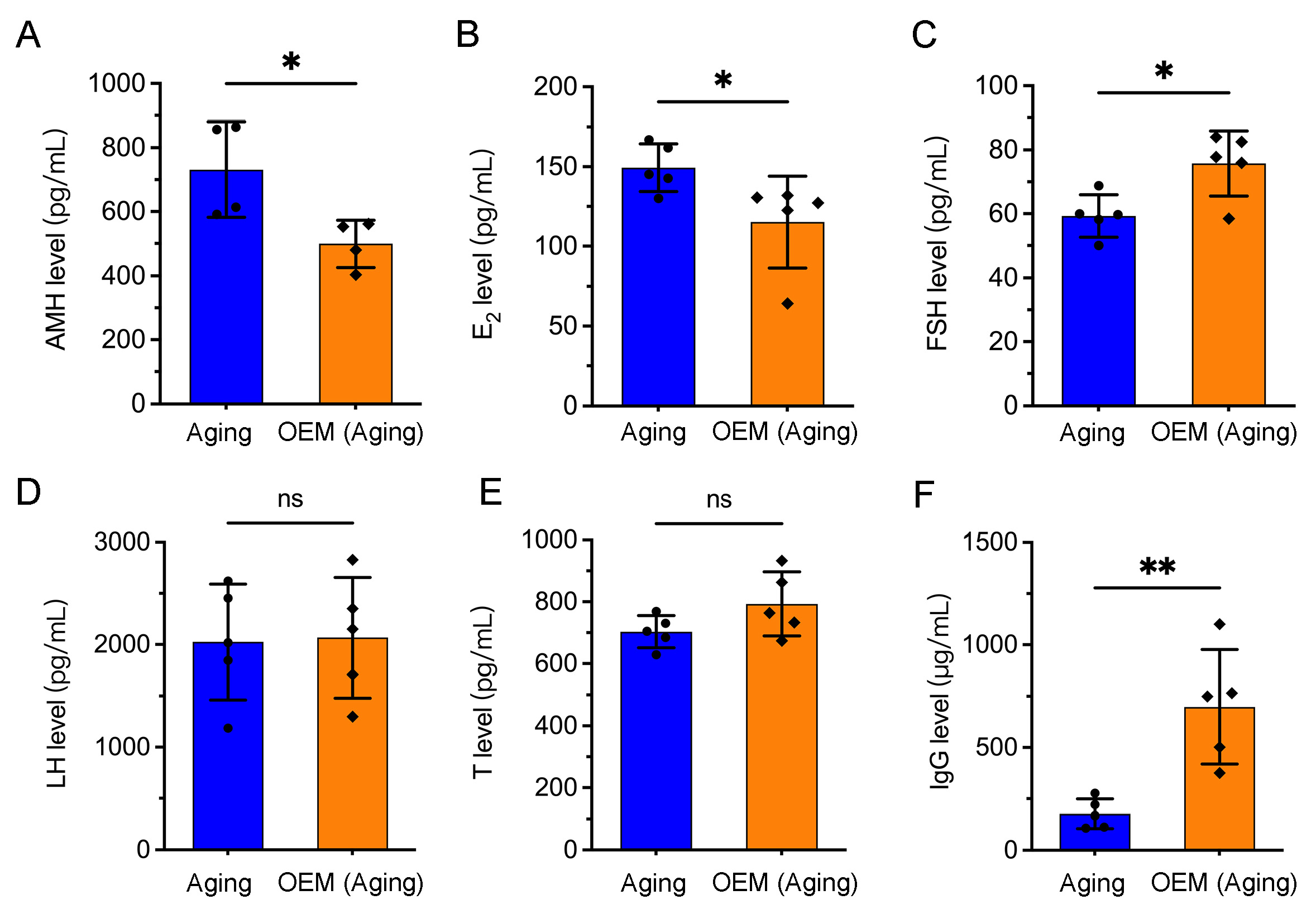
2.4. Premature Hormonal Aging and Increased IgG Levels in OEM
2.5. Premature Osteoporosis Induced by OEM in Perimenopausal Women
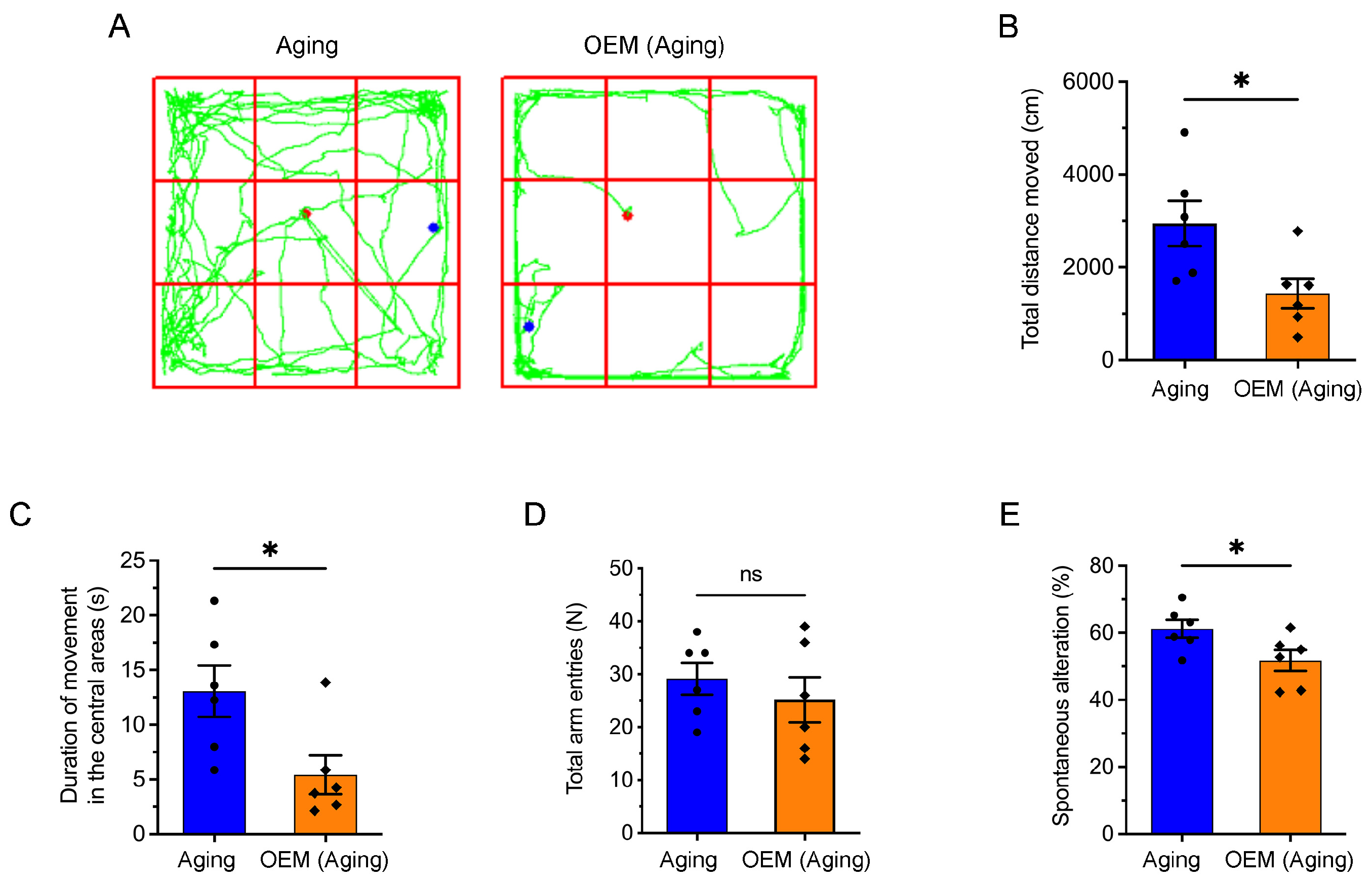
2.6. Acceleration of Anxiety and Cognitive Impairment in Aging Mice Induced by OEM
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. RNA Sequencing and Analysis
4.3. Estrous Cycle Detection
4.4. Histological Staining and Follicle Counting
4.5. Serum Hormone Measurement
4.6. Micro-Computed Tomography (Micro-CT) Analysis
4.7. Open-Field Test
4.8. Y-Maze Test for Spontaneous Alternation
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vercellini, P.; Vigano, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Greene, R.; Stratton, P.; Cleary, S.D.; Ballweg, M.L.; Sinaii, N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil. Steril. 2009, 91, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, S.; Ghorbani, M.; Abdolahzadeh, M.; Chehrazi, M.; Jorsaraei, S.G.; Mirabi, P. Stages of endometriosis: Does it affect oocyte quality, embryo development and fertilization rate? JBRA Assist. Reprod. 2022, 26, 620–626. [Google Scholar] [CrossRef]
- Takeuchi, A.; Koga, K.; Satake, E.; Makabe, T.; Taguchi, A.; Miyashita, M.; Takamura, M.; Harada, M.; Hirata, T.; Hirota, Y.; et al. Endometriosis Triggers Excessive Activation of Primordial Follicles via PI3K-PTEN-Akt-Foxo3 Pathway. J. Clin. Endocrinol. Metab. 2019, 104, 5547–5554. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Dolmans, M.M.; Donnez, O.; Masuzaki, H.; Soares, M.; Donnez, J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014, 101, 1031–1037. [Google Scholar] [CrossRef]
- Ge, L.; Yang, Y.; Gao, Y.; Xiao, T.; Chang, W.; Wang, H.; Xiao, Z.; Chen, J.; Li, M.; Yu, M.; et al. Ovarian Endometrioma Disrupts Oocyte-Cumulus Communication and Mitochondrial Function. Cell Prolif. 2025, e13800. [Google Scholar] [CrossRef]
- Sreerangaraja Urs, D.B.; Wu, W.H.; Komrskova, K.; Postlerova, P.; Lin, Y.F.; Tzeng, C.R.; Kao, S.H. Mitochondrial Function in Modulating Human Granulosa Cell Steroidogenesis and Female Fertility. Int. J. Mol. Sci. 2020, 21, 3592. [Google Scholar] [CrossRef] [PubMed]
- Kasapoglu, I.; Ata, B.; Uyaniklar, O.; Seyhan, A.; Orhan, A.; Yildiz Oguz, S.; Uncu, G. Endometrioma-related reduction in ovarian reserve (ERROR): A prospective longitudinal study. Fertil. Steril. 2018, 110, 122–127. [Google Scholar] [CrossRef]
- Nelson, S.M.; Davis, S.R.; Kalantaridou, S.; Lumsden, M.A.; Panay, N.; Anderson, R.A. Anti-Mullerian hormone for the diagnosis and prediction of menopause: A systematic review. Hum. Reprod. Update 2023, 29, 327–346. [Google Scholar] [CrossRef]
- Muzii, L.; Di Tucci, C.; Di Feliciantonio, M.; Galati, G.; Di Donato, V.; Musella, A.; Palaia, I.; Panici, P.B. Antimullerian hormone is reduced in the presence of ovarian endometriomas: A systematic review and meta-analysis. Fertil. Steril. 2018, 110, 932–940.E1. [Google Scholar] [CrossRef]
- Guo, X.; She, Y.; Liu, Q.; Qin, J.; Wang, L.; Xu, A.; Qi, B.; Sun, C.; Xie, Y.; Ma, Y.; et al. Osteoporosis and depression in perimenopausal women: From clinical association to genetic causality. J. Affect. Disord. 2024, 356, 371–378. [Google Scholar] [CrossRef]
- Duralde, E.R.; Sobel, T.H.; Manson, J.E. Management of perimenopausal and menopausal symptoms. BMJ 2023, 382, e072612. [Google Scholar] [CrossRef]
- Muka, T.; Oliver-Williams, C.; Kunutsor, S.; Laven, J.S.; Fauser, B.C.; Chowdhury, R.; Kavousi, M.; Franco, O.H. Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol. 2016, 1, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, M.B.; Sampaio, O.G.M.; Camara, F.E.A.; Schneider, A.; de Avila, B.M.; Prosczek, J.; Masternak, M.M.; Campos, A.R. Ovarian aging in humans: Potential strategies for extending reproductive lifespan. Geroscience 2023, 45, 2121–2133. [Google Scholar] [CrossRef]
- Beevors, L.I.; Sundar, S.; Foster, P.A. Steroid metabolism and hormonal dynamics in normal and malignant ovaries. Essays Biochem. 2024, 68, 491–507. [Google Scholar] [CrossRef]
- Ma, S.; Ji, Z.; Zhang, B.; Geng, L.; Cai, Y.; Nie, C.; Li, J.; Zuo, Y.; Sun, Y.; Xu, G.; et al. Spatial transcriptomic landscape unveils immunoglobin-associated senescence as a hallmark of aging. Cell 2024, 187, 7025–7044.e34. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.R.; Jannausch, M.; McConnell, D.; Little, R.; Greendale, G.A.; Finkelstein, J.S.; Neer, R.M.; Johnston, J.; Ettinger, B. Hormone predictors of bone mineral density changes during the menopausal transition. J. Clin. Endocrinol. Metab. 2006, 91, 1261–1267. [Google Scholar] [CrossRef]
- Fisher, D.W.; Bennett, D.A.; Dong, H. Sexual dimorphism in predisposition to Alzheimer’s disease. Neurobiol. Aging 2018, 70, 308–324. [Google Scholar] [CrossRef]
- Xiong, J.; Kang, S.S.; Wang, Z.; Liu, X.; Kuo, T.C.; Korkmaz, F.; Padilla, A.; Miyashita, S.; Chan, P.; Zhang, Z.; et al. FSH blockade improves cognition in mice with Alzheimer’s disease. Nature 2022, 603, 470–476. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Pankhurst, M.W. Hyperactivation of dormant primordial follicles in ovarian endometrioma patients. Reproduction 2020, 160, R145–R153. [Google Scholar] [CrossRef]
- Telfer, E.E.; Grosbois, J.; Odey, Y.L.; Rosario, R.; Anderson, R.A. Making a good egg: Human oocyte health, aging, and in vitro development. Physiol. Rev. 2023, 103, 2623–2677. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Gong, X.; Wang, C.C.; Zhang, T.; Huang, J. Diminished Ovarian Reserve in Endometriosis: Insights from In Vitro, In Vivo, and Human Studies-A Systematic Review. Int. J. Mol. Sci. 2023, 24, 15967. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Murphy, A.R.; Campo, H.; Kim, J.J. Strategies for modelling endometrial diseases. Nat. Rev. Endocrinol. 2022, 18, 727–743. [Google Scholar] [CrossRef]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Benaglia, L.; Paffoni, A.; Busnelli, A.; Vigano, P.; Vercellini, P. Risks of conservative management in women with ovarian endometriomas undergoing IVF. Hum. Reprod. Update 2015, 21, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Gazzo, I.; Crosa, M.; Rosato, F.P.; Barra, F.; Leone Roberti Maggiore, U. Impact of surgery for endometriosis on the outcomes of in vitro fertilization. Best. Pract. Res. Clin. Obstet. Gynaecol. 2024, 95, 102496. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, J.; Zhang, C. Synthesis, Regulatory Factors, and Signaling Pathways of Estrogen in the Ovary. Reprod. Sci. 2023, 30, 350–360. [Google Scholar] [CrossRef]
- Fiorentino, G.; Cimadomo, D.; Innocenti, F.; Soscia, D.; Vaiarelli, A.; Ubaldi, F.M.; Gennarelli, G.; Garagna, S.; Rienzi, L.; Zuccotti, M. Biomechanical forces and signals operating in the ovary during folliculogenesis and their dysregulation: Implications for fertility. Hum. Reprod. Update 2023, 29, 1–23. [Google Scholar] [CrossRef]
- Umehara, T.; Winstanley, Y.E.; Andreas, E.; Morimoto, A.; Williams, E.J.; Smith, K.M.; Carroll, J.; Febbraio, M.A.; Shimada, M.; Russell, D.L.; et al. Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary. Sci. Adv. 2022, 8, eabn4564. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Nakamura, T.; Motooka, Y.; Ito, F.; Jiang, L.; Akatsuka, S.; Iwase, A.; Kajiyama, H.; Kikkawa, F.; Toyokuni, S. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice. Redox Biol. 2020, 37, 101726. [Google Scholar] [CrossRef]
- Briley, S.M.; Jasti, S.; McCracken, J.M.; Hornick, J.E.; Fegley, B.; Pritchard, M.T.; Duncan, F.E. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction 2016, 152, 245–260. [Google Scholar] [CrossRef] [PubMed]
- di Clemente, N.; Racine, C.; Pierre, A.; Taieb, J. Anti-Mullerian Hormone in Female Reproduction. Endocr. Rev. 2021, 42, 753–782. [Google Scholar] [CrossRef] [PubMed]
- Cedars, M.I. Evaluation of Female Fertility-AMH and Ovarian Reserve Testing. J. Clin. Endocrinol. Metab. 2022, 107, 1510–1519. [Google Scholar] [CrossRef]
- Vignali, M.; Mabrouk, M.; Ciocca, E.; Alabiso, G.; Barbasetti di Prun, A.; Gentilini, D.; Busacca, M. Surgical excision of ovarian endometriomas: Does it truly impair ovarian reserve? Long term anti-Mullerian hormone (AMH) changes after surgery. J. Obstet. Gynaecol. Res. 2015, 41, 1773–1778. [Google Scholar] [CrossRef]
- Younis, J.S.; Shapso, N.; Ben-Sira, Y.; Nelson, S.M.; Izhaki, I. Endometrioma surgery—A systematic review and meta-analysis of the effect on antral follicle count and anti-Mullerian hormone. Am. J. Obstet. Gynecol. 2022, 226, 33–51.E7. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Leng, J.; Cui, Q.; Lang, J. Follicle loss after laparoscopic treatment of ovarian endometriotic cysts. Int. J. Gynaecol. Obstet. 2011, 115, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Muzii, L.; Di Tucci, C.; Di Feliciantonio, M.; Marchetti, C.; Perniola, G.; Panici, P.B. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Scala, C.; Racca, A.; Calanni, L.; Remorgida, V.; Venturini, P.L.; Leone Roberti Maggiore, U. Second surgery for recurrent unilateral endometriomas and impact on ovarian reserve: A case-control study. Fertil. Steril. 2015, 103, 1236–1243. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef] [PubMed]
- As-Sanie, S.; Black, R.; Giudice, L.C.; Gray Valbrun, T.; Gupta, J.; Jones, B.; Laufer, M.R.; Milspaw, A.T.; Missmer, S.A.; Norman, A.; et al. Assessing research gaps and unmet needs in endometriosis. Am. J. Obstet. Gynecol. 2019, 221, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wan, Q.; Liu, Q.; Fan, Y.; Zhou, Q.; Skowronski, A.A.; Wang, S.; Shao, Z.; Liao, C.Y.; Ding, L.; et al. IgG is an aging factor that drives adipose tissue fibrosis and metabolic decline. Cell Metab. 2024, 36, 793–807.E5. [Google Scholar] [CrossRef]
- Pascoal, E.; Wessels, J.M.; Aas-Eng, M.K.; Abrao, M.S.; Condous, G.; Jurkovic, D.; Espada, M.; Exacoustos, C.; Ferrero, S.; Guerriero, S.; et al. Strengths and limitations of diagnostic tools for endometriosis and relevance in diagnostic test accuracy research. Ultrasound Obstet. Gynecol. 2022, 60, 309–327. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Gao, Y.; Shi, S.; Zhao, D.; Cao, H.; Fu, T.; Cai, X.; Xiao, J. LncRNA-AK137033 inhibits the osteogenic potential of adipose-derived stem cells in diabetic osteoporosis by regulating Wnt signaling pathway via DNA methylation. Cell Prolif. 2022, 55, e13174. [Google Scholar] [CrossRef]
- Al-Daghestani, H.; Qaisar, R.; Al Kawas, S.; Ghani, N.; Rani, K.G.A.; Azeem, M.; Hasnan, H.K.; Kassim, N.K.; Samsudin, A.R. Pharmacological inhibition of endoplasmic reticulum stress mitigates osteoporosis in a mouse model of hindlimb suspension. Sci. Rep. 2024, 14, 4719. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Furuse, T.; Kimura, R.; Tucci, V.; Kaneda, H.; Wakana, S.; Osumi, N. Paternal Aging Affects Behavior in Pax6 Mutant Mice: A Gene/Environment Interaction in Understanding Neurodevelopmental Disorders. PLoS ONE 2016, 11, e0166665. [Google Scholar] [CrossRef]
- Carroll, J.C.; Rosario, E.R.; Chang, L.; Stanczyk, F.Z.; Oddo, S.; LaFerla, F.M.; Pike, C.J. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J. Neurosci. 2007, 27, 13357–13365. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, L.; Yang, Y.; Xiao, T.; Gao, Y.; Chang, W.; Du, F.; Yu, M.; Zhang, J.V. Ovarian Endometriosis Accelerates Premature Ovarian Failure and Contributes to Osteoporosis and Cognitive Decline in Aging Mice. Int. J. Mol. Sci. 2025, 26, 3313. https://doi.org/10.3390/ijms26073313
Ge L, Yang Y, Xiao T, Gao Y, Chang W, Du F, Yu M, Zhang JV. Ovarian Endometriosis Accelerates Premature Ovarian Failure and Contributes to Osteoporosis and Cognitive Decline in Aging Mice. International Journal of Molecular Sciences. 2025; 26(7):3313. https://doi.org/10.3390/ijms26073313
Chicago/Turabian StyleGe, Lei, Yali Yang, Tianxia Xiao, Yuqing Gao, Wakam Chang, Feifei Du, Ming Yu, and Jian V. Zhang. 2025. "Ovarian Endometriosis Accelerates Premature Ovarian Failure and Contributes to Osteoporosis and Cognitive Decline in Aging Mice" International Journal of Molecular Sciences 26, no. 7: 3313. https://doi.org/10.3390/ijms26073313
APA StyleGe, L., Yang, Y., Xiao, T., Gao, Y., Chang, W., Du, F., Yu, M., & Zhang, J. V. (2025). Ovarian Endometriosis Accelerates Premature Ovarian Failure and Contributes to Osteoporosis and Cognitive Decline in Aging Mice. International Journal of Molecular Sciences, 26(7), 3313. https://doi.org/10.3390/ijms26073313





