Mdm2-Mediated Ubiquitination Plays a Pivotal Role in Differentiating the Endocytic Roles of GRK2 and Arrestin3
Abstract
1. Introduction
2. Results
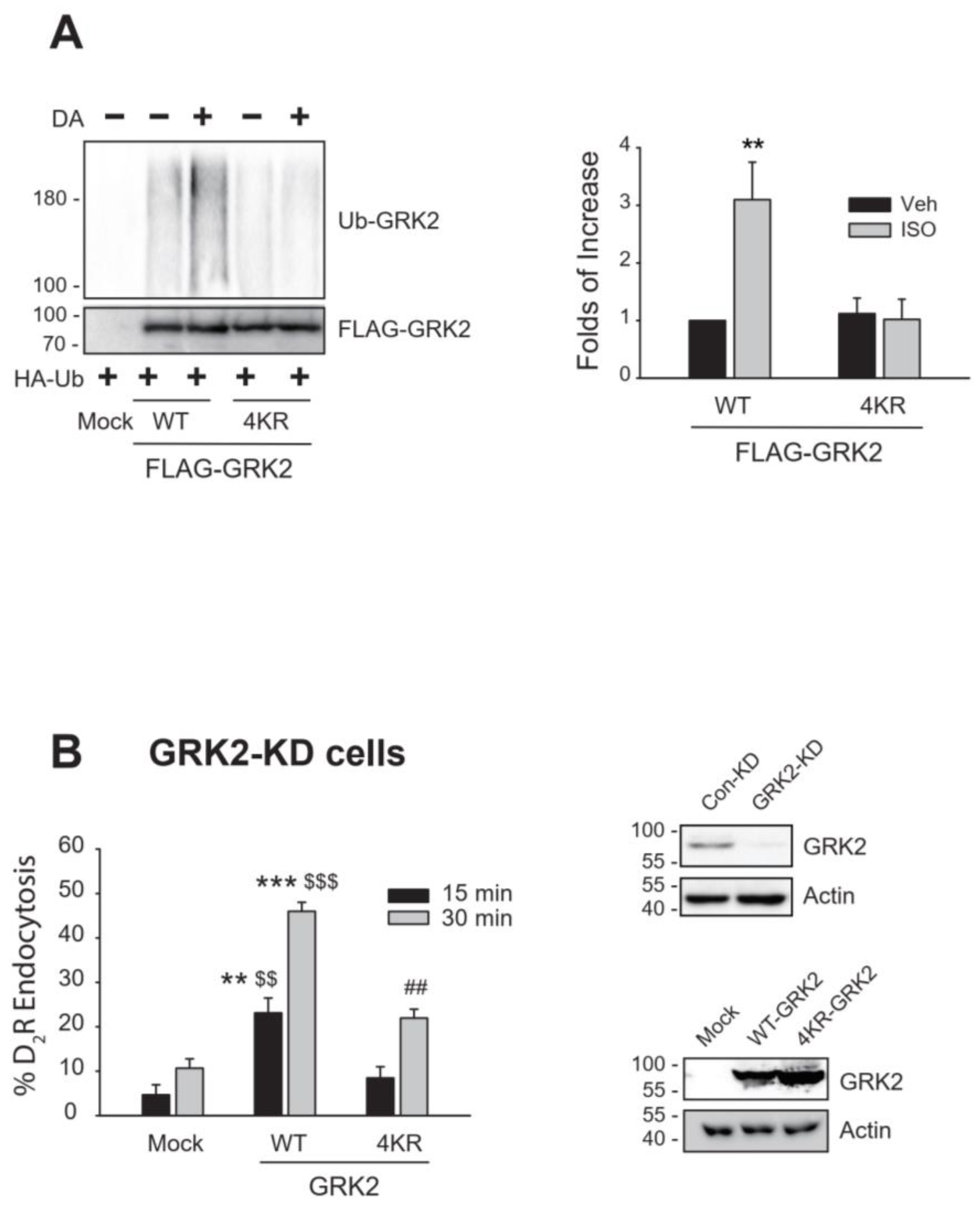
2.1. The Ubiquitination of GRK2 Mediated by Mdm2 Is Associated with Its Endocytic Activity
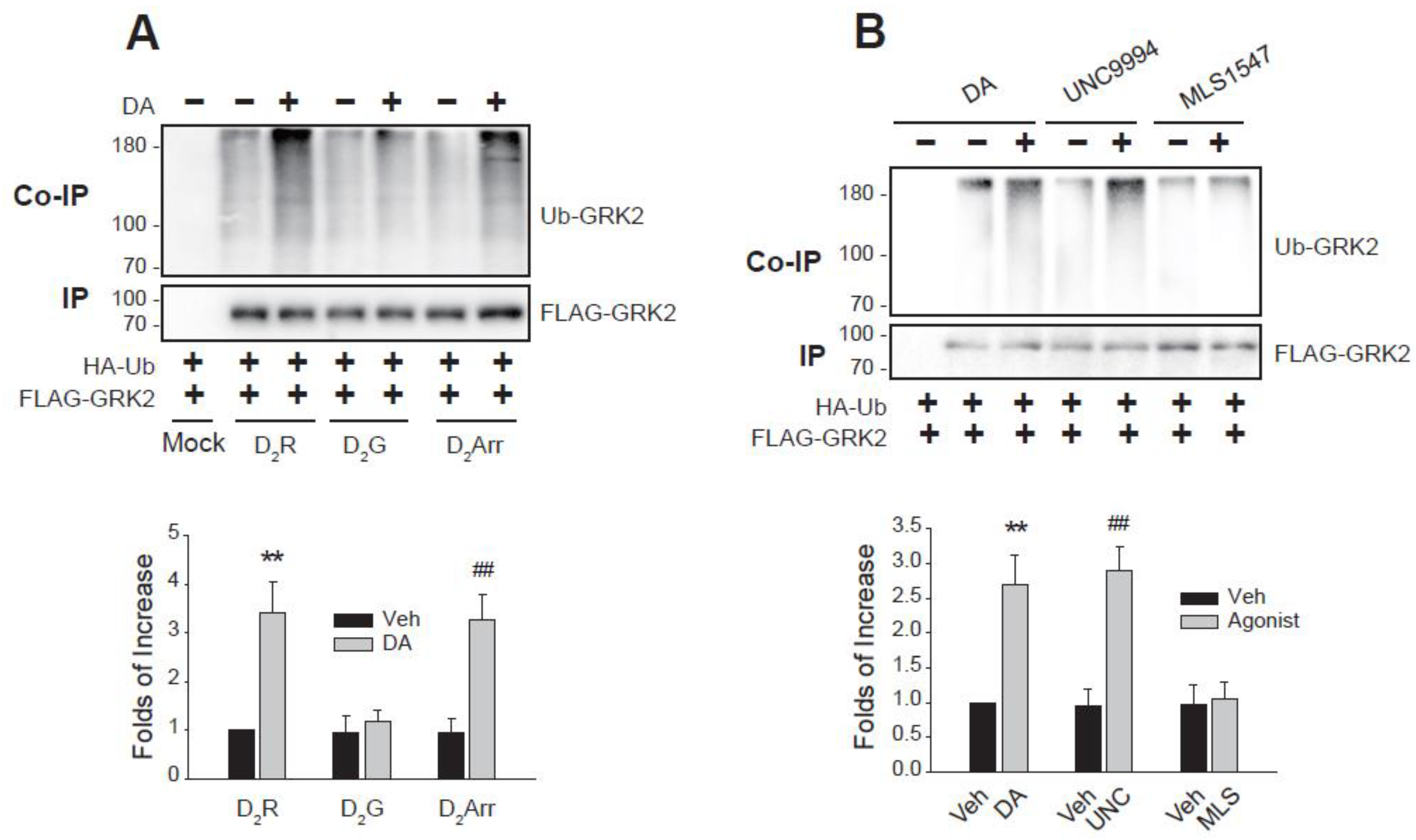
2.2. Arrestin-Biased Signaling Pathway Is Involved in the GRK2 Ubiquitination
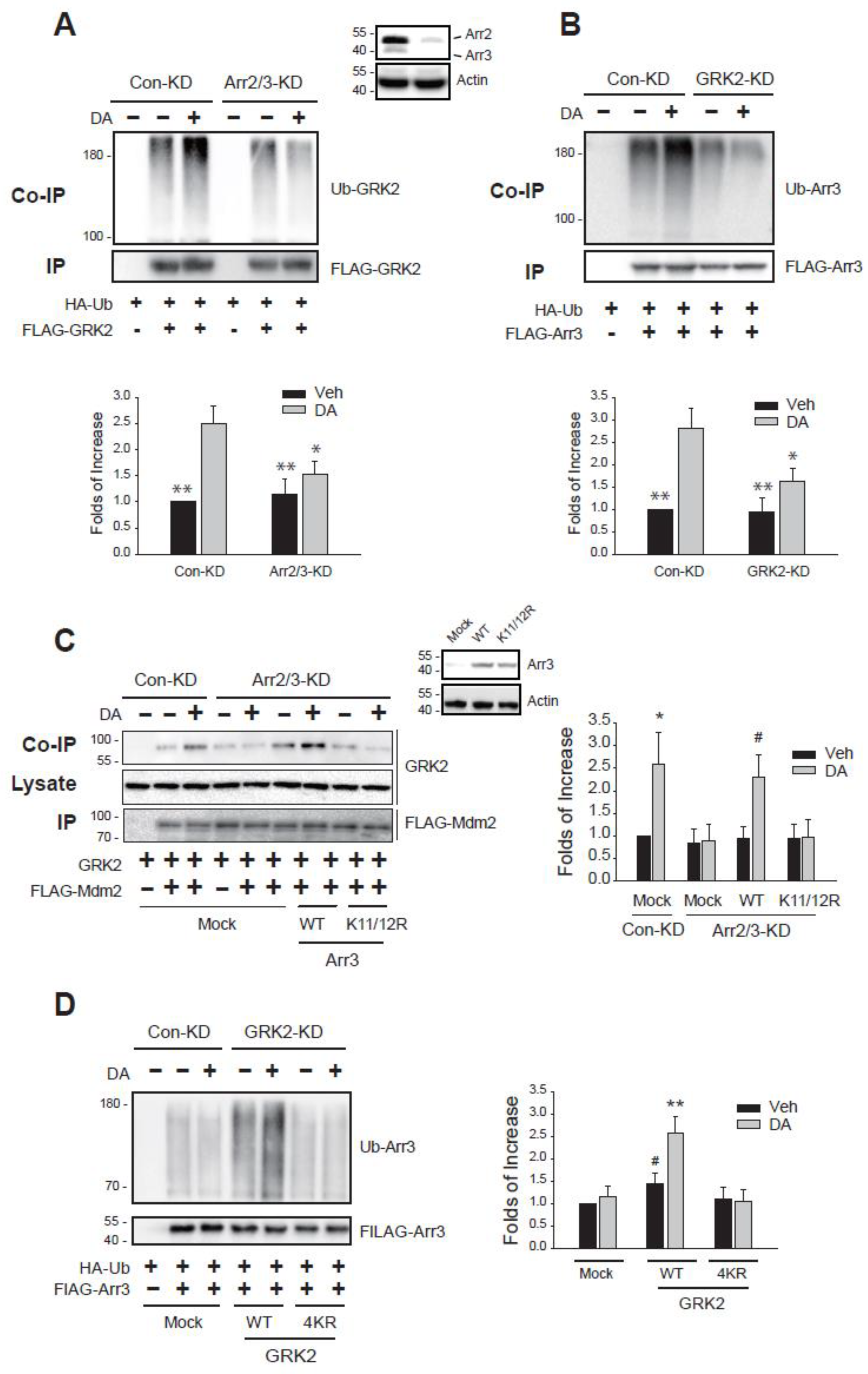
2.3. Ubiquitination of arrestin3 and GRK2 Is Mutually Related
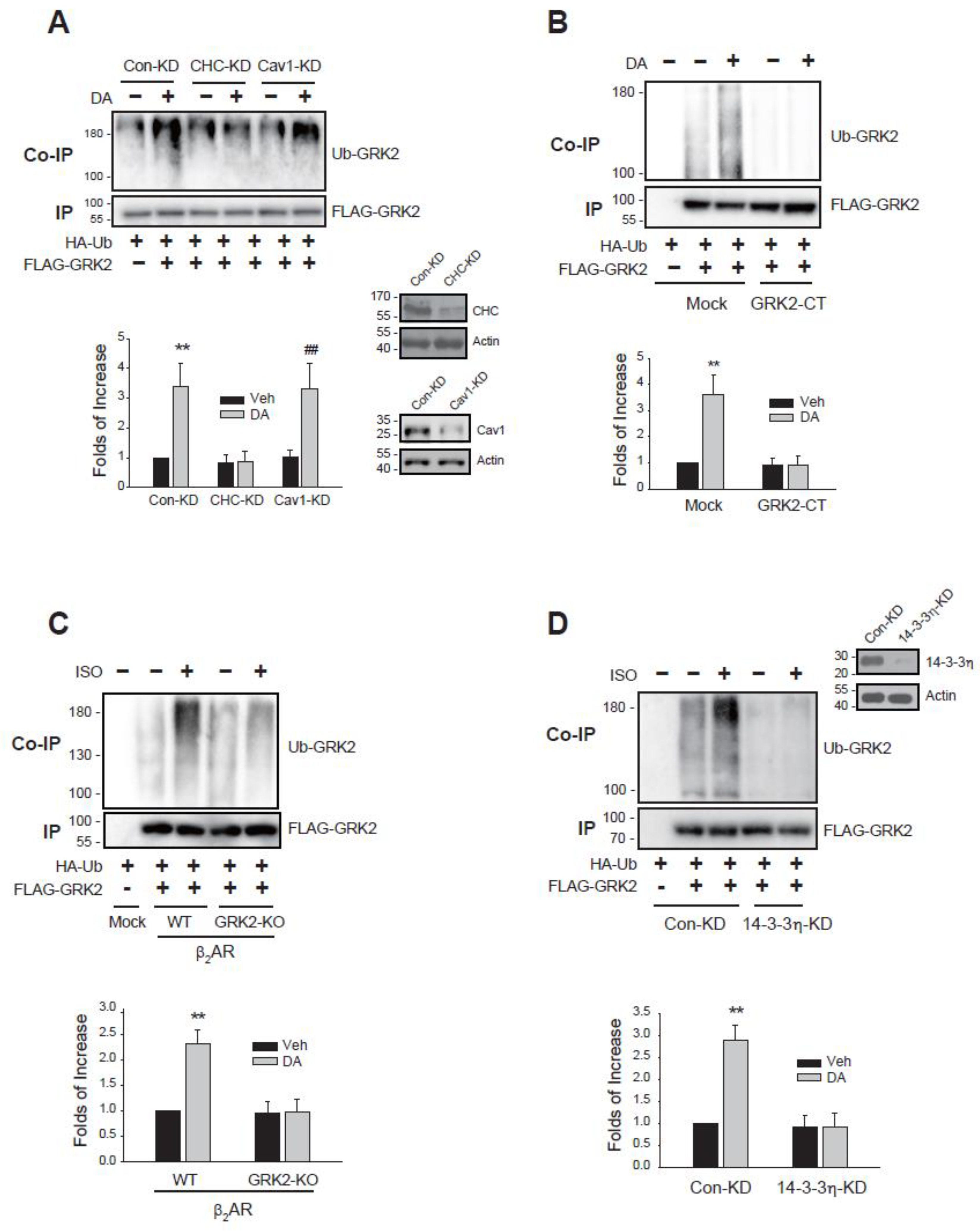
2.4. Cellular Components Responsible for the Ubiquitination of arrestin3 Are Also Involved in the Ubiquitination of GRK2
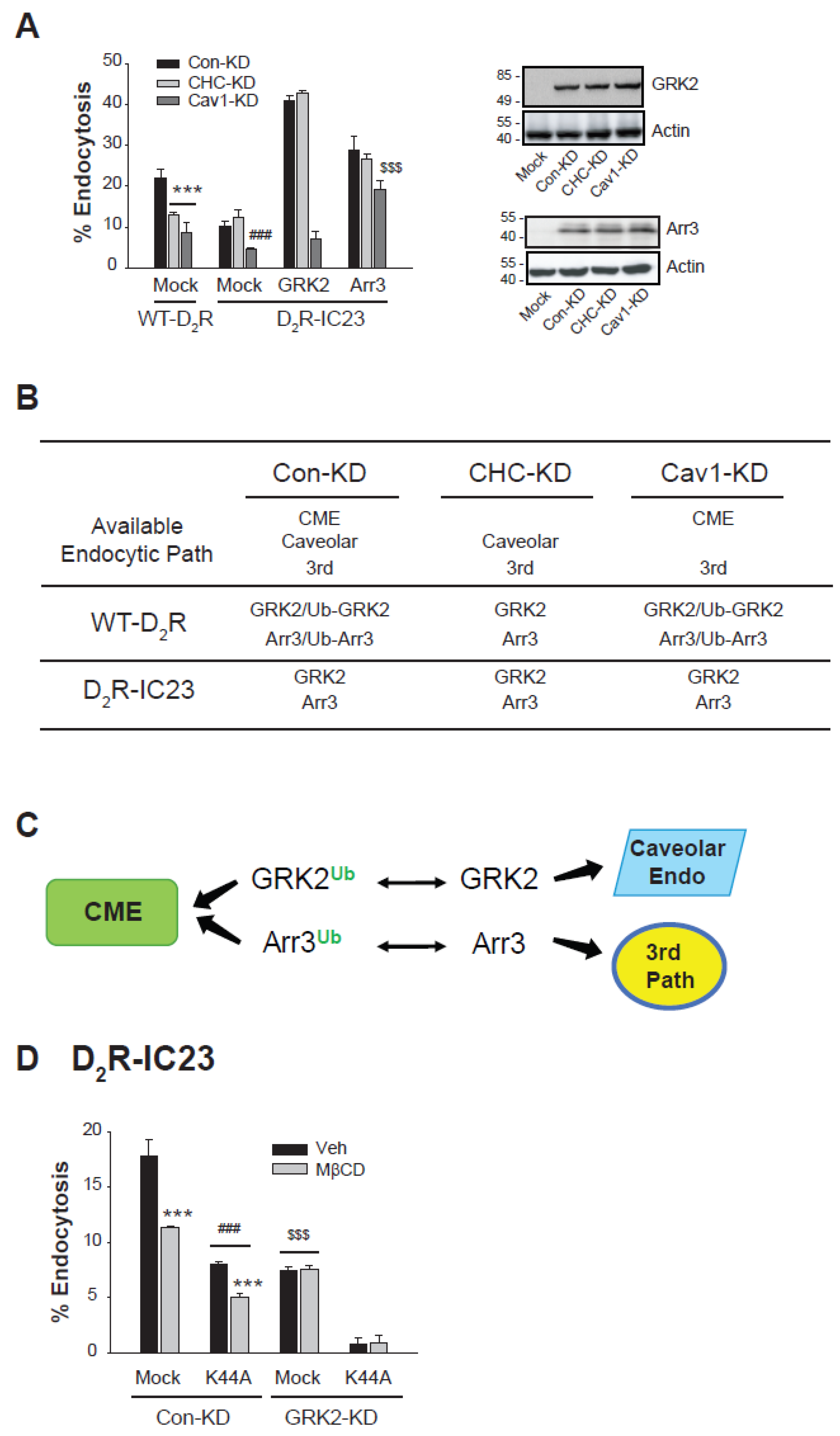
2.5. Differential Endocytic Pathways of D2R Are Regulated by the Ubiquitination Status of GRK2 and arrestin3
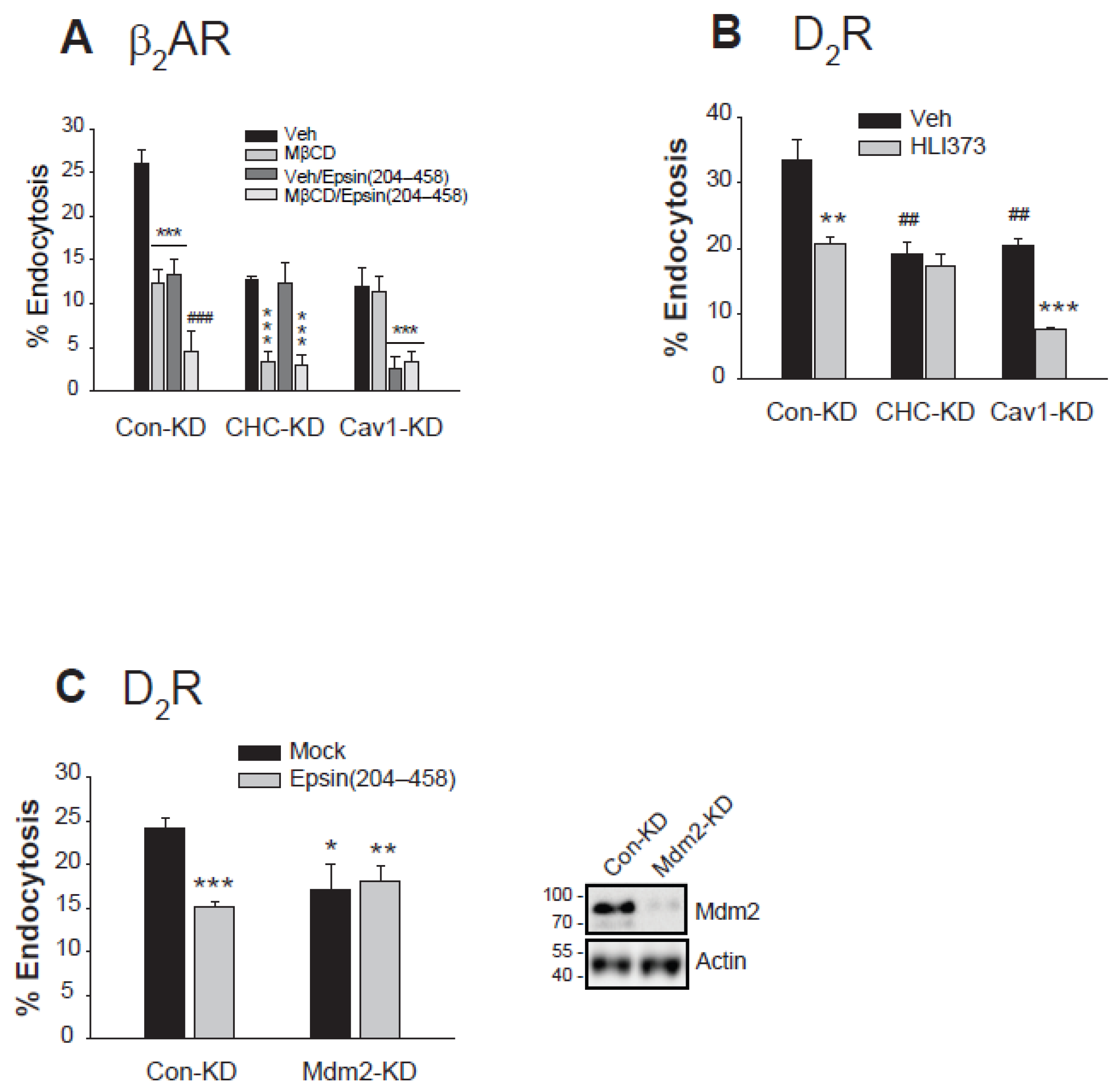
2.6. Mdm2-Mediated Ubiquitination Is Involved in the Clathrin-Mediated Endocytosis of Dopamine D2 Receptor and β2 Adrenoceptor
2.7. Interdependent Relationship Between GRK2/arrestin3 Ubiquitination and D2R Endocytic Pathways
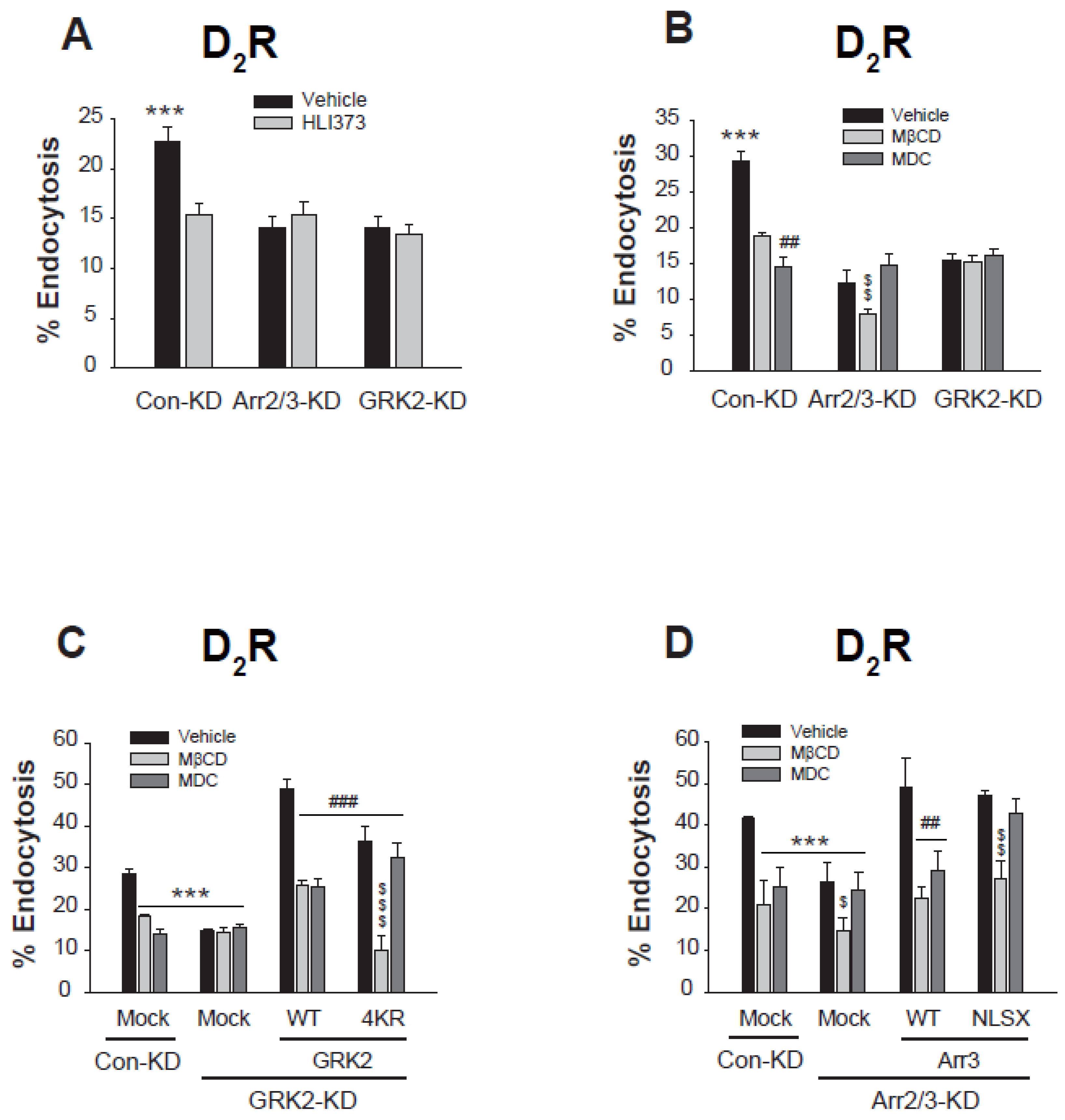
2.8. Ubiquitination-Dependent Protein Interactions of arrestin3 and GRK2 Determine Their Selective Contribution to Distinct Endocytic Pathways
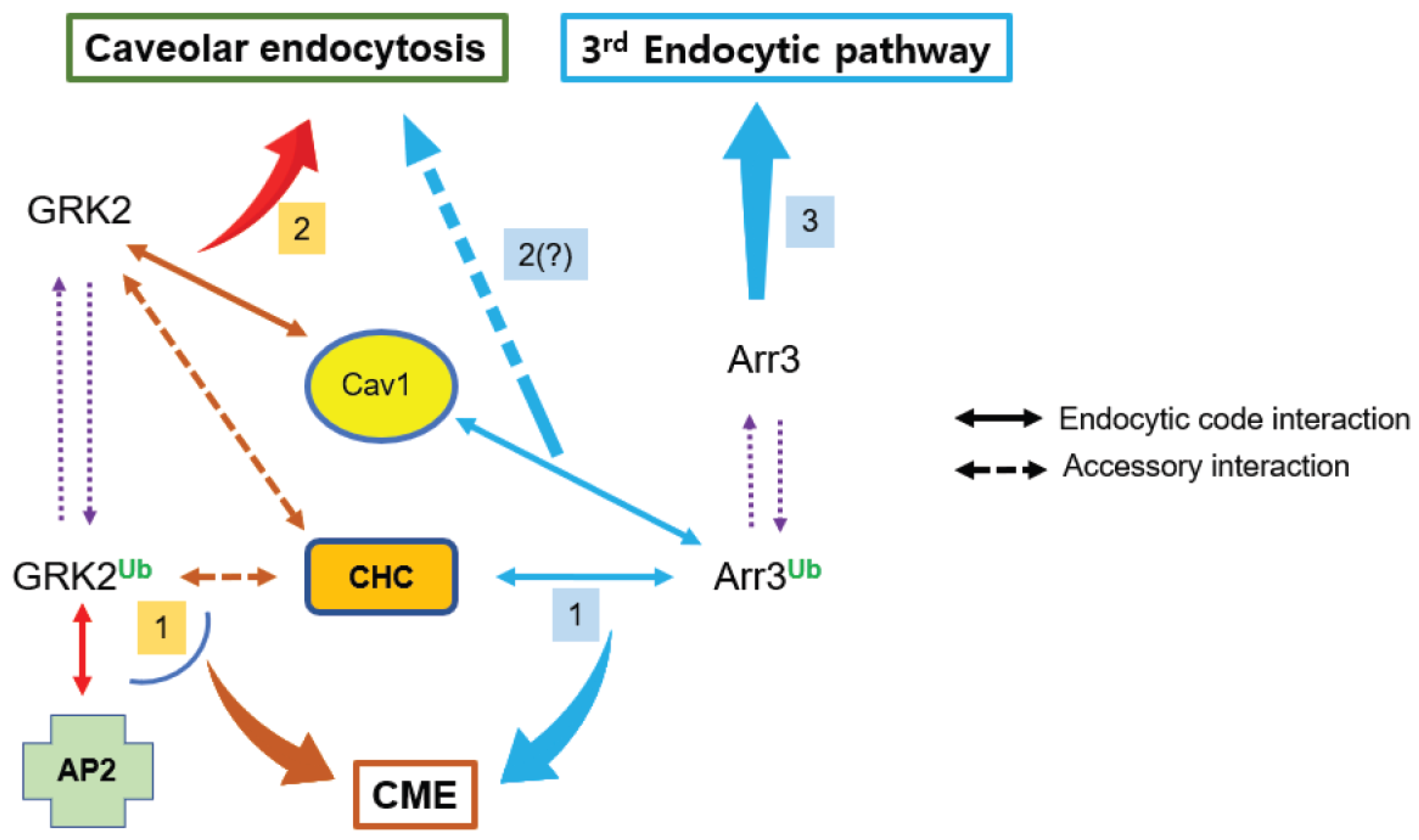
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. DNA Constructs
4.3. Cell Culture
4.4. Immunoprecipitation and Immunoblotting
4.5. Detection of Protein Ubiquitination
4.6. Receptor Endocytosis
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Kim, K.M. Multifactorial Regulation of G Protein-Coupled Receptor Endocytosis. Biomol. Ther. 2017, 25, 26–43. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, X.; Zheng, M.; Zhang, X.; Min, C.; Wang, Z.; Cheon, S.H.; Oak, M.H.; Nah, S.Y.; Kim, K.M. Selectivity of commonly used inhibitors of clathrin-mediated and caveolae-dependent endocytosis of G protein-coupled receptors. Biochim. Biophys. Acta 2015, 1848, 2101–2110. [Google Scholar] [CrossRef]
- Shih, W.; Gallusser, A.; Kirchhausen, T. A clathrin-binding site in the hinge of the beta 2 chain of mammalian AP-2 complexes. J. Biol. Chem. 1995, 270, 31083–31090. [Google Scholar] [CrossRef]
- Ferguson, S.S.; Downey, W.E., 3rd; Colapietro, A.M.; Barak, L.S.; Menard, L.; Caron, M.G. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 1996, 271, 363–366. [Google Scholar] [CrossRef]
- Schmid, S.L. Clathrin-coated vesicle formation and protein sorting: An integrated process. Annu. Rev. Biochem. 1997, 66, 511–548. [Google Scholar] [CrossRef]
- van der Bliek, A.M.; Redelmeier, T.E.; Damke, H.; Tisdale, E.J.; Meyerowitz, E.M.; Schmid, S.L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 1993, 122, 553–563. [Google Scholar]
- Nabi, I.R.; Le, P.U. Caveolae/raft-dependent endocytosis. J. Cell Biol. 2003, 161, 673–677. [Google Scholar] [CrossRef]
- Henley, J.R.; Krueger, E.W.; Oswald, B.J.; McNiven, M.A. Dynamin-mediated internalization of caveolae. J. Cell Biol. 1998, 141, 85–99. [Google Scholar]
- Gilman, A.G. G proteins: Transducers of receptor-generated signals. Annu. Rev. Biochem. 1987, 56, 615–649. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, J.A.; Inglese, J.; Higgins, J.B.; Arriza, J.L.; Casey, P.J.; Kim, C.; Benovic, J.L.; Kwatra, M.M.; Caron, M.G.; Lefkowitz, R.J. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science 1992, 257, 1264–1267. [Google Scholar] [PubMed]
- Touhara, K.; Inglese, J.; Pitcher, J.A.; Shaw, G.; Lefkowitz, R.J. Binding of G protein beta gamma-subunits to pleckstrin homology domains. J. Biol. Chem. 1994, 269, 10217–10220. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, J.A.; Fredericks, Z.L.; Stone, W.C.; Premont, R.T.; Stoffel, R.H.; Koch, W.J.; Lefkowitz, R.J. Phosphatidylinositol 4,5-bisphosphate (PIP2)-enhanced G protein-coupled receptor kinase (GRK) activity. Location, structure, and regulation of the PIP2 binding site distinguishes the GRK subfamilies. J. Biol. Chem. 1996, 271, 24907–24913. [Google Scholar] [CrossRef] [PubMed]
- Penela, P.; Ribas, C.; Sanchez-Madrid, F.; Mayor, F., Jr. G protein-coupled receptor kinase 2 (GRK2) as a multifunctional signaling hub. Cell Mol. Life Sci. 2019, 76, 4423–4446. [Google Scholar] [CrossRef]
- Boguth, C.A.; Singh, P.; Huang, C.C.; Tesmer, J.J. Molecular basis for activation of G protein-coupled receptor kinases. EMBO J. 2010, 29, 3249–3259. [Google Scholar] [CrossRef]
- Penela, P.; Elorza, A.; Sarnago, S.; Mayor, F., Jr. Beta-arrestin- and c-Src-dependent degradation of G-protein-coupled receptor kinase 2. EMBO J. 2001, 20, 5129–5138. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, X.; Guo, S.; Le, H.T.; Zhang, X.; Kim, K.M. Molecular mechanisms involved in epidermal growth factor receptor-mediated inhibition of dopamine D3 receptor signaling. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1187–1200. [Google Scholar] [CrossRef]
- Houslay, M.D.; Baillie, G.S. Phosphodiesterase-4 gates the ability of protein kinase A to phosphorylate G-protein receptor kinase-2 and influence its translocation. Biochem. Soc. Trans. 2006, 34, 474–475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krasel, C.; Dammeier, S.; Winstel, R.; Brockmann, J.; Mischak, H.; Lohse, M.J. Phosphorylation of GRK2 by protein kinase C abolishes its inhibition by calmodulin. J. Biol. Chem. 2001, 276, 1911–1915. [Google Scholar] [CrossRef]
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef]
- Ardley, H.C.; Robinson, P.A. E3 ubiquitin ligases. Essays Biochem. 2005, 41, 15–30. [Google Scholar] [CrossRef]
- Shenoy, S.K.; McDonald, P.H.; Kohout, T.A.; Lefkowitz, R.J. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 2001, 294, 1307–1313. [Google Scholar] [CrossRef]
- Zhang, X.; Min, X.; Wang, S.; Sun, N.; Kim, K.M. Mdm2-mediated ubiquitination of beta-arrestin2 in the nucleus occurs in a Gbetagamma- and clathrin-dependent manner. Biochem. Pharmacol. 2020, 178, 114049. [Google Scholar] [CrossRef]
- Salcedo, A.; Mayor, F., Jr.; Penela, P. Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2. EMBO J. 2006, 25, 4752–4762. [Google Scholar] [CrossRef]
- Penela, P.; Ruiz-Gomez, A.; Castano, J.G.; Mayor, F., Jr. Degradation of the G protein-coupled receptor kinase 2 by the proteasome pathway. J. Biol. Chem. 1998, 273, 35238–35244. [Google Scholar] [PubMed]
- Pack, T.F.; Orlen, M.I.; Ray, C.; Peterson, S.M.; Caron, M.G. The dopamine D2 receptor can directly recruit and activate GRK2 without G protein activation. J. Biol. Chem. 2018, 293, 6161–6171. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, L.; Kim, K.M. Biased Dopamine D(2) Receptors Exhibit Distinct Intracellular Trafficking Properties and ERK Activation in Different Subcellular Domains. Biomol. Ther. 2024, 32, 56–64. [Google Scholar] [CrossRef]
- Peterson, S.M.; Pack, T.F.; Wilkins, A.D.; Urs, N.M.; Urban, D.J.; Bass, C.E.; Lichtarge, O.; Caron, M.G. Elucidation of G-protein and beta-arrestin functional selectivity at the dopamine D2 receptor. Proc. Natl. Acad. Sci. USA 2015, 112, 7097–7102. [Google Scholar] [CrossRef]
- Allen, J.A.; Yost, J.M.; Setola, V.; Chen, X.; Sassano, M.F.; Chen, M.; Peterson, S.; Yadav, P.N.; Huang, X.P.; Feng, B.; et al. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl. Acad. Sci. USA 2011, 108, 18488–18493. [Google Scholar] [CrossRef]
- Free, R.B.; Chun, L.S.; Moritz, A.E.; Miller, B.N.; Doyle, T.B.; Conroy, J.L.; Padron, A.; Meade, J.A.; Xiao, J.; Hu, X.; et al. Discovery and characterization of a G protein-biased agonist that inhibits beta-arrestin recruitment to the D2 dopamine receptor. Mol. Pharmacol. 2014, 86, 96–105. [Google Scholar] [CrossRef]
- Kundu, D.; Min, X.; Zhang, X.; Tian, X.; Wang, S.; Kim, K.M. The Ubiquitination of Arrestin3 within the Nucleus Triggers the Nuclear Export of Mdm2, Which, in Turn, Mediates the Ubiquitination of GRK2 in the Cytosol. Int. J. Mol. Sci. 2024, 25, 9644. [Google Scholar] [CrossRef]
- Shenoy, S.K.; Lefkowitz, R.J. Receptor-specific ubiquitination of beta-arrestin directs assembly and targeting of seven-transmembrane receptor signalosomes. J. Biol. Chem. 2005, 280, 15315–15324. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Barbash, S.; Kongsamut, S.; Eishingdrelo, A.; Sakmar, T.P.; Eishingdrelo, H. 14-3-3 signal adaptor and scaffold proteins mediate GPCR trafficking. Sci. Rep. 2019, 9, 11156. [Google Scholar] [CrossRef]
- Koch, W.J.; Inglese, J.; Stone, W.C.; Lefkowitz, R.J. The binding site for the beta gamma subunits of heterotrimeric G proteins on the beta-adrenergic receptor kinase. J. Biol. Chem. 1993, 268, 8256–8260. [Google Scholar] [PubMed]
- Nobles, K.N.; Xiao, K.; Ahn, S.; Shukla, A.K.; Lam, C.M.; Rajagopal, S.; Strachan, R.T.; Huang, T.Y.; Bressler, E.A.; Hara, M.R.; et al. Distinct phosphorylation sites on the beta(2)-adrenergic receptor establish a barcode that encodes differential functions of beta-arrestin. Sci. Signal. 2011, 4, ra51. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, M.; Kim, K.M. GRK2-mediated receptor phosphorylation and Mdm2-mediated beta-arrestin2 ubiquitination drive clathrin-mediated endocytosis of G protein-coupled receptors. Biochem. Biophys. Res. Commun. 2020, 533, 383–390. [Google Scholar] [CrossRef]
- Cho, D.; Zheng, M.; Min, C.; Ma, L.; Kurose, H.; Park, J.H.; Kim, K.M. Agonist-induced endocytosis and receptor phosphorylation mediate resensitization of dopamine D(2) receptors. Mol. Endocrinol. 2010, 24, 574–586. [Google Scholar] [CrossRef]
- Kirchhausen, T. Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol. 1999, 15, 705–732. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, K.M.; Koch, W.J. GRK2 in cardiovascular disease and its potential as a therapeutic target. J. Mol. Cell Cardiol. 2022, 172, 14–23. [Google Scholar] [CrossRef]
- Jean-Charles, P.Y.; Yu, S.M.; Abraham, D.; Kommaddi, R.P.; Mao, L.; Strachan, R.T.; Zhang, Z.S.; Bowles, D.E.; Brian, L.; Stiber, J.A.; et al. Mdm2 regulates cardiac contractility by inhibiting GRK2-mediated desensitization of beta-adrenergic receptor signaling. JCI Insight 2017, 2, e95998. [Google Scholar] [CrossRef]
- Yu, S.S.; Lefkowitz, R.J.; Hausdorff, W.P. Beta-adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J. Biol. Chem. 1993, 268, 337–341. [Google Scholar]
- Pathak, C.; Vaidya, F.U.; Waghela, B.N.; Jaiswara, P.K.; Gupta, V.K.; Kumar, A.; Rajendran, B.K.; Ranjan, K. Insights of Endocytosis Signaling in Health and Disease. Int. J. Mol. Sci. 2023, 24, 2971. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Valenzano, K.J.; Robinson, S.R.; Yao, W.D.; Barak, L.S.; Caron, M.G. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. J. Biol. Chem. 2001, 276, 37409–37414. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.J.; Hawes, B.E.; Inglese, J.; Luttrell, L.M.; Lefkowitz, R.J. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling. J. Biol. Chem. 1994, 269, 6193–6197. [Google Scholar]
- Laporte, S.A.; Miller, W.E.; Kim, K.M.; Caron, M.G. beta-Arrestin/AP-2 interaction in G protein-coupled receptor internalization: Identification of a beta-arrestin binging site in beta 2-adaptin. J. Biol. Chem. 2002, 277, 9247–9254. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhang, X.; Guo, S.; Zhang, X.; Choi, H.J.; Lee, M.Y.; Kim, K.M. PKCbetaII inhibits the ubiquitination of beta-arrestin2 in an autophosphorylation-dependent manner. FEBS Lett. 2015, 589, 3929–3937. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, X.; Zhang, X.; Kim, K.M. The EGF receptor inhibits the signaling of dopamine D3 receptor through the phosphorylation of GRK2 on tyrosine residues. Biochem. Biophys. Res. Commun. 2017, 489, 515–522. [Google Scholar] [CrossRef]
- Min, C.; Zheng, M.; Zhang, X.; Caron, M.G.; Kim, K.M. Novel roles for beta-arrestins in the regulation of pharmacological sequestration to predict agonist-induced desensitization of dopamine D3 receptors. Br. J. Pharmacol. 2013, 170, 1112–1129. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Kundu, D.; Zhang, X.; Tian, X.; Peng, L.; Kim, K.-M. Mdm2-Mediated Ubiquitination Plays a Pivotal Role in Differentiating the Endocytic Roles of GRK2 and Arrestin3. Int. J. Mol. Sci. 2025, 26, 3238. https://doi.org/10.3390/ijms26073238
Wang S, Kundu D, Zhang X, Tian X, Peng L, Kim K-M. Mdm2-Mediated Ubiquitination Plays a Pivotal Role in Differentiating the Endocytic Roles of GRK2 and Arrestin3. International Journal of Molecular Sciences. 2025; 26(7):3238. https://doi.org/10.3390/ijms26073238
Chicago/Turabian StyleWang, Shujie, Dooti Kundu, Xiaohan Zhang, Xinru Tian, Lulu Peng, and Kyeong-Man Kim. 2025. "Mdm2-Mediated Ubiquitination Plays a Pivotal Role in Differentiating the Endocytic Roles of GRK2 and Arrestin3" International Journal of Molecular Sciences 26, no. 7: 3238. https://doi.org/10.3390/ijms26073238
APA StyleWang, S., Kundu, D., Zhang, X., Tian, X., Peng, L., & Kim, K.-M. (2025). Mdm2-Mediated Ubiquitination Plays a Pivotal Role in Differentiating the Endocytic Roles of GRK2 and Arrestin3. International Journal of Molecular Sciences, 26(7), 3238. https://doi.org/10.3390/ijms26073238





