Tri-Phenyl-Phosphonium-Based Nano Vesicles: A New In Vitro Nanomolar-Active Weapon to Eradicate PLX-Resistant Melanoma Cells
Abstract
1. Introduction
1.1. Main Causes of CM
1.2. Possible Treatments for Melanoma
1.3. The Study
2. Results and Discussion
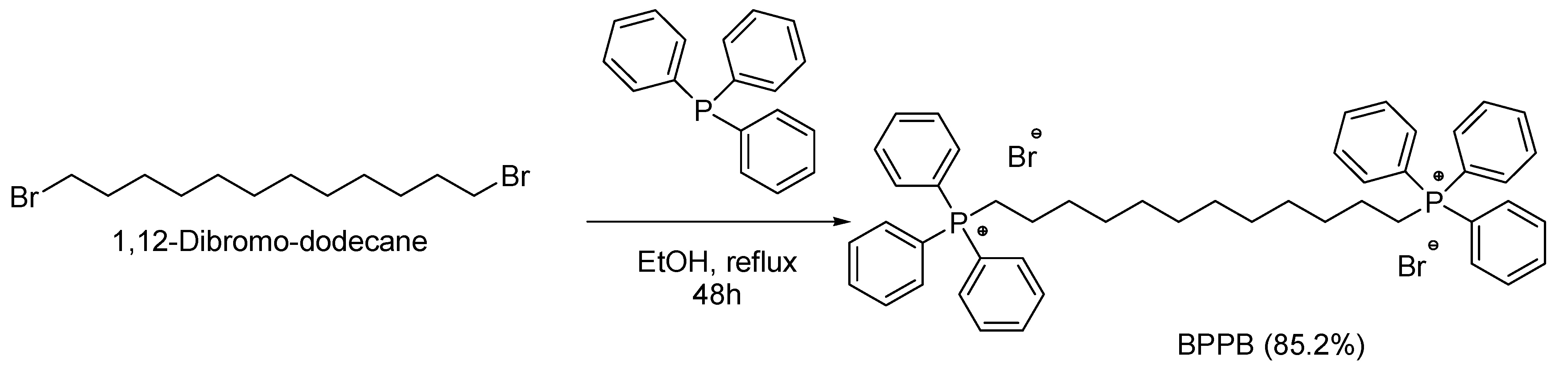
2.1. 1,1-(1,12-Dodecanediyl)bis [1,1,1]-triphenylphosphonium di-bromide (BPPB)
2.2. Biological Effects of BPPB on Tumoral and Not Tumoral Human Cells Models
2.2.1. The Rationale of the Study
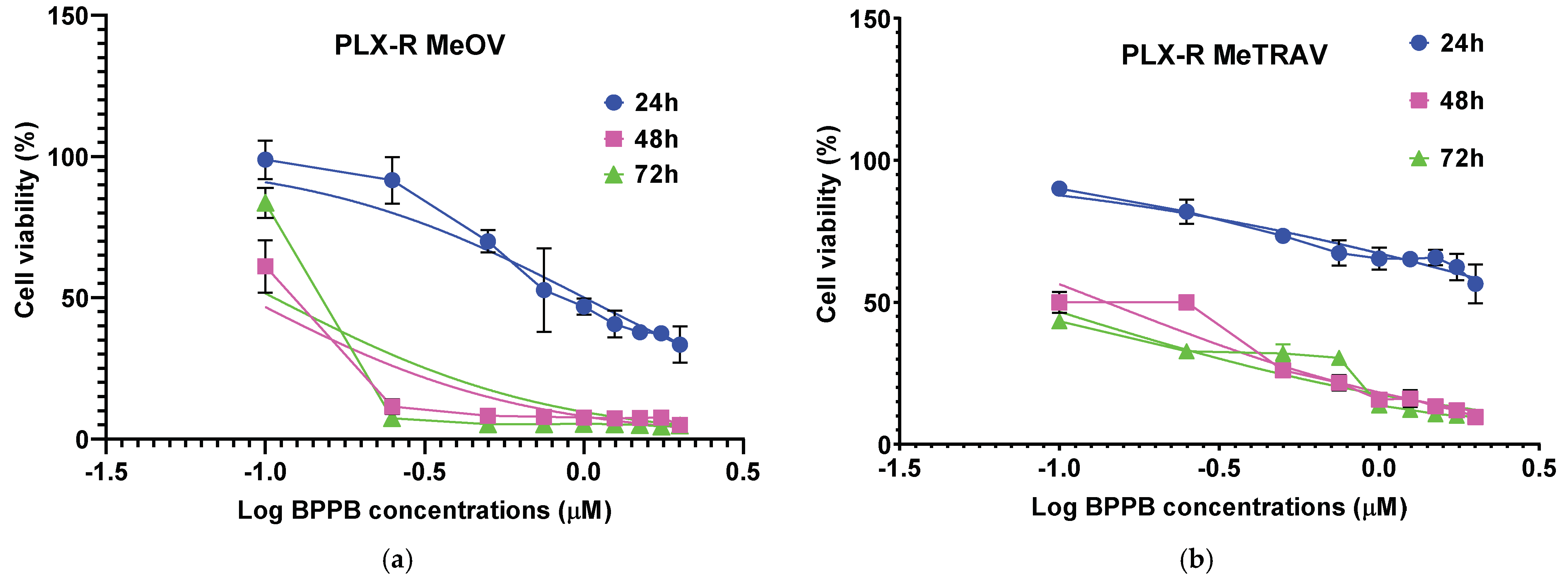
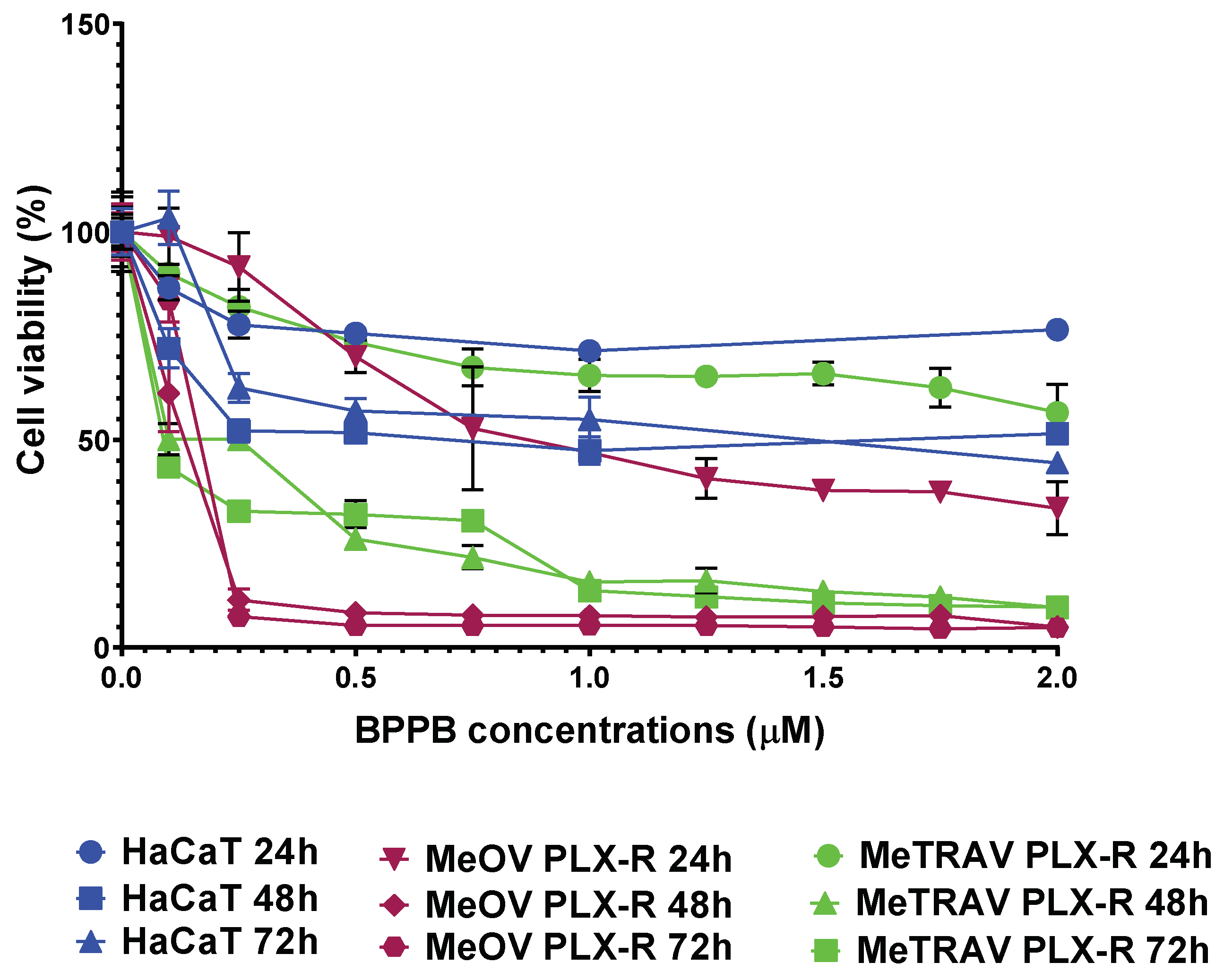
2.2.2. Concentration- and Time-Dependent Effects of BPPB on PLX-R MeOV and MeTRAV Cell Viability
Correlation Between BPPB Cytotoxic Effects and BPPB Concentrations
Correlation Between BPPB Cytotoxic Effects and Time of Exposure
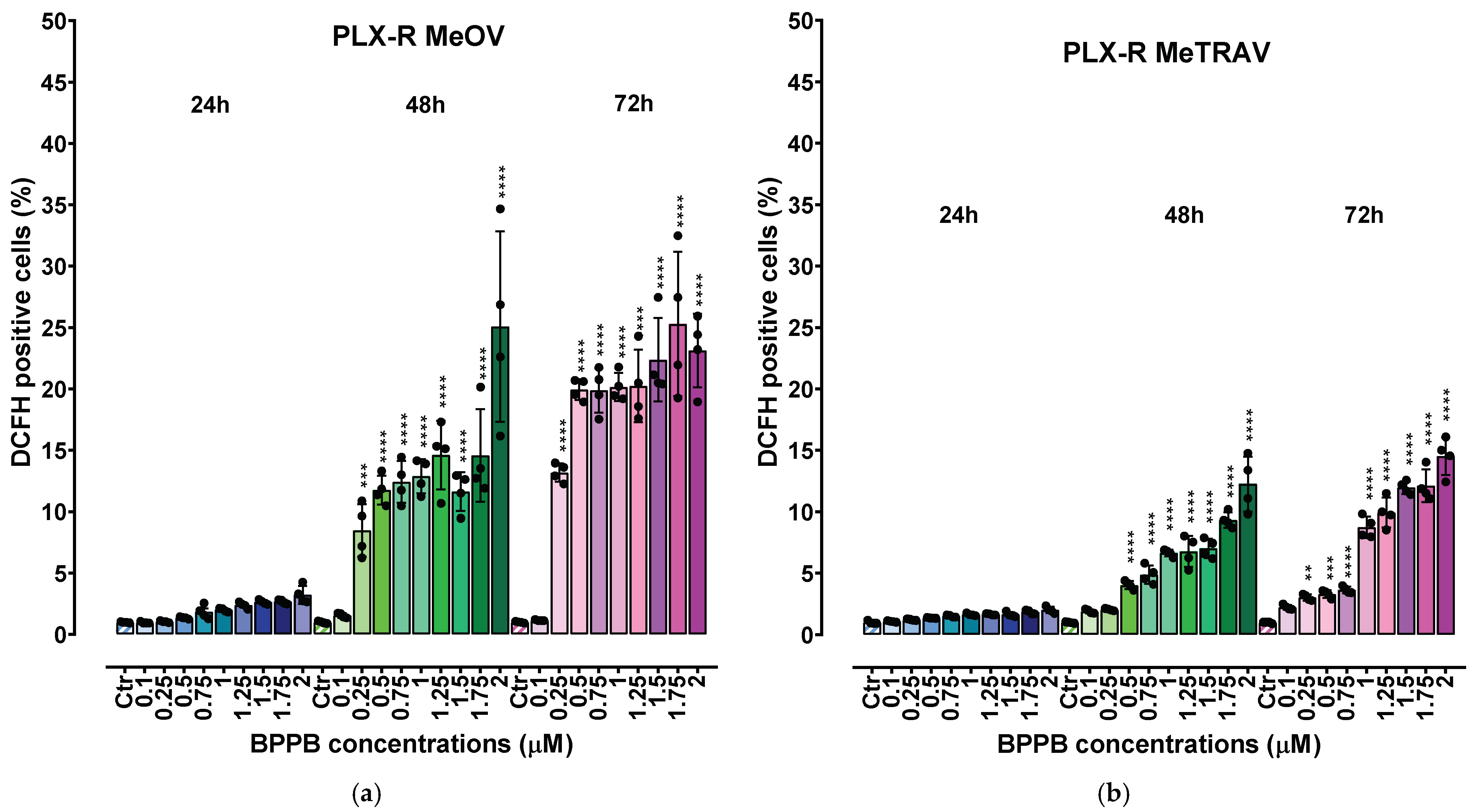
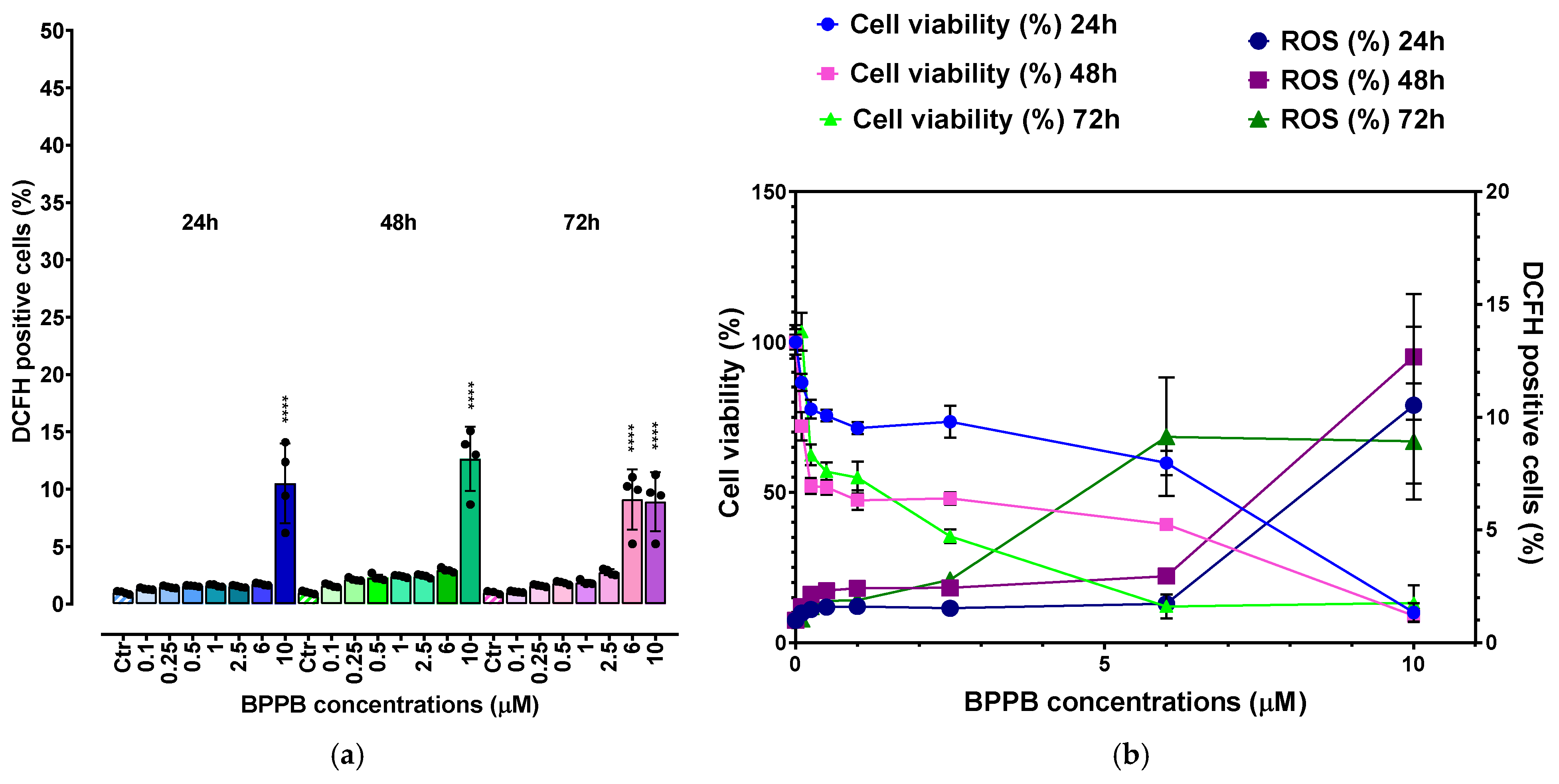
2.2.3. Concentration- and Time-Dependent Effects of BPPB on ROS Production in PLX-R Cells
Correlation Between ROS Production Increase and BPPB Concentrations
Correlation Between ROS Production Increase and Exposure Timing
Correlation Between BPPB Cytotoxic Effects (Cell Viability %) and ROS Overproduction
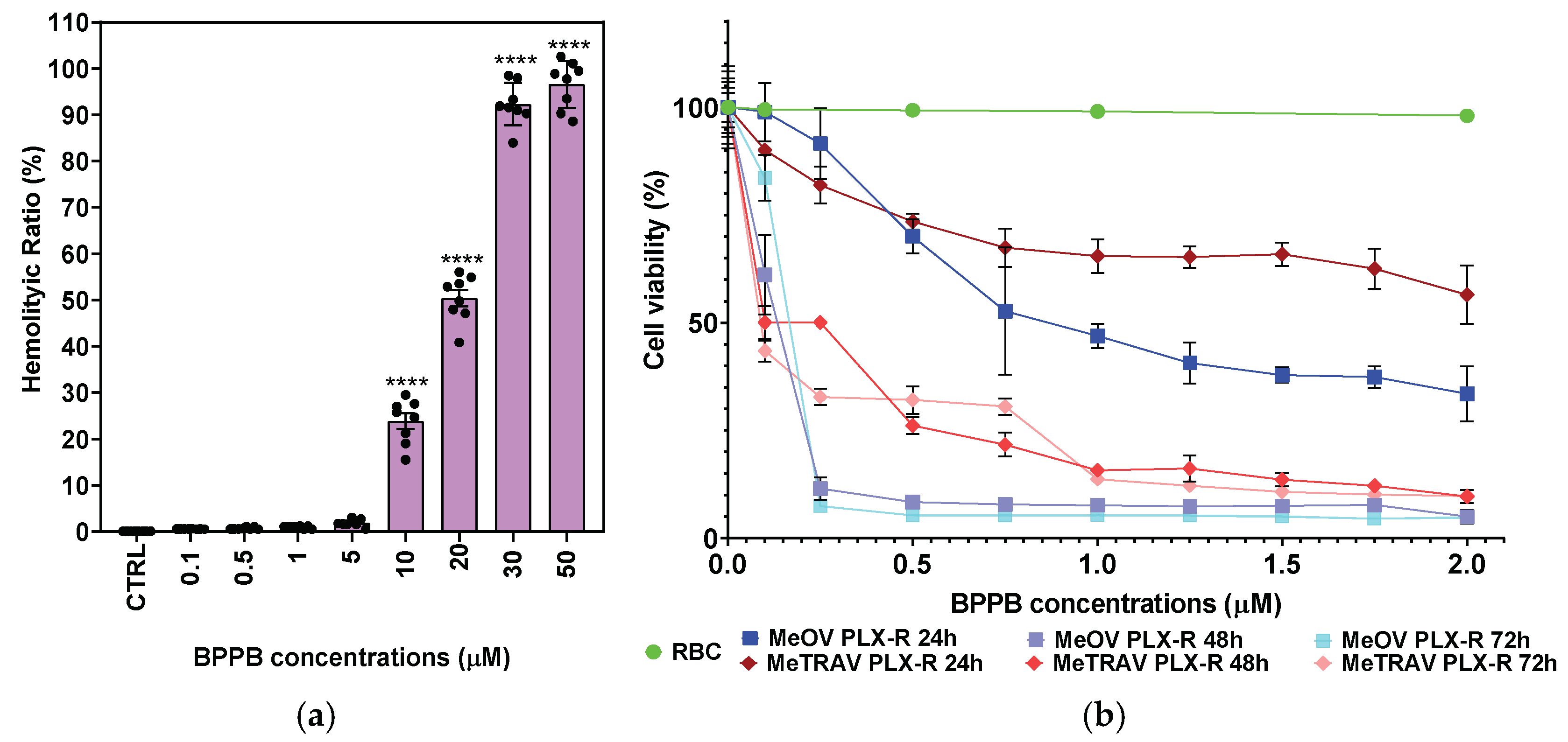
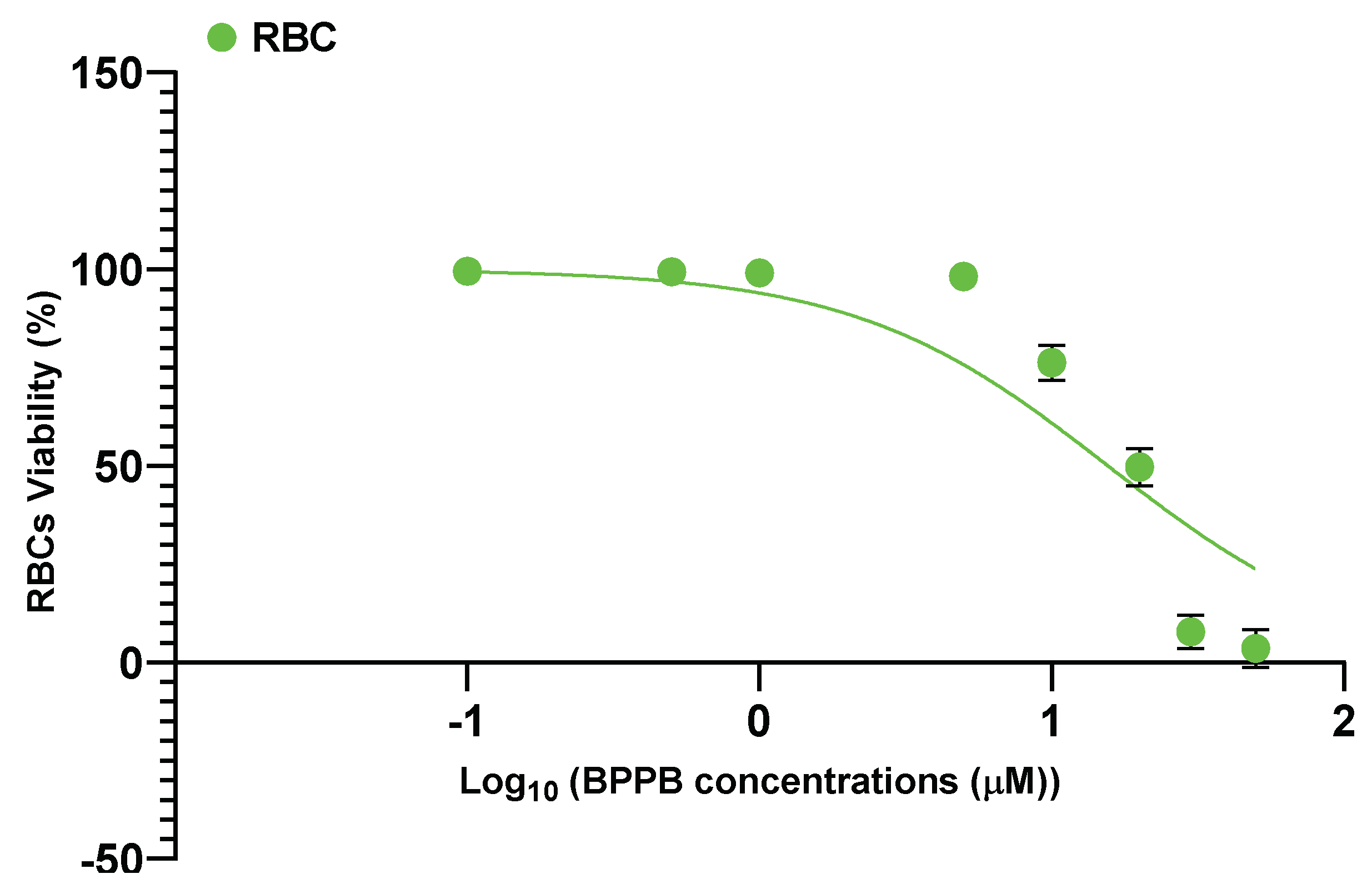
2.2.4. In Vitro Hemolytic Toxicity of BPPB on Red Blood Cells (RBC)
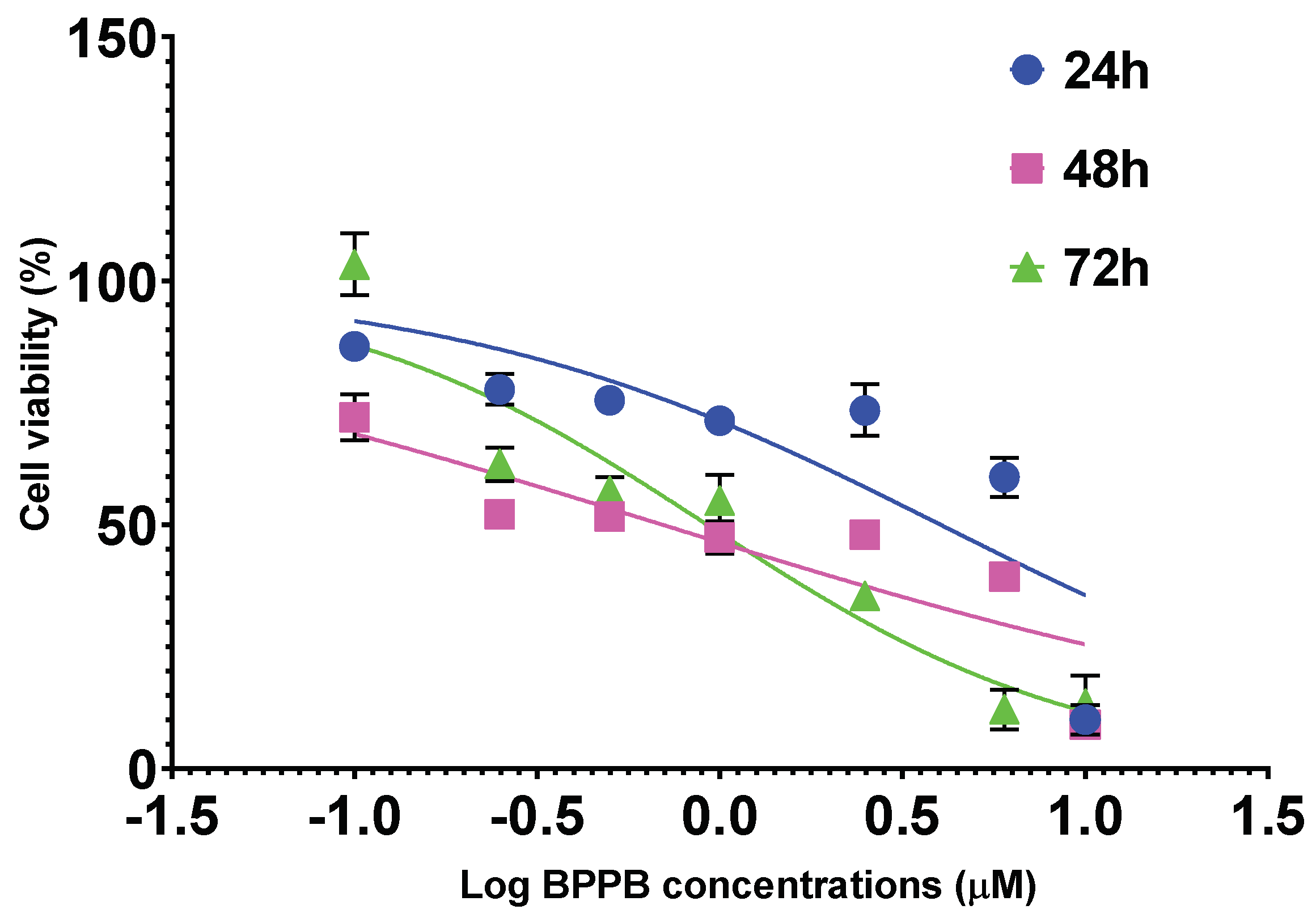
2.2.5. Concentration and Time-Dependent Effects of BPPB on HaCaT Cell Viability
2.2.6. Concentration and Time-Dependent Effects of BPPB on ROS Production by HaCaT Cells
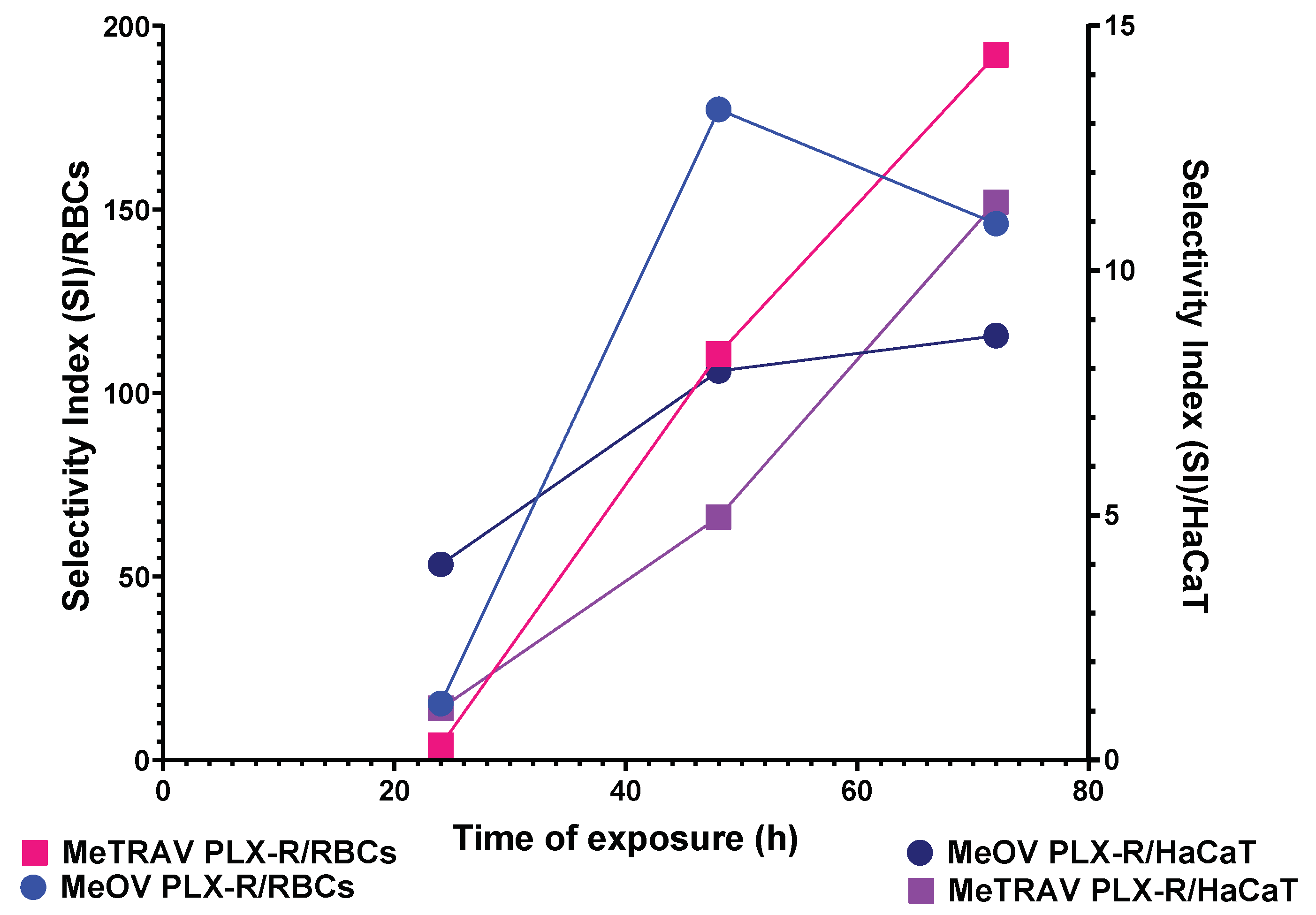
2.2.7. Selectivity Index
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. BPPB Cytotoxicity Evaluation on PLX-Resistant CMM Cells
3.2.1. Cell Lines and Culture Conditions
3.2.2. Treatments
3.2.3. Cell Viability Assay
3.3. In Vitro Hemolytic Toxicity of BPPB Using Red Blood Cells (RBCs)
3.4. Evaluation of Cytotoxicity of BPPB on Human Keratinocites (HaCaT)
3.4.1. Cell Culture
3.4.2. Treatments
3.4.3. Viability Assay
3.5. ROS Production
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drugs in Clinical Development for Melanoma. Pharm. Med. 2012, 26, 171–183. [CrossRef]
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous Melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef]
- Schadendorf, D.; Fisher, D.E.; Garbe, C.; Gershenwald, J.E.; Grob, J.-J.; Halpern, A.; Herlyn, M.; Marchetti, M.A.; McArthur, G.; Ribas, A.; et al. Melanoma. Nat. Rev. Dis. Primers 2015, 1, 15003. [Google Scholar] [CrossRef]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global Cancer Statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/en (accessed on 21 January 2025).
- Bastian, B.C. The Molecular Pathology of Melanoma: An Integrated Taxonomy of Melanocytic Neoplasia. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 239–271. [Google Scholar] [CrossRef]
- Keim, U.; Gandini, S.; Amaral, T.; Katalinic, A.; Holleczek, B.; Flatz, L.; Leiter, U.; Whiteman, D.; Garbe, C. Cutaneous Melanoma Attributable to UVR Exposure in Denmark and Germany. Eur. J. Cancer 2021, 159, 98–104. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and Number of Cancer Cases and Deaths Attributable to Potentially Modifiable Risk Factors in the United States. CA A Cancer J. Clin. 2017, 68, 31–54. [Google Scholar] [CrossRef]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Abeni, D.; Boyle, P.; Melchi, C.F. Meta-Analysis of Risk Factors for Cutaneous Melanoma: I. Common and Atypical Naevi. Eur. J. Cancer 2005, 41, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Bliss, J.M.; Ford, D.; Swerdlow, A.J.; Armstrong, B.K.; Cristofolini, M.; Elwood, J.M.; Green, A.; Holly, E.A.; Mack, T.; Mackie, R.M.; et al. Risk of Cutaneous Melanoma Associated with Pigmentation Characteristics and Freckling: Systematic Overview of 10 Case-control Studies. Int. J. Cancer 1995, 62, 367–376. [Google Scholar] [CrossRef]
- Kubica, A.W.; Brewer, J.D. Melanoma in Immunosuppressed Patients. Mayo Clin. Proc. 2012, 87, 991–1003. [Google Scholar] [CrossRef]
- Bradford, P.T.; Goldstein, A.M.; Tamura, D.; Khan, S.G.; Ueda, T.; Boyle, J.; Oh, K.-S.; Imoto, K.; Inui, H.; Moriwaki, S.-I.; et al. Cancer and Neurologic Degeneration in Xeroderma Pigmentosum: Long Term Follow-up Characterises the Role of DNA Repair. J. Med. Genet. 2010, 48, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Berk-Krauss, J.; Stein, J.A.; Weber, J.; Polsky, D.; Geller, A.C. New Systematic Therapies and Trends in Cutaneous Melanoma Deaths Among US Whites, 1986–2016. Am. J. Public Health 2020, 110, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Erdei, E.; Torres, S.M. A New Understanding in the Epidemiology of Melanoma. Expert Rev. Anticancer Ther. 2010, 10, 1811–1823. [Google Scholar] [CrossRef]
- Leary, M.; Heerboth, S.; Lapinska, K.; Sarkar, S. Sensitization of Drug Resistant Cancer Cells: A Matter of Combination Therapy. Cancers 2018, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Holderfield, M.; Deuker, M.M.; McCormick, F.; McMahon, M. Targeting RAF Kinases for Cancer Therapy: BRAF-Mutated Melanoma and Beyond. Nat. Rev. Cancer 2014, 14, 455–467. [Google Scholar] [CrossRef]
- Haq, R.; Fisher, D.E.; Widlund, H.R. Molecular Pathways: BRAF Induces Bioenergetic Adaptation by Attenuating Oxidative Phosphorylation. Clin. Cancer Res. 2014, 20, 2257–2263. [Google Scholar] [CrossRef]
- Bollag, G.; Hirth, P.; Tsai, J.; Zhang, J.; Ibrahim, P.N.; Cho, H.; Spevak, W.; Zhang, C.; Zhang, Y.; Habets, G.; et al. Clinical Efficacy of a RAF Inhibitor Needs Broad Target Blockade in BRAF-Mutant Melanoma. Nature 2010, 467, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Hallmeyer, S.; Gonzalez, R.; Lawson, D.H.; Cranmer, L.D.; Linette, G.P.; Puzanov, I.; Taback, B.; Cowey, C.L.; Ribas, A.; Daniels, G.A.; et al. Vemurafenib Treatment for Patients with Locally Advanced, Unresectable Stage IIIC or Metastatic Melanoma and Activating Exon 15 BRAF Mutations Other than V600E. Melanoma Res. 2017, 27, 585–590. [Google Scholar] [CrossRef]
- Joseph, R.; Swaika, A.; Crozier, J.A. Vemurafenib: An Evidence-Based Review of Its Clinical Utility in the Treatment of Metastatic Melanoma. Drug Des. Dev. Ther. 2014, 8, 775–787. [Google Scholar] [CrossRef][Green Version]
- Tanda, E.T.; Vanni, I.; Boutros, A.; Andreotti, V.; Bruno, W.; Ghiorzo, P.; Spagnolo, F. Current State of Target Treatment in BRAF Mutated Melanoma. Front. Mol. Biosci. 2020, 7, 154. [Google Scholar] [CrossRef]
- Alfei, S.; Zuccari, G.; Bacchetti, F.; Torazza, C.; Milanese, M.; Siciliano, C.; Athanassopoulos, C.M.; Piatti, G.; Schito, A.M. Synthesized Bis-Triphenyl Phosphonium-Based Nano Vesicles Have Potent and Selective Antibacterial Effects on Several Clinically Relevant Superbugs. Nanomaterials 2024, 14, 1351. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Zuccari, G.; Athanassopoulos, C.M.; Domenicotti, C.; Marengo, B. Strongly ROS-Correlated, Time-Dependent, and Selective Antiproliferative Effects of Synthesized Nano Vesicles on BRAF Mutant Melanoma Cells and Their Hyaluronic Acid-Based Hydrogel Formulation. Int. J. Mol. Sci. 2024, 25, 10071. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Giannoni, P.; Signorello, M.G.; Torazza, C.; Zuccari, G.; Athanassopoulos, C.M.; Domenicotti, C.; Marengo, B. The Remarkable and Selective In Vitro Cytotoxicity of Synthesized Bola-Amphiphilic Nanovesicles on Etoposide-Sensitive and -Resistant Neuroblastoma Cells. Nanomaterials 2024, 14, 1505. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, O.; Valenti, G.E.; Monteleone, L.; Pietra, G.; Mingari, M.C.; Benzi, A.; Bruzzone, S.; Ravera, S.; Leardi, R.; Farinini, E.; et al. PLX4032 Resistance of Patient-Derived Melanoma Cells: Crucial Role of Oxidative Metabolism. Front. Oncol. 2023, 13, 1210130. [Google Scholar] [CrossRef]
- Nuraje, N.; Bai, H.; Su, K. Bolaamphiphilic Molecules: Assembly and Applications. Prog. Polym. Sci. 2013, 38, 302–343. [Google Scholar] [CrossRef]
- Li, K.; Xiao, G.; Richardson, J.J.; Tardy, B.L.; Ejima, H.; Huang, W.; Guo, J.; Liao, X.; Shi, B. Targeted Therapy against Metastatic Melanoma Based on Self-Assembled Metal-Phenolic Nanocomplexes Comprised of Green Tea Catechin. Adv. Sci. 2019, 6, 1801688. [Google Scholar] [CrossRef]
- Heinisch, O. Steel, R. G. D., and J. H. Torrie: Principles and Procedures of Statistics. (With Special Reference to the Biological Sciences.) McGraw-Hill Book Company, New York, Toronto, London 1960, 481 S., 15 Abb.; 81 s 6 d. Biom. Z 1962, 4, 207–208. [Google Scholar] [CrossRef]
- Bobbitt, Z. What Is a Good R-Squared Value? Available online: https://www.statology.org/good-r-squared-value/#:~:text=For%20example%2C%20in%20scientific%20studies%2C%20the%20R-squared%20may,if%20there%20is%20extreme%20variability%20in%20the%20dataset (accessed on 22 January 2025).
- Valutazione Della Bontà Di Adattamento per Modelli Non Lineari: Metodi, Parametri e Considerazioni Pratiche. Available online: https://www.geeksforgeeks.org/evaluating-goodness-of-fit-for-nonlinear-models-methods-metrics-and-practical-considerations/ (accessed on 22 January 2025).
- Ceccacci, F.; Sennato, S.; Rossi, E.; Proroga, R.; Sarti, S.; Diociaiuti, M.; Casciardi, S.; Mussi, V.; Ciogli, A.; Bordi, F.; et al. Aggregation Behaviour of Triphenylphosphonium Bolaamphiphiles. J. Colloid Interface Sci. 2018, 531, 451–462. [Google Scholar] [CrossRef]
- Chandrasekhar, B.; Gor, R.; Ramalingam, S.; Thiagarajan, A.; Sohn, H.; Madhavan, T. Repurposing FDA-Approved Compounds to Target JAK2 for Colon Cancer Treatment. Discov. Oncol. 2024, 15, 1–18. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Ölschläger, V.; Schrader, A.; Hockertz, S. Comparison of Primary Human Fibroblasts and Keratinocytes with Immortalized Cell Lines Regarding Their Sensitivity to Sodium Dodecyl Sulfate in a Neutral Red Uptake Cytotoxicity Assay. Arzneimittelforschung 2009, 59, 146–152. [Google Scholar] [CrossRef]
- Schoop, V.M.; Fusenig, N.E.; Mirancea, N. Epidermal Organization and Differentiation of HaCaT Keratinocytes in Organotypic Coculture with Human Dermal Fibroblasts. J. Investig. Dermatol. 1999, 112, 343–353. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Davoren, M.; Byrne, H.J. In Vitro Mammalian Cytotoxicological Study of PAMAM Dendrimers — Towards Quantitative Structure Activity Relationships. Toxicol. Vitr. 2010, 24, 169–177. [Google Scholar] [CrossRef]
- Krzywik, J.; Mozga, W.; Aminpour, M.; Janczak, J.; Maj, E.; Wietrzyk, J.; Tuszyński, J.A.; Huczyński, A. Synthesis, Antiproliferative Activity and Molecular Docking Studies of Novel Doubly Modified Colchicine Amides and Sulfonamides as Anticancer Agents. Molecules 2020, 25, 1789. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Duan, H.; Tong, X.; Hsu, P.; Han, L.; Morris-Natschke, S.L.; Yang, S.; Liu, W.; Lee, K.-H. Cytotoxicity, Hemolytic Toxicity, and Mechanism of Action of Pulsatilla Saponin D and Its Synthetic Derivatives. J. Nat. Prod. 2017, 81, 465–474. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Kulenty, L.; Załuska-Ogryzek, K.; Góralczyk, A.; Łuszczki, J.J. Screening of the Antimelanoma Activity of Monoterpenes—In Vitro Experiments on Four Human Melanoma Lines. Curr. Issues Mol. Biol. 2025, 47, 97. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants 2020, 9, 50. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef]
- Colla, R.; Izzotti, A.; De Ciucis, C.; Fenoglio, D.; Ravera, S.; Speciale, A.; Ricciarelli, R.; Furfaro, A.L.; Pulliero, A.; Passalacqua, M.; et al. Glutathione-Mediated Antioxidant Response and Aerobic Metabolism: Two Crucial Factors Involved in Determining the Multi-Drug Resistance of High-Risk Neuroblastoma. Oncotarget 2016, 7, 70715–70737. [Google Scholar] [CrossRef]
- Wojtala, A.; Bonora, M.; Malinska, D.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Methods to Monitor ROS Production by Fluorescence Microscopy and Fluorometry. Methods Enzymol. 2014, 542, 243–262. [Google Scholar] [PubMed]


| Exposure Time (Hours) | IC50 MeOV PLX-R (µM) | IC50 MeTRAV PLX-R (µM) |
|---|---|---|
| 24 | 1.0060 ± 0.1253 | N.D. |
| 48 | 0.0878 ± 0.0187 | 0.1406 ± 0.0271 |
| 72 | 0.1065 ± 0.0343 | 0.0809 ± 0.0268 |
| Cells | IC50 24 h (µM) | IC50 48 h (µM) | IC50 72 h (µM) | HC50 Experiment Time (µM) |
|---|---|---|---|---|
| RBCs | N.A.Q. | N.A.Q. | N.A.Q. | 15.56 ± 12.13 |
| PLX-R MeOV | 1.0060 ± 0.1253 | 0.0878 ± 0.0187 | 0.1065 ± 0.0343 | N.A. |
| PLX-R MeTRAV | 3.7970 ± 1.0880 | 0.1406 ± 0.0271 | 0.0810 ± 0.0268 | N.A. |
| HaCaT | 4.0210 ± 2.1590 | 0.6981 ± 0.3030 | 0.9238 ± 0.2487 | N.A. |
| Cells | SI 24 h a | SI 48 h a | SI 72 h a |
|---|---|---|---|
| RBCs * | 15.46 | 177.22 | 146.10 |
| RBCs ** | 4.10 | 110.67 | 192.10 |
| HaCaT * | 4.00 | 7.95 | 8.67 |
| HaCaT ** | 1.06 | 4.97 | 11.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S.; Torazza, C.; Bacchetti, F.; Signorello, M.G.; Passalacqua, M.; Domenicotti, C.; Marengo, B. Tri-Phenyl-Phosphonium-Based Nano Vesicles: A New In Vitro Nanomolar-Active Weapon to Eradicate PLX-Resistant Melanoma Cells. Int. J. Mol. Sci. 2025, 26, 3227. https://doi.org/10.3390/ijms26073227
Alfei S, Torazza C, Bacchetti F, Signorello MG, Passalacqua M, Domenicotti C, Marengo B. Tri-Phenyl-Phosphonium-Based Nano Vesicles: A New In Vitro Nanomolar-Active Weapon to Eradicate PLX-Resistant Melanoma Cells. International Journal of Molecular Sciences. 2025; 26(7):3227. https://doi.org/10.3390/ijms26073227
Chicago/Turabian StyleAlfei, Silvana, Carola Torazza, Francesca Bacchetti, Maria Grazia Signorello, Mario Passalacqua, Cinzia Domenicotti, and Barbara Marengo. 2025. "Tri-Phenyl-Phosphonium-Based Nano Vesicles: A New In Vitro Nanomolar-Active Weapon to Eradicate PLX-Resistant Melanoma Cells" International Journal of Molecular Sciences 26, no. 7: 3227. https://doi.org/10.3390/ijms26073227
APA StyleAlfei, S., Torazza, C., Bacchetti, F., Signorello, M. G., Passalacqua, M., Domenicotti, C., & Marengo, B. (2025). Tri-Phenyl-Phosphonium-Based Nano Vesicles: A New In Vitro Nanomolar-Active Weapon to Eradicate PLX-Resistant Melanoma Cells. International Journal of Molecular Sciences, 26(7), 3227. https://doi.org/10.3390/ijms26073227










