Engineering Exosomes for CNS Disorders: Advances, Challenges, and Therapeutic Potential
Abstract
1. Introduction
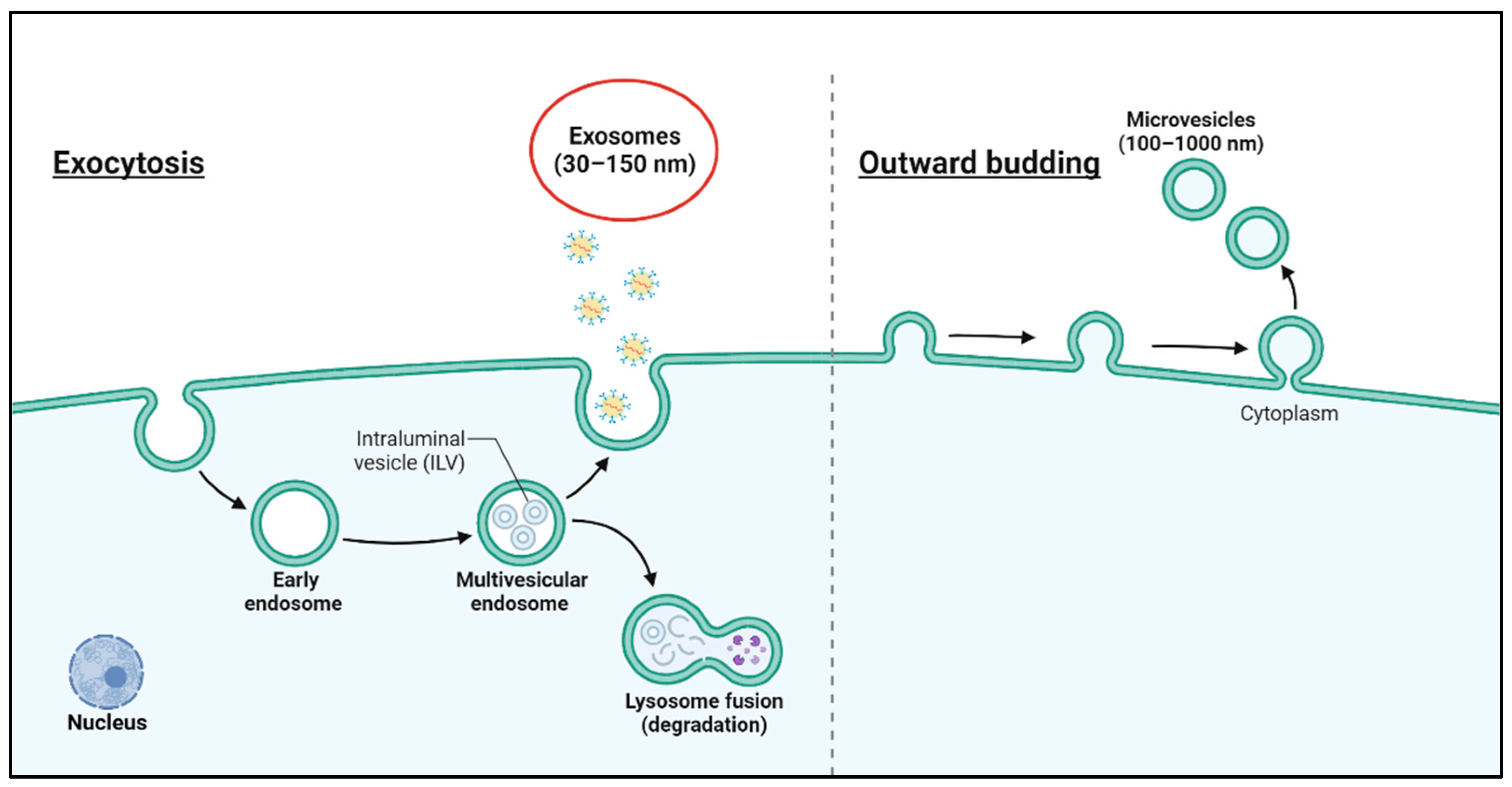
2. Characteristics of Exosomes
3. Engineering Techniques to Enhance Exosome Function
3.1. Cargo Packaging
3.2. Chemical Modification
3.3. Membrane Fusion
3.4. Accumulation
4. Application of Exosomes for the Treatment of Neurological Diseases
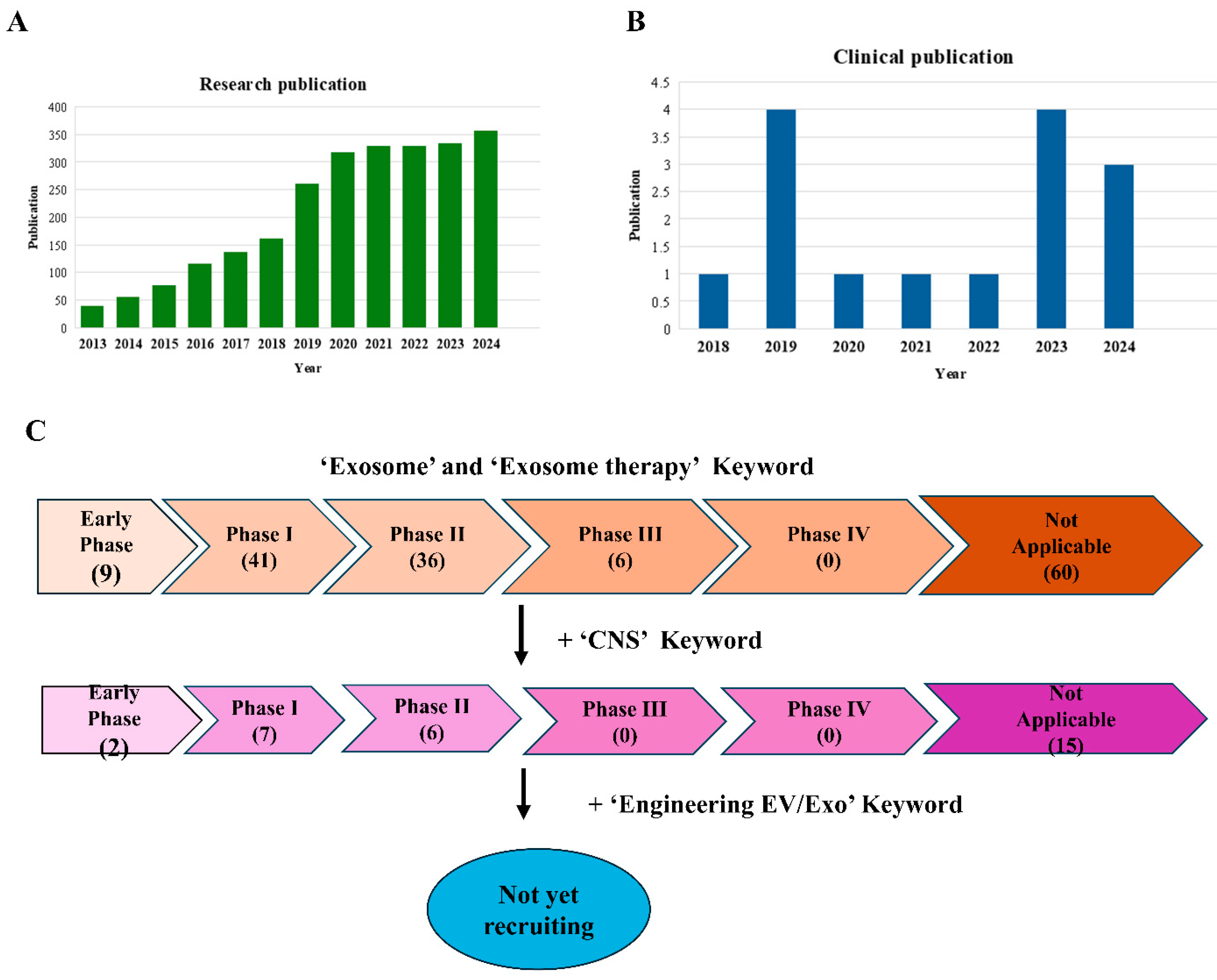
5. Exosome Application in Clinical Trials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ni, Z.; Zhou, S.; Li, S.; Kuang, L.; Chen, H.; Luo, X.; Ouyang, J.; He, M.; Du, X.; Chen, L. Exosomes: Roles and therapeutic potential in osteoarthritis. Bone Res. 2020, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Beit-Yannai, E.; Tabak, S.; Stamer, W.D. Physical exosome:exosome interactions. J. Cell. Mol. Med. 2018, 22, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Mebarek, S.; Buchet, R.; Pikula, S.; Strzelecka-Kiliszek, A.; Brizuela, L.; Corti, G.; Collacchi, F.; Anghieri, G.; Magrini, A.; Ciancaglini, P.; et al. Do Media Extracellular Vesicles and Extracellular Vesicles Bound to the Extracellular Matrix Represent Distinct Types of Vesicles? Biomolecules 2023, 14, 42. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Kolonics, F.; Szeifert, V.; Timár, C.I.; Ligeti, E.; Lőrincz, Á.M. The Functional Heterogeneity of Neutrophil-Derived Extracellular Vesicles Reflects the Status of the Parent Cell. Cells 2020, 9, 2718. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, S.; Liu, L.; Dang, P.; Liu, Y.; Sun, Z.; Qiao, B.; Wang, C. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 124. [Google Scholar] [CrossRef]
- Ratajczak, K.; Grel, H.; Olejnik, P.; Jakiela, S.; Stobiecka, M. Current progress, strategy, and prospects of PD-1/PDL-1 immune checkpoint biosensing platforms for cancer diagnostics, therapy monitoring, and drug screening. Biosens. Bioelectron. 2023, 240, 115644. [Google Scholar] [CrossRef]
- Hazrati, A.; Mirsanei, Z.; Heidari, N.; Malekpour, K.; Rahmani-Kukia, N.; Abbasi, A.; Soudi, S. The potential application of encapsulated exosomes: A new approach to increase exosomes therapeutic efficacy. Biomed. Pharmacother. 2023, 162, 114615. [Google Scholar] [CrossRef] [PubMed]
- Namiot, E.D.; Sokolov, A.V.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B. Nanoparticles in Clinical Trials: Analysis of Clinical Trials, FDA Approvals and Use for COVID-19 Vaccines. Int. J. Mol. Sci. 2023, 24, 787. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Ezban, M.; Hermit, M.B.; Persson, E. FIXing postinfusion monitoring: Assay experiences with N9-GP(nonacog beta pegol; Refixia®; Rebinyn®). Haemophilia 2019, 25, 154–161. [Google Scholar] [CrossRef]
- Hussen, B.M.; Faraj, G.S.H.; Rasul, M.F.; Hidayat, H.J.; Salihi, A.; Baniahmad, A.; Taheri, M.; Ghafouri-Frad, S. Strategies to overcome the main challenges of the use of exosomes as drug carrier for cancer therapy. Cancer Cell Int. 2022, 22, 323. [Google Scholar] [CrossRef]
- Alotaibi, A.S.; A Mahroos, R.; Al Yateem, S.S.; Menezes, R.G. Central Nervous System Causes of Sudden Unexpected Death: A Comprehensive Review. Cureus 2022, 14, e20944. [Google Scholar] [CrossRef] [PubMed]
- Charvériat, M.; Lafon, V.; Mouthon, F.; Zimmer, L. Innovative approaches in CNS drug discovery. Therapies 2021, 76, 101–109. [Google Scholar] [CrossRef]
- Patel, N.C. Methods to optimize CNS exposure of drug candidates. Bioorganic Med. Chem. Lett. 2020, 30, 127503. [Google Scholar] [CrossRef] [PubMed]
- Tonge, P.J. Drug-Target Kinetics in Drug Discovery. ACS Chem. Neurosci. 2018, 9, 29–39. [Google Scholar] [CrossRef]
- Gebeyehu, A.; Kommineni, N.; Meckes, D.G.; Sachdeva, M.S. Role of Exosomes for Delivery of Chemotherapeutic Drugs. Crit. Rev. Ther. Drug Carr. Syst. 2021, 38, 53–97. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Wang, L.; Lu, M.; Chopp, M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 2001, 56, 1666–1672. [Google Scholar] [CrossRef]
- Conaty, P.; Sherman, L.S.; Naaldijk, Y.; Ulrich, H.; Stolzing, A.; Rameshwar, P. Methods of Mesenchymal Stem Cell Homing to the Blood-Brain Barrier. Methods Mol. Biol. 2018, 1842, 81–91. [Google Scholar] [PubMed]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, F. Exosomal microRNAs in cancer: Potential biomarkers and immunotherapeutic targets for immune checkpoint molecules. Front. Genet. 2023, 14, 1052731. [Google Scholar] [CrossRef]
- Challagundla, K.B.; Wise, P.M.; Neviani, P.; Chava, H.; Murtadha, M.; Xu, T.; Kennedy, R.; Ivan, C.; Zhang, X.; Vannini, I.; et al. Exosome-Mediated Transfer of microRNAs Within the Tumor Microenvironment and Neuroblastoma Resistance to Chemotherapy. JNCI J. Natl. Cancer Inst. 2015, 107, djv135. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal. 2022, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation—Efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar]
- Jella, K.K.; Nasti, T.H.; Li, Z.; Malla, S.R.; Buchwald, Z.S.; Khan, M.K. Exosomes, Their Biogenesis and Role in Inter-Cellular Communication, Tumor Microenvironment and Cancer Immunotherapy. Vaccines 2018, 6, 69. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes—Vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef]
- He, J.; Ren, W.; Wang, W.; Han, W.; Jiang, L.; Zhang, D.; Guo, M. Exosomal targeting and its potential clinical application. Drug Deliv. Transl. Res. 2022, 12, 2385–2402. [Google Scholar] [CrossRef]
- Xu, M.; Feng, T.; Liu, B.; Qiu, F.; Xu, Y.; Zhao, Y.; Zheng, Y. Engineered exosomes: Desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics 2021, 11, 8926–8944. [Google Scholar] [CrossRef] [PubMed]
- Parada, N.; Romero-Trujillo, A.; Georges, N.; Alcayaga-Miranda, F. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J. Adv. Res. 2021, 31, 61–74. [Google Scholar] [CrossRef]
- Lara, P.; Chan, A.B.; Cruz, L.J.; Quest, A.F.G.; Kogan, M.J. Exploiting the Natural Properties of Extracellular Vesicles in Targeted Delivery towards Specific Cells and Tissues. Pharmaceutics 2020, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Kastelowitz, N.; Yin, H. Exosomes and Microvesicles: Identification and Targeting By Particle Size and Lipid Chemical Probes. ChemBioChem 2014, 15, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Van den Boorn, J.G.; Schlee, M.; Coch, C.; Hartmann, G. SiRNA delivery with exosome nanoparticles. Nat. Biotechnol. 2011, 29, 325–326. [Google Scholar] [CrossRef]
- Hood, J.L. Post Isolation Modification of Exosomes for Nanomedicine Applications. Nanomedicine 2016, 11, 1745–1756. [Google Scholar] [CrossRef]
- Hornung, S.; Dutta, S.; Bitan, G. CNS-Derived Blood Exosomes as a Promising Source of Biomarkers: Opportunities and Challenges. Front. Mol. Neurosci. 2020, 13, 38. [Google Scholar] [CrossRef]
- Yim, N.; Ryu, S.-W.; Choi, K.; Lee, K.R.; Lee, S.; Choi, H.; Kim, J.; Shaker, M.R.; Sun, W.; Park, J.-H.; et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat. Commun. 2016, 7, 12277. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, T.; Zhao, C.; Li, G. The Regulation of Exosome Generation and Function in Physiological and Pathological Processes. Int. J. Mol. Sci. 2023, 25, 255. [Google Scholar] [CrossRef]
- Lopes, D.; Lopes, J.; Pereira-Silva, M.; Peixoto, D.; Rabiee, N.; Veiga, F.; Moradi, O.; Guo, Z.-H.; Wang, X.-D.; Conde, J.; et al. Bioengineered exosomal-membrane-camouflaged abiotic nanocarriers: Neurodegenerative diseases, tissue engineering and regenerative medicine. Mil. Med Res. 2023, 10, 19. [Google Scholar] [CrossRef]
- Choi, H.; Choi, K.; Kim, D.-H.; Oh, B.-K.; Yim, H.; Jo, S.; Choi, C. Strategies for Targeted Delivery of Exosomes to the Brain: Advantages and Challenges. Pharmaceutics 2022, 14, 672. [Google Scholar] [CrossRef]
- Huang, L.; Wu, E.; Liao, J.; Wei, Z.; Wang, J.; Chen, Z. Research Advances of Engineered Exosomes as Drug Delivery Carrier. ACS Omega 2023, 8, 43374–43387. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Mehra, A.; Arora, S.; Gugulothu, D.; Vora, L.K.; Prasad, R.; Khatri, D.K. Exosome-mediated delivery and regulation in neurological disease progression. Int. J. Biol. Macromol. 2024, 264, 130728. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Fueller, J.; Herbst, K.; Meurer, M.; Gubicza, K.; Kurtulmus, B.; Knopf, J.D.; Kirrmaier, D.; Buchmuller, B.C.; Pereira, G.; Lemberg, M.K.; et al. CRISPR-Cas12a–assisted PCR tagging of mammalian genes. J. Cell Biol. 2020, 219, 69. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Dai, X.; Xu, Y.; Xing, G.; Liu, H.; Lu, T.; Chen, Y.; Zhang, Y. Drug repositioning: Progress and challenges in drug discovery for various diseases. Eur. J. Med. Chem. 2022, 234, 114239. [Google Scholar] [CrossRef]
- Di Santo, R.; Niccolini, B.; Romanò, S.; Vaccaro, M.; Di Giacinto, F.; De Spirito, M.; Ciasca, G. Advancements in Mid-Infrared spectroscopy of extracellular vesicles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 305, 123346. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Migneault, F.; Dieudé, M.; Turgeon, J.; Beillevaire, D.; Hardy, M.-P.; Brodeur, A.; Thibodeau, N.; Perreault, C.; Hébert, M.-J. Apoptotic exosome-like vesicles regulate endothelial gene expression, inflammatory signaling, and function through the NF-κB signaling pathway. Sci. Rep. 2020, 10, 12562. [Google Scholar] [CrossRef]
- Wang, J.; Jing, J.; Zhou, C.; Fan, Y. Emerging roles of exosomes in oral diseases progression. Int. J. Oral Sci. 2024, 16, 4. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Qasim, M.; Kim, J.-H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.B.; Shon, O.-J.; Seo, M.-S.; Choi, Y.; Park, W.T.; Lee, G.W. Mesenchymal Stem Cell-Derived Exosomes and Their Therapeutic Potential for Osteoarthritis. Biology 2021, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Foldager, C.B.; Pei, M.; Hui, J.H.P. Advances in Mesenchymal Stem Cell-based Strategies for Cartilage Repair and Regeneration. Stem Cell Rev. Rep. 2014, 10, 686–696. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-P.; Lin, Z.-X.; Jiang, X.-Y.; Yu, X.-Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018, 39, 542–551. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Gong, W.; Gong, W.; Li, R.; Li, R.; Li, L.; Li, L.; Wang, J.; Wang, J. Preparation of genetically or chemically engineered exosomes and their therapeutic effects in bone regeneration and anti-inflammation. Front. Bioeng. Biotechnol. 2024, 12, 1329388. [Google Scholar] [CrossRef]
- Oshchepkova, A.; Zenkova, M.; Vlassov, V. Extracellular Vesicles for Therapeutic Nucleic Acid Delivery: Loading Strategies and Challenges. Int. J. Mol. Sci. 2023, 24, 7287. [Google Scholar] [CrossRef]
- Lin, T.-L.; Lin, Y.-H.; Lee, A.K.-X.; Kuo, T.-Y.; Chen, C.-Y.; Chen, K.-H.; Chou, Y.-T.; Chen, Y.-W.; Shie, M.-Y. The exosomal secretomes of mesenchymal stem cells extracted via 3D-printed lithium-doped calcium silicate scaffolds promote osteochondral regeneration. Mater. Today Bio 2023, 22, 100728. [Google Scholar] [CrossRef]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.-A.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.P.K.; Holme, M.N.; Stevens, M.M. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano 2017, 11, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chen, Y.; Peng, M.; Zheng, J.H.; Zuo, C. Exploring the potential of exosomes in diagnosis and drug delivery for pancreatic ductal adenocarcinoma. Int. J. Cancer 2022, 152, 110–122. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Gong, C.; Wang, Z.; Xia, Q.; Gu, F.; Hu, C.; Zhang, L.; Guo, H.; Gao, S. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine 2018, 14, 1973–1985. [Google Scholar] [CrossRef]
- Viñas, J.L.; Spence, M.; Gutsol, A.; Knoll, W.; Burger, D.; Zimpelmann, J.; Allan, D.S.; Burns, K.D. Receptor-Ligand Interaction Mediates Targeting of Endothelial Colony Forming Cell-derived Exosomes to the Kidney after Ischemic Injury. Sci. Rep. 2018, 8, 16320. [Google Scholar] [CrossRef]
- Choi, E.S.; Song, J.; Kang, Y.Y.; Mok, H. Mannose-Modified Serum Exosomes for the Elevated Uptake to Murine Dendritic Cells and Lymphatic Accumulation. Macromol. Biosci. 2019, 19, e1900042. [Google Scholar] [CrossRef]
- Martín-Durán, J.M.; Hejnol, A. A developmental perspective on the evolution of the nervous system. Dev. Biol. 2021, 475, 181–192. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, J.; Wang, P.; Liu, X.; Liu, P.; Cheng, X.; Cao, L.; Wu, H.; Chen, J.; Zhou, L. Neural stem cell-derived exosomes and regeneration: Cell-free therapeutic strategies for traumatic brain injury. Stem Cell Res. Ther. 2023, 14, 198. [Google Scholar] [CrossRef]
- Johnstone, R.M. Maturation of reticulocytes: Formation of exosomes as a mechanism for shedding membrane proteins. Biochem. Cell Biol. 1992, 70, 179–190. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Du, X.; Li, X.; Liu, S.; Xu, Y. The Emerging Role of Neural Cell-Derived Exosomes in Intercellular Communication in Health and Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 738442. [Google Scholar] [CrossRef]
- Huang, X.; Ding, J.; Li, Y.; Liu, W.; Ji, J.; Wang, H.; Wang, X. Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Exp. Cell Res. 2018, 371, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Niazi, S.K. Non-Invasive Drug Delivery across the Blood–Brain Barrier: A Prospective Analysis. Pharmaceutics 2023, 15, 2599. [Google Scholar] [CrossRef]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar]
- Du, W.; Feng, Y.; Wang, X.; Piao, X.; Cui, Y.; Chen, L.; Lei, X.; Sun, X.; Liu, X.; Wang, H.; et al. Curcumin Suppresses Malignant Glioma Cells Growth and Induces Apoptosis by Inhibition of SHH/GLI1 Signaling Pathway in Vitro and Vivo. CNS Neurosci. Ther. 2013, 19, 926–936. [Google Scholar] [CrossRef]
- Lee, E.C.; Ha, T.W.; Lee, D.-H.; Hong, D.-Y.; Park, S.-W.; Lee, J.Y.; Lee, M.R.; Oh, J.S. Utility of Exosomes in Ischemic and Hemorrhagic Stroke Diagnosis and Treatment. Int. J. Mol. Sci. 2022, 23, 8367. [Google Scholar] [CrossRef]
- Ferguson, S.W.; Nguyen, J. Exosomes as therapeutics: The implications of molecular composition and exosomal heterogeneity. J. Control Release 2016, 228, 179–190. [Google Scholar] [CrossRef]
- Lai, J.J.; Chau, Z.L.; Chen, S.; Hill, J.J.; Korpany, K.V.; Liang, N.; Lin, L.; Lin, Y.; Liu, J.K.; Liu, Y.; et al. Exosome Processing and Characterization Approaches for Research and Technology Development. Adv. Sci. 2022, 9, e2103222. [Google Scholar] [CrossRef]
- Fu, J.M.; Song, W.; Hao, Z.; Fan, M.M.; Li, Y.M. Research trends and hotspots of exosomes in respiratory diseases. Medicine 2023, 102, e35381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-X.; Sun, C.; Wang, L.; Guo, X.-L. New insight into isolation, identification techniques and medical applications of exosomes. J. Control Release 2019, 308, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Li, H.; Xu, G.; Hu, Y.; Zhang, W.; Tian, K. Current Knowledge and Future Perspectives of Exosomes as Nanocarriers in Diagnosis and Treatment of Diseases. Int. J. Nanomed. 2023, 18, 4751–4778. [Google Scholar] [CrossRef]
- Kim, M.; Lee, Y.; Lee, M. Hypoxia-specific anti-RAGE exosomes for nose-to-brain delivery of anti-miR-181a oligonucleotide in an ischemic stroke model. Nanoscale 2021, 13, 14166–14178. [Google Scholar] [CrossRef]
- Guo, S.; Perets, N.; Betzer, O.; Ben-Shaul, S.; Sheinin, A.; Michaelevski, I.; Popovtzer, R.; Offen, D.; Levenberg, S. Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury. ACS Nano 2019, 13, 10015–10028. [Google Scholar] [CrossRef]
- Foo, J.B.; Looi, Q.H.; Chong, P.P.; Hassan, N.H.; Yeo, G.E.C.; Ng, C.Y.; Koh, B.; How, C.W.; Lee, S.H.; Law, J.X. Comparing the Therapeutic Potential of Stem Cells and their Secretory Products in Regenerative Medicine. Stem Cells Int. 2021, 2021, 2616807. [Google Scholar] [CrossRef]
- Song, J.; Song, B.; Yuan, L.; Yang, G. Multiplexed strategies toward clinical translation of extracellular vesicles. Theranostics 2022, 12, 6740–6761. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.; Nam, H.; Moon, S.; Kwon, Y.J.; Lee, J.B. Engineered extracellular vesicles and their mimetics for clinical translation. Methods 2020, 177, 80–94. [Google Scholar] [CrossRef]
- Silva, A.K.; Morille, M.; Piffoux, M.; Arumugam, S.; Mauduit, P.; Larghero, J.; Bianchi, A.; Aubertin, K.; Blanc-Brude, O.; Noël, D.; et al. Development of extracellular vesicle-based medicinal products: A position paper of the group “Extracellular Vesicle translatiOn to clinicaL perspectiVEs—EVOLVE France”. Adv. Drug Deliv. Rev. 2021, 179, 114001. [Google Scholar] [CrossRef] [PubMed]
- Brezgin, S.; Danilik, O.; Yudaeva, A.; Kachanov, A.; Kostyusheva, A.; Karandashov, I.; Ponomareva, N.; Zamyatnin, A.A.; Parodi, A.; Chulanov, V.; et al. Basic Guide for Approaching Drug Delivery with Extracellular Vesicles. Int. J. Mol. Sci. 2024, 25, 10401. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Chin, C.S.H.; Lim, Z.F.S.; Ng, S.K. HEK293 Cell Line as a Platform to Produce Recombinant Proteins and Viral Vectors. Front. Bioeng. Biotechnol. 2021, 9, 796991. [Google Scholar] [CrossRef]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zeng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Minh, A.D.; Kamen, A.A. Critical Assessment of Purification and Analytical Technologies for Enveloped Viral Vector and Vaccine Processing and Their Current Limitations in Resolving Co-Expressed Extracellular Vesicles. Vaccines 2021, 9, 823. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Barabadi, M.; Tan, J.L.; Morton, D.A.V.; Frith, J.E.; Lim, R. To Protect and to Preserve: Novel Preservation Strategies for Extracellular Vesicles. Front. Pharmacol. 2018, 9, 1199. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Zeng, H.; Guo, S.; Ren, X.; Wu, Z.; Liu, S.; Yao, X. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells 2023, 12, 1416. [Google Scholar] [CrossRef]
- Maroto, R.; Zhao, Y.; Jamaluddin, M.; Popov, V.L.; Wang, H.; Kalubowilage, M.; Zhang, Y.; Luisi, J.; Sun, H.; Culbertson, C.T.; et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell. Vesicles 2017, 6, 1359478. [Google Scholar] [CrossRef]
- Muthu, S.; Bapat, A.; Jain, R.; Jeyaraman, N.; Jeyaraman, M. Exosomal therapy—A new frontier in regenerative medicine. Stem Cell Investig. 2021, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Harn, H.-J.; Chen, Y.-S.; Lin, E.-Y.; Chiou, T.-W. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Tzu Chi Med. J. 2020, 32, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tsai, T.; Lee, C. Regulation of exosomes as biologic medicines: Regulatory challenges faced in exosome development and manufacturing processes. Clin. Transl. Sci. 2024, 17, e13904. [Google Scholar] [CrossRef]
- Liu, W.-Z.; Ma, Z.-J.; Kang, X.-W. Current status and outlook of advances in exosome isolation. Anal. Bioanal. Chem. 2022, 414, 7123–7141. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Lu, J.; Ng, I.O.-L. Exosomes and cancer—Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 2022, 11, 54. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y. Understanding exosomes: Part 1-Characterization, quantification and isolation techniques. Periodontol. 2000 2024, 94, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 11503. [Google Scholar] [CrossRef]
- Wang, W.; Sun, H.; Duan, H.; Sheng, G.; Tian, N.; Liu, D.; Sun, Z. Isolation and usage of exosomes in central nervous system diseases. CNS Neurosci. Ther. 2024, 30, e14677. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, M.I.; Khan, M.U.; Khan, N.M.; Bungau, S.; Hassan, S.S.U. Applications of Extracellular Vesicles in Nervous System Disorders: An Overview of Recent Advances. Bioengineering 2022, 10, 51. [Google Scholar] [CrossRef]
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as Naturally Occurring Vehicles for Delivery of Biopharmaceuticals: Insights from Drug Delivery to Clinical Perspectives. Nanomaterials 2021, 11, 1481. [Google Scholar] [CrossRef]
- Isaac, R.; Reis, F.C.G.; Ying, W.; Olefsky, J.M. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021, 33, 1744–1762. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fu, X.; Kou, Y.; Han, N. Engineering strategies and optimized delivery of exosomes for theranostic application in nerve tissue. Theranostics 2023, 13, 4266–4286. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- EL Andaloussi, S.; Lakhal, S.; Mäger, I.; Wood, M.J. Exosomes for targeted siRNA delivery across biological barriers. Adv. Drug Deliv. Rev. 2013, 65, 391–397. [Google Scholar] [CrossRef]
- Ingato, D.; Lee, J.U.; Sim, S.J.; Kwon, Y.J. Good things come in small packages: Overcoming challenges to harness extracellular vesicles for therapeutic delivery. J. Control Release 2016, 241, 174–185. [Google Scholar] [CrossRef]

| Disease | Donor Cell | Therapeutic Molecule | Intended Recipient Cells/Cargo | Drug Loading Method/Drug Route | Animal |
|---|---|---|---|---|---|
| Stroke | adipose-derived stem cells (ADSCs) | PEDF | unknown | Transfection/intravenous | Rats |
| bone marrow mesenchymal stem cells (BMSC) | miR-124 | Lamp2b-RVG | Electroporation/intravenous | Mice | |
| mesenchymal stem cells (MSCs) | miR-223-3p | unknown | Transfection/intravenous | Rats | |
| Brain tumor | RAW 264.7 | curcumin and SPIONs | RGE-peptide | Electroporation/intravenous | Mice |
| Spinal cord injury (SCI) | mesenchymal stem cells (MSC) | PTEN-siRNA | unknown | Co-incubation/intranasal | Rats |
| Parkinson’s disease | HEK-293T cells | Aptamer F5R1 | Microglia, neurons, astrocytes | Co-incubation/intraperitoneal | Mice |
| Alzheimer’s disease | Astrocyte | miR-29 | Neuron, Glia | Transfection/Intracerebral | Rats |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.C.; Choi, D.; Lee, D.-H.; Oh, J.S. Engineering Exosomes for CNS Disorders: Advances, Challenges, and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 3137. https://doi.org/10.3390/ijms26073137
Lee EC, Choi D, Lee D-H, Oh JS. Engineering Exosomes for CNS Disorders: Advances, Challenges, and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(7):3137. https://doi.org/10.3390/ijms26073137
Chicago/Turabian StyleLee, Eun Chae, Dongsic Choi, Dong-Hun Lee, and Jae Sang Oh. 2025. "Engineering Exosomes for CNS Disorders: Advances, Challenges, and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 7: 3137. https://doi.org/10.3390/ijms26073137
APA StyleLee, E. C., Choi, D., Lee, D.-H., & Oh, J. S. (2025). Engineering Exosomes for CNS Disorders: Advances, Challenges, and Therapeutic Potential. International Journal of Molecular Sciences, 26(7), 3137. https://doi.org/10.3390/ijms26073137






