O-GlcNAcylation and Phosphorylation Crosstalk in Vascular Smooth Muscle Cells: Cellular and Therapeutic Significance in Cardiac and Vascular Pathologies
Abstract
1. Introduction
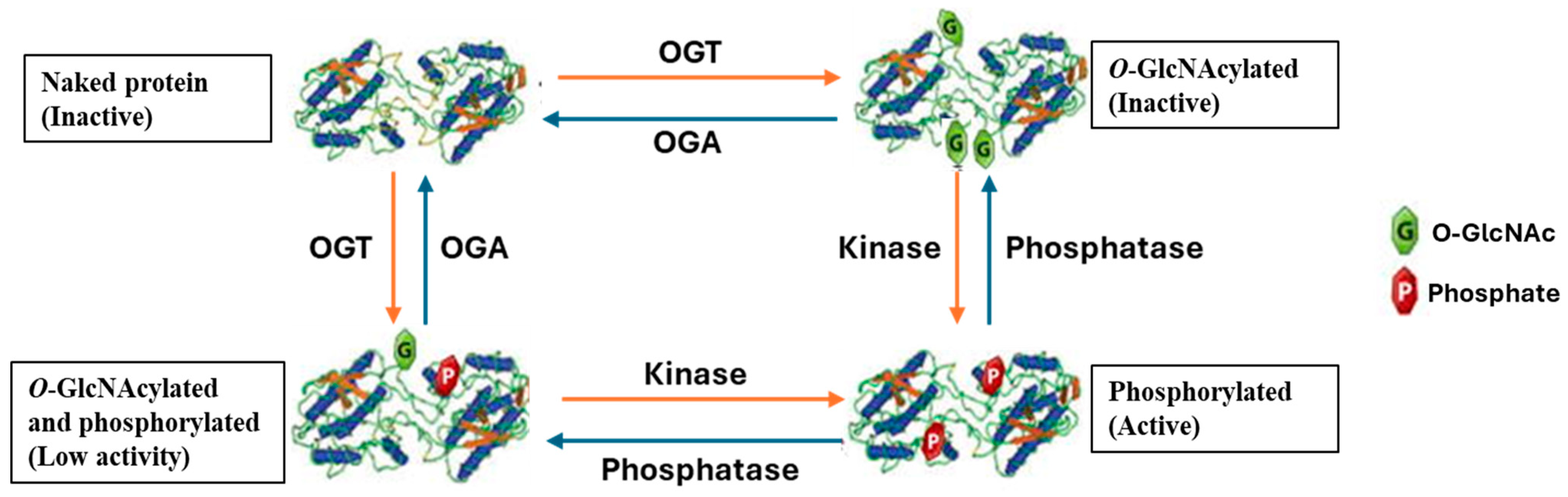
2. O-GlcNAcylation and Phosphorylation Crosstalk
2.1. O-GlcNAcylation and Phosphorylation Operating as Friends
2.2. O-GlcNAcylation and Phosphorylation Operating as Foes
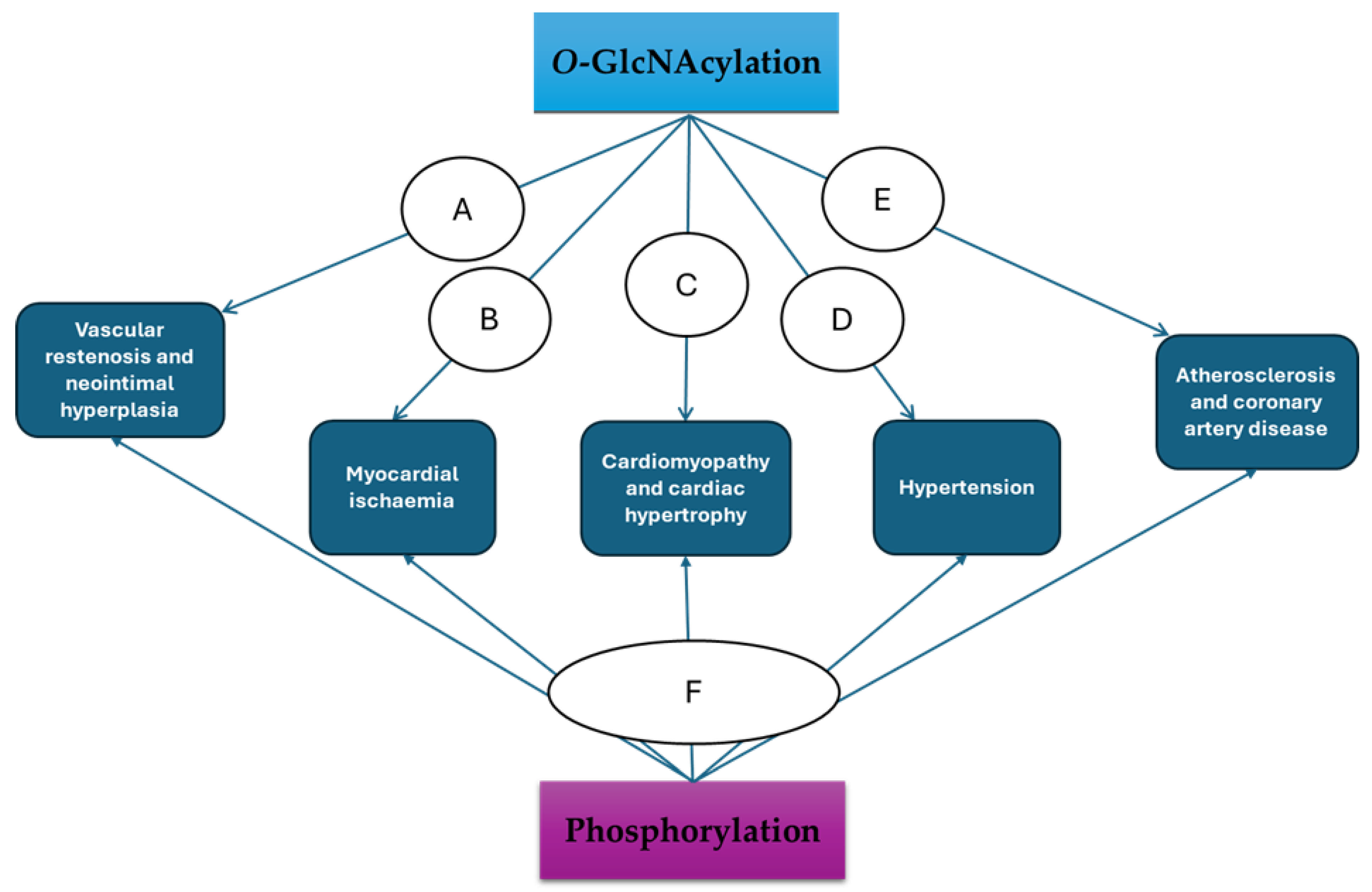
3. Effects of O-GlcNAcylation and Phosphorylation Crosstalk on Regulatory Proteins Expressed by VSMCs
4. Exploring the O-GlcNAcylation and Phosphorylation Crosstalk in VSMCs for Therapeutic Gains in CVDs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALDH2 | Acetaldehyde dehydrogenase 2 |
| Ca2+/CAM-PK | Calcium-calmodulin protein kinase |
| CaMKIV | Calcium/calmodulin-dependent protein kinase IV |
| CHK1 | Checkpoint kinase 1 |
| CVD | Cardiovascular disease |
| DOCA | Deoxycorticosterone acetate |
| eIF-2 | Eukaryotic initiation factor-2 |
| eNOS | Endothelial nitric oxide synthase |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| FOXP1 | Forkhead box protein P1 |
| GFAT | Glutamine-fructose-6P amidotransferase |
| GlcN-6-P | Glucosamine-6-phosphate |
| GNPNAT | Glucosamine-6-phosphate N-acetyltransferase |
| GSK-3 | Glycogen synthase kinase-3 |
| MAPKs | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappaB |
| OGA | O-GlcNAcase |
| O-GlcNAc | β-D-N-acetylglucosamine |
| OGT | O-GlcNAc transferase |
| PGM3 | GlcNAc phosphomutase |
| PI3K | Phosphoinositide 3-kinases |
| PKC | Protein kinase C |
| PKG | cGMP-dependent protein kinase |
| PLC-β1 | Phospholipase C-beta 1 |
| PTKs | Tyrosine protein kinases |
| PTM | Post-translational modification |
| PUGNAc | O-(2-acetamido-2-deoxy-d-glucopyranosylidene)-amino-N-phenylcarbamate |
| SERCA2 | Sarco/endoplasmic reticulum calcium (Ca2+)-ATPase |
| Sp1 | Specificity protein 1 |
| T2DM | Type 2 diabetes mellitus |
| TGF-β | Transforming growth factor beta |
| UAP/AGX1 | UDP-N-acetylhexosamine pyrophosphorylase 1 |
| UDP-GlcNAc | Uridine diphosphate-N-acetylglucosamine |
| VSMCs | Vascular smooth muscle cells |
References
- Yang, X.; Qian, K. Protein O-GlcNAcylation: Emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2017, 18, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.R.; Hart, G.W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 1984, 259, 3308–3317. [Google Scholar] [CrossRef] [PubMed]
- Chatham, J.C.; Young, M.E.; Zhang, J. Role of O-linked N-acetylglucosamine (O-GlcNAc) modification of proteins in diabetic cardiovascular complications. Curr. Opin. Pharmacol. 2021, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.N.; Collins, H.E.; Wende, A.R.; Chatham, J.C. O-GlcNAcylation and cardiovascular disease. Biochem. Soc. Trans. 2017, 45, 545–553. [Google Scholar] [CrossRef]
- Bolanle, I.O.; Riches-Suman, K.; Williamson, R.; Palmer, T.M. Emerging roles of protein O-GlcNAcylation in cardiovascular diseases: Insights and novel therapeutic targets. Pharmacol. Res. 2021, 165, 105467. [Google Scholar] [CrossRef]
- Laczy, B.; Hill, B.G.; Wang, K.; Paterson, A.J.; White, C.R.; Xing, D.; Chen, Y.F.; Darley-Usmar, V.; Oparil, S.; Chatham, J.C. Protein O-GlcNAcylation: A new signaling paradigm for the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H13–H28. [Google Scholar] [CrossRef]
- Bolanle, I.O.; Riches-Suman, K.; Loubani, M.; Williamson, R.; Palmer, T.M. Revascularisation of type 2 diabetics with coronary artery disease: Insights and therapeutic targeting of O-GlcNAcylation. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1349–1356. [Google Scholar] [CrossRef]
- Marsh, S.A.; Collins, H.E.; Chatham, J.C. Protein O-GlcNAcylation and cardiovascular (patho)physiology. J. Biol. Chem. 2014, 289, 34449–34456. [Google Scholar] [CrossRef]
- Dassanayaka, S.; Jones, S.P. O-GlcNAc and the cardiovascular system. Pharmacol. Ther. 2014, 142, 62–71. [Google Scholar] [CrossRef]
- Jensen, R.V.; Andreadou, I.; Hausenloy, D.J.; Bøtker, H.E. The Role of O-GlcNAcylation for Protection against Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2019, 20, 404. [Google Scholar] [CrossRef]
- Fülöp, N.; Marchase, R.B.; Chatham, J.C. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc. Res. 2007, 73, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; Crea, F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.D.; Sakabe, K.; Housley, M.P.; Dias, W.B.; Hart, G.W. O-linked beta-N-acetylglucosaminyltransferase substrate specificity is regulated by myosin phosphatase targeting and other interacting proteins. J. Biol. Chem. 2008, 283, 33935–33941. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, F.; Kudlow, J.E. Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: Coupling protein O-GlcNAcylation to transcriptional repression. Cell 2002, 110, 69–80. [Google Scholar] [CrossRef]
- Kim, Y.H.; Song, M.; Oh, Y.S.; Heo, K.; Choi, J.W.; Park, J.M.; Kim, S.H.; Lim, S.; Kwon, H.M.; Ryu, S.H.; et al. Inhibition of phospholipase C-beta1-mediated signaling by O-GlcNAc modification. J. Cell. Physiol. 2006, 207, 689–696. [Google Scholar] [CrossRef]
- Nishi, H.; Shaytan, A.; Panchenko, A.R. Physicochemical mechanisms of protein regulation by phosphorylation. Front. Genet. 2014, 5, 270. [Google Scholar] [CrossRef]
- Cohen, P. The regulation of protein function by multisite phosphorylation—A 25 year update. Trends Biochem. Sci. 2000, 25, 596–601. [Google Scholar] [CrossRef]
- Rapundalo, S.T. Cardiac protein phosphorylation: Functional and pathophysiological correlates. Cardiovasc. Res. 1998, 38, 559–588. [Google Scholar] [CrossRef]
- Lindemann, J.P.; Watanabe, A.M. Phosphorylation of phospholamban in intact myocardium. Role of Ca2+-calmodulin-dependent mechanisms. J. Biol. Chem. 1985, 260, 4516–4525. [Google Scholar] [CrossRef]
- Vittone, L.; Mundiña, C.; Chiappe de Cingolani, G.; Mattiazzi, A. Role of Ca2+-calmodulin dependent phospholamban phosphorylation on the relaxant effect of beta-adrenergic agonists. Mol. Cell. Biochem. 1993, 124, 33–42. [Google Scholar] [CrossRef]
- Parekh, D.B.; Ziegler, W.; Parker, P.J. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000, 19, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Talosi, L.; Kranias, E.G. Effect of alpha-adrenergic stimulation on activation of protein kinase C and phosphorylation of proteins in intact rabbit hearts. Circ. Res. 1992, 70, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Huggins, J.P.; Cook, E.A.; Piggott, J.R.; Mattinsley, T.J.; England, P.J. Phospholamban is a good substrate for cyclic GMP-dependent protein kinase in vitro, but not in intact cardiac or smooth muscle. Biochem. J. 1989, 260, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Sabine, B.; Willenbrock, R.; Haase, H.; Karczewski, P.; Wallukat, G.; Dietz, R.; Krause, E.G. Cyclic GMP-mediated phospholamban phosphorylation in intact cardiomyocytes. Biochem. Biophys. Res. Commun. 1995, 214, 75–80. [Google Scholar] [CrossRef]
- Srivastava, A.K. Protein tyrosine phosphorylation in cardiovascular system. Mol. Cell. Biochem. 1995, 149, 87–94. [Google Scholar] [CrossRef]
- Foncea, R.; Andersson, M.; Ketterman, A.; Blakesley, V.; Sapag-Hagar, M.; Sugden, P.H.; LeRoith, D.; Lavandero, S. Insulin-like growth factor-I rapidly activates multiple signal transduction pathways in cultured rat cardiac myocytes. J. Biol. Chem. 1997, 272, 19115–19124. [Google Scholar] [CrossRef]
- Clerk, A.; Gillespie-Brown, J.; Fuller, S.J.; Sugden, P.H. Stimulation of phosphatidylinositol hydrolysis, protein kinase C translocation, and mitogen-activated protein kinase activity by bradykinin in rat ventricular myocytes: Dissociation from the hypertrophic response. Biochem. J. 1996, 317, 109–118. [Google Scholar] [CrossRef]
- Clerk, A.; Sugden, P.H. Cell stress-induced phosphorylation of ATF2 and c-Jun transcription factors in rat ventricular myocytes. Biochem. J. 1997, 325, 801–810. [Google Scholar] [CrossRef]
- van der Laarse, S.A.M.; Leney, A.C.; Heck, A.J.R. Crosstalk between phosphorylation and O-GlcNAcylation: Friend or foe. FEBS J. 2018, 285, 3152–3167. [Google Scholar] [CrossRef]
- Khoury, G.A.; Baliban, R.C.; Floudas, C.A. Proteome-wide post-translational modification statistics: Frequency analysis and curation of the swiss-prot database. Sci. Rep. 2011, 13, 90. [Google Scholar] [CrossRef]
- Hu, P.; Shimoji, S.; Hart, G.W. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation. FEBS Lett. 2010, 584, 2526–2538. [Google Scholar] [CrossRef] [PubMed]
- Trinidad, J.C.; Barkan, D.T.; Gulledge, B.F.; Thalhammer, A.; Sali, A.; Schoepfer, R.; Burlingame, A.L. Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol. Cell. Proteom. 2012, 11, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Tomašič, T.; Sharif, S.; Brouwer, A.J.; Anderluh, M.; Ruijtenbeek, R.; Pieters, R.J. Peptide microarray analysis of the cross-talk between O-GlcNAcylation and tyrosine phosphorylation. FEBS Lett. 2017, 591, 1872–1883. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pandey, A.; Hart, G.W. Dynamic interplay between O-linked N-acetylglucosaminylation and glycogen synthase kinase-3-dependent phosphorylation. Mol. Cell. Proteom. 2007, 6, 1365–1379. [Google Scholar] [CrossRef]
- Hart, G.W.; Slawson, C.; Ramirez-Correa, G.; Lagerlof, O. Cross talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011, 80, 825–858. [Google Scholar] [CrossRef]
- Ng, Y.H.; Okolo, C.A.; Erickson, J.R.; Baldi, J.C.; Jones, P.P. Protein O-GlcNAcylation in the heart. Acta Physiol. 2021, 233, e13696. [Google Scholar] [CrossRef]
- Bacakova, L.; Travnickova, M.; Filova, E.; Matějka, R.; Stepanovska, J.; Musilkova, J.; Zarubova, J.; Molitor, M.; Bacakova, L.; Travnickova, M.; et al. The Role of Vascular Smooth Muscle Cells in the Physiology and Pathophysiology of Blood Vessels. In Muscle Cell and Tissue-Current Status of Research Field; IntechOpen: London, UK, 2018; pp. 1–13. [Google Scholar] [CrossRef]
- Chatham, J.C.; Patel, R.P. Protein glycosylation in cardiovascular health and disease. Nat. Rev. Cardiol. 2024, 21, 525–544. [Google Scholar] [CrossRef]
- Constable, S.; Lim, J.M.; Vaidyanathan, K.; Wells, L. O-GlcNAc transferase regulates transcriptional activity of human Oct4. Glycobiology 2017, 27, 927–937. [Google Scholar] [CrossRef]
- Rexach, J.E.; Clark, P.M.; Hsieh-Wilson, L.C. Chemical approaches to understanding O-GlcNAc glycosylation in the brain. Nat. Chem. Biol. 2008, 4, 97–106. [Google Scholar] [CrossRef]
- Wu, D.; Cai, Y.; Jin, J. Potential coordination role between O-GlcNAcylation and epigenetics. Protein Cell 2017, 8, 713–723. [Google Scholar] [CrossRef]
- Wells, L.; Vosseller, K.; Hart, G.W. Glycosylation of nucleocytoplasmic proteins: Signal transduction and O-GlcNAc. Science 2001, 291, 2376–2378. [Google Scholar] [CrossRef] [PubMed]
- Tarbet, H.J.; Toleman, C.A.; Boyce, M. A Sweet Embrace: Control of Protein-Protein Interactions by O-Linked β-N-Acetylglucosamine. Biochemistry 2018, 57, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.C.; Lee, K.Y.; Park, J.E.; Do, S.I. OGT functions as a catalytic chaperone under heat stress response: A unique defense role of OGT in hyperthermia. Biochem. Biophys. Res. Commun. 2004, 322, 1045–1051. [Google Scholar] [CrossRef]
- Hart, G.W.; Greis, K.D.; Dong, L.Y.; Blomberg, M.A.; Chou, T.Y.; Jiang, M.S.; Roquemore, E.P.; Snow, D.M.; Kreppel, L.K.; Cole, R.N.; et al. O-linked N-acetylglucosamine: The “yin-yang” of Ser/Thr phosphorylation? Nuclear and cytoplasmic glycosylation. Adv. Exp. Med. Biol. 1995, 376, 115–123. [Google Scholar] [CrossRef]
- Wang, Z.; Gucek, M.; Hart, G.W. Cross-talk between GlcNAcylation and phosphorylation: Site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc. Proc. Natl. Acad. Sci. USA 2008, 105, 13793–13798. [Google Scholar] [CrossRef]
- Bolanle, I.O.; Palmer, T.M. Targeting Protein O-GlcNAcylation, a Link between Type 2 Diabetes Mellitus and Inflammatory Disease. Cells 2022, 11, 705. [Google Scholar] [CrossRef]
- Haller, H.; Baur, E.; Quass, P.; Behrend, M.; Lindschau, C.; Distler, A.; Luft, F.C. High glucose concentrations and protein kinase C isoforms in vascular smooth muscle cells. Kidney Int. 1995, 47, 1057–1067. [Google Scholar] [CrossRef]
- Williams, B.; Schrier, R.W. Characterization of glucose-induced in situ protein kinase C activity in cultured vascular smooth muscle cells. Diabetes 1992, 41, 1464–1472. [Google Scholar] [CrossRef]
- Griffith, L.S.; Schmitz, B. O-linked N-acetylglucosamine levels in cerebellar neurons respond reciprocally to pertubations of phosphorylation. Eur. J. Biochem. 1999, 262, 824–831. [Google Scholar] [CrossRef]
- Alexander, S.; Smith, E.; Davis, L.; Gooley, A.; Por, S.B.; Browne, L.; Williams, K.L. Characterization of an antigenically related family of cell-type specific proteins implicated in slug migration in Dictyostelium discoideum. Differentiation 1988, 38, 82–90. [Google Scholar] [CrossRef]
- Comer, F.I.; Hart, G.W. Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry 2001, 40, 7845–7852. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.Y.; Hart, G.W.; Dang, C.V. c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J. Biol. Chem. 1995, 270, 18961–18965. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Cole, R.N.; Zaia, J.; Hart, G.W. Alternative O-glycosylation/O-phosphorylation of the murine estrogen receptor beta. Biochemistry 2000, 39, 11609–11620. [Google Scholar] [CrossRef] [PubMed]
- Musicki, B.; Kramer, M.F.; Becker, R.E.; Burnett, A.L. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc. Natl. Acad. Sci. USA 2005, 102, 11870–11875. [Google Scholar] [CrossRef]
- Lefebvre, T.; Alonso, C.; Mahboub, S.; Dupire, M.J.; Zanetta, J.P.; Caillet-Boudin, M.L.; Michalski, J.C. Effect of okadaic acid on O-linked N-acetylglucosamine levels in a neuroblastoma cell line. Biochim. Biophys. Acta BBA-Gen. Subj. 1999, 1472, 71–81. [Google Scholar] [CrossRef]
- Vosseller, K.; Wells, L.; Lane, M.D.; Hart, G.W. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 5313–5318. [Google Scholar] [CrossRef]
- Umapathi, P.; Mesubi, O.O.; Banerjee, P.S.; Abrol, N.; Wang, Q.; Luczak, E.D.; Wu, Y.; Granger, J.M.; Wei, A.C.; Reyes Gaido, O.E.; et al. Excessive O-GlcNAcylation Causes Heart Failure and Sudden Death. Circulation 2021, 143, 1687–1703, Erratum in Circulation 2021, 143, e892. https://doi.org/10.1161/CIR.0000000000000976. [Google Scholar] [CrossRef]
- Clark, R.J.; McDonough, P.M.; Swanson, E.; Trost, S.U.; Suzuki, M.; Fukuda, M.; Dillmann, W.H. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J. Biol. Chem. 2003, 278, 44230–44237. [Google Scholar] [CrossRef]
- Hu, Y.; Belke, D.; Suarez, J.; Swanson, E.; Clark, R.; Hoshijima, M.; Dillmann, W.H. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ. Res. 2005, 96, 1006–1013. [Google Scholar] [CrossRef]
- Andrés, V.; Ureña, J.; Poch, E.; Chen, D.; Goukassian, D. Role of Sp1 in the induction of p27 gene expression in vascular smooth muscle cells in vitro and after balloon angioplasty. Arter. Thromb. Vasc. Biol. 2001, 21, 342–347. [Google Scholar] [CrossRef]
- Takahashi-Yanaga, F. Roles of Glycogen Synthase Kinase-3 (GSK-3) in Cardiac Development and Heart Disease. J. UOEH 2018, 40, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Leney, A.C.; El Atmioui, D.; Wu, W.; Ovaa, H.; Heck, A.J.R. Elucidating crosstalk mechanisms between phosphorylation and O-GlcNAcylation. Proc. Natl. Acad. Sci. USA 2017, 114, E7255–E7261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Udeshi, N.D.; Slawson, C.; Compton, P.D.; Sakabe, K.; Cheung, W.D.; Shabanowitz, J.; Hunt, D.F.; Hart, G.W. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates cytokinesis. Sci. Signal. 2010, 3, ra2. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Gao, C.; Peng, B.; Xue, J.; Xia, D.; Yang, L.; Zhang, J.; Gao, X.; Hu, Y.; Lin, S.; et al. FOXP1 phosphorylation antagonizes its O-GlcNAcylation in regulating ATR activation in response to replication stress. EMBO J. 2025, 44, 457–483. [Google Scholar] [CrossRef]
- Jiang, K.; Deng, M.; Du, W.; Liu, T.; Li, J.; Zhou, Y. Functions and inhibitors of CHK1 in cancer therapy. Med. Drug Discov. 2024, 28, 100185. [Google Scholar] [CrossRef]
- Zachara, N.E.; Hart, G.W. O-GlcNAc a sensor of cellular state: The role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim. Biophys. Acta BBA-Gen. Subj. 2004, 1673, 13–28. [Google Scholar] [CrossRef]
- Slawson, C.; Housley, M.P.; Hart, G.W. O-GlcNAc cycling: How a single sugar post-translational modification is changing the way we think about signaling networks. J. Cell. Biochem. 2006, 97, 71–83. [Google Scholar] [CrossRef]
- Zeidan, Q.; Hart, G.W. The intersections between O-GlcNAcylation and phosphorylation: Implications for multiple signaling pathways. J. Cell Sci. 2010, 123, 13–22. [Google Scholar] [CrossRef]
- Slawson, C.; Lakshmanan, T.; Knapp, S.; Hart, G.W. A mitotic GlcNAcylation/phosphorylation signaling complex alters the posttranslational state of the cytoskeletal protein vimentin. Mol. Biol. Cell. 2008, 19, 4130–4140. [Google Scholar] [CrossRef]
- Wells, L.; Kreppel, L.K.; Comer, F.I.; Wadzinski, B.E.; Hart, G.W. O-GlcNAc transferase is in a functional complex with protein phosphatase 1 catalytic subunits. J. Biol. Chem. 2004, 279, 38466–38470. [Google Scholar] [CrossRef]
- Gandy, J.C.; Rountree, A.E.; Bijur, G.N. Akt1 is dynamically modified with O-GlcNAc following treatments with PUGNAc and insulin-like growth factor-1. FEBS Lett. 2006, 580, 3051–3058. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.A.; Acevedo-Duncan, M.; Potter, R.L. Selective decrease of membrane-associated PKC-alpha and PKC-epsilon in response to elevated intracellular O-GlcNAc levels in transformed human glial cells. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2005, 1743, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Soesanto, Y.; McClain, D.A. Protein modification by O-linked GlcNAc reduces angiogenesis by inhibiting Akt activity in endothelial cells. Arter. Thromb. Vasc. Biol. 2008, 28, 651–657. [Google Scholar] [CrossRef]
- Maione, A.S.; Cipolletta, E.; Sorriento, D.; Borriello, F.; Soprano, M.; Rusciano, M.R.; D’Esposito, V.; Markabaoui, A.K.; De Palma, G.D.; Martino, G.; et al. Cellular subtype expression and activation of CaMKII regulate the fate of atherosclerotic plaque. Atherosclerosis 2017, 256, 53–61. [Google Scholar] [CrossRef]
- Naz, H.; Tarique, M.; Suhail, M.; Shankar, H.; Muhammad, N.; Usmani, D.; Ashraf, M.; Zughaibi, T.A. Calcium-/calmodulin-dependent protein kinase IV (CAMKIV): A multifunctional enzyme and its role in various cancer: An update. Curr. Mol. Biol. Rep. 2020, 6, 139–147. [Google Scholar] [CrossRef]
- Chen, S.; Crother, T.R.; Arditi, M. Emerging role of IL-17 in atherosclerosis. J. Innate Immun. 2010, 2, 325–333. [Google Scholar] [CrossRef]
- Dias, W.B.; Cheung, W.D.; Wang, Z.; Hart, G.W. Regulation of calcium/calmodulin-dependent kinase IV by O-GlcNAc modification. J. Biol. Chem. 2009, 284, 21327–21337. [Google Scholar] [CrossRef]
- Song, M.; Kim, H.S.; Park, J.M.; Kim, S.H.; Kim, I.H.; Ryu, S.H.; Suh, P.G. O-GlcNAc transferase is activated by CaMKIV-dependent phosphorylation under potassium chloride-induced depolarization in NG-108-15 cells. Cell. Signal. 2008, 20, 94–104. [Google Scholar] [CrossRef]
- Cao, G.; Xuan, X.; Li, Y.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. Single-cell RNA sequencing reveals the vascular smooth muscle cell phenotypic landscape in aortic aneurysm. Cell Commun. Signal. 2023, 21, 113. [Google Scholar] [CrossRef]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef]
- Chappell, J.; Harman, J.L.; Narasimhan, V.M.; Yu, H.; Foote, K.; Simons, B.D.; Bennett, M.R.; Jørgensen, H.F. Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models. Circ. Res. 2016, 119, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal. 2022, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Hartweck, L.M.; Scott, C.L.; Olszewski, N.E. Two O-linked N-acetylglucosamine transferase genes of Arabidopsis thaliana L. Heynh. have overlapping functions necessary for gamete and seed development. Genetics 2002, 161, 1279–1291. [Google Scholar] [CrossRef]
- Goldberg, H.J.; Whiteside, C.I.; Hart, G.W.; Fantus, I.G. Posttranslational, reversible O-glycosylation is stimulated by high glucose and mediates plasminogen activator inhibitor-1 gene expression and Sp1 transcriptional activity in glomerular mesangial cells. Endocrinology 2006, 147, 222–231. [Google Scholar] [CrossRef]
- Zhang, X.; Li, R.; Qin, X.; Wang, L.; Xiao, J.; Song, Y.; Sheng, X.; Guo, M.; Ji, X. Sp1 Plays an Important Role in Vascular Calcification Both In Vivo and In Vitro. J. Am. Heart Assoc. 2018, 7, e007555. [Google Scholar] [CrossRef]
- Ding, J.; Fayyaz, A.I.; Ding, Y.; Liang, D.; Luo, M. Role of Specificity Protein 1 (SP1) in Cardiovascular Diseases: Pathological Mechanisms and Therapeutic Potentials. Biomolecules 2024, 14, 807. [Google Scholar] [CrossRef]
- Mehrhof, F.B.; Schmidt-Ullrich, R.; Dietz, R.; Scheidereit, C. Regulation of vascular smooth muscle cell proliferation: Role of NF-kappaB revisited. Circ. Res. 2005, 96, 958–964. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef]
- James, L.R.; Tang, D.; Ingram, A.; Ly, H.; Thai, K.; Cai, L.; Scholey, J.W. Flux through the hexosamine pathway is a determinant of nuclear factor kappaB- dependent promoter activation. Diabetes 2002, 51, 1146–1156. [Google Scholar] [CrossRef]
- Cui, X.; Pan, G.; Chen, Y.; Guo, X.; Liu, T.; Zhang, J.; Yang, X.; Cheng, M.; Gao, H.; Jiang, F. The p53 pathway in vasculature revisited: A therapeutic target for pathological vascular remodeling? Pharmacol. Res. 2021, 169, 105683. [Google Scholar] [CrossRef] [PubMed]
- Fiordaliso, F.; Leri, A.; Cesselli, D.; Limana, F.; Safai, B.; Nadal-Ginard, B.; Anversa, P.; Kajstura, J. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes 2001, 50, 2363–2375. [Google Scholar] [CrossRef] [PubMed]
- Men, H.; Cai, H.; Cheng, Q.; Zhou, W.; Wang, X.; Huang, S.; Zheng, Y.; Cai, L. The regulatory roles of p53 in cardiovascular health and disease. Cell Mol. Life Sci. 2021, 78, 2001–2018. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.D.; Bungard, D.; Lytton, J. Regulation of SERCA Ca2+ pump expression by cytoplasmic [Ca2+] in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2001, 280, C843–C851. [Google Scholar] [CrossRef]
- Gustavsson, M.; Verardi, R.; Mullen, D.G.; Mote, K.R.; Traaseth, N.J.; Gopinath, T.; Veglia, G. Allosteric regulation of SERCA by phosphorylation-mediated conformational shift of phospholamban. Proc. Natl. Acad. Sci. USA 2013, 110, 17338–17343. [Google Scholar] [CrossRef]
- Bobik, A. Transforming growth factor-betas and vascular disorders. Arter. Thromb. Vasc. Biol. 2006, 26, 1712–1720. [Google Scholar] [CrossRef]
- Goumans, M.J.; Liu, Z.; ten Dijke, P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009, 19, 116–127. [Google Scholar] [CrossRef]
- Azhar, M.; Schultz Jel, J.; Grupp, I.; Dorn II, G.W.; Meneton, P.; Molin, D.G.; Gittenberger-de Groot, A.C.; Doetschman, T. Transforming growth factor beta in cardiovascular development and function. Cytokine Growth Factor Rev. 2003, 14, 391–407. [Google Scholar] [CrossRef]
- Ringvold, H.C.; Khalil, R.A. Protein Kinase C as Regulator of Vascular Smooth Muscle Function and Potential Target in Vascular Disorders. Adv. Pharmacol. 2017, 78, 203–301. [Google Scholar] [CrossRef]
- Federici, M.; Menghini, R.; Mauriello, A.; Hribal, M.L.; Ferrelli, F.; Lauro, D.; Sbraccia, P.; Spagnoli, L.G.; Sesti, G.; Lauro, R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation 2002, 106, 466–472. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, J.; Yu, H.; Jin, X. AKT phosphorylation sites of Ser473 and Thr308 regulate AKT degradation. Biosci. Biotechnol. Biochem. 2019, 83, 429–435. [Google Scholar] [CrossRef]
- Abeyrathna, P.; Su, Y. The critical role of Akt in cardiovascular function. Vasc. Pharmacol. 2015, 74, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Goncharova, E.A.; Ammit, A.J.; Irani, C.; Carroll, R.G.; Eszterhas, A.J.; Panettieri, R.A.; Krymskaya, V.P. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L354–L363. [Google Scholar] [CrossRef] [PubMed]
- Naga Prasad, S.V.; Perrino, C.; Rockman, H.A. Role of phosphoinositide 3-kinase in cardiac function and heart failure. Trends Cardiovasc. Med. 2003, 13, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Morello, F.; Perino, A.; Hirsch, E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc. Res. 2009, 82, 261–271. [Google Scholar] [CrossRef]
- Ray, M.K.; Datta, B.; Chakraborty, A.; Chattopadhyay, A.; Meza-Keuthen, S.; Gupta, N.K. The eukaryotic initiation factor 2-associated 67-kDa polypeptide (p67) plays a critical role in regulation of protein synthesis initiation in animal cells. Proc. Natl. Acad. Sci. USA 1992, 89, 539–543. [Google Scholar] [CrossRef]
- Proud, C.G. eIF2 and the control of cell physiology. Semin. Cell Dev. Biol. 2005, 16, 3–12. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, X.; Li, W.; Wang, T.; Cui, S.; Li, T.; Wang, Y.; Xu, W.; Ma, Y.; Yang, B.; et al. eIF2α-mediated integrated stress response links multiple intracellular signaling pathways to reprogram vascular smooth muscle cell fate in carotid artery plaque. Heliyon 2024, 10, e26904. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef]
- Lazarus, B.D.; Love, D.C.; Hanover, J.A. O-GlcNAc cycling: Implications for neurodegenerative disorders. Int. J. Biochem. Cell Biol. 2009, 41, 2134–2146. [Google Scholar] [CrossRef]
- Lima, V.V.; Giachini, F.R.; Hardy, D.M.; Webb, R.C.; Tostes, R.C. O-GlcNAcylation: A novel pathway contributing to the effects of endothelin in the vasculature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R236–R250. [Google Scholar] [CrossRef] [PubMed]
- Loaeza-Reyes, K.J.; Zenteno, E.; Moreno-Rodríguez, A.; Torres-Rosas, R.; Argueta-Figueroa, L.; Salinas-Marín, R.; Castillo-Real, L.M.; Pina-Canseco, S.; Cervera, Y.P. An Overview of Glycosylation and its Impact on Cardiovascular Health and Disease. Front. Mol. Biosci. 2021, 8, 751637. [Google Scholar] [CrossRef]
- Zachara, N.E.; Hart, G.W. Cell signaling, the essential role of O-GlcNAc! Biochim. Biophys Acta BBA-Mol. Cell Biol. Lipids 2006, 1761, 599–617. [Google Scholar] [CrossRef]
- Hart, G.W.; Housley, M.P.; Slawson, C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007, 446, 1017–1022. [Google Scholar] [CrossRef]
- Gallant, C.; You, J.Y.; Sasaki, Y.; Grabarek, Z.; Morgan, K.G. MARCKS is a major PKC-dependent regulator of calmodulin targeting in smooth muscle. J. Cell Sci. 2005, 118, 3595–3605. [Google Scholar] [CrossRef]
- Hardy, D.; Tostes, R.; Webb, C. O-Glcnacylation augments protein kinase C mediated vasoconstriction. FASEB J. 2010, 24, 603–608. [Google Scholar] [CrossRef]
- Lima, V.V.; Giachini, F.R.; Carneiro, F.S.; Carneiro, Z.N.; Fortes, Z.B.; Carvalho, M.H.; Webb, R.C.; Tostes, R.C. Increased vascular O-GlcNAcylation augments reactivity to constrictor stimuli—Vasoactive Peptide Symposium. J. Am. Soc. Hypertens. 2008, 2, 410–417. [Google Scholar] [CrossRef]
- Lima, V.V.; Giachini, F.R.; Choi, H.; Carneiro, F.S.; Carneiro, Z.N.; Fortes, Z.B.; Carvalho, M.H.; Webb, R.C.; Tostes, R.C. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein O-GlcNAcylation. Hypertension 2009, 53, 166–174. [Google Scholar] [CrossRef]
- Rigsby, C.S.; Lima, V.V.; Webb, R.C.; Tostes, R.C. Increased O-GlcNAcylation in Renal Arteries from Angiotensin Ii/Salt-Hypertensive Rats. In Hypertension; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009; Volume 54, p. E117. [Google Scholar]
- Kim, J.H.; Hwang, J.; Jung, J.H.; Lee, H.J.; Lee, D.Y.; Kim, S.H. Molecular networks of FOXP family: Dual biologic functions, interplay with other molecules and clinical implications in cancer progression. Mol. Cancer. 2019, 18, 180. [Google Scholar] [CrossRef]
- Bot, P.T.; Grundmann, S.; Goumans, M.J.; de Kleijn, D.; Moll, F.; de Boer, O.; van der Wal, A.C.; van Soest, A.; de Vries, J.P.; van Royen, N.; et al. Forkhead box protein P1 as a downstream target of transforming growth factor-β induces collagen synthesis and correlates with a more stable plaque phenotype. Atherosclerosis 2011, 218, 33–43. [Google Scholar] [CrossRef]
- Xing, T.; Du, L.; Zhuang, X.; Zhang, L.; Hao, J.; Wang, J. Upregulation of microRNA-206 induces apoptosis of vascular smooth muscle cells and decreases risk of atherosclerosis through modulating FOXP1. Exp. Ther. Med. 2017, 14, 4097–4103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McKavanagh, P.; Yanagawa, B.; Zawadowski, G.; Cheema, A. Management and Prevention of Saphenous Vein Graft Failure: A Review. Cardiol. Ther. 2017, 6, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Motwani, J.G.; Topol, E.J. Aortocoronary saphenous vein graft disease: Pathogenesis, predisposition, and prevention. Circulation 1998, 97, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Campeau, L.; Lespérance, J.; Hermann, J.; Corbara, F.; Grondin, C.M.; Bourassa, M.G. Loss of the improvement of angina between 1 and 7 years after aortocoronary bypass surgery: Correlations with changes in vein grafts and in coronary arteries. Circulation 1979, 60, 1–5. [Google Scholar] [CrossRef]
- Ferron, M.; Denis, M.; Persello, A.; Rathagirishnan, R.; Lauzier, B. Protein O-GlcNAcylation in Cardiac Pathologies: Past, Present, Future. Front. Endocrinol. 2019, 9, 819. [Google Scholar] [CrossRef]
- Lenzen, S.; Panten, U. Alloxan: History and mechanism of action. Diabetologia 1988, 31, 337–342. [Google Scholar] [CrossRef]
- Advani, S.B.; Sam, J. Potential anticancer and antiviral agents. Substituted 3-[1′(2′,3′,4′-tri-O-benzoyl-β-d-ribopyranosyl)]-2-benzoxazolinones. J. Heterocycl. Chem. 1968, 5, 119–122. [Google Scholar] [CrossRef]
- Gloster, T.M.; Zandberg, W.F.; Heinonen, J.E.; Shen, D.L.; Deng, L.; Vocadlo, D.J. Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat. Chem. Biol. 2011, 7, 174–181. [Google Scholar] [CrossRef]
- Ortiz-Meoz, R.F.; Jiang, J.; Lazarus, M.B.; Orman, M.; Janetzko, J.; Fan, C.; Duveau, D.Y.; Tan, Z.W.; Thomas, C.J.; Walker, S. A small molecule that inhibits OGT activity in cells. ACS Chem. Biol. 2015, 10, 1392–1397. [Google Scholar] [CrossRef]
- Martin, S.E.S.; Tan, Z.W.; Itkonen, H.M.; Duveau, D.Y.; Paulo, J.A.; Janetzko, J.; Boutz, P.L.; Törk, L.; Moss, F.A.; Thomas, C.J.; et al. Structure-Based Evolution of Low Nanomolar O-GlcNAc Transferase Inhibitors. J. Am. Chem. Soc. 2018, 140, 13542–13545. [Google Scholar] [CrossRef]
- Souza-Silva, L.; Alves-Lopes, R.; Silva Miguez, J.; Dela Justina, V.; Neves, K.B.; Mestriner, F.L.; Tostes, R.C.; Giachini, F.R.; Lima, V.V. Glycosylation with O-linked β-N-acetylglucosamine induces vascular dysfunction via production of superoxide anion/reactive oxygen species. Can. J. Physiol. Pharmacol. 2018, 96, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Cencioni, C.; Fasanaro, P.; Zaccagnini, G.; Greco, S.; Sarra-Ferraris, G.; Antonini, A.; Martelli, F.; Capogrossi, M.C. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Greco, S.; Gaetano, C.; Martelli, F. Oxidative Stress and MicroRNAs in Vascular Diseases. Int. J. Mol. Sci. 2013, 14, 17319–17346. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Feng, W.; Nöt, L.G.; Miller, A.P.; Zhang, Y.; Chen, Y.F.; Majid-Hassan, E.; Chatham, J.C.; Oparil, S. Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H335–H342. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Li, M.; Yuan, Q.; Xue, M.; Xu, F.; Chen, Y. Inhibition of ALDH2 by O-GlcNAcylation contributes to the hyperglycemic exacerbation of myocardial ischemia/reperfusion injury. Oncotarget 2017, 8, 19413–19426. [Google Scholar] [CrossRef]
- Yan, K.; Wang, K.; Li, P. The role of post-translational modifications in cardiac hypertrophy. J. Cell. Mol. Med. 2019, 23, 3795–3807. [Google Scholar] [CrossRef]
- Giachini, F.R.; Zemse, S.M.; Carneiro, F.S.; Lima, V.V.; Carneiro, Z.N.; Callera, G.E.; Ergul, A.; Webb, R.C.; Tostes, R.C. Interleukin-10 attenuates vascular responses to endothelin-1 via effects on ERK1/2-dependent pathway. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H489–H496. [Google Scholar] [CrossRef]
- Raman, P.; Krukovets, I.; Marinic, T.E.; Bornstein, P.; Stenina, O.I. Glycosylation mediates up-regulation of a potent antiangiogenic and proatherogenic protein, thrombospondin-1, by glucose in vascular smooth muscle cells. J. Biol. Chem. 2007, 282, 5704–5714. [Google Scholar] [CrossRef]

| Protein | Cardiovascular Function | Effects of Increased O-GlcNAcylation | Effects of Phosphorylation | Net Effects of O-GlcNAcylation and Phosphorylation Crosstalk | References |
|---|---|---|---|---|---|
| Sp1 | Transcription factor involved in the regulation of vascular calcification, endothelial dysfunction, fibrosis, and regulation of gene expression | Enhanced activity | Activation | Decreased activity | [59,61,86,87,88] |
| NF-κB | Regulation of inflammatory response and proliferation of VSMCs | Enhanced activity | Activation | Decreased activity | [89,90,91] |
| p53 | Modulation of metabolism, cell cycle arrest, pro-programmed cell death, and anti-angiogenesis | Enhanced activity | Activation | Decreased activity | [92,93,94] |
| SERCA2 | Regulation of calcium homeostasis | Reduced expression | Enhanced activity | Unknown | [59,95,96] |
| TGF-β | Vascular remodelling and regulation of the renal renin-angiotensin system | Increased expression | Activation | Decreased activity | [97,98,99] |
| PKCα,ε | Regulator of cardiac contractility and vascular tone | Altered translocation and expression | Activation | Decreased activity | [74,100] |
| Akt | Regulator of growth, proliferation, migration, and metabolism of vascular cells | Decreased response to agonists such as insulin | Activation | Decreased activity | [101,102,103] |
| PI3K | Regulator of cardiac and vascular contractility and growth | Decreased activity and phosphorylation | Activation | Decreased activity | [101,104,105,106] |
| eIF-2 | mRNA translation and regulation | Reduced function | Activation | Decreased activity | [107,108,109] |
| MAPKs (p38 and ERK1/2) | Regulations of contraction, migration, adhesion, collagen deposition, cell proliferation, differentiation, and survival of vascular smooth muscle cells | Enhance activity | Activation | Enhanced activity | [110,111,112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolanle, I.O.; Palmer, T.M. O-GlcNAcylation and Phosphorylation Crosstalk in Vascular Smooth Muscle Cells: Cellular and Therapeutic Significance in Cardiac and Vascular Pathologies. Int. J. Mol. Sci. 2025, 26, 3303. https://doi.org/10.3390/ijms26073303
Bolanle IO, Palmer TM. O-GlcNAcylation and Phosphorylation Crosstalk in Vascular Smooth Muscle Cells: Cellular and Therapeutic Significance in Cardiac and Vascular Pathologies. International Journal of Molecular Sciences. 2025; 26(7):3303. https://doi.org/10.3390/ijms26073303
Chicago/Turabian StyleBolanle, Israel O., and Timothy M. Palmer. 2025. "O-GlcNAcylation and Phosphorylation Crosstalk in Vascular Smooth Muscle Cells: Cellular and Therapeutic Significance in Cardiac and Vascular Pathologies" International Journal of Molecular Sciences 26, no. 7: 3303. https://doi.org/10.3390/ijms26073303
APA StyleBolanle, I. O., & Palmer, T. M. (2025). O-GlcNAcylation and Phosphorylation Crosstalk in Vascular Smooth Muscle Cells: Cellular and Therapeutic Significance in Cardiac and Vascular Pathologies. International Journal of Molecular Sciences, 26(7), 3303. https://doi.org/10.3390/ijms26073303







