The Leucine-Rich Repeat Kinase 2 Variant LRRK2G2019S Up-Regulates L-Type (CaV1.3) Calcium Channel via the CaVβ3 Subunit: Possible Role in the Pathogenesis of Parkinson’s Disease
Abstract
1. Introduction
2. Results
2.1. The Subunits of L-Type CaV1.3α Channels May Contain Motifs for Regulation by LRRK2
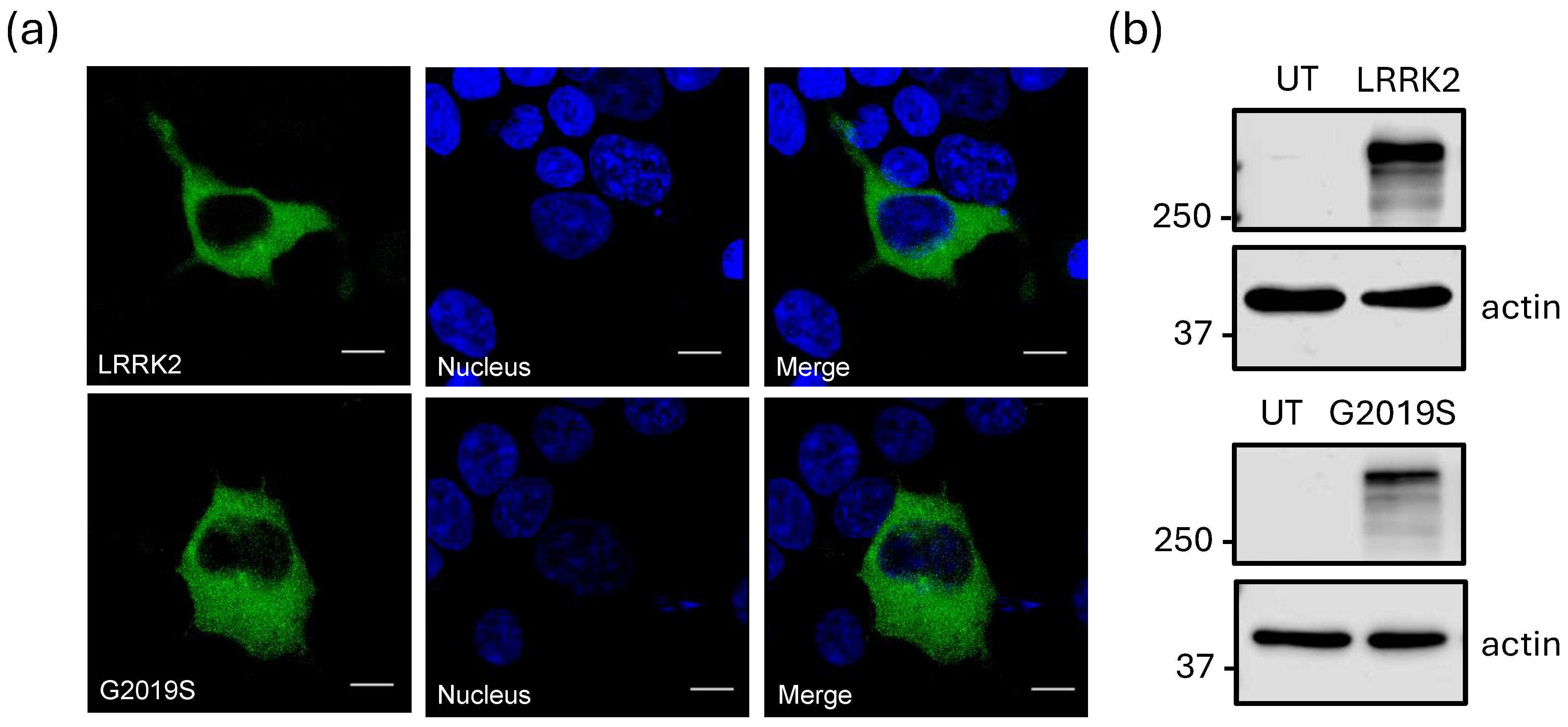
2.2. Expression of LRRK2 and Its Increased Activity Mutant LRRK2G2019S
2.3. LRRK2 Interacts with CaVβ3 Subunit in HEK-293 Cells
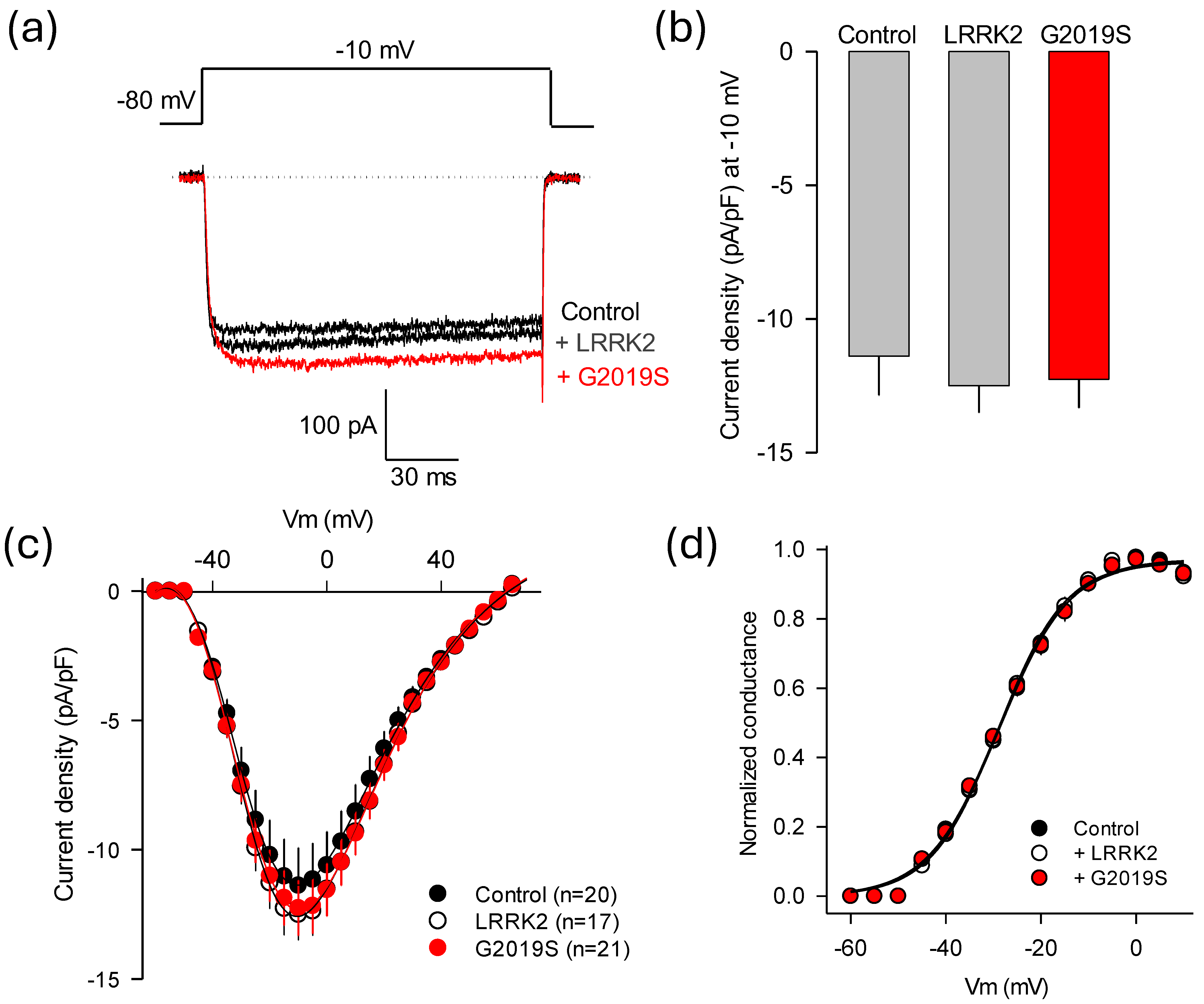
2.4. The Mutant LRRK2 Regulates L-Type CaV1.3 Channel Functional Expression
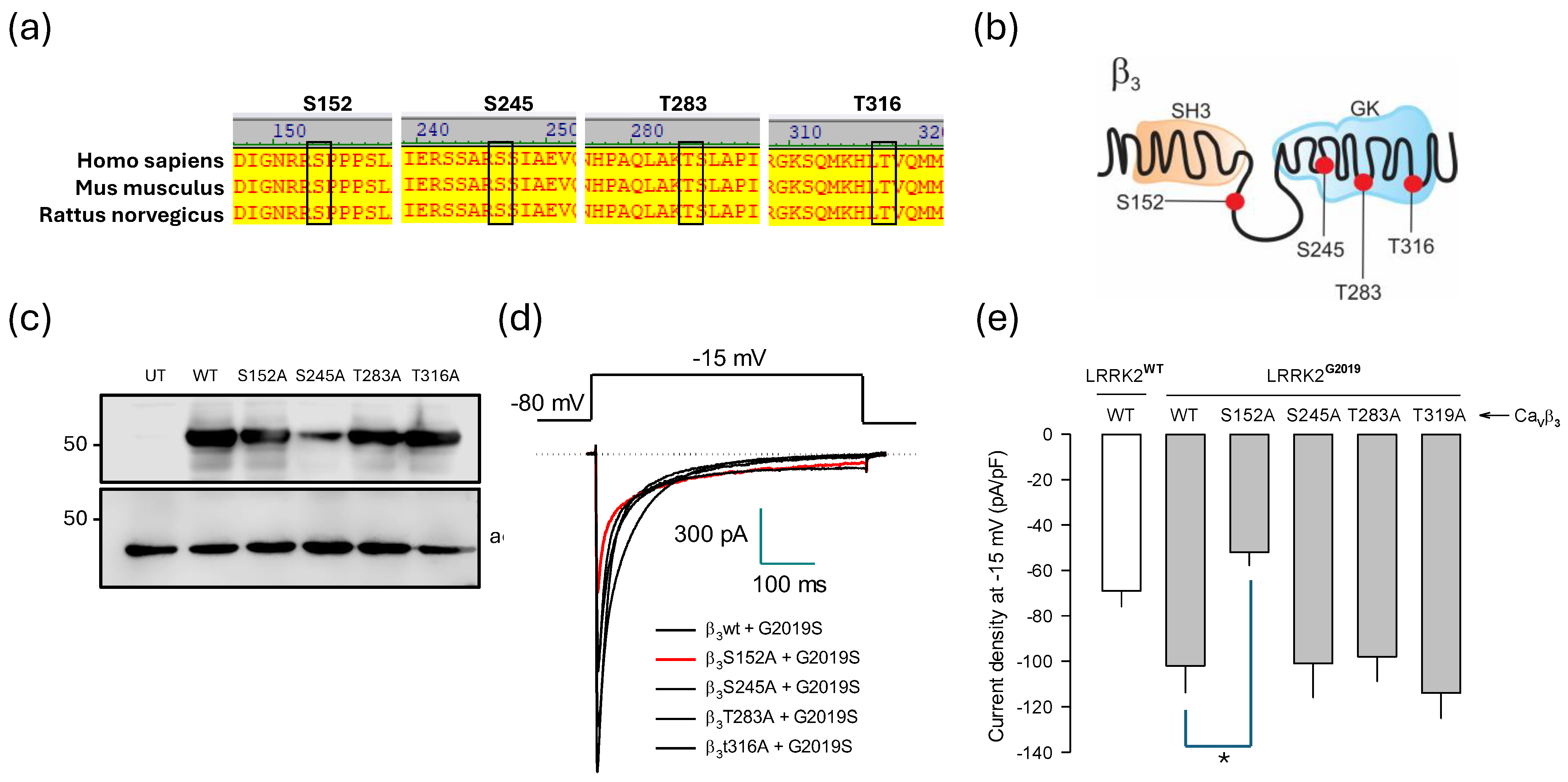
2.5. Relevance of the CaVβ3 Subunit in the Effects of LRRK2G2019S
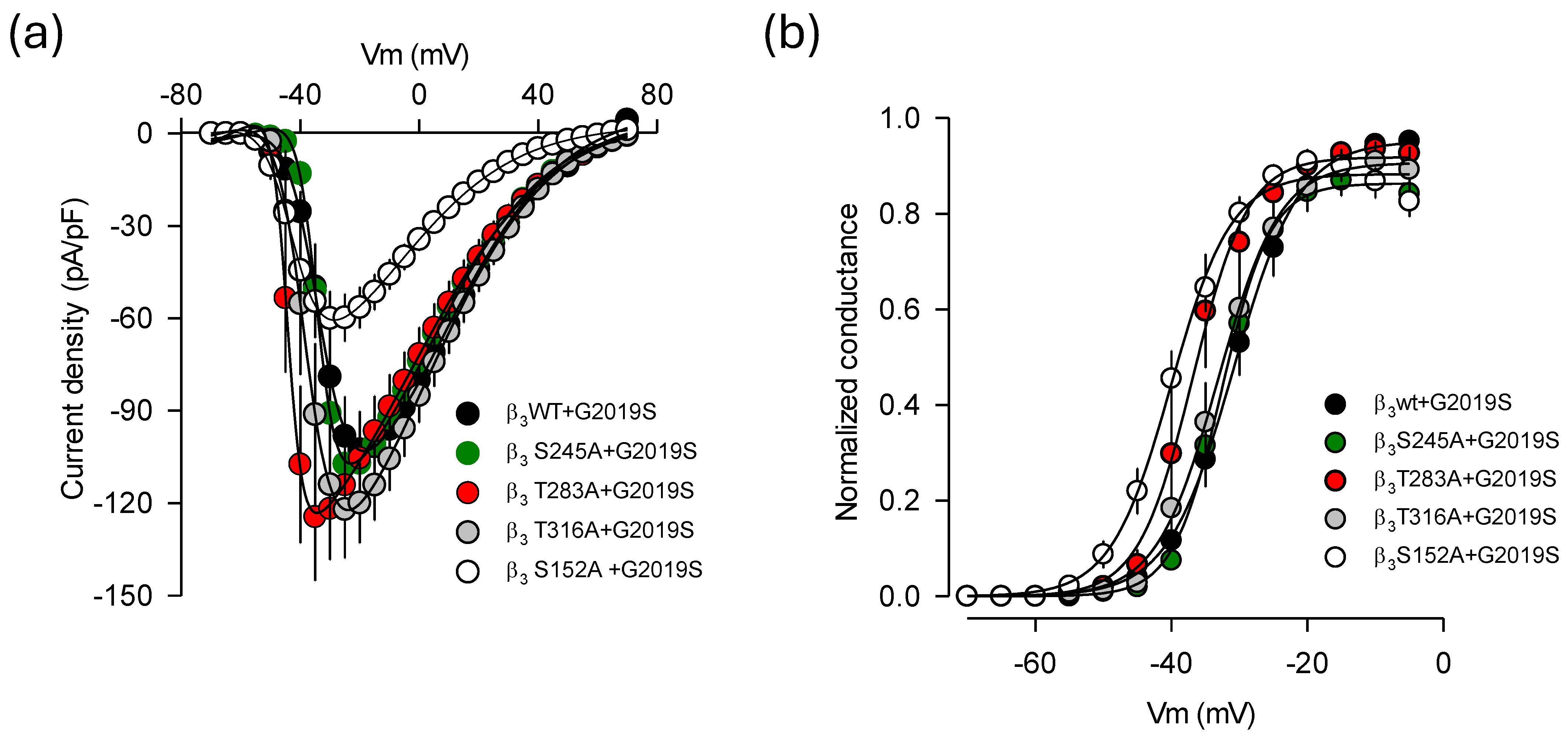
2.6. Identification of Molecular Elements Implicated in the Regulation of CaV1.3 Channels by LRRK2
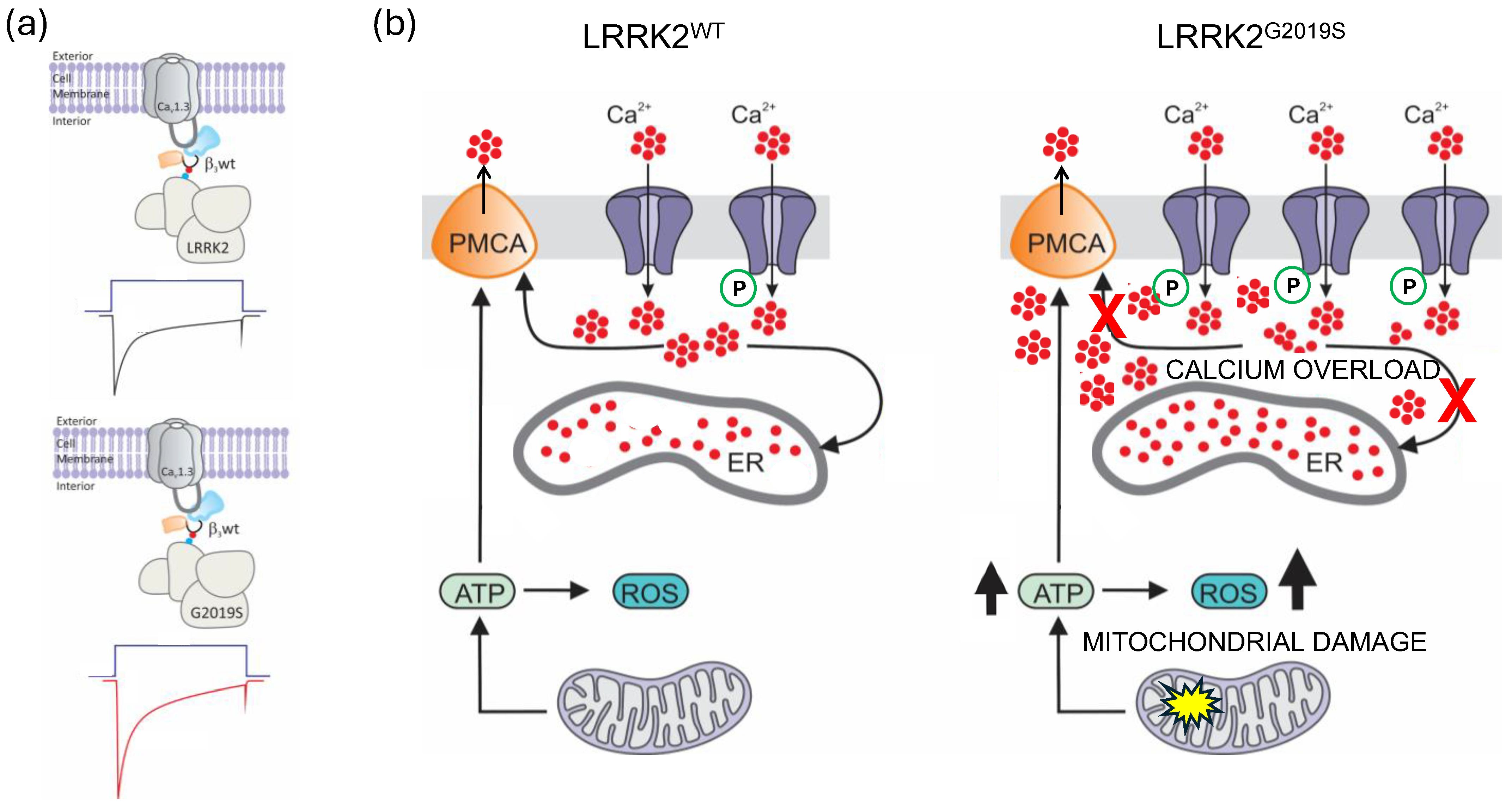
3. Discussion
4. Materials and Methods
4.1. Bioinformatics
4.2. Cell Culture and Transfection
4.3. Immunofluorescence
4.4. Site-Directed Mutagenesis
4.5. Western Blotting
4.6. Immunoprecipitation
4.7. Electrophysiology
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef]
- Gandini, M.A.; Sandoval, A.; Felix, R. Toxins targeting voltage-activated Ca2+ channels and their potential biomedical applications. Curr. Top. Med. Chem. 2015, 15, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.E.; Couette, B.; Bourinet, E.; Platzer, J.; Reimer, D.; Striessnig, J.; Nargeot, J. Functional role of L-type CaV1.3 Ca2+ channels in cardiac pacemaker activity. Proc. Natl. Acad. Sci. USA 2003, 100, 5543–5548. [Google Scholar] [CrossRef]
- Vandael, D.H.; Marcantoni, A.; Carbone, E. CaV1.3 channels as key regulators of neuron-like firings and catecholamine release in chromaffin cells. Curr. Mol. Pharmacol. 2015, 8, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Pinggera, A.; Striessnig, J. CaV1.3 (CACNA1D) L-type Ca2+ channel dysfunction in CNS disorders. J. Physiol. 2016, 594, 5839–5849. [Google Scholar] [CrossRef]
- Platzer, J.; Engel, J.; Schrott-Fischer, A.; Stephan, K.; Bova, S.; Chen, H.; Zheng, H.; Striessnig, J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 2000, 102, 89–97. [Google Scholar] [CrossRef]
- Sandoval, A.; Duran, P.; Corzo-López, A.; Fernández-Gallardo, M.; Muñoz-Herrera, D.; Leyva-Leyva, M.; González-Ramírez, R.; Felix, R. The role of voltage-gated calcium channels in the pathogenesis of Parkinson’s disease. Int. J. Neurosci. 2024, 134, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Bedford, C.; Sears, C.; Perez-Carrion, M.; Piccoli, G.; Condliffe, S.B. LRRK2 Regulates voltage-gated calcium channel function. Front. Mol. Neurosci. 2016, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Guaitoli, G.; Gilsbach, B.K.; Raimondi, F.; Gloeckner, C.J. First model of dimeric LRRK2: The challenge of unrevealing the structure of a multidomain Parkinson’s-associated protein. Biochem. Soc. Trans. 2016, 44, 1635–1641. [Google Scholar] [CrossRef]
- Gilsbach, B.K.; Kortholt, A. Structural biology of the LRRK2 GTPase and kinase domains: Implications for regulation. Front. Mol. Neurosci. 2014, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Ríos, P.; Gómez-Suaga, P.; Fernández, B.; Madero-Pérez, J.; Schwab, A.J.; Ebert, A.D.; Hilfiker, S. Alterations in late endocytic trafficking related to the pathobiology of LRRK2-linked Parkinson’s disease. Biochem. Soc. Trans. 2015, 43, 390–395. [Google Scholar] [CrossRef]
- Yan, J.; Almilaji, A.; Schmid, E.; Elvira, B.; Shimshek, D.R.; van der Putten, H.; Wagner, C.A.; Shumilina, E.; Lang, F. Leucine-rich repeat kinase 2-sensitive Na+/Ca2+ exchanger activity in dendritic cells. FASEB J. 2015, 29, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Pungaliya, P.P.; Bai, Y.; Lipinski, K.; Anand, V.S.; Sen, S.; Brown, E.L.; Bates, B.; Reinhart, P.H.; West, A.B.; Hirst, W.D.; et al. Identification and characterization of a leucine-rich repeat kinase 2 (LRRK2) consensus phosphorylation motif. PLoS ONE 2010, 5, e13672. [Google Scholar] [CrossRef]
- Liu, Z.; Bryant, N.; Kumaran, R.; Beilina, A.; Abeliovich, A.; Cookson, M.R.; West, A.B. LRRK2 phosphorylates membrane-bound Rabs and is activated by GTP-bound Rab7L1 to promote recruitment to the trans-Golgi network. Hum. Mol. Genet. 2018, 27, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Shani, V.; Safory, H.; Szargel, R.; Wang, N.; Cohen, T.; Elghani, F.A.; Hamza, H.; Savyon, M.; Radzishevsky, I.; Shaulov, L.; et al. Physiological and pathological roles of LRRK2 in the nuclear envelope integrity. Hum. Mol. Genet. 2019, 28, 3982–3996. [Google Scholar] [CrossRef]
- West, A.B.; Moore, D.J.; Biskup, S.; Bugayenko, A.; Smith, W.W.; Ross, C.A.; Dawson, V.L.; Dawson, T.M. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl. Acad. Sci. USA 2005, 102, 16842–16847. [Google Scholar] [CrossRef] [PubMed]
- Lesage, S.; Houot, M.; Mangone, G.; Tesson, C.; Bertrand, H.; Forlani, S.; Anheim, M.; Brefel-Courbon, C.; Broussolle, E.; Thobois, S.; et al. Genetic and phenotypic basis of autosomal dominant Parkinson’s disease in a large multi-center cohort. Front. Neurol. 2020, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- El Otmani, H.; Daghi, M.; Tahiri Jouti, N.; Lesage, S. An overview of the worldwide distribution of LRRK2 mutations in Parkinson’s disease. Neurodegener. Dis. Manag. 2023, 13, 335–350. [Google Scholar] [CrossRef]
- Kmiecik, M.J.; Micheletti, S.; Coker, D.; Heilbron, K.; Shi, J.; Stagaman, K.; Filshtein Sonmez, T.; Fontanillas, P.; Shringarpure, S.; Wetzel, M.; et al. Genetic analysis and natural history of Parkinson’s disease due to the LRRK2 G2019S variant. Brain 2024, 147, 1996–2008. [Google Scholar] [CrossRef]
- Gandini, M.A.; Sandoval, A.; González-Ramírez, R.; Mori, Y.; de Waard, M.; Felix, R. Functional coupling of Rab3-interacting molecule 1 (RIM1) and L-type Ca2+ channels in insulin release. J. Biol. Chem. 2011, 286, 15757–15765. [Google Scholar] [CrossRef] [PubMed]
- Church, P.J.; Stanley, E.F. Single L-type calcium channel conductance with physiological levels of calcium in chick ciliary ganglion neurons. J. Physiol. 1996, 496 Pt 1, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.W.Z.; Liu, C.; Soong, T.W.; Hu, Z. β subunits of voltage-gated calcium channels in cardiovascular diseases. Front. Cardiovasc. Med. 2023, 10, 1119729. [Google Scholar] [CrossRef] [PubMed]
- Brunet, S.; Emrick, M.A.; Sadilek, M.; Scheuer, T.; Catterall, W.A. Phosphorylation sites in the Hook domain of CaVβ subunits differentially modulate CaV1.2 channel function. J. Mol. Cell. Cardiol. 2015, 87, 248–256. [Google Scholar] [CrossRef][Green Version]
- Baldelli, P.; Hernández-Guijo, J.M.; Carabelli, V.; Novara, M.; Cesetti, T.; Andrés-Mateos, E.; Montiel, C.; Carbone, E. Direct and remote modulation of L-channels in chromaffin cells: Distinct actions on α1C and α1D subunits? Mol. Neurobiol. 2004, 29, 73–96. [Google Scholar] [CrossRef]
- Sandoval, A.; Duran, P.; Gandini, M.A.; Andrade, A.; Almanza, A.; Kaja, S.; Felix, R. Regulation of L-type Cav1.3 channel activity and insulin secretion by the cGMP-PKG signaling pathway. Cell Calcium. 2017, 66, 1–9. [Google Scholar] [CrossRef]
- Raifman, T.K.; Kumar, P.; Haase, H.; Klussmann, E.; Dascal, N.; Weiss, S. Protein kinase C enhances plasma membrane expression of cardiac L-type calcium channel, CaV1.2. Channels 2017, 11, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.; Calderón-Rivera, A.; Sandoval, A.; González-Ramírez, R.; Vargas-Parada, A.; Ojeda-Alonso, J.; Granados-Soto, V.; Delgado-Lezama, R.; Felix, R. Cdk5-Dependent phosphorylation of CaV3.2 T-Type channels: Possible role in nerve ligation-induced neuropathic allodynia and the compound action potential in primary afferent C fibers. J. Neurosci. 2020, 40, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Loya-López, S.; Sandoval, A.; González-Ramírez, R.; Calderón-Rivera, A.; Ávalos-Fuentes, A.; Rodríguez-Sánchez, M.; Caballero, R.; Tovar-Soto, D.; Felix, R.; Florán, B. Cdk5 phosphorylates CaV1.3 channels and regulates GABAA-mediated miniature inhibitory post-synaptic currents in striato-nigral terminals. Biochem. Biophys. Res. Commun. 2020, 524, 255–261. [Google Scholar] [CrossRef]
- Liu, H.; Felix, R.; Gurnett, C.A.; De Waard, M.; Witcher, D.R.; Campbell, K.P. Expression and subunit interaction of voltage-dependent Ca2+ channels in PC12 cells. J. Neurosci. 1996, 16, 7557–7565. [Google Scholar] [CrossRef] [PubMed]
- Grimaldo, L.; Sandoval, A.; Duran, P.; Gómez Flores-Ramos, L.; Felix, R. The ubiquitin E3 ligase Parkin regulates neuronal CaV1.3 channel functional expression. J. Neurophysiol. 2022, 128, 1555–1564. [Google Scholar] [CrossRef]
- Andrade, A.; Sandoval, A.; González-Ramírez, R.; Lipscombe, D.; Campbell, K.P.; Felix, R. The CaVα2δ subunit augments functional expression and modifies the pharmacology of CaV1.3 L-type channels. Cell Calcium. 2009, 46, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Koschak, A.; Reimer, D.; Huber, I.; Grabner, M.; Glossmann, H.; Engel, J.; Striessnig, J. α1D (CaV1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J. Biol. Chem. 2001, 276, 22100–22106. [Google Scholar] [CrossRef]
- Chan, C.S.; Guzman, J.N.; Ilijic, E.; Mercer, J.N.; Rick, C.; Tkatch, T.; Meredith, G.E.; Surmeier, D.J. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature 2007, 447, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Hall, D.D.; Hell, J.W. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol. Rev. 2009, 89, 411–452. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Dascal, N. Molecular aspects of modulation of L-type calcium channels by protein kinase C. Curr. Mol. Pharmacol. 2015, 8, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zamponi, G.W. Regulation of voltage gated calcium channels by GPCRs and post-translational modification. Curr. Opin. Pharmacol. 2017, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferron, L.; Guderyan, S.D.; Smith, E.J.; Zamponi, G.W. CaVβ-subunit dependence of forward and reverse trafficking of CaV1.2 calcium channels. Mol. Brain. 2022, 15, 43. [Google Scholar] [CrossRef]
- Felix, R.; Weiss, N. Ubiquitination and proteasome-mediated degradation of voltage-gated Ca2+ channels and potential pathophysiological implications. Gen. Physiol. Biophys. 2017, 36, 1–5. [Google Scholar] [CrossRef]
- Liss, B.; Striessnig, J. The potential of L-Type calcium channels as a drug target for neuroprotective therapy in Parkinson’s Disease. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 263–289. [Google Scholar] [CrossRef]
- Hurley, M.J.; Brandon, B.; Gentleman, S.M.; Dexter, D.T. Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins. Brain 2013, 136, 2077–2097. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.M.; Bartsch, D. The role of L-type voltage-gated calcium channels CaV1.2 and CaV1.3 in normal and pathological brain function. Cell Tissue Res. 2014, 357, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Ilijic, E.; Guzman, J.N.; Surmeier, D.J. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol. Dis. 2011, 43, 364–471. [Google Scholar] [CrossRef] [PubMed]
- Meredith, G.E.; Kang, U.J. Behavioral models of Parkinson’s disease in rodents: A new look at an old problem. Mov. Disord. 2006, 21, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Xu, Y.Y.; Liu, S.; Ma, Z.G. Isradipine attenuates MPTP-induced dopamine neuron degeneration by inhibiting up-regulation of L-type calcium channels and iron accumulation in the substantia nigra of mice. Oncotarget 2017, 8, 47284–47295. [Google Scholar] [CrossRef] [PubMed]
- Putzier, I.; Kullmann, P.H.; Horn, J.P.; Levitan, E.S. CaV1.3 channel voltage dependence, not Ca2+ selectivity, drives pacemaker activity and amplifies bursts in nigral dopamine neurons. J. Neurosci. 2009, 29, 15414–15419. [Google Scholar] [CrossRef] [PubMed]
- Parkinson Study Group STEADY-PD III Investigators. Isradipine versus placebo in early Parkinson disease: A Randomized trial. Ann. Intern. Med. 2020, 172, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lipscombe, D. Neuronal CaV1.3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J. Neurosci. 2001, 21, 5944–5951. [Google Scholar] [CrossRef]
- Gandini, M.A.; Sandoval, A.; Felix, R. Patch-clamp recording of voltage-sensitive Ca2+ channels. Cold Spring Harb. Protoc. 2014, 2014, 329–335. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval, A.; Corzo-López, A.; Duran, P.; Tovar-Soto, D.; Vargas-Caballero, B.; Galicia-Saldaña, V.; González-Ramírez, R.; Felix, R. The Leucine-Rich Repeat Kinase 2 Variant LRRK2G2019S Up-Regulates L-Type (CaV1.3) Calcium Channel via the CaVβ3 Subunit: Possible Role in the Pathogenesis of Parkinson’s Disease. Int. J. Mol. Sci. 2025, 26, 3229. https://doi.org/10.3390/ijms26073229
Sandoval A, Corzo-López A, Duran P, Tovar-Soto D, Vargas-Caballero B, Galicia-Saldaña V, González-Ramírez R, Felix R. The Leucine-Rich Repeat Kinase 2 Variant LRRK2G2019S Up-Regulates L-Type (CaV1.3) Calcium Channel via the CaVβ3 Subunit: Possible Role in the Pathogenesis of Parkinson’s Disease. International Journal of Molecular Sciences. 2025; 26(7):3229. https://doi.org/10.3390/ijms26073229
Chicago/Turabian StyleSandoval, Alejandro, Alejandra Corzo-López, Paz Duran, Diana Tovar-Soto, Bryan Vargas-Caballero, Valeria Galicia-Saldaña, Ricardo González-Ramírez, and Ricardo Felix. 2025. "The Leucine-Rich Repeat Kinase 2 Variant LRRK2G2019S Up-Regulates L-Type (CaV1.3) Calcium Channel via the CaVβ3 Subunit: Possible Role in the Pathogenesis of Parkinson’s Disease" International Journal of Molecular Sciences 26, no. 7: 3229. https://doi.org/10.3390/ijms26073229
APA StyleSandoval, A., Corzo-López, A., Duran, P., Tovar-Soto, D., Vargas-Caballero, B., Galicia-Saldaña, V., González-Ramírez, R., & Felix, R. (2025). The Leucine-Rich Repeat Kinase 2 Variant LRRK2G2019S Up-Regulates L-Type (CaV1.3) Calcium Channel via the CaVβ3 Subunit: Possible Role in the Pathogenesis of Parkinson’s Disease. International Journal of Molecular Sciences, 26(7), 3229. https://doi.org/10.3390/ijms26073229





