Interleukin-1β Inhibits Ovarian Cancer Cell Proliferation and Metastasis Through the MAPK/MMP12 Pathway
Abstract
1. Introductions
2. Results
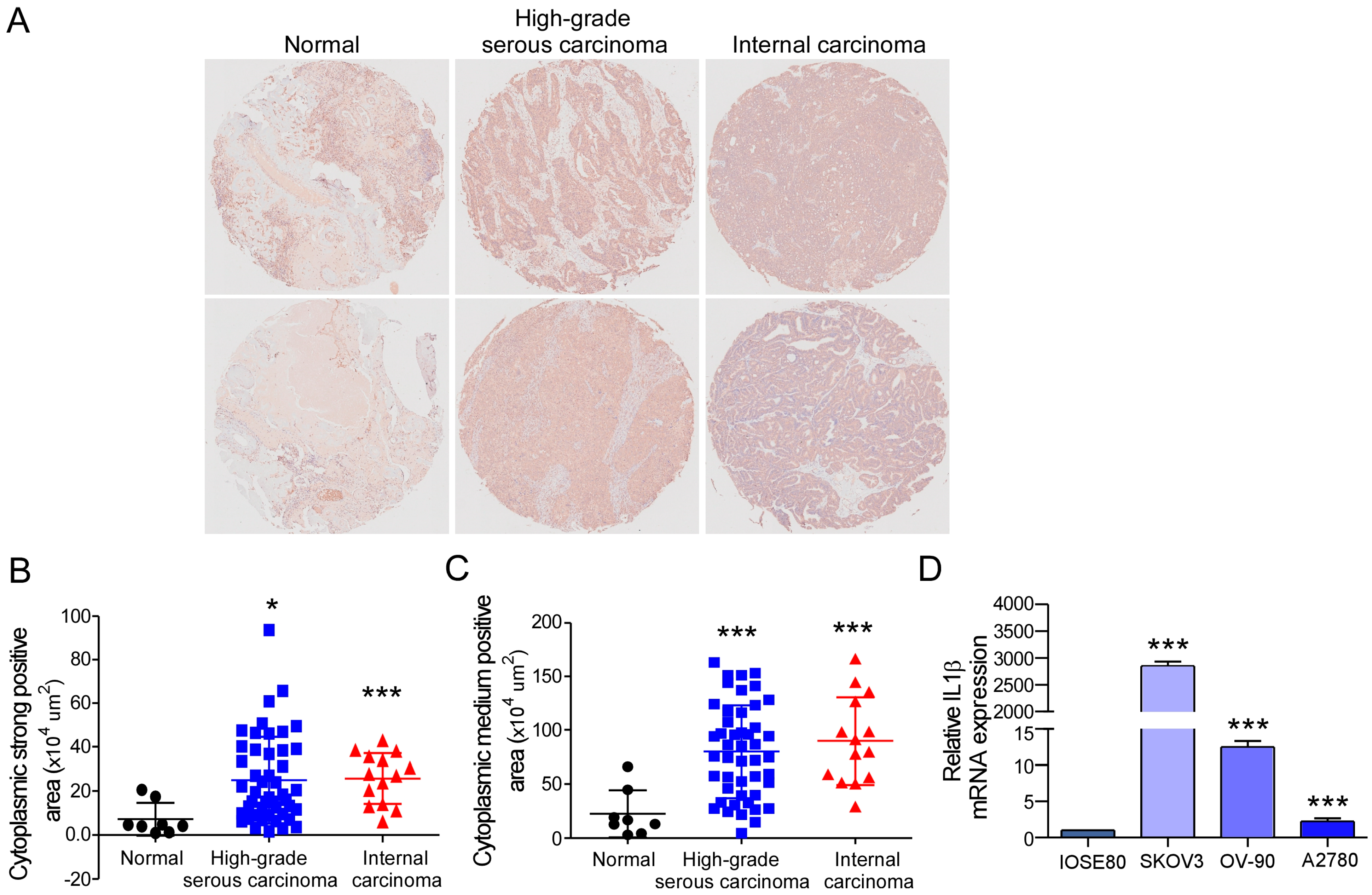
2.1. IL-1β Expression Is Increased Abnormally in Ovarian Cancer Tissues and Cells
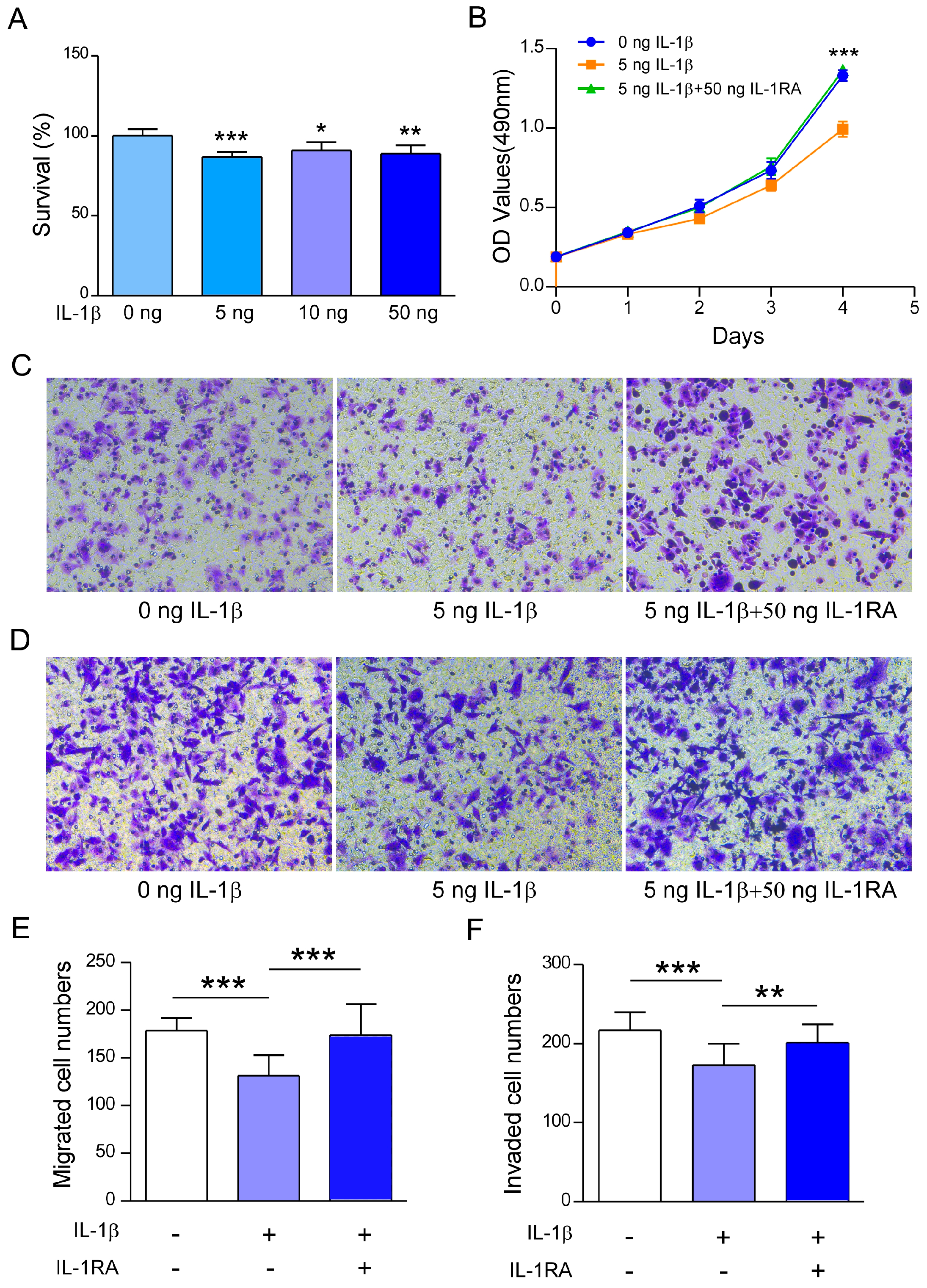
2.2. Recombinant IL-1β Inhibits A2780 Cell Survival, Migration, and Invasion
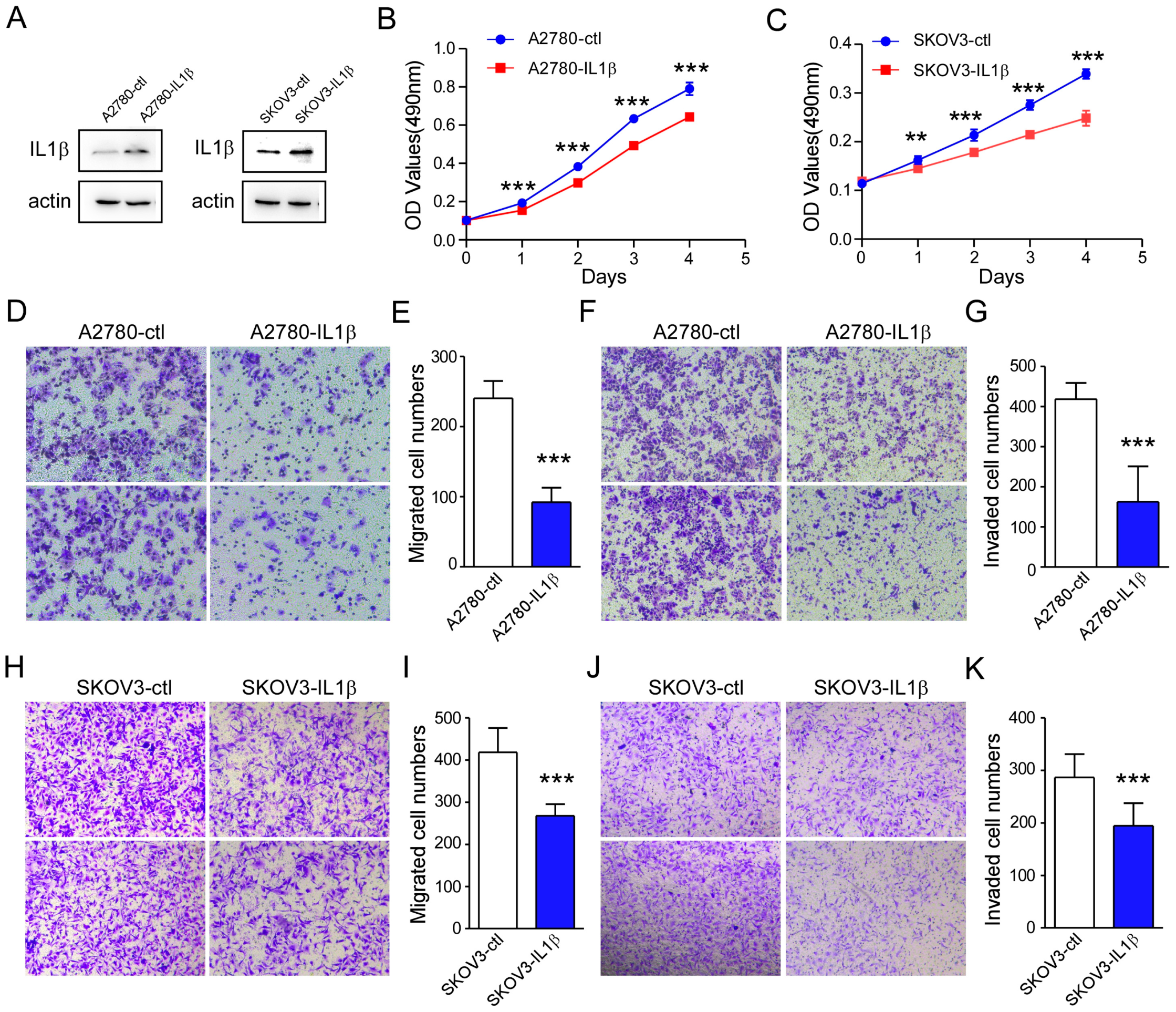
2.3. IL-1β Overexpression Is Related to a Reduction in Ovarian Cancer Cell Survival and Metastasis
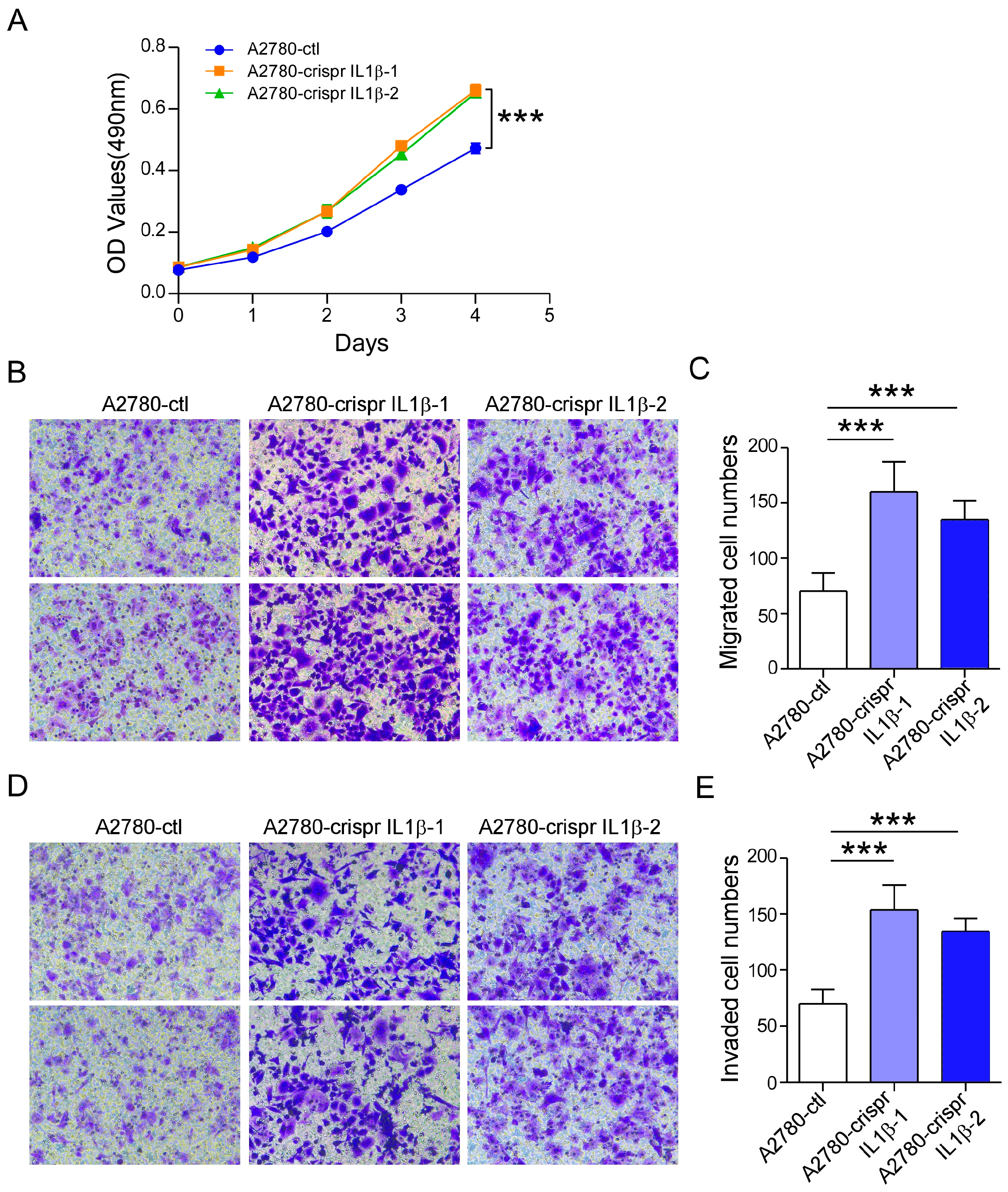
2.4. IL-1β Knockdown Promotes A2780 Cell Survival and Metastasis
2.5. Screening for Differentially Expressed Genes Regulated by IL-1β
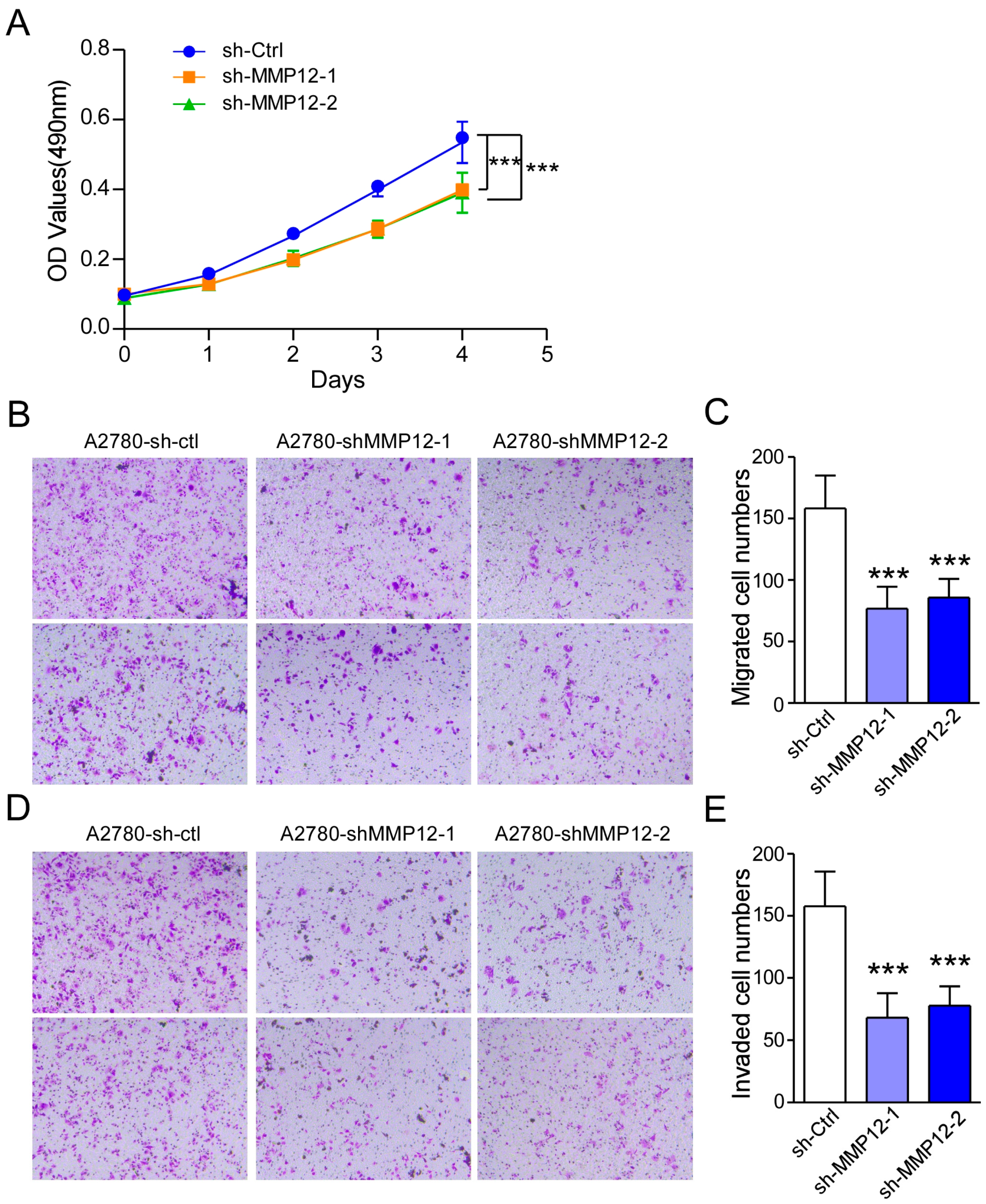
2.6. IL-1β Regulates MMP12 Through the MAPK/AP-1 Signaling Pathway, and Reduced MMP12 Suppresses A2780 Cell Proliferation and Metastasis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Immunohistochemistry of the Cancer Tissue Microarray
4.3. Recombinant IL-1β and IL-1RA Treatment
4.4. MTT Assay
4.5. Transwell Assay
4.6. Stable IL-1β-Overexpression and IL-1β-Knockdown Cells
4.7. MMP12-Knockdown Cells
4.8. RNA Sequencing Analysis
4.9. RNA Extraction and qRT-PCR
4.10. Western Blotting Analysis
4.11. Dual Luciferase Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Li, X.M.; Yang, K.; Tong, W.H. Advancements in ovarian cancer immunodiagnostics and therapeutics via phage display technology. Front. Immunol. 2024, 15, 1402862. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; Jordan, S.J. Global epidemiology of epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 2024, 21, 389–400. [Google Scholar] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [PubMed]
- Cabasag, C.J.; Fagan, P.J.; Ferlay, J.; Vignat, J.; Laversanne, M.; Liu, L.; Aa, M.A.; Bray, F.; Soerjomataram, I. Ovarian cancer today and tomorrow: A global assessment by world region and Human Development Index using GLOBOCAN 2020. Int. J. Cancer 2022, 151, 1535–1541. [Google Scholar] [CrossRef]
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S.; Basha, R. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar]
- Simon, K.; Teichmann, S.A. Universal and tissue-specific fibroblasts in chronic inflammation and cancer. Cancer Cell 2024, 42, 1648–1650. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Erdman, S.E.; Poutahidis, T. Cancer inflammation and regulatory T cells. Int. J. Cancer 2010, 127, 768–779. [Google Scholar] [CrossRef]
- Boccardi, V.; Marano, L. Aging, cancer, and inflammation: The telomerase connection. Int. J. Mol. Sci. 2024, 25, 8542. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta-A friend or foe in malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.S.; Ghoreschi, K. The interleukin-1 family. Adv. Exp. Med. Biol. 2016, 941, 21–29. [Google Scholar] [PubMed]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [PubMed]
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol. Rev. 2018, 281, 57–61. [Google Scholar]
- Apte, R.N.; Krelin, Y.; Song, X.; Dotan, S.; Recih, E.; Elkabets, M.; Carmi, Y.; Dvorkin, T.; White, R.M.; Gayvoronsky, L.; et al. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour-host interactions. Eur. J. Cancer 2006, 42, 751–759. [Google Scholar]
- Apte, R.N.; Dotan, S.; Elkabets, M.; White, M.R.; Reich, E.; Carmi, Y.; Song, X.; Dvozkin, T.; Krelin, Y.; Voronov, E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006, 25, 387–408. [Google Scholar]
- Hajek, E.; Krebs, F.; Bent, R.; Haas, K.; Bast, A.; Steinmetz, I.; Tuettenberg, A.; Grabbe, S.; Bros, M. BRAF inhibitors stimulate inflammasome activation and interleukin 1 beta production in dendritic cells. Oncotarget 2018, 9, 28294–28308. [Google Scholar]
- Rébé, C.; Ghiringhelli, F. Interleukin-1beta and cancer. Cancers 2020, 12, 1791. [Google Scholar]
- Malkova, A.M.; Gubal, A.R.; Petrova, A.L.; Voronov, E.; Apte, R.N.; Semenov, K.N.; Sharoyko, V.V. Pathogenetic role and clinical significance of interleukin-1β in cancer. Immunology 2023, 168, 203–216. [Google Scholar]
- Latham, S.L.; O’Donnell, Y.E.I.; Croucher, D.R. Non-kinase targeting of oncogenic c-Jun N-terminal kinase (JNK) signaling: The future of clinically viable cancer treatments. Biochem. Soc. Trans. 2022, 50, 1823–1836. [Google Scholar]
- Abdelrahman, K.S.; Hassan, H.A.; Abdel-Aziz, S.A.; Marzouk, A.A.; Narumi, A.; Konno, H.; Abdel-Aziz, M. JNK signaling as a target for anticancer therapy. Pharmacol. Rep. 2021, 73, 405–434. [Google Scholar] [PubMed]
- Banerjee, S.; Lo, W.C.; Majumder, P.; Roy, D.; Ghorai, M.; Shaikh, N.K.; Kant, N.; Shekhawat, M.S.; Gadekar, V.S.; Ghosh, S.; et al. Multiple roles for basement membrane proteins in cancer progression and EMT. Eur. J. Cell Biol. 2022, 101, 151220. [Google Scholar]
- Marcos-Jubilar, M.; Orbe, J.; Roncal, C.; Machado, F.J.D.; Rodriguez, J.A.; Fernández-Montero, A.; Colina, I.; Rodil, R.; Pastrana, J.C.; Páramo, J.A. Association of SDF1 and MMP12 with atherosclerosis and inflammation: Clinical and experimental study. Life 2021, 11, 414. [Google Scholar] [CrossRef]
- Guan, P.P.; Ding, W.Y.; Wang, P. The roles of prostaglandin F2 in regulating the expression of matrix metalloproteinase-12 via an insulin growth factor-2-dependent mechanism in sheared chondrocytes. Signal Transduct. Target. Ther. 2018, 3, 27. [Google Scholar]
- Guo, Z.Y.; Jiang, L.P. Matrix metalloproteinase 12 (MMP12) as an adverse prognostic biomarker of vascular invasion in hepatic cell carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2238–2249. [Google Scholar]
- Hung, W.Y.; Lee, W.J.; Cheng, G.Z.; Tsai, C.H.; Yang, Y.C.; Lai, T.C.; Chen, J.Q.; Chung, C.L.; Chang, J.H.; Chien, M.H. Blocking MMP-12-modulated epithelial-mesenchymal transition by repurposing penfluridol restrains lung adenocarcinoma metastasis via uPA/uPAR/TGF-beta/Akt pathway. Cell Oncol. 2021, 44, 1087–1103. [Google Scholar]
- Lv, F.Z.; Wang, J.L.; Wu, Y.; Chen, H.F.; Shen, X.Y. Knockdown of MMP12 inhibits the growth and invasion of lung adenocarcinoma cells. Int. J. Immunopathol. Pharmacol. 2015, 28, 77–84. [Google Scholar]
- Zeng, L.; Qian, J.; Zhu, F.; Wu, F.; Zhao, H.; Zhu, H. The prognostic values of matrix metalloproteinases in ovarian cancer. J. Int. Med. Res. 2020, 48, 300060519825983. [Google Scholar]
- Noh, J.M.; Shen, C.; Kim, S.J.; Kim, M.R.; Kim, S.H.; Kim, J.H.; Park, B.H.; Park, J.H. Interleukin-1β increases Angptl4 (FIAF) expression via the JNK signaling pathway in osteoblastic MC3T3-E1 cells. Exp. Clin. Endocrinol. Diabetes 2015, 123, 445–460. [Google Scholar]
- Song, M.; Deng, M.; Peng, Z.; Dai, F.; Wang, Y.; Shu, W.; Zhou, X.; Zhang, J.; Hou, Y.; Yu, B. Granulocyte colony-stimulating factor mediates bone loss via the activation of IL-1β/JNK signaling pathway in murine Staphylococcus aureus-induced osteomyelitis. Int. Immunopharmacol. 2024, 141, 112959. [Google Scholar]
- Woolery, K.T.; Hoffman, M.S.; Kraft, J.; Nicosia, S.V.; Kumar, A.; Kruk, P.A. Urinary interleukin-1β levels among gynecological patients. J. Ovarian Res. 2014, 7, 104. [Google Scholar]
- Schauer, I.G.; Zhang, J.; Xing, Z.; Guo, X.; Mercado-Uribe, I.; Sood, A.K.; Huang, P.; Liu, J. Interleukin-1β promotes ovarian tumorigenesis through a p53/NF-κB-mediated inflammatory response in stromal fibroblasts. Neoplasia 2013, 15, 409–420. [Google Scholar] [PubMed]
- Li, G.S.; Tang, Y.X.; Zhang, W.; Li, J.D.; Huang, H.Q.; Liu, J.; Fu, Z.W.; He, R.Q.; Kong, J.L.; Zhou, H.F.; et al. MMP12 is a potential predictive and prognostic biomarker of various cancers including lung adenocarcinoma. Cancer Control 2024, 31, 10732748241235468. [Google Scholar]
- Macciò, A.; Madeddu, C. Inflammation and ovarian cancer. Cytokine 2012, 58, 133–147. [Google Scholar] [PubMed]
- Allen, I.C.; TeKippe, E.M.; Woodford, R.M.; Uronis, J.M.; Holl, E.K.; Rogers, A.B.; Herfarth, H.H.; Jobin, C.; Ting, J.P. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 2010, 207, 1045–1056. [Google Scholar]
- Haabeth, O.A.; Lorvik, K.B.; Hammarstrom, C.; Donaldson, I.M.; Haraldsen, G.; Bogen, B.; Corthay, A. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat. Commun. 2011, 2, 240. [Google Scholar]
- Haabeth, O.A.; Lorvik, K.B.; Yagita, H.; Bogen, B.; Corthay, A. Interleukin-1 is required for cancer eradication mediated by tumor-specific Th1 cells. Oncoimmunology 2016, 5, e1039763. [Google Scholar]
- McLoed, A.G.; Sherrill, T.P.; Cheng, N.S.; Han, W.; Saxon, J.A.; Gleaves, L.A.; Wu, P.; Polosukhin, V.V.; Karin, M.; Yull, F.E.; et al. Neutrophil-Derived IL-1β impairs the efficacy of NF-κB inhibitors against lung cancer. Cell Rep. 2016, 16, 120–132. [Google Scholar]
- Kim, J.W.; Koh, Y.; Kim, N.W.; Ahn, Y.O.; Kim, T.M.; Han, S.W.; Oh, Y.; Lee, S.H.; Im, S.A.; Kim, T.Y.; et al. Clinical implications of VEGF, TGF-beta1, and IL-1beta in patients with advanced non-small cell lung cancer. Cancer Res. Treat. 2013, 45, 325–333. [Google Scholar]
- Matamoros, J.A.; Silva, M.I.F.; Moura, P.M.M.F.; Leitão, M.D.C.G.; Coimbra, E.C. Reduced expression of IL-1β and IL-18 proinflammatory interleukins increases the risk of developing cervical cancer. Asian Pac. J. Cancer Prev. 2019, 20, 2715–2721. [Google Scholar]
- Martínez-Reza, I.; Díaz, L.; Barrera, D.; Segovia-Mendoza, M.; Pedraza-Sánchez, S.; Soca-Chafre, G.; Larrea, F.; García-Becerra, R. Calcitriol inhibits the proliferation of Triple-Negative breast cancer cells through a mechanism involving the proinflammatory cytokines IL-1β and TNF-α. J. Immunol. Res. 2019, 10, 6384278. [Google Scholar]
- Chen, L.C.; Wang, L.J.; Tsang, N.M.; Ojcius, D.M.; Chen, C.C.; Ouyang, C.N.; Hsueh, C.; Liang, Y.; Chang, K.P.; Chen, C.C.; et al. Tumour inflammasome-derived IL-1β recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol. Med. 2012, 4, 1276–1293. [Google Scholar] [PubMed]
- Santiago, A.E.; Paula, S.O.C.; Carvalho, A.T.; Cândido, E.B.; Furtado, R.; Filho, A.L.S. Systemic inflammatory patterns in ovarian cancer patients: Analysis of cytokines, chemokines, and microparticles. Rev. Bras. Ginecol. Obstet. 2023, 45, e780–e789. [Google Scholar] [PubMed]
- Mustea, A.; Pirvulescu, C.; Könsgen, D.; Braicu, E.I.; Yuan, S.; Sun, P.; Lichtenegger, W.; Sehouli, J. Decreased IL-1 RA concentration in ascites is associated with a significant improvement in overall survival in ovarian cancer. Cytokine 2008, 42, 77–84. [Google Scholar] [PubMed]
- Stadlmann, S.; Pollheimer, J.; Moser, P.; Raggi, A.; Amberger, A.; Margreiter, R.; Offner, F.; Mikuz, G.; Dirnhofer, S.; Moch, H. Cytokine-regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. Eur. J. Cancer. 2003, 39, 2499–2505. [Google Scholar]
- Liu, W.; Wang, L.; Zhang, J.; Cheng, K.; Zheng, W.; Ma, Z. CC Chemokine 2 promotes ovarian cancer progression through the MEK/ERK/MAP3K19 signaling pathway. Int. J. Mol. Sci. 2023, 24, 10652. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, C.; Fu, B.; Xie, J.; Li, W.; Zhang, G.; Ma, Z.; Jiao, P. E3 ubiquitin ligase ANKIB1 attenuates antiviral immune responses by promoting K48-linked polyubiquitination of MAVS. Cell Rep. 2024, 43, 114687. [Google Scholar]
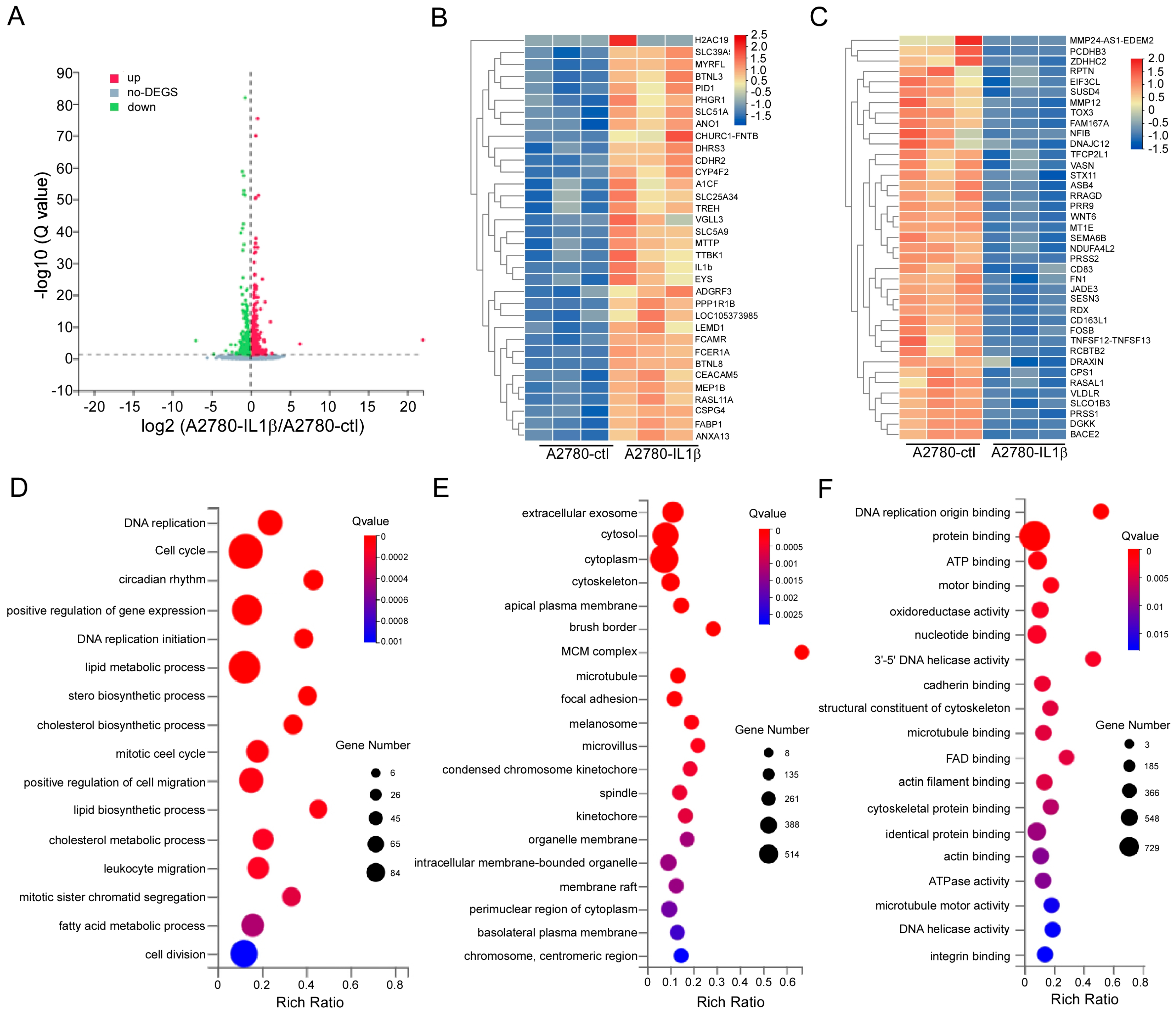
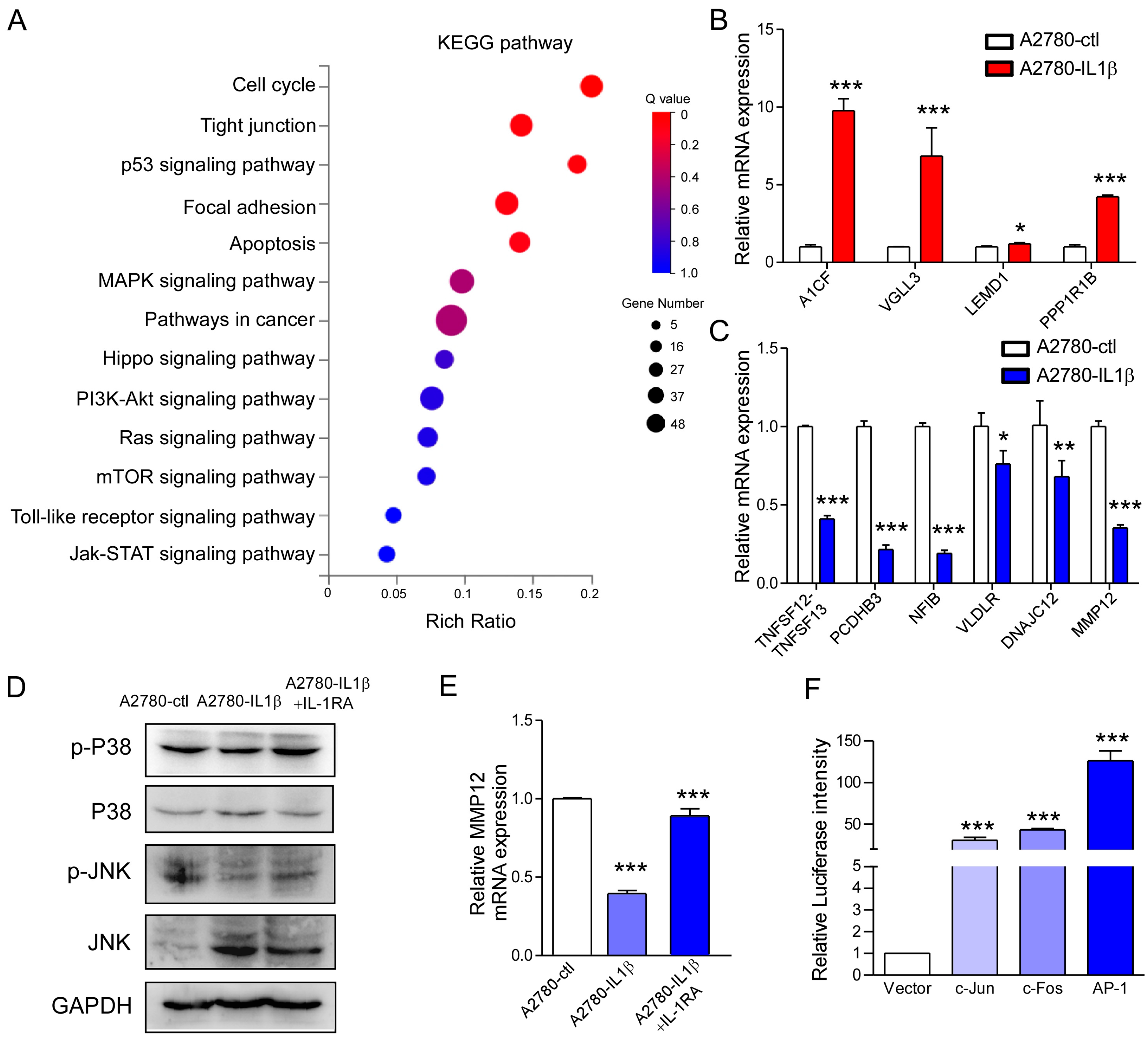
| Gene ID | Gene Name | Description | Fold Change/log2(IL1β/ctl) |
|---|---|---|---|
| 29974 | A1CF | Apobec-1 Complementation Factor | 2.71 |
| 389136 | VGLL3 | Vestigial-like family member 3 | 1.93 |
| 93273 | LEMD1 | LEM domain containing 1 | 1.93 |
| 84752 | PPP1R1B | Protein phosphatase 1 regulatory inhibitor subunit 1B | 1.83 |
| 407977 | TNFSF12-13 | Tumor necrosis factor superfamily members | −6.99 |
| 56132 | PCDHB3 | Protocadgerin beta 3 | −2.52 |
| 4781 | NFIB | Nuclear factor IB | −2.38 |
| 7436 | VLDLR | Very low density lipoprotein receptor | −1.94 |
| 56521 | DNAJC12 | DnaJ heat shock protein family (HSP40) member C12 | −1.87 |
| 4321 | MMP12 | Matrix Metallopeptidase 12 | −1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Zhang, J.; Li, Z.; Zhu, Y.; Han, X.; Lei, L.; Cheng, K.; Liu, W. Interleukin-1β Inhibits Ovarian Cancer Cell Proliferation and Metastasis Through the MAPK/MMP12 Pathway. Int. J. Mol. Sci. 2025, 26, 3287. https://doi.org/10.3390/ijms26073287
Ma Z, Zhang J, Li Z, Zhu Y, Han X, Lei L, Cheng K, Liu W. Interleukin-1β Inhibits Ovarian Cancer Cell Proliferation and Metastasis Through the MAPK/MMP12 Pathway. International Journal of Molecular Sciences. 2025; 26(7):3287. https://doi.org/10.3390/ijms26073287
Chicago/Turabian StyleMa, Zhenling, Jiajia Zhang, Zhenzhen Li, Yiyang Zhu, Xulu Han, Lanxiang Lei, Kun Cheng, and Wei Liu. 2025. "Interleukin-1β Inhibits Ovarian Cancer Cell Proliferation and Metastasis Through the MAPK/MMP12 Pathway" International Journal of Molecular Sciences 26, no. 7: 3287. https://doi.org/10.3390/ijms26073287
APA StyleMa, Z., Zhang, J., Li, Z., Zhu, Y., Han, X., Lei, L., Cheng, K., & Liu, W. (2025). Interleukin-1β Inhibits Ovarian Cancer Cell Proliferation and Metastasis Through the MAPK/MMP12 Pathway. International Journal of Molecular Sciences, 26(7), 3287. https://doi.org/10.3390/ijms26073287






