Role of Mitochondrial Dysfunction in Neuropathy
Abstract
1. Introduction
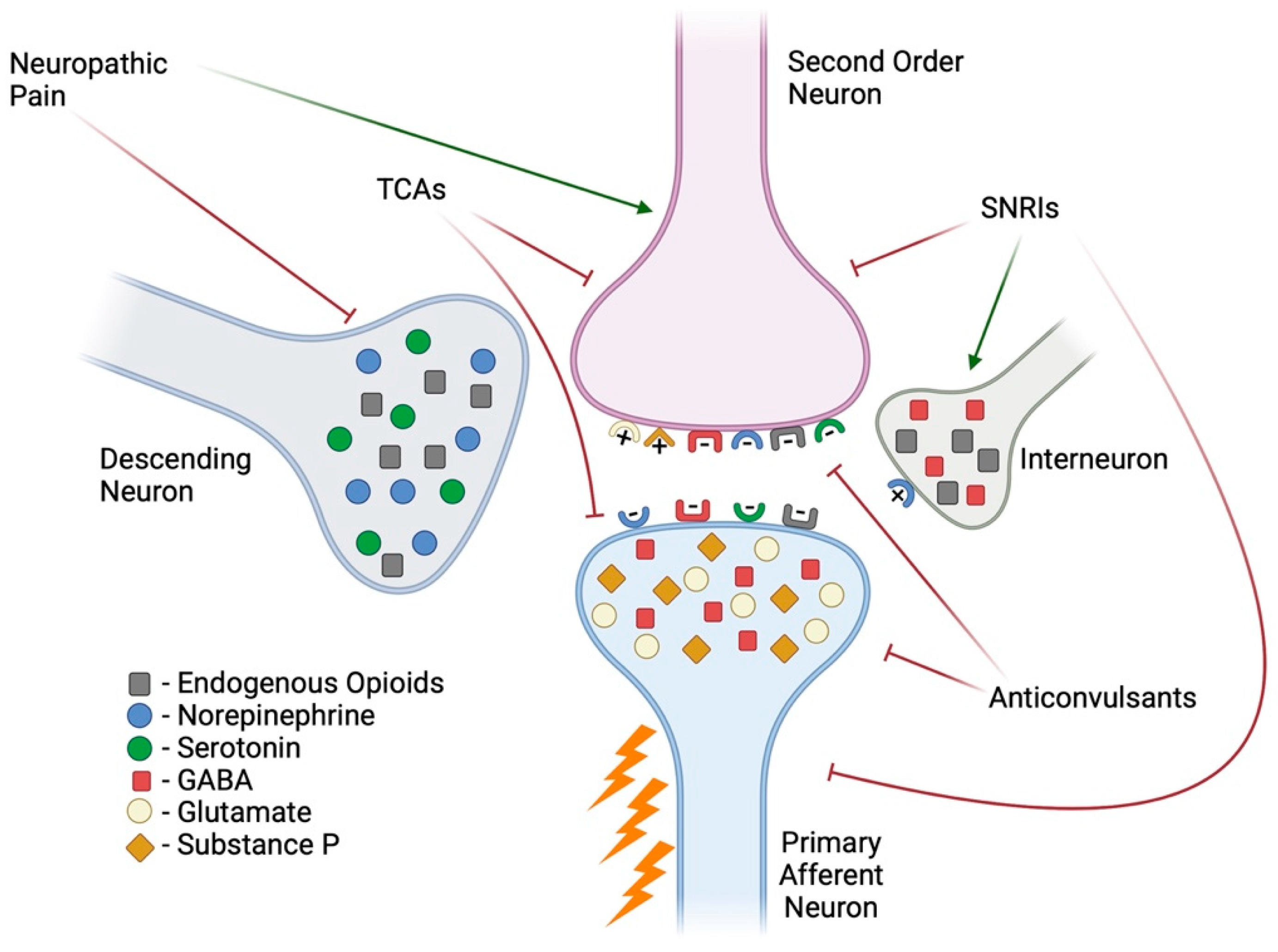
2. Neuropathic Pain
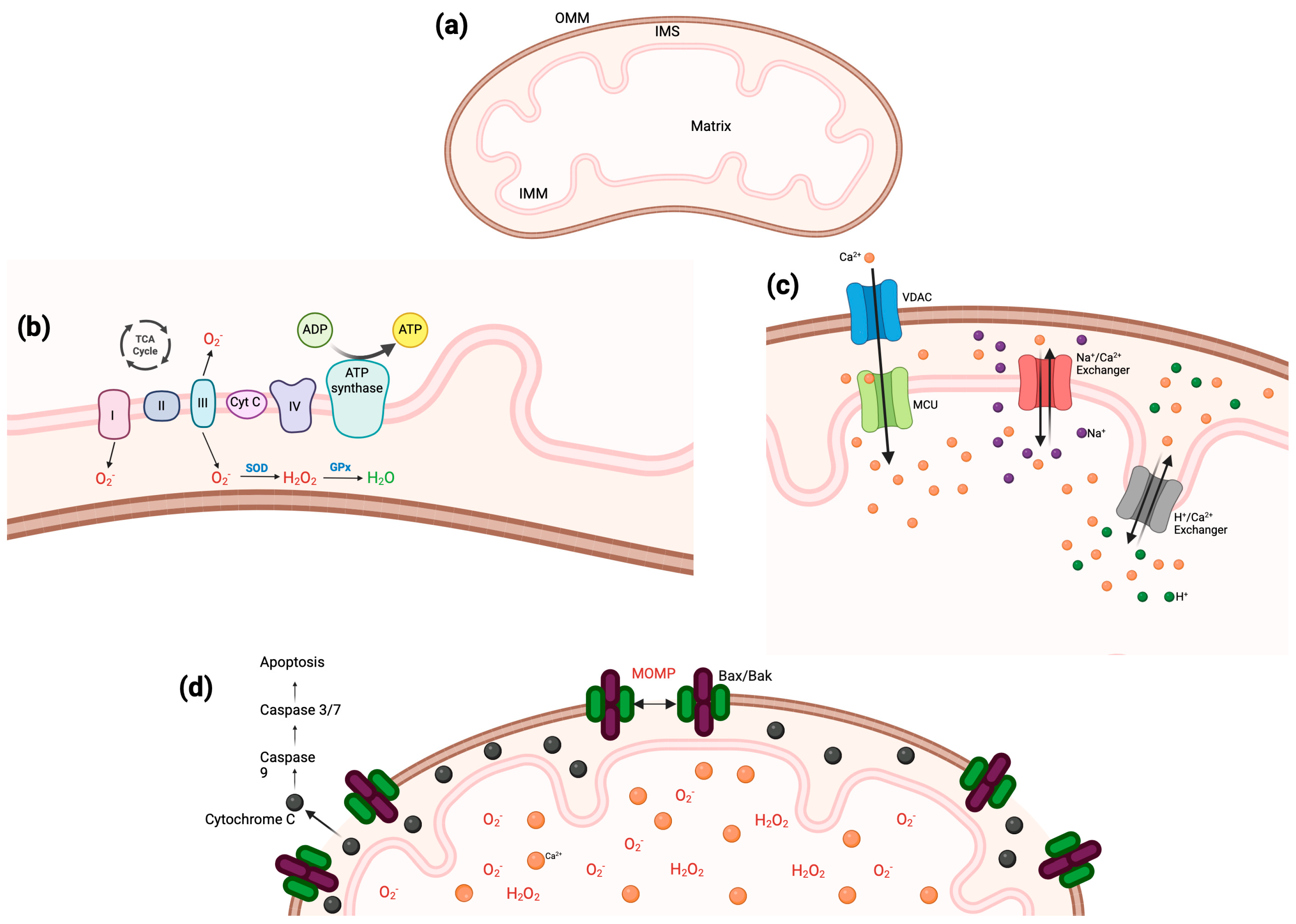
3. Importance of the Mitochondria
3.1. Electron Trasport Chain
3.2. Reactive Oxygen Species
3.3. Calcium Homeostasis
3.4. Apoptosis
4. Mitochondrial Dysfunction and DPN
4.1. ETC and DPN
4.2. ROS and DPN
4.3. Calcium and DPN
| Mitochondrial Dysfunction | Consequence | Reversible? | Available Treatment? |
|---|---|---|---|
| Deficient ETC | Increased hypersensitivity, reduced ETC complex activity and protein levels, high levels of ROS and inflammation [96,97] | Yes, to some extent | None |
| ROS Overload | Sciatic nerve lesions, increased pain sensitivity, deformations in sciatic nerve, slower motor and sensory nerve conduction, decreased blood flow to sciatic nerve, increased apoptosis rate [86,98,99] | Yes, to some extent | None Studies evaluating the effect of potential antioxidants are under way [100] |
| Disrupted Calcium Homeostasis | Lower threshold for neuron excitation, increased apoptosis rate, Schwann cell demyelination, motor deficits, hypersensitivity, increased T-type channel density [101,102,103] | Yes, to some extent | None Calcium infusions were studied, but no promising results [104] Vitamin D also studied, but no results shared [105] |
| Dysregulated Apoptosis | Reduced activity of NGF/Akt, GSK3β signaling pathway, increased levels of BAX, decreased BCL2 levels, axonal shrinkage, neuron degeneration, increased hypersensitivity [106,107] | Yes, to some extent | None |
4.4. Apoptosis and DPN
5. Conclusions
Funding
Conflicts of Interest
References
- Rikard, S.M.; Strahan, A.E.; Schmit, K.M.; Guy, G.P. Chronic Pain Among Adults—United States, 2019–2021; MMWR and Morbidity and Mortality Weekly Report; CDC: Atlanta, GA, USA, 2023; pp. 379–385.
- Armstrong, S.A.; Herr, M.J. Physiology, Nociception; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Willemen, H.L.D.M.; Santos Ribeiro, P.S.; Broeks, M.; Meijer, N.; Versteeg, S.; Tiggeler, A.; de Boer, T.P.; Małecki, J.M.; Falnes, P.Ø.; Jans, J.; et al. Inflammation-induced mitochondrial and metabolic disturbances in sensory neurons control the switch from acute to chronic pain. Cell Rep. Med. 2023, 4, 101265. [Google Scholar] [CrossRef]
- Zeilhofer, H.U.; Wildner, H.; Yévenes, G.E. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev. 2012, 92, 193–235. [Google Scholar] [PubMed]
- Zang, Y.; Jiang, D.; Zhuang, X.; Chen, S. Changes in the central nervous system in diabetic neuropathy. Heliyon 2023, 9, e18368. [Google Scholar] [PubMed]
- Tender, G.C.; Li, Y.Y.; Cui, J.G. Brain-derived neurotrophic factor redistribution in the dorsal root ganglia correlates with neuropathic pain inhibition after resiniferatoxin treatment. Spine J. 2010, 10, 715–720. [Google Scholar]
- Clark, A.K.; Malcangio, M. Fractalkine/CX3CR1 signaling during neuropathic pain. Front. Cell. Neurosci. 2014, 8, 121. [Google Scholar]
- Fischer, T.Z.; Waxman, S.G. Neuropathic pain in diabetes—Evidence for a central mechanism. Nat. Rev. Neurol. 2010, 6, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef]
- Pontchartrain Orthopedics & Sports Medicine. What is Neuropathic Pain? 2025. Available online: https://posm.org/types-of-neuropathic-pain-and-how-its-treated/ (accessed on 28 January 2025).
- Staff, N.P.; AGrisold, G.W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef]
- Colvin, L.A. Chemotherapy-induced peripheral neuropathy: Where are we now? Pain 2019, 160 (Suppl. S1), S1–S10. [Google Scholar]
- Lemanska, A.; Harkin, A.; Iveson, T.; Kelly, C.; Saunders, M.; Faithfull, S. The association of clinical and patient factors with chemotherapy-induced peripheral neuropathy (CIPN) in colorectal cancer: Secondary analysis of the SCOT trial. ESMO Open 2023, 8, 102063. [Google Scholar]
- Yang, H.; Sloan, G.; Ye, Y.; Wang, S.; Duan, B.; Tesfaye, S.; Gao, L. New Perspective in Diabetic Neuropathy: From the Periphery to the Brain, a Call for Early Detection, and Precision Medicine. Front. Endocrinol. 2019, 10, 929. [Google Scholar]
- American Diabetes Association Professional Practice Committee. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [PubMed]
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diabetes Rep. 2019, 19, 86. [Google Scholar] [CrossRef]
- Ziegler, D.; Fonseca, V. From guideline to patient: A review of recent recommendations for pharmacotherapy of painful diabetic neuropathy. J. Diabetes Complicat. 2015, 29, 146–156. [Google Scholar]
- Jang, H.N.; Oh, T.J. Pharmacological and Nonpharmacological Treatments for Painful Diabetic Peripheral Neuropathy. Diabetes Metab. J. 2023, 47, 743–756. [Google Scholar]
- Sloan, G.; Selvarajah, D.; Tesfaye, S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 2021, 17, 400–420. [Google Scholar]
- Monzel, A.S.; Enríquez, J.A.; Picard, M. Multifaceted mitochondria: Moving mitochondrial science beyond function and dysfunction. Nat. Metab. 2023, 5, 546–562. [Google Scholar]
- Benaroya, H. Mitochondria and MICOS—function and modeling. Rev. Neurosci. 2024, 35, 503–531. [Google Scholar]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar]
- Gatliff, J.; Campanella, M. TSPO: Kaleidoscopic 18-kDa amid biochemical pharmacology, control and targeting of mitochondria. Biochem. J. 2016, 473, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R. Translocator protein (18kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar]
- Gatliff, J.; East, D.; Crosby, J.; Abeti, R.; Harvey, R.; Craigen, W.; Parker, P.; Campanella, M. TSPO interacts with VDAC1 and triggers a ROS-mediated inhibition of mitochondrial quality control. Autophagy 2014, 10, 2279–2296. [Google Scholar] [PubMed]
- Gatliff, J.; East, D.A.; Singh, A.; Alvarez, M.S.; Frison, M.; Matic, I.; Ferraina, C.; Sampson, N.; Turkheimer, F.; Campanella, M. A role for TSPO in mitochondrial Ca. Cell Death Dis. 2017, 8, e2896. [Google Scholar]
- Batarseh, A.; Papadopoulos, V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol. Cell. Endocrinol. 2010, 327, 1–12. [Google Scholar]
- Nutma, E.; Ceyzériat, K.; Amor, S.; Tsartsalis, S.; Millet, P.; Owen, D.R.; Papadopoulos, V.; Tournier, B.B. Cellular sources of TSPO expression in healthy and diseased brain. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 146–163. [Google Scholar] [PubMed]
- Guilarte, T.R. TSPO in diverse CNS pathologies and psychiatric disease: A critical review and a way forward. Pharmacol. Ther. 2019, 194, 44–58. [Google Scholar] [CrossRef]
- El Chemali, L.; Boutary, S.; Liu, S.; Liu, G.J.; Middleton, R.J.; Banati, R.B.; Bahrenberg, G.; Rupprecht, R.; Schumacher, M.; Massaad-Massade, L. GRT-X Stimulates Dorsal Root Ganglia Axonal Growth in Culture via TSPO and Kv7.2/3 Potassium Channel Activation. Int. J. Mol. Sci. 2024, 25, 7327. [Google Scholar] [CrossRef]
- Biswas, L.; Farhan, F.; Reilly, J.; Bartholomew, C.; Shu, X. TSPO Ligands Promote Cholesterol Efflux and Suppress Oxidative Stress and Inflammation in Choroidal Endothelial Cells. Int. J. Mol. Sci. 2018, 19, 3740. [Google Scholar] [CrossRef]
- Cooper, G.M. The Cell: A Molecular Approach; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial fission and fusion: A dynamic role in aging and potential target for age-related disease. Mech. Ageing Dev. 2020, 186, 111212. [Google Scholar]
- Cogliati, S.; Cabrera-Alarcón, J.L.; Enriquez, J.A. Regulation and functional role of the electron transport chain supercomplexes. Biochem. Soc. Trans. 2021, 49, 2655–2668. [Google Scholar] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell: The Mitochondrion; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Garza, S.; Chen, L.; Galano, M.; Cheung, G.; Sottas, C.; Li, L.; Li, Y.; Zirkin, B.R.; Papadopoulos, V. Mitochondrial dynamics, Leydig cell function, and age-related testosterone deficiency. FASEB J. 2022, 36, e22637. [Google Scholar]
- Mendelsohn, R.; Garcia, G.C.; Bartol, T.M.; Lee, C.T.; Khandelwal, P.; Liu, E.; Spencer, D.J.; Husar, A.; Bushong, E.A.; Phan, S.; et al. Morphological principles of neuronal mitochondria. J. Comp. Neurol. 2022, 530, 886–902. [Google Scholar]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Azad, M.B.; Gibson, S.B. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009, 16, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Springer, M.Z.; Macleod, K.F. In Brief: Mitophagy: Mechanisms and role in human disease. J. Pathol. 2016, 240, 253–255. [Google Scholar]
- Hushmandi, K.; Einollahi, B.; Aow, R.; Suhairi, S.B.; Klionsky, D.J.; Aref, A.R.; Reiter, R.J.; Makvandi, P.; Rabiee, N.; Xu, Y.; et al. Investigating the interplay between mitophagy and diabetic neuropathy: Uncovering the hidden secrets of the disease pathology. Pharmacol. Res. 2024, 208, 107394. [Google Scholar]
- Lennicke, C.; Cochemé, H.M. Redox regulation of the insulin signalling pathway. Redox Biol. 2021, 42, 101964. [Google Scholar]
- Movafagh, S.; Crook, S.; Vo, K. Regulation of hypoxia-inducible factor-1a by reactive oxygen species: New developments in an old debate. J. Cell. Biochem. 2015, 116, 696–703. [Google Scholar] [CrossRef]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell. Res. 2011, 21, 103–115. [Google Scholar]
- Oswald, M.C.W.; Garnham, N.; Sweeney, S.T.; Landgraf, M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018, 592, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Alexander, K.; Francis, M.M. Reactive Oxygen Species: Angels and Demons in the Life of a Neuron. NeuroSci 2022, 3, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Hameed, L.S.; Berg, D.A.; Belnoue, L.; Jensen, L.D.; Cao, Y.; Simon, A. Environmental changes in oxygen tension reveal ROS-dependent neurogenesis and regeneration in the adult newt brain. eLife 2015, 4, e08422. [Google Scholar] [PubMed]
- Dickinson, B.C.; Peltier, J.; Stone, D.; Schaffer, D.V.; Chang, C.J. Nox2 redox signaling maintains essential cell populations in the brain. Nat. Chem. Biol. 2011, 7, 106–112. [Google Scholar] [CrossRef]
- Wilson, C.; Núñez, M.T.; González-Billault, C. Contribution of NADPH oxidase to the establishment of hippocampal neuronal polarity in culture. J. Cell. Sci. 2015, 128, 2989–2995. [Google Scholar]
- Klann, E.; Roberson, E.D.; Knapp, L.T.; Sweatt, J.D. A role for superoxide in protein kinase C activation and induction of long-term potentiation. J. Biol. Chem. 1998, 273, 4516–4522. [Google Scholar]
- Romero-Garcia, S.; Prado-Garcia, H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer (Review). Int. J. Oncol. 2019, 54, 1155–1167. [Google Scholar] [CrossRef]
- Báthori, G.; Csordás, G.; Garcia-Perez, C.; Davies, E.; Hajnóczky, G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC). J. Biol. Chem. 2006, 281, 17347–17358. [Google Scholar]
- Matuz-Mares, D.; González-Andrade, M.; Araiza-Villanueva, M.G.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Mitochondrial Calcium: Effects of Its Imbalance in Disease. Antioxidants 2022, 11, 801. [Google Scholar] [CrossRef]
- Ge, W.D.; Du, T.T.; Wang, C.Y.; Sun, L.N.; Wang, Y.Q. Calcium signaling crosstalk between the endoplasmic reticulum and mitochondria, a new drug development strategies of kidney diseases. Biochem. Pharmacol. 2024, 225, 116278. [Google Scholar]
- Issop, L.; Fan, J.; Lee, S.; Rone, M.B.; Basu, K.; Mui, J.; Papadopoulos, V. Mitochondria-associated membrane formation in hormone-stimulated Leydig cell steroidogenesis: Role of ATAD3. Endocrinology 2015, 156, 334–345. [Google Scholar]
- Shoshan-Barmatz, V.; Pittala, S.; Mizrachi, D. VDAC1 and the TSPO: Expression, Interactions, and Associated Functions in Health and Disease States. Int. J. Mol. Sci. 2019, 20, 3348. [Google Scholar] [CrossRef] [PubMed]
- Territo, P.R.; Mootha, V.K.; French, S.A.; Balaban, R.S. Ca2+ activation of heart mitochondrial oxidative phosphorylation: Role of the F(0)/F(1)-ATPase. Am. J. Physiol. Cell. Physiol. 2000, 278, C423–C435. [Google Scholar] [PubMed]
- Han, X.J.; Lu, Y.F.; Li, S.A.; Kaitsuka, T.; Sato, Y.; Tomizawa, K.; Nairn, A.C.; Takei, K.; Matsui, H.; Matsushita, M. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J. Cell. Biol. 2008, 182, 573–585. [Google Scholar]
- Kremneva, E.; Kislin, M.; Kang, X.; Khiroug, L. Motility of astrocytic mitochondria is arrested by Ca2+-dependent interaction between mitochondria and actin filaments. Cell Calcium 2013, 53, 85–93. [Google Scholar] [PubMed]
- Gandhi, S.; Wood-Kaczmar, A.; Yao, Z.; Plun-Favreau, H.; Deas, E.; Klupsch, K.; Downward, J.; Latchman, D.S.; Tabrizi, S.J.; Wood, N.W.; et al. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol. Cell. 2009, 33, 627–638. [Google Scholar]
- Zhang, I.X.; Herrmann, A.; Leon, J.; Jeyarajan, S.; Arunagiri, A.; Arvan, P.; Gilon, P.; Satin, L.S. ER stress increases expression of intracellular calcium channel RyR1 to modify Ca. J. Biol. Chem. 2023, 299, 105065. [Google Scholar] [CrossRef]
- Jeyarajan, S.; Zhang, I.X.; Arvan, P.; Lentz, S.I.; Satin, L.S. Simultaneous Measurement of Changes in Mitochondrial and Endoplasmic Reticulum Free Calcium in Pancreatic Beta Cells. Biosensors 2023, 13, 382. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Bouchier-Hayes, L.; Green, D.R. Mitochondrial outer membrane permeabilization during apoptosis: The innocent bystander scenario. Cell Death Differ. 2006, 13, 1396–1402. [Google Scholar] [CrossRef]
- Moon, D.O. Calcium’s Role in Orchestrating Cancer Apoptosis: Mitochondrial-Centric Perspective. Int. J. Mol. Sci. 2023, 24, 8982. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Wei, S.; Nguyen, T.H.; Jo, Y.; Zhang, Y.; Park, W.; Gariani, K.; Oh, C.M.; Kim, H.H.; Ha, K.T.; et al. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease. Exp. Mol. Med. 2023, 55, 1595–1619. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell. Biol. 2020, 21, 85–100. [Google Scholar]
- Benítez-Guzmán, A.; Arriaga-Pizano, L.; Morán, J.; Gutiérrez-Pabello, J.A. Endonuclease G takes part in AIF-mediated caspase-independent apoptosis in Mycobacterium bovis-infected bovine macrophages. Vet Res. 2018, 49, 69. [Google Scholar] [CrossRef] [PubMed]
- Barohn, R.J.; Gajewski, B.; Pasnoor, M.; Brown, A.; Herbelin, L.L.; Kimminau, K.S.; Mudaranthakam, D.P.; Jawdat, O.; Dimachkie, M.M.; Patient Assisted Intervention for Neuropathy: Comparison of Treatment in Real Life Situations (PAIN-CONTRoLS) Study Team; et al. Patient Assisted Intervention for Neuropathy: Comparison of Treatment in Real Life Situations (PAIN-CONTRoLS): Bayesian Adaptive Comparative Effectiveness Randomized Trial. JAMA Neurol. 2021, 78, 68–76. [Google Scholar] [CrossRef]
- Smith, E.M.; Pang, H.; Cirrincione, C.; Fleishman, S.; Paskett, E.D.; Ahles, T.; Bressler, L.R.; Fadul, C.E.; Knox, C.; Le-Lindqwister, N.; et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: A randomized clinical trial. JAMA 2013, 309, 1359–1367. [Google Scholar] [CrossRef]
- Schreiber, A.K.; Nones, C.F.; Reis, R.C.; Chichorro, J.G.; Cunha, J.M. Diabetic neuropathic pain: Physiopathology and treatment. World J. Diabetes 2015, 6, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins Medicine. Diabetic Neuropathy. 2025. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/diabetes/diabetic-neuropathy-nerve-problems#:~:text=Diabetic%20Polyneuropathy,feet%20—%20are%20affected%20the%20most (accessed on 30 January 2025).
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Chowdhury, S.K.; Zherebitskaya, E.; Smith, D.R.; Akude, E.; Chattopadhyay, S.; Jolivalt, C.G.; Calcutt, N.A.; Fernyhough, P. Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment. Diabetes 2010, 59, 1082–1091. [Google Scholar] [CrossRef]
- Kalvala, A.K.; Kumar, R.; Sherkhane, B.; Gundu, C.; Arruri, V.K.; Kumar, A. Bardoxolone Methyl Ameliorates Hyperglycemia Induced Mitochondrial Dysfunction by Activating the keap1-Nrf2-ARE Pathway in Experimental Diabetic Neuropathy. Mol. Neurobiol. 2020, 57, 3616–3631. [Google Scholar] [CrossRef]
- Berti-Mattera, L.N.; Larkin, B.; Hourmouzis, Z.; Kern, T.S.; Siegel, R.E. NF-κB subunits are differentially distributed in cells of lumbar dorsal root ganglia in naïve and diabetic rats. Neurosci. Lett. 2011, 490, 41–45. [Google Scholar] [PubMed]
- Saleh, A.; Roy Chowdhury, S.K.; Smith, D.R.; Balakrishnan, S.; Tessler, L.; Martens, C.; Morrow, D.; Schartner, E.; Frizzi, K.E.; Calcutt, N.A.; et al. Ciliary neurotrophic factor activates NF-κB to enhance mitochondrial bioenergetics and prevent neuropathy in sensory neurons of streptozotocin-induced diabetic rodents. Neuropharmacology 2013, 65, 65–73. [Google Scholar]
- Sleeman, M.W.; Anderson, K.D.; Lambert, P.D.; Yancopoulos, G.D.; Wiegand, S.J. The ciliary neurotrophic factor and its receptor, CNTFR alpha. Pharm. Acta Helv. 2000, 74, 265–272. [Google Scholar]
- Aghanoori, M.R.; Aghanoori, M.R.; Agarwal, P.; Gauvin, E.; Nagalingam, R.S.; Bonomo, R.; Yathindranath, V.; Smith, D.R.; Hai, Y.; Lee, S.; et al. CEBPβ regulation of endogenous IGF-1 in adult sensory neurons can be mobilized to overcome diabetes-induced deficits in bioenergetics and axonal outgrowth. Cell. Mol. Life Sci. 2022, 79, 193. [Google Scholar]
- Chen, X.; Shen, W.B.; Yang, P.; Dong, D.; Sun, W.; Yang, P. High Glucose Inhibits Neural Stem Cell Differentiation Through Oxidative Stress and Endoplasmic Reticulum Stress. Stem Cells Dev. 2018, 27, 745–755. [Google Scholar]
- Oyenihi, A.B.; Ayeleso, A.O.; Mukwevho, E.; Masola, B. Antioxidant strategies in the management of diabetic neuropathy. Biomed. Res. Int. 2015, 2015, 515042. [Google Scholar]
- Zhang, Q.; Song, W.; Zhao, B.; Xie, J.; Sun, Q.; Shi, X.; Yan, B.; Tian, G.; Liang, X. Quercetin Attenuates Diabetic Peripheral Neuropathy by Correcting Mitochondrial Abnormality via Activation of AMPK/PGC-1α Pathway. Front. Neurosci. 2021, 15, 636172. [Google Scholar]
- Kiasalari, Z.; Rahmani, T.; Mahmoudi, N.; Baluchnejadmojarad, T.; Roghani, M. Diosgenin ameliorates development of neuropathic pain in diabetic rats: Involvement of oxidative stress and inflammation. Biomed. Pharmacother. 2017, 86, 654–661. [Google Scholar]
- El-Baz, F.K.; Salama, A.; Salama, R.A.A. Attenuates Diabetic Neuropathy Induced by STZ in Rats: Involvement of Thioredoxin. Biomed. Res. Int. 2020, 2020, 1295492. [Google Scholar]
- Ou, X.; Wang, Z.; Yu, D.; Guo, W.; Zvyagin, A.V.; Lin, Q.; Qu, W. VEGF-loaded ROS-responsive nanodots improve the structure and function of sciatic nerve lesions in type II diabetic peripheral neuropathy. Biomaterials 2025, 315, 122906. [Google Scholar]
- Mandel, N.; Büttner, M.; Poschet, G.; Kuner, R.; Agarwal, N. SUMOylation Modulates Reactive Oxygen Species (ROS) Levels and Acts as a Protective Mechanism in the Type 2 Model of Diabetic Peripheral Neuropathy. Cells 2023, 12, 2511. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [PubMed]
- Yang, K.; Wang, Y.; Li, Y.W.; Chen, Y.G.; Xing, N.; Lin, H.B.; Zhou, P.; Yu, X.P. Progress in the treatment of diabetic peripheral neuropathy. Biomed. Pharmacother. 2022, 148, 112717. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Calcium control of neurotransmitter release. Cold Spring Harb. Perspect. Biol. 2012, 4, a011353. [Google Scholar]
- Timic Stamenic, T.; Todorovic, S.M. Thalamic T-Type Calcium Channels as Targets for Hypnotics and General Anesthetics. Int. J. Mol. Sci. 2022, 23, 2349. [Google Scholar] [CrossRef]
- Messinger, R.B.; Naik, A.K.; Jagodic, M.M.; Nelson, M.T.; Lee, W.Y.; Choe, W.J.; Orestes, P.; Latham, J.R.; Todorovic, S.M.; Jevtovic-Todorovic, V. In vivo silencing of the Ca(V)3.2 T-type calcium channels in sensory neurons alleviates hyperalgesia in rats with streptozocin-induced diabetic neuropathy. Pain 2009, 145, 184–195. [Google Scholar]
- Serra, J.; Duan, W.R.; Locke, C.; Solà, R.; Liu, W.; Nothaft, W. Effects of a T-type calcium channel blocker, ABT-639, on spontaneous activity in C-nociceptors in patients with painful diabetic neuropathy: A randomized controlled trial. Pain 2015, 156, 2175–2183. [Google Scholar]
- George, D.S.; Hackelberg, S.; Jayaraj, N.D.; Ren, D.; Edassery, S.L.; Rathwell, C.A.; Miller, R.E.; Malfait, A.M.; Savas, J.N.; Miller, R.J.; et al. Mitochondrial calcium uniporter deletion prevents painful diabetic neuropathy by restoring mitochondrial morphology and dynamics. Pain 2022, 163, 560–578. [Google Scholar]
- Lim, T.K.; Rone, M.B.; Lee, S.; Antel, J.P.; Zhang, J. Mitochondrial and bioenergetic dysfunction in trauma-induced painful peripheral neuropathy. Mol. Pain 2015, 11, 58. [Google Scholar]
- Chowdhury, S.K.; Smith, D.R.; Fernyhough, P. The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol. Dis. 2013, 51, 56–65. [Google Scholar] [CrossRef]
- Bachewal, P.; Gundu, C.; Yerra, V.G.; Kalvala, A.K.; Areti, A.; Kumar, A. Morin exerts neuroprotection via attenuation of ROS induced oxidative damage and neuroinflammation in experimental diabetic neuropathy. Biofactors 2018, 44, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Kishore, L.; Kaur, N.; Singh, R. Effect of Kaempferol isolated from seeds of Eruca sativa on changes of pain sensitivity in Streptozotocin-induced diabetic neuropathy. Inflammopharmacology 2018, 26, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Ain Shams Univesity, Role of Curcumin in Paclitaxel Induced PN. 2023. Available online: https://clinicaltrials.gov/study/NCT05966441?term=Role%20of%20Curcumin%20in%20Paclitaxel%20Induced%20&rank=1&tab=table (accessed on 24 January 2025).
- Chen, S.R.; Zhang, J.; Chen, H.; Pan, H.L. Streptozotocin-Induced Diabetic Neuropathic Pain Is Associated with Potentiated Calcium-Permeable AMPA Receptor Activity in the Spinal Cord. J. Pharmacol. Exp. Ther. 2019, 371, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Jagodic, M.M.; Pathirathna, S.; Nelson, M.T.; Mancuso, S.; Joksovic, P.M.; Rosenberg, E.R.; Bayliss, D.A.; Jevtovic-Todorovic, V.; Todorovic, S.M. Cell-specific alterations of T-type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. J. Neurosci. 2007, 27, 3305–3316. [Google Scholar] [CrossRef]
- Tricaud, N.; Gautier, B.; Berthelot, J.; Gonzalez, S.; Van Hameren, G. Traumatic and Diabetic Schwann Cell Demyelination Is Triggered by a Transient Mitochondrial Calcium Release through Voltage Dependent Anion Channel 1. Biomedicines 2022, 10, 1447. [Google Scholar] [CrossRef]
- Beth Israel Medical Center. Calcium and Magnesium Infusion for the Prevention of Taxmen Induced Neuropathy. 2014. Available online: https://clinicaltrials.gov/study/NCT01682499?cond=neuropathy&term=calcium%20infusions&rank=1 (accessed on 30 January 2025).
- Basit, A. Vitamin D Treatment for Painful Diabetic Neuropathy. Baqai Institute of Diabetology and Endocrinology. 2016. Available online: https://clinicaltrials.gov/study/NCT02737423?term=Vitamin%20D%20Treatment%20for%20Painful%20Diabetic%20Neuropathy&rank=1 (accessed on 24 January 2025).
- Li, K.; Shi, X.; Luo, M.; Inam-U-Llah; Wu, P.; Zhang, M.; Zhang, C.; Li, Q.; Wang, Y.; Piao, F. Taurine protects against myelin damage of sciatic nerve in diabetic peripheral neuropathy rats by controlling apoptosis of schwann cells via NGF/Akt/GSK3β pathway. Exp. Cell. Res. 2019, 383, 111557. [Google Scholar] [CrossRef]
- Nazarnezhad, S.; Rahmati, M.; Shayannia, A.; Abbasi, Z.; Salehi, M.; Khaksari, M. Nesfatin-1 protects PC12 cells against high glucose-induced cytotoxicity via inhibiting oxidative stress, autophagy and apoptosis. Neurotoxicology 2019, 74, 196–202. [Google Scholar] [CrossRef]
- Srinivasan, S.; Stevens, M.; Wiley, J.W. Diabetic peripheral neuropathy: Evidence for apoptosis and associated mitochondrial dysfunction. Diabetes 2000, 49, 1932–1938. [Google Scholar] [CrossRef]
- Liu, Y.P.; Shao, S.J.; Guo, H.D. Schwann cells apoptosis is induced by high glucose in diabetic peripheral neuropathy. Life Sci. 2020, 248, 117459. [Google Scholar]
- Ye, S.; Cheng, Z.; Zhuo, D.; Liu, S. Different Types of Cell Death in Diabetic Neuropathy: A Focus on Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 8126. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhang, L.L.; Li, B.; Sun, F.X.; Wang, Z.D. BMP5 ameliorates diabetic peripheral neuropathy by augmenting mitochondrial function and inhibiting apoptosis in Schwann cells. Biochem. Biophys. Res. Commun. 2023, 643, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Q.; Liu, S.; Xin, H.; Zhang, X.; Guo, N. Maltol Improves Peripheral Nerve Function by Inhibiting Schwann Cell Apoptosis via the PERK/eIF2α/CHOP Pathway and MME Upregulation in Diabetic Peripheral Neuropathy. Pharmaceuticals 2024, 17, 1139. [Google Scholar] [CrossRef]
- Luo, Q.; Feng, Y.; Xie, Y.; Shao, Y.; Wu, M.; Deng, X.; Yuan, W.E.; Chen, Y.; Shi, X. Nanoparticle-microRNA-146a-5p polyplexes ameliorate diabetic peripheral neuropathy by modulating inflammation and apoptosis. Nanomedicine 2019, 17, 188–197. [Google Scholar]
- Zhang, Y.; Li, C.; Wang, Z.; Wang, T.; Zhou, Y.; Zheng, L. Blocking CXC Motif Chemokine Ligand 2 Ameliorates Diabetic Peripheral Neuropathy via Inhibiting Apoptosis and NLRP3 Inflammasome Activation. Biol. Pharm. Bull. 2023, 46, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Pyroptosis in Diabetic Peripheral Neuropathy and its Therapeutic Regulation. J. Inflamm. Res. 2024, 17, 3839–3864. [Google Scholar]
- Belavgeni, A.; Maremonti, F.; Stadtmüller, M.; Bornstein, S.R.; Linkermann, A. Schwann cell necroptosis in diabetic neuropathy. Proc. Natl. Acad. Sci. USA 2022, 119, e2204049119. [Google Scholar]
- Wu, K.Y.; Deng, F.; Mao, X.Y.; Zhou, D.; Shen, W.G. Ferroptosis involves in Schwann cell death in diabetic peripheral neuropathy. Open Med. 2023, 18, 20230809. [Google Scholar]
- Zhan, L.; Li, R.; Sun, Y.; Dou, M.; Yang, W.; He, S.; Zhang, Y. Effect of mito-TEMPO, a mitochondria-targeted antioxidant, in rats with neuropathic pain. Neuroreport 2018, 29, 1275–1281. [Google Scholar]
- Dai, C.Q.; Guo, Y.; Chu, X.Y. Neuropathic Pain: The Dysfunction of Drp1, Mitochondria, and ROS Homeostasis. Neurotox. Res. 2020, 38, 553–563. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza, N.; Papadopoulos, V. Role of Mitochondrial Dysfunction in Neuropathy. Int. J. Mol. Sci. 2025, 26, 3195. https://doi.org/10.3390/ijms26073195
Espinoza N, Papadopoulos V. Role of Mitochondrial Dysfunction in Neuropathy. International Journal of Molecular Sciences. 2025; 26(7):3195. https://doi.org/10.3390/ijms26073195
Chicago/Turabian StyleEspinoza, Nidia, and Vassilios Papadopoulos. 2025. "Role of Mitochondrial Dysfunction in Neuropathy" International Journal of Molecular Sciences 26, no. 7: 3195. https://doi.org/10.3390/ijms26073195
APA StyleEspinoza, N., & Papadopoulos, V. (2025). Role of Mitochondrial Dysfunction in Neuropathy. International Journal of Molecular Sciences, 26(7), 3195. https://doi.org/10.3390/ijms26073195







