Ortholog Analysis and Transformation of Glycoside Hydrolase Genes in Hyperthermophilic Archaeal Thermococcus Species
Abstract
1. Introduction
2. Results
2.1. Ortholog Detection in Thermococcus Species
2.2. Incorporation of the Tpa-GH Gene Cluster in the Genome of T. onnurineus NA1
| Species | Strain | Accession | OG0000069 | OG0001332 | OG0002060 | OG0002308 | OG0002385 | OG0002611 | OG0003709 | Total Genes |
|---|---|---|---|---|---|---|---|---|---|---|
| T. aciditolerans | SY113 | GCF_008152015 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 5 |
| T. aggregans | TY | GCF_024022995 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| T. alcaliphilus | AEDII12 | GCF_024054535 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| T. argininiproducens | IOH2 | GCF_023746595 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| T. barophilus | MP | GCF_000151105 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 3 |
| T. barossii | SHCK-94 | GCF_002214465 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 4 |
| T. bergensis | T7324 | GCF_020386975 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| T. camini | IRI35c | GCF_904067545 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 4 |
| T. celer | Vu13 | GCF_002214365 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| T. celericrescens | DSM17994 | GCF_001484195 | 2 | 1 | 1 | 0 | 1 | 0 | 0 | 5 |
| T. chitonophagus | GC74 | GCF_002214605 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 4 |
| T. cleftensis | CL1 | GCF_000265525 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 |
| T. eurythermalis | A501 | GCF_000769655 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 3 |
| T. gammatolerans | EJ3 | GCF_000022365 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| T. gorgonarius | W-12 | GCF_002214385 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| T. guaymasensis | DSM11113 | GCF_000816105 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| T. henrietii | EXT12c | GCF_900198835 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 3 |
| T. indicus | IOH1 | GCF_006274605 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 3 |
| T. kodakarensis | KOD1 | GCF_000009965 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 4 |
| T. litoralis | DSM 5473 | GCF_000246985 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| T. nautili | 01-30 | GCF_000585495 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 3 |
| T. onnurineus | NA1 | GCF_000018365 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| T. pacificus | P-4 | GCF_002214485 | 4 | 1 | 1 | 2 | 0 | 1 | 0 | 9 |
| T. paralvinellae | ES1 | GCF_000517445 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| T. peptonophilus | OG-1 | GCF_001592435 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| T. piezophilus | CDGS | GCF_001647085 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| T. profundus | DT5432 | GCF_002214585 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
| T. radiotolerans | EJ2 | GCF_002214565 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
| T. sibiricus | MM739 | GCF_000022545 | 3 | 0 | 0 | 3 | 0 | 1 | 0 | 7 |
| T. siculi | RG-20 | GCF_002214505 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 4 |
| T. stetteri | DSM5262 | GCF_017873335 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 5 |
| T. thermotolerans | 813A4 | GCF_024707485 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 3 |
| T. thioreducens | OGL-20P | GCF_900109425 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 3 |
| T. waiotapuensis | WT1 | GCF_032304395 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 5 |
| T. zilligii | AN1 | GCF_000258515 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
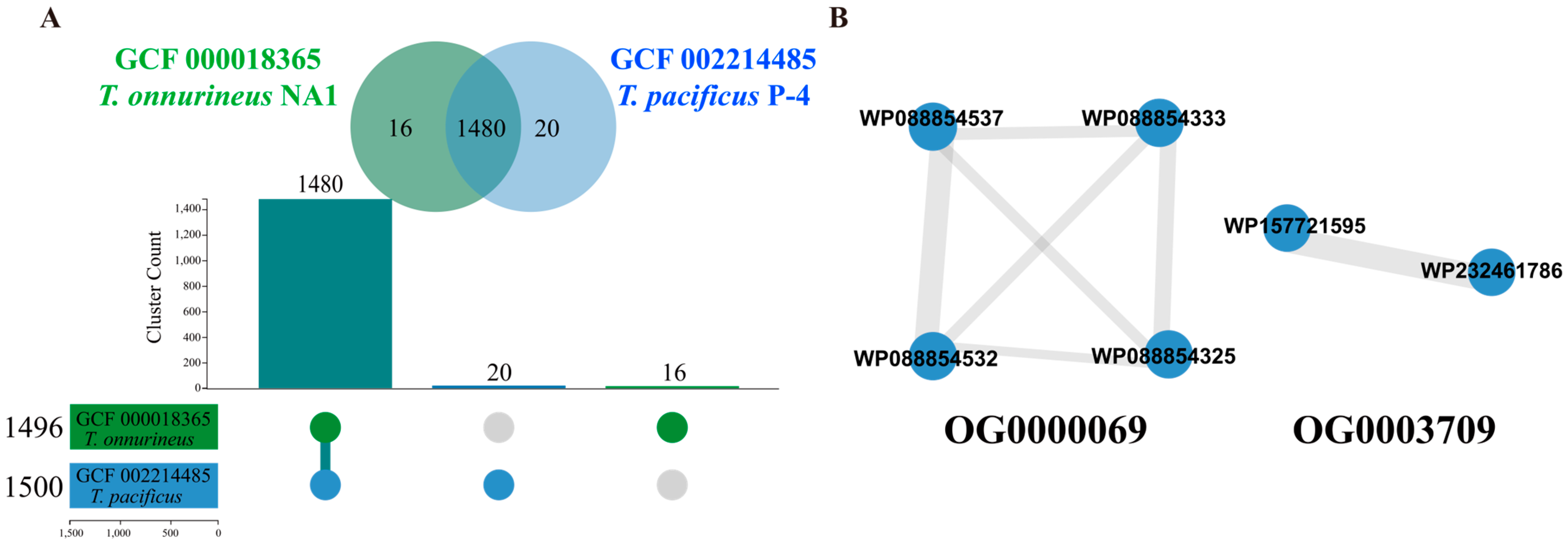
| Orthogroup (Gene Cluster) | OG0000069 | OG0001332 | OG0002060 | OG0002385 | OG0002611 | OG0003709 | OG0002308 |
|---|---|---|---|---|---|---|---|
| Gene family | GH1 family | CE4 family | GH38, GH57 family | GH4 family | GH16 family | GH5, GH12 family | Unknown GH domain |
| Number of species (spp.) | 29 | 29 | 13 | 8 | 6 | 2 | 6 |
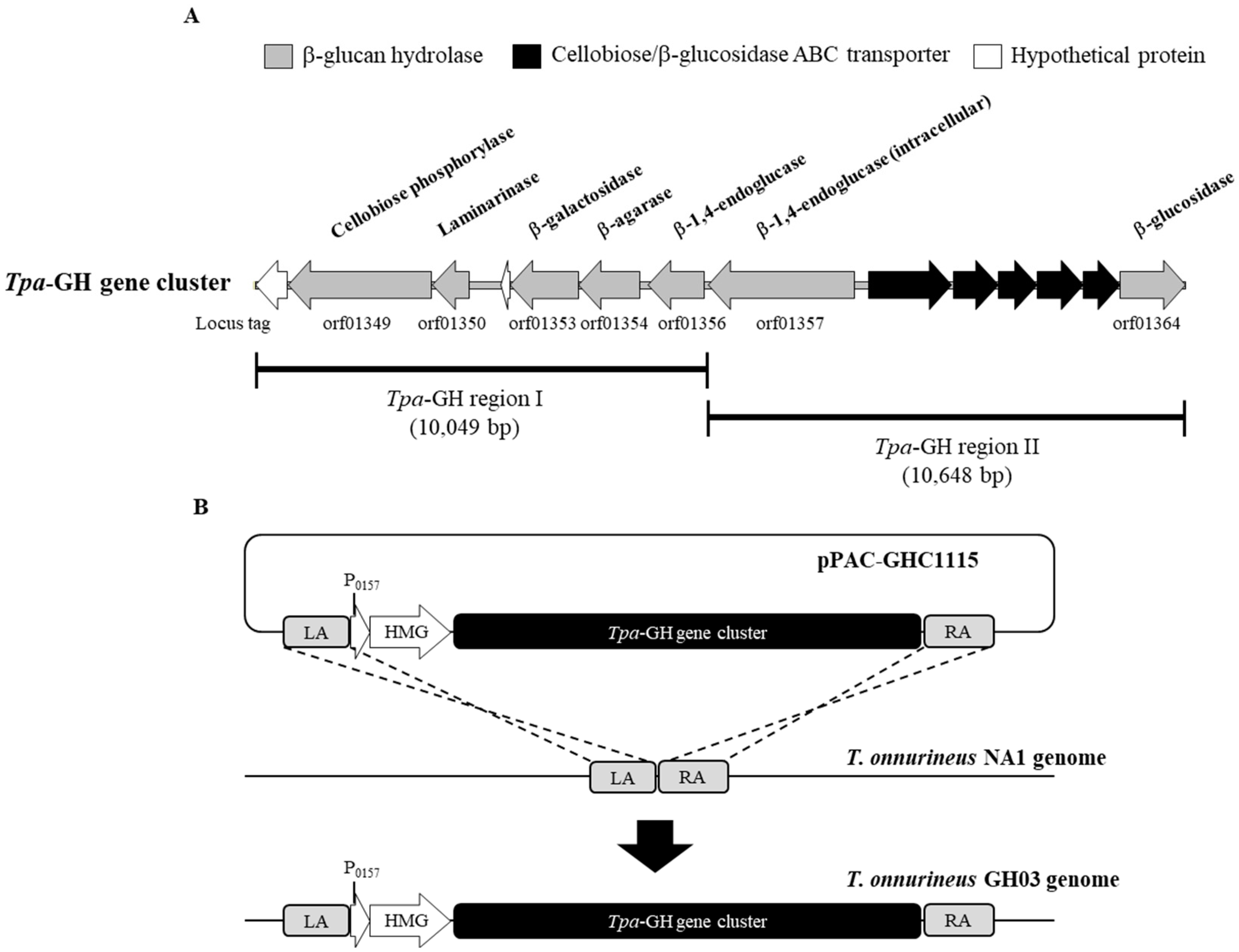
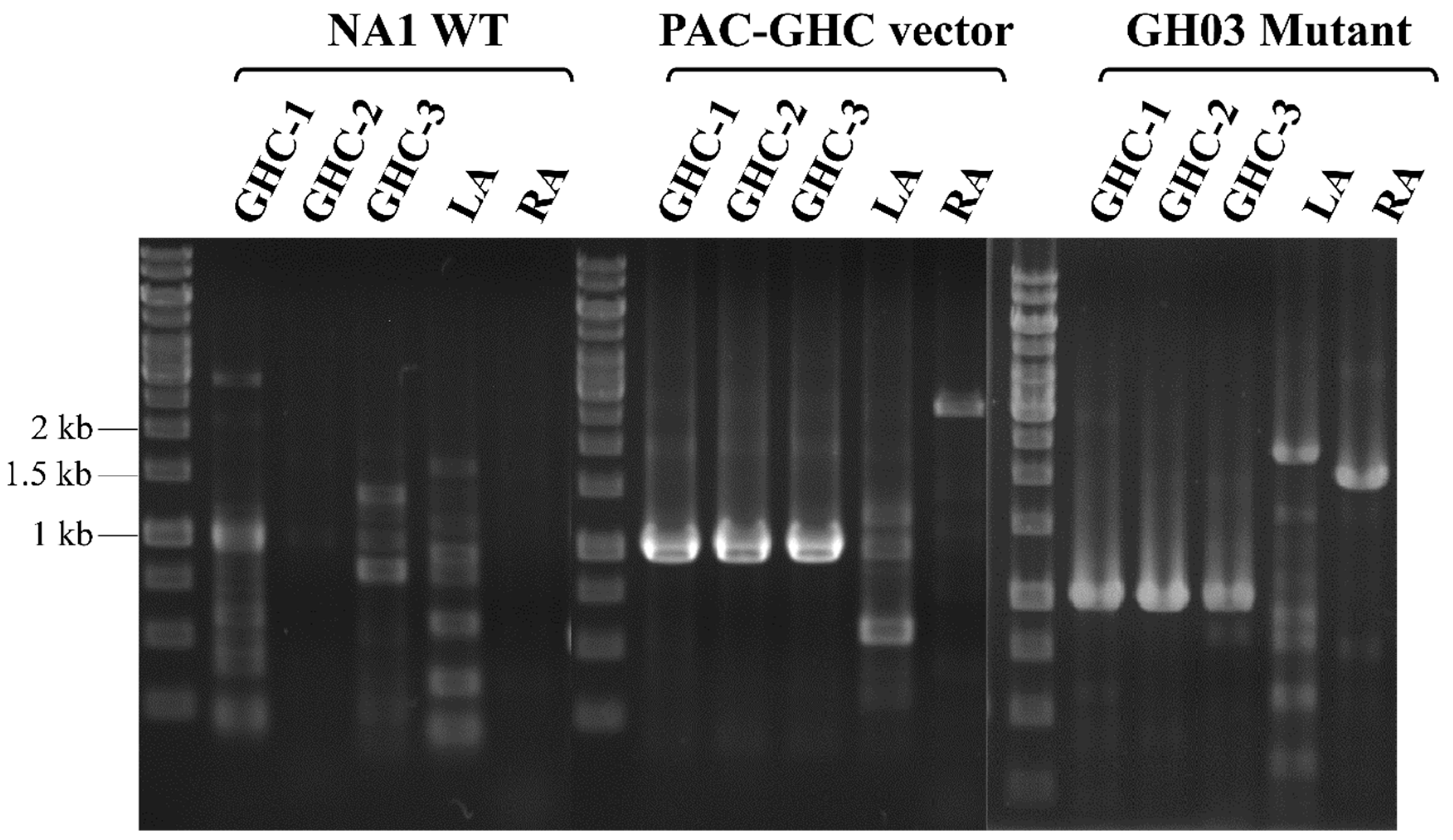
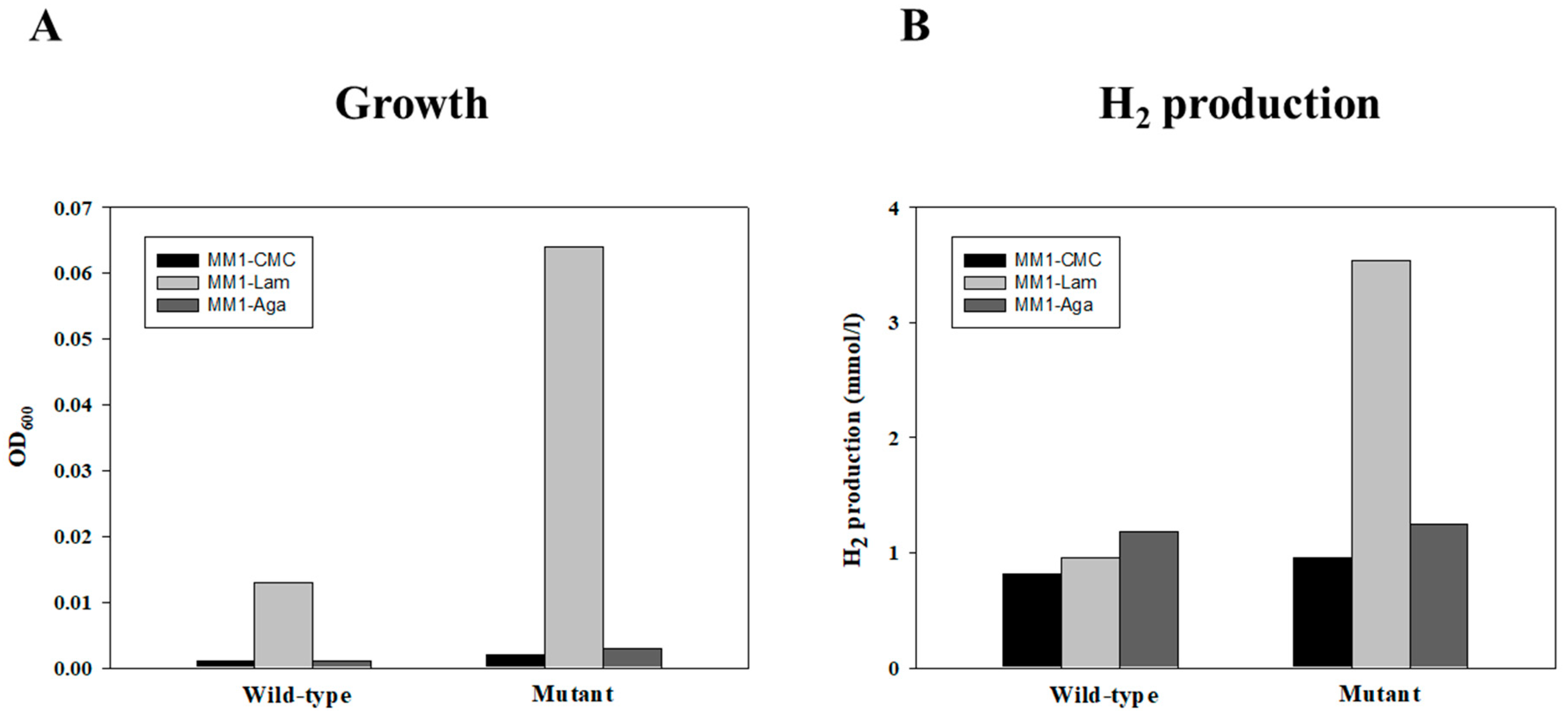
2.3. Enhanced Growth and Hydrogen Generation in the T. onnurineus GH03 Mutant
3. Discussion
4. Materials and Methods
4.1. Detection of Glycoside Hydrolase from Thermococcus Genomic Data
4.2. Strains and Cell Culture Conditions
4.3. Cloning and Mutant Construction
4.4. Growth and Hydrogen Production Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavicchioli, R. Archaea—Timeline of the third domain. Nat. Rev. Microbiol. 2011, 9, 51–61. [Google Scholar] [CrossRef]
- Shu, W.S.; Huang, L.N. Microbial diversity in extreme environments. Nat. Rev. Microbiol. 2022, 20, 219–235. [Google Scholar] [CrossRef]
- Rampelotto, P.H. Extremophiles and extreme environments. Life 2013, 3, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Berde, C.P.; Berde, V.B. Role of Archaea in the Remediation of Contaminated Soils. In Bioremediation and Phytoremediation Technologies in Sustainable Soil Management, 1st ed.; Apple Academic Press: Florida, FL, USA, 2022; pp. 33–52. [Google Scholar]
- Oyewusi, H.A.; Wahab, R.A.; Huyop, F. Dehalogenase-producing halophiles and their potential role in bioremediation. Mar. Pollut. Bull. 2020, 160, 111603. [Google Scholar] [CrossRef] [PubMed]
- Wegener, G.; Laso-Pérez, R.; Orphan, V.J.; Boetius, A. Anaerobic degradation of alkanes by marine archaea. Annu. Rev. Microbiol. 2022, 76, 553–577. [Google Scholar] [CrossRef]
- Jia, R.; Yang, D.; Xu, D.; Gu, T. Carbon steel biocorrosion at 80 °C by a thermophilic sulfate reducing archaeon biofilm provides evidence for its utilization of elemental iron as electron donor through extracellular electron transfer. Commun. Biol. 2018, 145, 47–54. [Google Scholar] [CrossRef]
- Fashola, M.; Ngole-Jeme, V.; Babalola, O. Diversity of acidophilic bacteria and archaea and their roles in bioremediation of acid mine drainage. Br. Microbiol. Res. J. 2015, 8, 443–456. [Google Scholar] [CrossRef]
- DeLong, E.F.; Wu, K.Y.; Prézelin, B.B.; Jovine, R.V. High abundance of Archaea in Antarctic marine picoplankton. Nature 1994, 371, 695–697. [Google Scholar] [CrossRef]
- Massana, R.; Murray, A.E.; Preston, C.M.; DeLong, E.F. Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara Channel. Appl. Environ. Microbiol. 1997, 63, 50–56. [Google Scholar] [CrossRef]
- Hutchins, D.A.; Capone, D.G. The marine nitrogen cycle: New developments and global change. Nat. Rev. Microbiol. 2022, 20, 401–414. [Google Scholar] [CrossRef]
- Worden, A.Z.; Follows, M.J.; Giovannoni, S.J.; Wilken, S.; Zimmerman, A.E.; Keeling, P.J. Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes. Science 2015, 347, 1257594. [Google Scholar] [CrossRef]
- Verma, D.; Kumar, V.; Satyanarayana, T. Genomic attributes of thermophilic and hyperthermophilic bacteria and archaea. World J. Microbiol. Biotechnol. 2022, 38, 135. [Google Scholar] [CrossRef]
- Brock, T.D. The value of basic research: Discovery of Thermus aquaticus and other extreme thermophiles. Genetics 1997, 146, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Stetter, K.O.; Thomm, M.; Winter, J.; Wildgruber, G.; Huber, H.; Zillig, W.; Jané-Covic, D.; König, H.; Palm, P.; Wunderl, S. Methanothermus fervidus, sp. nov., a novel extremely thermophilic methanogen isolated from an Icelandic hot spring. Zentralbl. Bakteriol. Mikrobiol. Hyg. C 1981, 2, 166–178. [Google Scholar] [CrossRef]
- Stetter, K.O. Ultrathin mycelia-forming organisms from submarine volcanic areas having an optimum growth temperature of 105 °C. Nature 1982, 300, 258–260. [Google Scholar] [CrossRef]
- Stetter, K.O. History of discovery of the first hyperthermophiles. Extremophiles 2006, 10, 357–362. [Google Scholar] [CrossRef]
- Bertoldo, C.; Antranikian, G. The order thermococcales. In The Prokaryotes, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 3, pp. 69–81. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, L.; Ning, K. Pan-genome study of Thermococcales reveals extensive genetic diversity and genetic evidence of thermophilic adaption. Environ. Microbiol. 2021, 23, 3599–3613. [Google Scholar] [CrossRef]
- Park, S.; Lee, B.; Park, K. Extremophilic carbohydrate active enzymes (CAZymes). J. Nutri. Health Food Eng. 2017, 7, 230–237. [Google Scholar] [CrossRef][Green Version]
- Klaus, T.; Ninck, S.; Albersmeier, A.; Busche, T.; Wibberg, D.; Jiang, J.; Elcheninov, A.G.; Zayulina, K.S.; Kaschani, F.; Bräsen, C. Activity-based protein profiling for the identification of novel carbohydrate-active enzymes involved in xylan degradation in the hyperthermophilic euryarchaeon Thermococcus sp. strain 2319x1E. Front. Microbiol. 2022, 12, 734039. [Google Scholar] [CrossRef]
- Bräsen, C.; Esser, D.; Rauch, B.; Siebers, B. Carbohydrate metabolism in Archaea: Current insights into unusual enzymes and pathways and their regulation. Microbiol. Mol. Biol. Rev. 2014, 78, 89–175. [Google Scholar] [CrossRef]
- Iacono, R.; De Lise, F.; Moracci, M.; Cobucci-Ponzano, B.; Strazzulli, A. Glycoside hydrolases from (hyper) thermophilic archaea: Structure, function, and applications. Essays Biochem. 2023, 67, 731–751. [Google Scholar] [CrossRef] [PubMed]
- Yennamalli, R.M.; Rader, A.J.; Wolt, J.D.; Sen, T.Z. Thermostability in endoglucanases is fold-specific. BMC Struct. Biol. 2011, 11, 10. [Google Scholar] [CrossRef]
- Dumorné, K.; Córdova, D.C.; Astorga-Eló, M.; Renganathan, P. Extremozymes: A potential source for industrial applications. J. Microbiol. Biotechnol. 2017, 27, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Henrissat, B. Structures and mechanisms of glycosyl hydrolases. Structure 1995, 3, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Naumoff, D. Hierarchical classification of glycoside hydrolases. Biochemistry 2011, 76, 622–635. [Google Scholar] [CrossRef]
- Ati, J.; Lafite, P.; Daniellou, R. Enzymatic synthesis of glycosides: From natural O-and N-glycosides to rare and S-glycosides. Beilstein J. Org. Chem. 2017, 13, 1857–1865. [Google Scholar] [CrossRef]
- Sjögren, J.; Collin, M. Bacterial glycosidases in pathogenesis and glycoengineering. Future Microbiol. 2014, 9, 1039–1051. [Google Scholar] [CrossRef]
- Vermassen, A.; Leroy, S.; Talon, R.; Provot, C.; Popowska, M.; Desvaux, M. Cell wall hydrolases in bacteria: Insight on the diversity of cell wall amidases, glycosidases and peptidases toward peptidoglycan. Front. Microbiol. 2019, 10, 331. [Google Scholar] [CrossRef]
- Pang, P.; Cao, L.C.; Liu, Y.H.; Xie, W.; Wang, Z. Structures of a glucose-tolerant β-glucosidase provide insights into its mechanism. J. Struct. Biol. 2017, 198, 154–162. [Google Scholar] [CrossRef]
- Sinha, S.K.; Datta, S. β-Glucosidase from the hyperthermophilic archaeon Thermococcus sp. is a salt-tolerant enzyme that is stabilized by its reaction product glucose. Appl. Microbiol. Biotechnol. 2016, 100, 8399–8409. [Google Scholar] [CrossRef]
- Albalawi, H.; Altayeb, H.N.; Iftikhar, S.; Al-Ghamdi, M.A.; Khan, J.A.; Nadeem, M.S. Biochemical and in silico evaluation of a recombinant, glucose tolerant, and highly thermostable β-glucosidase from Thermococcus radiotolerans DSM-15228. Electron. J. Biotechnol. 2023, 64, 10–17. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.E.; Lee, H.S.; Kwon, K.K.; Kang, S.G.; Lee, J.H. Novel substrate specificity of a thermostable β-glucosidase from the hyperthermophilic archaeon, Thermococcus pacificus P-4. Korean J. Microbiol. 2015, 51, 68–74. [Google Scholar] [CrossRef]
- Bae, S.S.; Kim, Y.J.; Yang, S.H.; Lim, J.K.; Jeon, J.H.; Lee, H.S.; Kang, S.G.; Kim, S.J.; Lee, J.H. Thermococcus onnurineus sp. nov., a hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent area at the PACMANUS field. J. Microbiol. Biotechnol. 2006, 16, 1826–1831. [Google Scholar]
- Lee, H.S.; Kang, S.G.; Bae, S.S.; Lim, J.K.; Cho, Y.; Kim, Y.J.; Jeon, J.H.; Cha, S.S.; Kwon, K.K.; Kim, H.T. The complete genome sequence of Thermococcus onnurineus NA1 reveals a mixed heterotrophic and carboxydotrophic metabolism. J. Bacteriol. 2008, 190, 7491–7499. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Bae, S.S.; Kim, Y.J.; Kim, T.W.; Lim, J.K.; Lee, S.H.; Choi, A.R.; Jeon, J.H.; Lee, J.H.; Lee, H.S. CO-dependent H2 production by genetically engineered Thermococcus onnurineus NA1. Appl. Environ. Microbiol. 2013, 79, 2048–2053. [Google Scholar] [CrossRef]
- Cho, S.; Kim, M.S.; Jeong, Y.; Lee, B.R.; Lee, J.H.; Kang, S.G.; Cho, B.K. Genome-wide primary transcriptome analysis of H2-producing archaeon Thermococcus onnurineus NA1. Sci. Rep. 2017, 7, 43044. [Google Scholar] [CrossRef]
- Sun, J.; Lu, F.; Luo, Y.; Bie, L.; Xu, L.; Wang, Y. OrthoVenn3: An integrated platform for exploring and visualizing orthologous data across genomes. Nucleic Acids Res. 2023, 51, W397–W403. [Google Scholar] [CrossRef]
- Moon, Y.J.; Kwon, J.; Yun, S.H.; Lim, H.L.; Kim, J.; Kim, S.J.; Kang, S.G.; Lee, J.H.; Kim, S.I.; Chung, Y.H. Proteomic insights into sulfur metabolism in the hydrogen-producing hyperthermophilic archaeon Thermococcus onnurineus NA1. Int. J. Mol. Sci. 2015, 16, 9167–9195. [Google Scholar] [CrossRef]
- Badawi, E.Y.; Elkharsa, R.A.; Abdelfattah, E.A. Value proposition of bio-hydrogen production from different biomass sources. Energy Nexus 2023, 10, 100194. [Google Scholar] [CrossRef]
- Lim, J.K.; Yang, J.I.; Kim, Y.J.; Park, Y.J.; Kim, Y.H. Bioconversion of CO to formate by artificially designed carbon monoxide: Formate oxidoreductase in hyperthermophilic archaea. Commun. Biol. 2022, 5, 539. [Google Scholar] [CrossRef]
- Beattie, A.; Hirst, E.; Percival, E. Studies on the metabolism of the Chrysophyceae. Comparative structural investigations on leucosin (chrysolaminarin) separated from diatoms and laminarin from the brown algae. Biochem. J. 1961, 79, 531. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, structure and biofunctional activities of laminarin from brown algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Kengen, S.W.; Luesink, E.J.; Stams, A.J.; Zehnder, A.J. Purification and characterization of an extremely thermostable β-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus. Eur. J. Biochem. 1993, 213, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.W.; Bylina, E.J.; Swanson, R.V.; Kelly, R.M. Comparison of a β-Glucosidase and a β-Mannosidase from the hyperthermophilic archaeon Pyrococcus furiosus: Purification, characterization, gene cloning, and sequence analysis. J. Biol. Chem. 1996, 271, 23749–23755. [Google Scholar] [CrossRef] [PubMed]
- Sakellaris, H.; Manners, J.M.; Pemberton, J.M. A gene encoding an exo-β-glucosidase from Cellvibrio mixtus. Curr. Microbiol. 1997, 35, 228–232. [Google Scholar] [CrossRef]
- Bauer, M.W.; Driskill, L.E.; Callen, W.; Snead, M.A.; Mathur, E.J.; Kelly, R.M. An endoglucanase, EglA, from the hyperthermophilic archaeon Pyrococcus furiosus hydrolyzes β-1, 4 bonds in mixed-linkage (1→ 3), (1→ 4)-β-d-glucans and cellulose. J. Bacteriol. 1999, 181, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Tebben, J.; Coffinet, S.; Wiltshire, K.; Iversen, M.H.; Harder, T.; Hinrichs, K.U.; Hehemann, J.H. Laminarin is a major molecule in the marine carbon cycle. Proc. Natl. Acad. Sci. USA 2020, 117, 6599–6607. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, S.; Lee, S.M.; Cha, J.; Lee, H.S.; Kang, S.G. Biohydrogen production from food waste using glucose-adapted hyperthermophilic archaeon. Waste Biomass Valori. 2023, 14, 2923–2930. [Google Scholar] [CrossRef]
- Kado, Y.; Inoue, T.; Ishikawa, K. Structure of hyperthermophilic β-glucosidase from Pyrococcus furiosus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 1473–1479. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.S.; Kang, S.G.; Lee, H.S. Biohydrogen production of obligate anaerobic archaeon Thermococcus onnurineus NA1 under oxic conditions via overexpression of frhAGB-encoding hydrogenase genes. Biotechnol. Biofuels. 2019, 12, 24. [Google Scholar] [CrossRef]
- Alderkamp, A.C.; Van Rijssel, M.; Bolhuis, H. Characterization of marine bacteria and the activity of their enzyme systems involved in degradation of the algal storage glucan laminarin. FEMS Microbiol. Ecol. 2007, 59, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Kim, E.Y.; Kim, Y.R.; Kim, J.K.; Lee, H.S.; Kong, I.S. Application of β-1, 3-glucanase from Pyrococcus furiosus for ethanol production using laminarin. J. Life Sci. 2011, 21, 68–73. [Google Scholar] [CrossRef]
- Ghosh, S. Assessment and update of status of pilot scale fermentative biohydrogen production with focus on candidate bioprocesses and decisive key parameters. Int. J. Hydrogen Energy. 2022, 47, 17161–17183. [Google Scholar] [CrossRef]
- Kim, T.W.; Bae, S.S.; Lee, J.W.; Lee, S.M.; Lee, J.H.; Lee, H.S.; Kang, S.G. A biological process efective for the conversion of CO-containing industrial waste gas to acetate. Bioresour. Technol. 2016, 211, 792–796. [Google Scholar] [CrossRef]
- Kim, M.S.; Choi, A.R.; Lee, S.H.; Jung, H.C.; Bae, S.S.; Yang, T.J.; Jeon, J.H.; Lim, J.K.; Youn, H.; Kim, T.W.; et al. A novel CO-responsive transcriptional regulator and enhanced H2 production by an engineered Thermococcus onnurineus NA1 strain. Appl. Environ. Microbiol. 2015, 81, 1708–1714. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.S.; Bae, S.S.; Choi, A.R.; Lee, J.W.; Kim, T.W.; Lee, J.H.; Lee, H.S.; Kang, S.G. Comparison of CO-dependent H2 production with strong promoters in Thermococcus onnurineus NA1. Appl. Microbiol. Biotechnol. 2014, 98, 979–986. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.S.; Lee, J.H.; Kim, T.W.; Bae, S.S.; Lee, S.M.; Jung, H.C.; Yang, T.J.; Choi, A.R.; Cho, Y.J.; et al. Adaptive engineering of a hyperthermophilic archaeon on CO and discovering the underlying mechanism by multi-omics analysis. Sci. Rep. 2016, 6, 22896. [Google Scholar] [CrossRef]
- Jung, H.C.; Lee, S.H.; Lee, S.M.; An, Y.J.; Lee, J.H.; Lee, H.S.; Kang, S.G. Adaptive evolution of a hyperthermophilic archaeon pinpoints a formate transporter as a critical factor for the growth enhancement on formate. Sci. Rep. 2017, 7, 6124. [Google Scholar] [CrossRef]
- Bae, S.S.; Lee, H.S.; Jeon, J.H.; Lee, J.H.; Kang, S.G.; Kim, T.W. Enhancing bio-hydrogen production from sodium formate by hyperthermophilic archaeon, Thermococcus onnurineus NA1. Bioprocess Biosyst. Eng. 2015, 38, 989–993. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Yang, G.; Sun, G.; Sage, V. A review of the enhancement of bio-hydrogen generation by chemicals addition. Catalysts 2019, 9, 353. [Google Scholar] [CrossRef]
- Sato, T.; Fukui, T.; Atomi, H.; Imanaka, T. Targeted gene disruption by homologous recombination in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J. Bacteriol. 2003, 185, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory: New York, NY, USA, 2001. [Google Scholar]
- Sokolova, T.G.; Jeanthon, C.; Kostrikina, N.A.; Chernyh, N.A.; Lebedinsky, A.V.; Stackebrandt, E.; Bonch-Osmolovskaya, E.A. The first evidence of anaerobic CO oxidation coupled with H2 production by a hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Extremophiles 2004, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, H.S.; Kim, E.S.; Bae, S.S.; Lim, J.K.; Matsumi, R.; Lebedinsky, A.V.; Sokolova, T.G.; Kozhevnikova, D.A.; Cha, S.S. Formate-driven growth coupled with H2 production. Nature 2010, 467, 352–355. [Google Scholar] [CrossRef]
- Holden, J.F.; Takai, K.; Summit, M.; Bolton, S.; Zyskowski, J.; Baross, J.A. Diversity among three novel groups of hyperthermophilic deep-sea Thermococcus species from three sites in the northeastern Pacific Ocean. FEMS Microbiol. Ecol. 2001, 36, 51–60. [Google Scholar] [CrossRef]
- Balch, W.E.; Wolfe, R. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl. Environ. Microbiol. 1976, 32, 781–791. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.W.; Lim, J.K.; Lee, H.S.; Kang, S.G.; Lee, J.-H.; Kwon, K.K.; Kim, Y.J. Ortholog Analysis and Transformation of Glycoside Hydrolase Genes in Hyperthermophilic Archaeal Thermococcus Species. Int. J. Mol. Sci. 2025, 26, 3305. https://doi.org/10.3390/ijms26073305
Lee JW, Lim JK, Lee HS, Kang SG, Lee J-H, Kwon KK, Kim YJ. Ortholog Analysis and Transformation of Glycoside Hydrolase Genes in Hyperthermophilic Archaeal Thermococcus Species. International Journal of Molecular Sciences. 2025; 26(7):3305. https://doi.org/10.3390/ijms26073305
Chicago/Turabian StyleLee, Jun Won, Jae Kyu Lim, Hyun Sook Lee, Sung Gyun Kang, Jung-Hyun Lee, Kae Kyoung Kwon, and Yun Jae Kim. 2025. "Ortholog Analysis and Transformation of Glycoside Hydrolase Genes in Hyperthermophilic Archaeal Thermococcus Species" International Journal of Molecular Sciences 26, no. 7: 3305. https://doi.org/10.3390/ijms26073305
APA StyleLee, J. W., Lim, J. K., Lee, H. S., Kang, S. G., Lee, J.-H., Kwon, K. K., & Kim, Y. J. (2025). Ortholog Analysis and Transformation of Glycoside Hydrolase Genes in Hyperthermophilic Archaeal Thermococcus Species. International Journal of Molecular Sciences, 26(7), 3305. https://doi.org/10.3390/ijms26073305






