The Potential of Peptide-Based Inhibitors in Disrupting Protein–Protein Interactions for Targeted Cancer Therapy
Abstract
1. Introduction
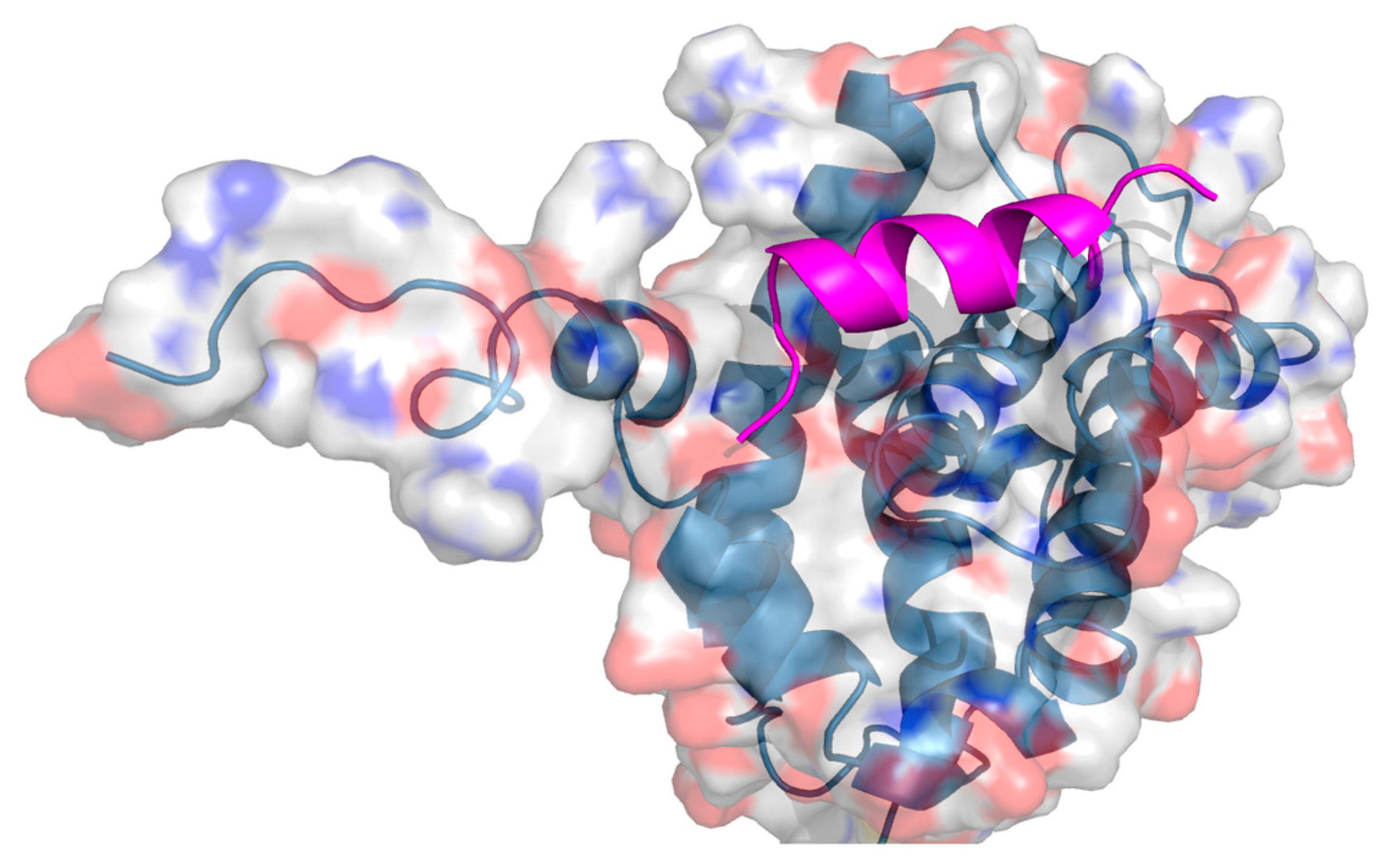
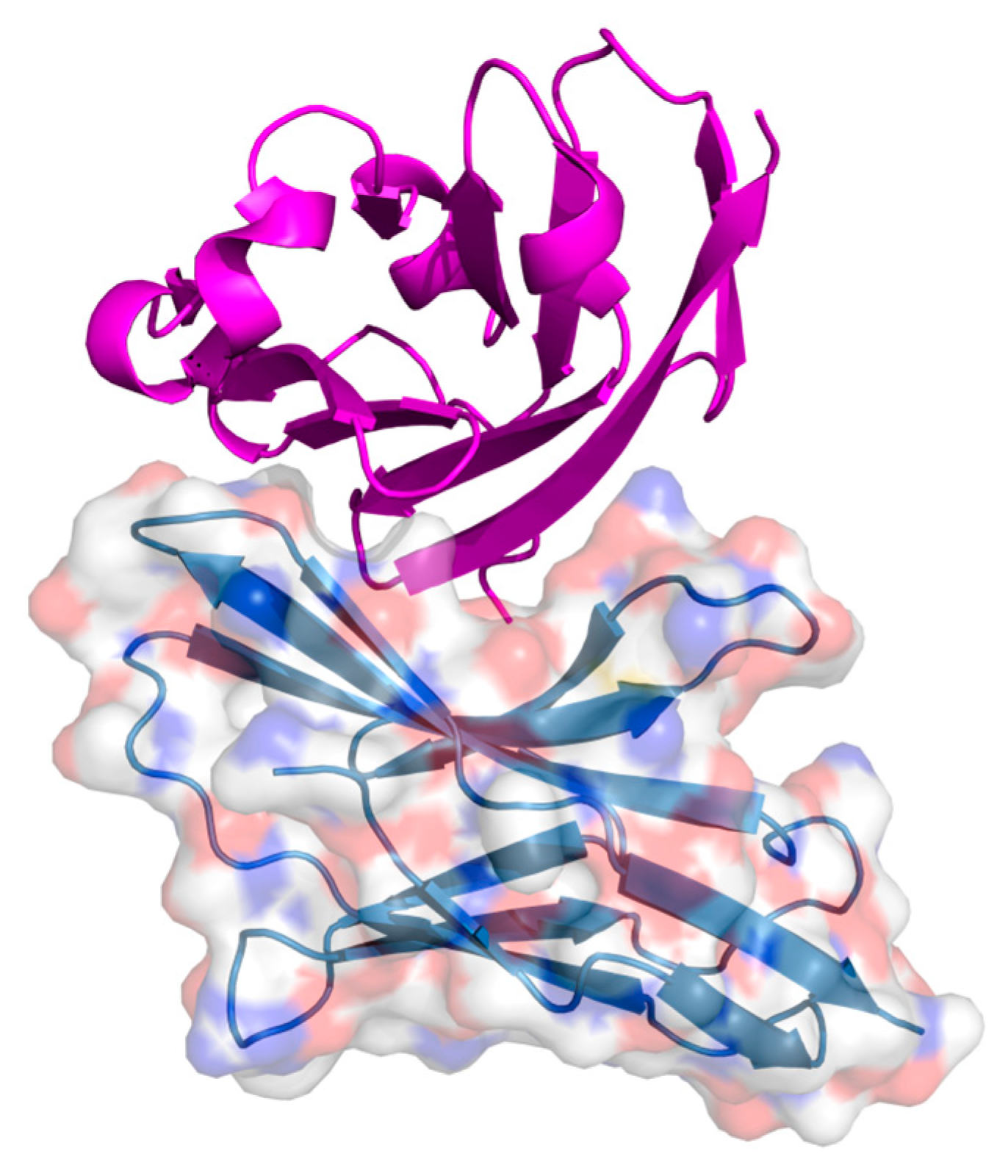
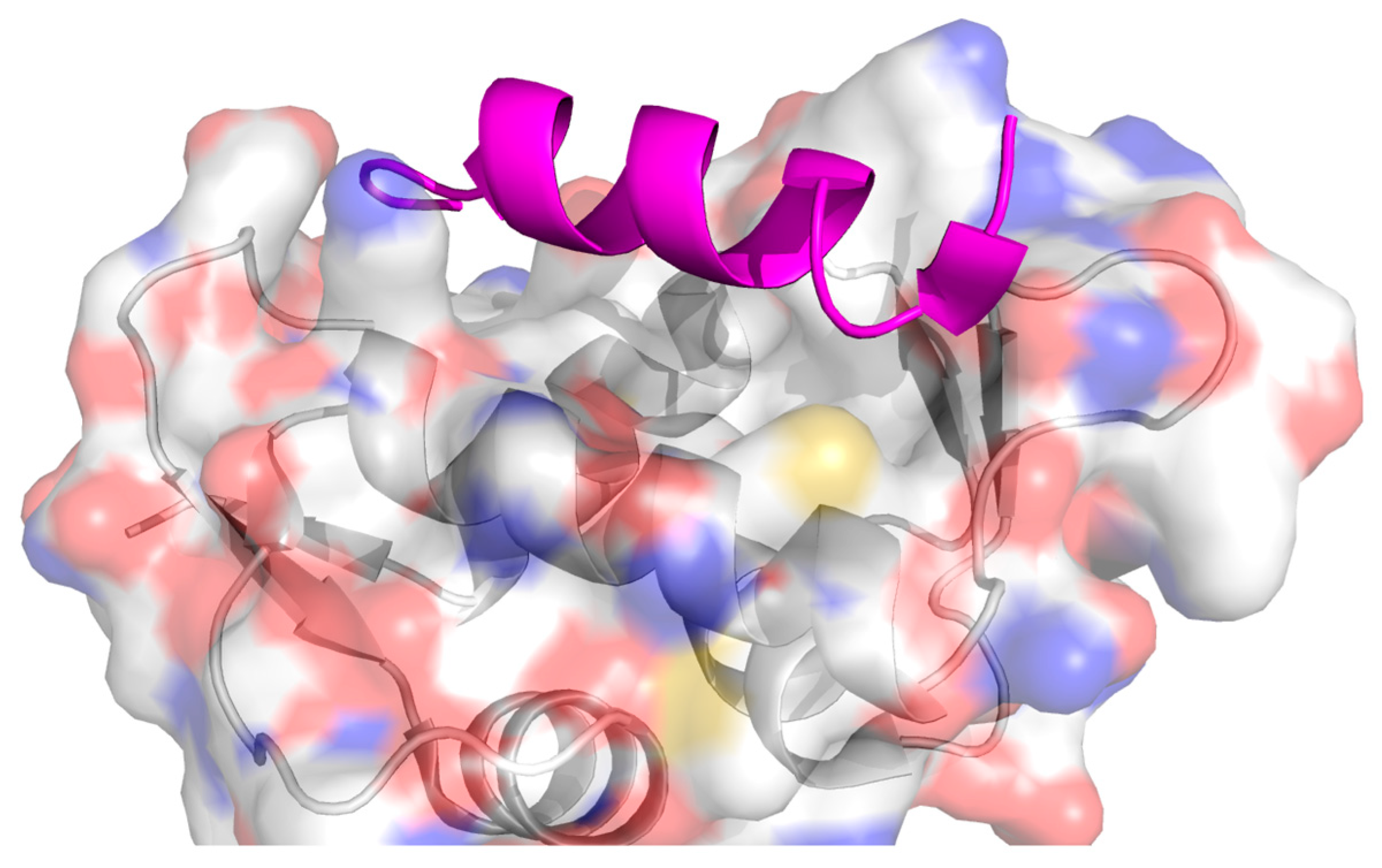
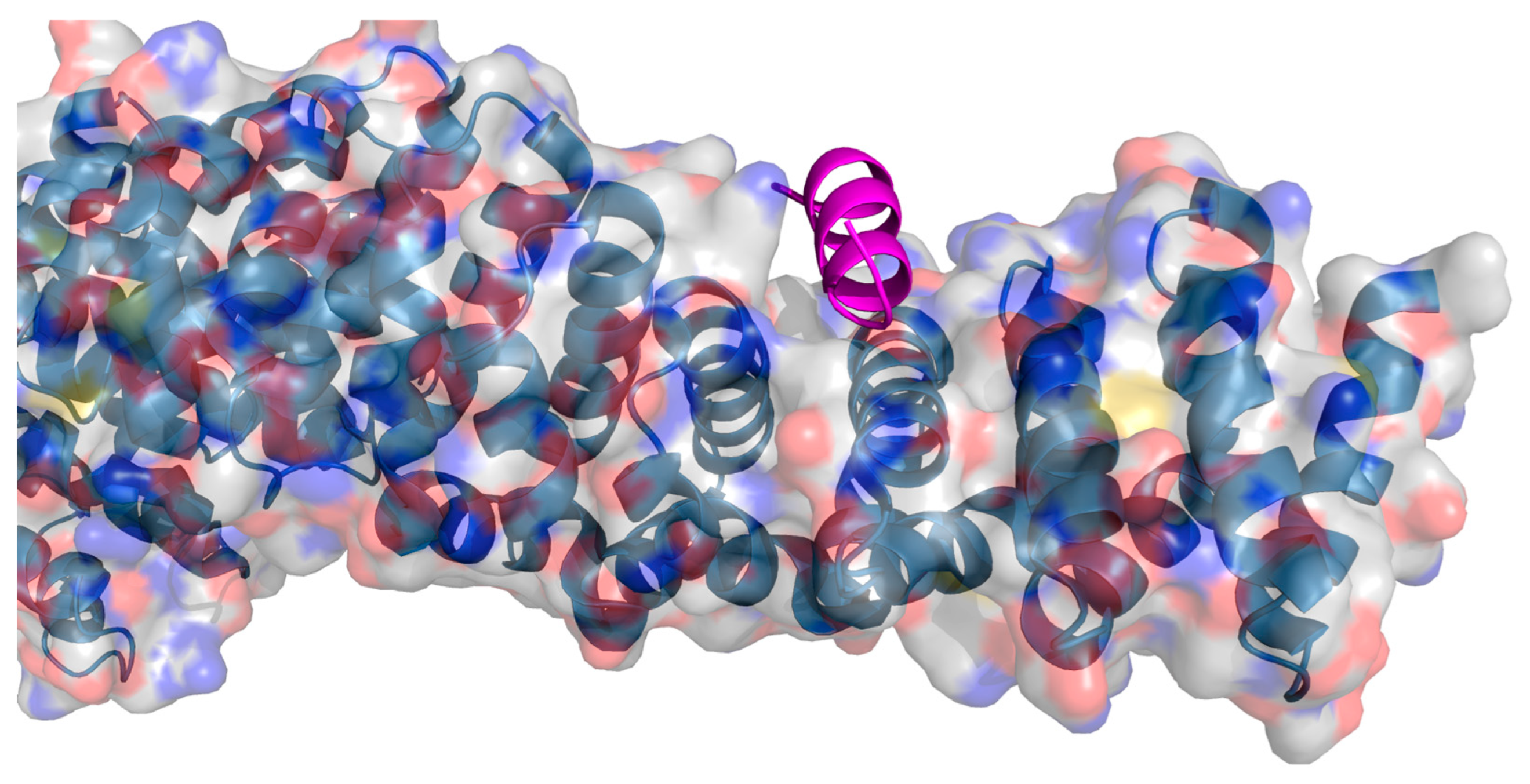
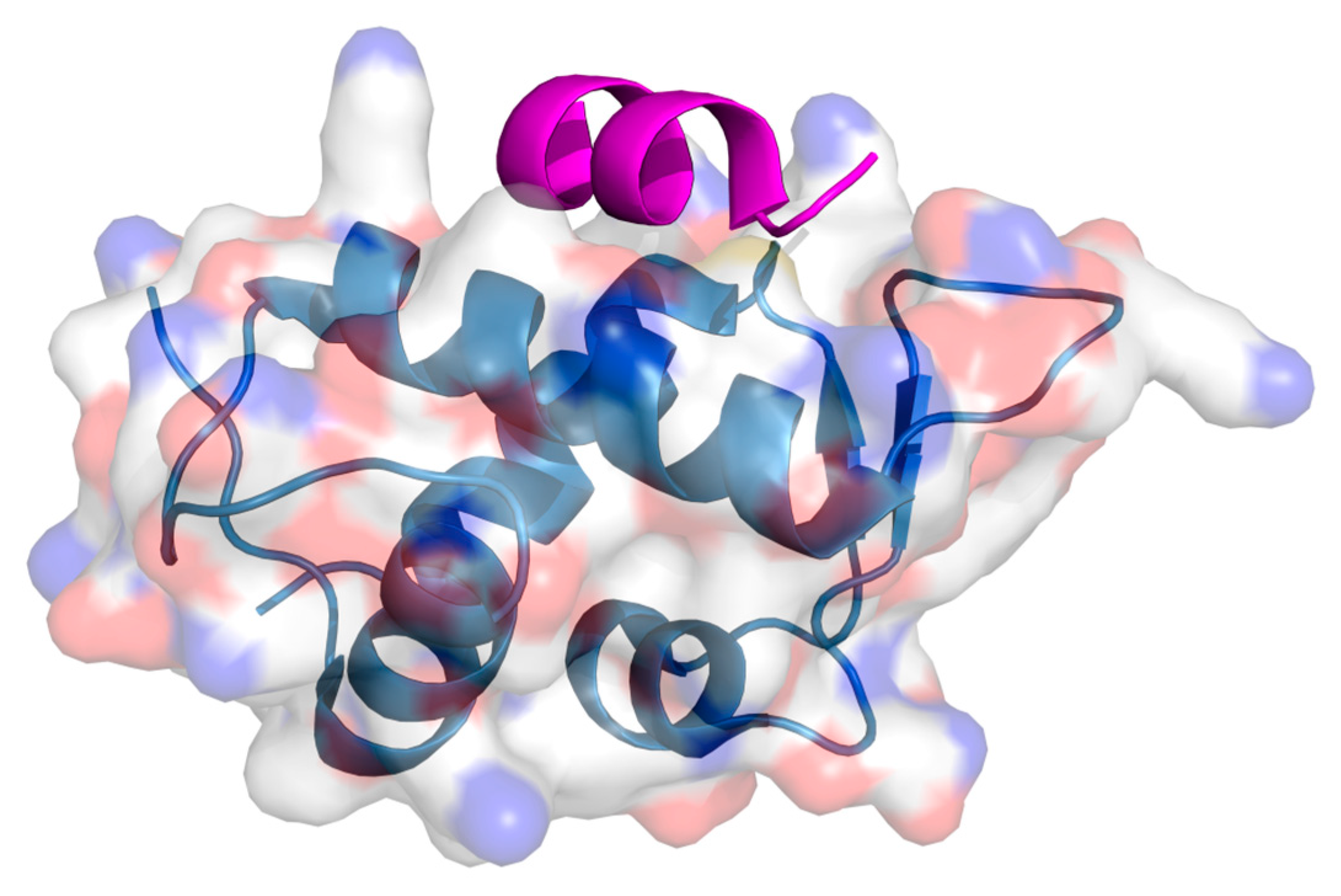
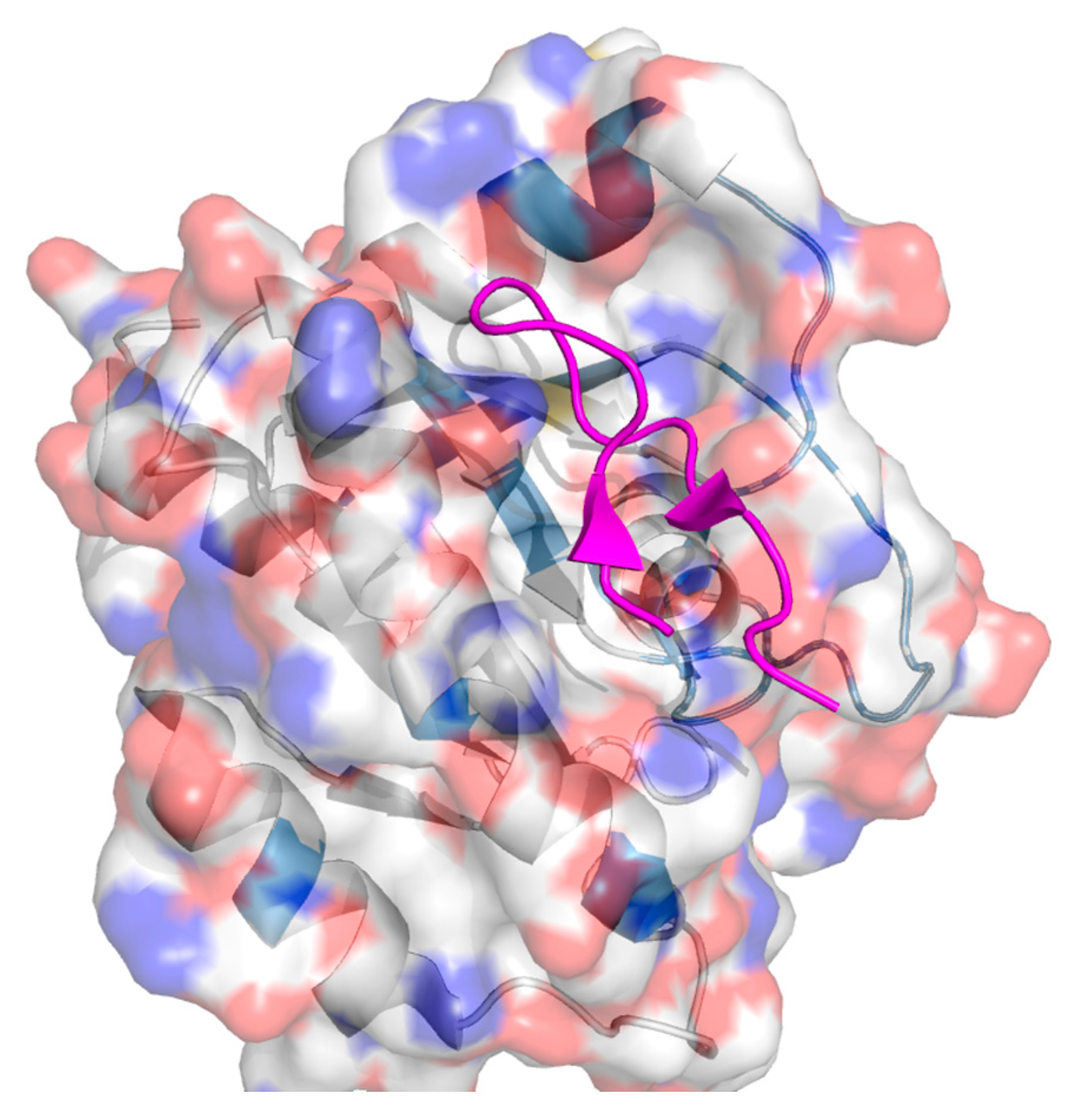
2. Protein–Protein Interactions: Specific Targeting and Challenges
3. Targeting PPIs as an Anticancer Strategy
4. Current Approaches to PPI Inhibition
5. Chemical Strategies to Overcome Peptide-Based Inhibitor Limitations
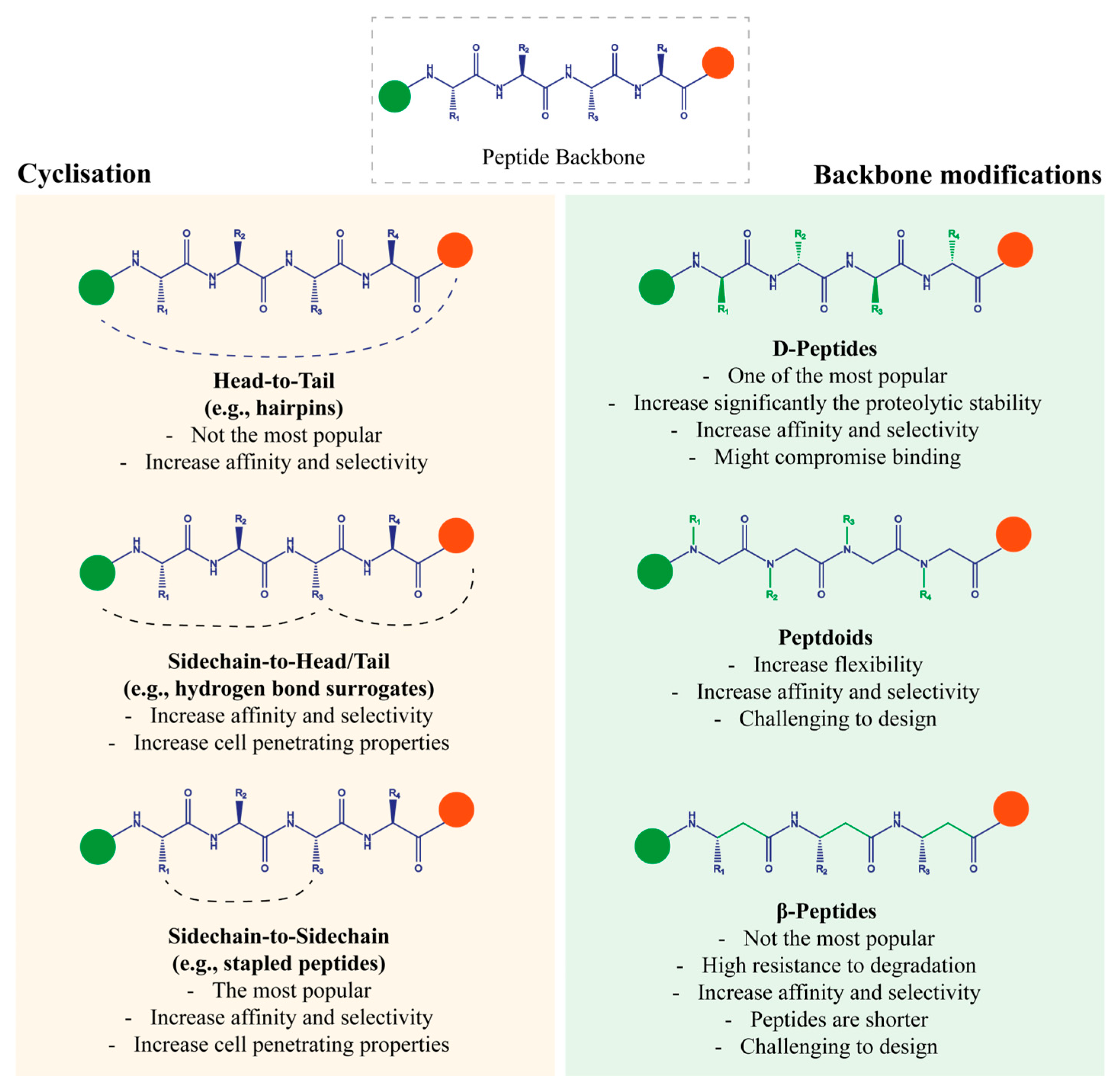
5.1. Cyclisation
5.1.1. Hydrogen Bond Surrogates (Sidechain-to-Head/Tail)
- Bcl-xL
- p53/MDM2
- Ras/sos
5.1.2. β-Hairpin (Head-to-Tail)
- p53/HDM2
- p53/MDM2
- PD-1/PD-L1
- BRCA1-BRCT
5.1.3. Stapled Peptides (Sidechain-to-Sidechain)
- p53/MDM2
- MDM2/MDM4
- Bcl-2
- β-catenin
- CYFIP
- Beclin-1
5.2. Backbone Modifications
5.2.1. Variation in Stereochemistry (D-Peptides)
- HER2/HER3
- ERG
- NRP1
- MYB-CBP/P300
5.2.2. Extension of Backbone (Incorporation of β-Amino acids)
5.2.3. Shift in the Sidechains to Nitrogen Atoms (Peptoids)
- PRMT1
- β-catenin
- Vimentin
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ala | Alanine |
| Asp | Aspartic Acid |
| Bcl-2 | B-cell lymphoma-2 |
| Bcl-xL | B-cell lymphoma-extra large |
| BRCA1 | Breast cancer type 1 |
| CLL | Chronic lymphocytic leukaemia |
| CYFIP1 | Cytoplasmic FMR1-interacting protein 1 |
| Cys | Cysteine |
| CXCR4 | C-X-C Motif Chemokine Receptor 4 |
| GC-C | Guanylate cyclase-C |
| EGFR | Epidermal growth factor receptor |
| FDA | Food and Drug Administration |
| Gly | Glycine |
| HBS | Hydrogen bond surrogate |
| HDM2 | Human MDM2 |
| HER2 | Human epidermal growth factor 2 |
| His | Histidine |
| IBAT | Ileal bile acid transporter |
| IGF-R1 | Insulin-like Growth Factor-1 Receptor |
| GnRH | Growth hormone-releasing hormone |
| GLP-1 | Glucagon-like peptide-1 |
| IM | Intramuscular |
| IV | Intravenous |
| KRAS | Kristen Rat Sarcoma Viral oncogene homolog |
| mAbs | Monoclonal antibodies |
| MCR | Melanocortin receptor |
| MW | Molecular weight |
| NOD2 | Nucleotide-binding oligomerization domain-containing protein 2 |
| NPR-B | Natriuretic peptide receptor B |
| NSCLC | Non-small cell lung carcinoma |
| O | Oral |
| OncoPPIs | Oncogenic PPIs |
| PK | Pharmacokinetic |
| PPIs | Protein–protein interactions |
| PRMT1 | Protein arginine methyltransferase 1 |
| PROTAC | Peptide with proteolysis-targeting chimera |
| PTH1R | Parathyroid hormone-related receptor-1 |
| Ras | Rat sarcoma virus |
| RI-EIP1 | Retroinverso-ERG inhibitory peptides |
| RTK | Receptor tyrosine kinase |
| SC | Subcutaneous |
| SMDs | Small-molecule drugs |
| Sos | Son of sevenless |
| SSTR | Somatostatin receptor |
| TCF | T-cell factor |
| Trp | Tryptophan |
| Tyr | Tyrosine |
| WASF1 | Wiskott–Aldrich syndrome protein family member 1 |
References
- OECD. OECD Indicators: Health at a Glance 2023. Available online: https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-2023_7a7afb35-en (accessed on 7 May 2024).
- Cancer IAfRo. IARC Biennial Report 2022–2023. Available online: https://www.iarc.who.int/news-events/iarc-biennial-report-2022-2023/ (accessed on 7 May 2024).
- Phi, L.T.H.; Sari, I.N.; Yang, Y.-G. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef] [PubMed]
- Printezi, M.I.; Kilgallen, A.B.; Bond, M.J.G. Toxicity and efficacy of chronomodulated chemotherapy: A systematic review. Lancet Oncol. 2022, 23, e129–e143. [Google Scholar] [CrossRef]
- Patel, H.; Palekar, S.; Patel, A. Ibrutinib amorphous solid dispersions with enhanced dissolution at colonic pH for the localized treatment of colorectal cancer. Int. J. Pharm. 2023, 641, 123056. [Google Scholar] [CrossRef] [PubMed]
- Shuel, S.L. Targeted cancer therapies: Clinical pearls for primary care. Can. Fam. Physician 2022, 68, 515–518. [Google Scholar] [CrossRef]
- Victoir, B.; Croix, C.; Gouilleux, F. Targeted Therapeutic Strategies for the Treatment of Cancer. Cancers 2024, 16, 461. [Google Scholar] [CrossRef]
- Zhang, J.; Joshua, A.M.; Li, Y. Targeted therapy, immunotherapy, and small molecules and peptidomimetics as emerging immunoregulatory agents for melanoma. Cancer Lett. 2024, 586, 216633. [Google Scholar] [CrossRef]
- Gálffy, G.; Morócz, É.; Korompay, R. Targeted therapeutic options in early and metastatic NSCLC-overview. Pathol. Oncol. Res. 2024, 30, 1611715. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Sun, Y.; Wei, Y. Advances in targeted therapy and biomarker research in thyroid cancer. Front. Endocrinol. 2024, 15, 1372553. [Google Scholar] [CrossRef]
- Berger, M.F.; Mardis, E.R. The emerging clinical relevance of genomics in cancer medicine. Nat. Rev. Clin. Oncol. 2018, 15, 353–365. [Google Scholar] [CrossRef]
- Liu, G.H.; Chen, T.; Zhang, X. Small molecule inhibitors targeting the cancers. MedComm 2022, 3, e181. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef]
- Vecchio, I.; Tornali, C.; Bragazzi, N.L. The Discovery of Insulin: An Important Milestone in the History of Medicine. Front. Endocrinol. 2018, 9, 613. [Google Scholar] [CrossRef]
- Rossino, G.; Marchese, E.; Galli, G.; Verde, F.; Finizio, M.; Serra, M.; Linciano, P.; Collina, S. Peptides as Therapeutic Agents: Challenges and Opportunities in the Green Transition Era. Molecules 2023, 28, 7165. [Google Scholar] [CrossRef]
- Keating, G.M. Exenatide. Drugs 2005, 65, 1681–1692, discussion 1693–1695. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.J.; Jobe, L.J.; Martin, R. Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Clin. Ther. 2005, 27, 1500–1512. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Sobel, J.D. Anidulafungin: A novel echinocandin. Clin. Infect. Dis. 2006, 43, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Andries, M.; Glintborg, D.; Kvistborg, A. A 12-month randomized crossover study on the effects of lanreotide Autogel and octreotide long-acting repeatable on GH and IGF-l in patients with acromegaly. Clin. Endocrinol. 2008, 68, 473–480. [Google Scholar] [CrossRef]
- Klotz, L.; Boccon-Gibod, L.; Shore, N.D. The efficacy and safety of degarelix: A 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008, 102, 1531–1538. [Google Scholar] [CrossRef]
- Riedl, M. Icatibant, a Selective Bradykinin B2 Receptor Antagonist, Proves Effective and Safe in Treating the Symptoms of Hereditary Angioedema (HAE) Attacks. J. Allergy Clin. Immunol. 2008, 121, S103. [Google Scholar] [CrossRef]
- Astrup, A.; Rössner, S.; Van Gaal, L. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet 2009, 374, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Kager, L.; Pötschger, U.; Bielack, S. Review of mifamurtide in the treatment of patients with osteosarcoma. Ther. Clin. Risk Manag. 2010, 6, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Piekarz, R.L.; Frye, R.; Turner, M. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J. Clin. Oncol. 2009, 27, 5410–5417. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Stein, G.E.; Johnson, L.B. Telavancin: A novel lipoglycopeptide. Clin. Infect. Dis. 2009, 49, 1908–1914. [Google Scholar] [CrossRef]
- Falutz, J.; Mamputu, J.C.; Potvin, D. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: A pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. J. Clin. Endocrinol. Metab. 2010, 95, 4291–4304. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, I.M.; McHutchison, J.G.; Dusheiko, G. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 2011, 364, 2405–2416. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Gilroy, R.; Pertkiewicz, M. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut 2011, 60, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Lembo, A.J.; Lavins, B.J. Linaclotide for irritable bowel syndrome with constipation: A 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am. J. Gastroenterol. 2012, 107, 1702–1712. [Google Scholar] [CrossRef]
- Colao, A.; Petersenn, S.; Newell-Price, J. A 12-month phase 3 study of pasireotide in Cushing’s disease. N. Engl. J. Med. 2012, 366, 914–924. [Google Scholar] [CrossRef]
- Herndon, T.M.; Deisseroth, A.; Kaminskas, E.; Kane, R.C.; Koti, K.M.; Rothmann, M.D.; Habtemariam, B.; Bullock, J.; Bray, J.D.; Hawes, J.; et al. Food and Drug Administration approval: Carfilzomib for the treatment of multiple myeloma. Clin. Cancer Res. 2013, 19, 4559–4563. [Google Scholar] [CrossRef]
- Petersen, A.B.; Knop, F.K.; Christensen, M. Lixisenatide for the treatment of type 2 diabetes. Drugs Today 2013, 49, 537–553. [Google Scholar] [CrossRef]
- Langendonk, J.G.; Balwani, M.; Anderson, K.E.; Bonkovsky, H.L.; Anstey, A.V.; Bissell, D.M.; Bloomer, J.; Edwards, C.; Neumann, N.J.; Parker, C.; et al. Afamelanotide for Erythropoietic Protoporphyria. N. Engl. J. Med. 2015, 373, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Boucher Helen, W.; Wilcox, M.; Talbot George, H. Once-Weekly Dalbavancin versus Daily Conventional Therapy for Skin Infection. N. Engl. J. Med. 2014, 370, 2169–2179. [Google Scholar] [CrossRef]
- Corey, G.R.; Kabler, H.; Mehra, P. Single-Dose Oritavancin in the Treatment of Acute Bacterial Skin Infections. N. Engl. J. Med. 2014, 370, 2180–2190. [Google Scholar] [CrossRef]
- Zeuzem, S.; Jacobson Ira, M.; Baykal, T. Retreatment of HCV with ABT-450/r–Ombitasvir and Dasabuvir with Ribavirin. N. Engl. J. Med. 2014, 370, 1604–1614. [Google Scholar] [CrossRef]
- Blair, H.A. Etelcalcetide: First Global Approval. Drugs 2016, 76, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D.; Hattersley, G.; Riis, B.J. Effect of Abaloparatide vs. Placebo on New Vertebral Fractures in Postmenopausal Women with Osteoporosis: A Randomized Clinical Trial. JAMA 2016, 316, 722–733. [Google Scholar] [CrossRef]
- Al-Salama, Z.T.; Syed, Y.Y. Plecanatide: First Global Approval. Drugs 2017, 77, 593–598. [Google Scholar] [CrossRef]
- Khanna, A.; English, S.W.; Wang, X.S.; Ham, K.; Tumlin, J.; Szerlip, H.; Busse, L.W.; Altaweel, L.; Albertson, T.E.; Mackey, C.; et al. Angiotensin II for the Treatment of Vasodilatory Shock. N. Engl. J. Med. 2017, 377, 419–430. [Google Scholar] [CrossRef]
- Sorli, C.; Harashima, S.I.; Tsoukas, G.M. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): A double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 251–260. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Kingsberg, S.A.; Clayton, A.H.; Portman, D. Bremelanotide for the Treatment of Hypoactive Sexual Desire Disorder: Two Randomized Phase 3 Trials. Obstet. Gynecol. 2019, 134, 899–908. [Google Scholar] [CrossRef]
- Clément, K.; van den Akker, E.; Argente, J. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: Single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020, 8, 960–970. [Google Scholar] [CrossRef]
- Topf, J.; Wooldridge, T.; McCafferty, K. Efficacy of Difelikefalin for the Treatment of Moderate to Severe Pruritus in Hemodialysis Patients: Pooled Analysis of KALM-1 and KALM-2 Phase 3 Studies. Kidney Med. 2022, 4, 100512. [Google Scholar] [CrossRef]
- Rovin, B.H.; Teng, Y.K.O.; Ginzler, E.M. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Odevixibat: First Approval. Drugs 2021, 81, 1781–1786. [Google Scholar] [CrossRef]
- Savarirayan, R.; Tofts, L.; Irving, M. Safe and persistent growth-promoting effects of vosoritide in children with achondroplasia: 2-year results from an open-label, phase 3 extension study. Genet. Med. 2021, 23, 2443–2447. [Google Scholar] [CrossRef]
- Nikitin, D.; Lin, G.A.; Campbell, J.D. The effectiveness and value of tirzepatide for type 2 diabetes mellitus. J. Manag. Care Spec. Pharm. 2022, 28, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Neul, J.L.; Percy, A.K.; Benke, T.A. Trofinetide for the treatment of Rett syndrome: A randomized phase 3 study. Nat. Med. 2023, 29, 1468–1475. [Google Scholar] [CrossRef]
- Hoy, S.M. Motixafortide: First Approval. Drugs 2023, 83, 1635–1643. [Google Scholar] [CrossRef]
- Smith, D.J.; Lambrou, A.; Patel, P. SARS-CoV-2 Rebound with and without Use of COVID-19 Oral Antivirals. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Correction: Zilucoplan: First Approval. Drugs 2024, 84, 373. [Google Scholar] [CrossRef]
- Kang, C. Danicopan: First Approval. Drugs 2024, 84, 613–618. [Google Scholar] [CrossRef]
- Thanasomboon, R.; Kalapanulak, S.; Netrphan, S. Exploring dynamic protein-protein interactions in cassava through the integrative interactome network. Sci. Rep. 2020, 10, 6510. [Google Scholar] [CrossRef]
- Titeca, K.; Lemmens, I.; Tavernier, J. Discovering cellular protein-protein interactions: Technological strategies and opportunities. Mass Spectrom. Rev. 2019, 38, 79–111. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanov, U.; Emili, A. Protein-protein interaction networks: Probing disease mechanisms using model systems. Genome Med. 2013, 5, 37. [Google Scholar] [CrossRef]
- Cheng, F.; Zhao, J.; Wang, Y. Comprehensive characterization of protein–protein interactions perturbed by disease mutations. Nat. Genet. 2021, 53, 342–353. [Google Scholar] [CrossRef]
- Ryanm, D.P.; Matthews, J.M. Protein-protein interactions in human disease. Curr. Opin. Struct. Biol. 2005, 15, 441–446. [Google Scholar] [CrossRef]
- Garner, A.L.; Janda, K.D. Protein-protein interactions and cancer: Targeting the central dogma. Curr. Top. Med. Chem. 2011, 11, 258–280. [Google Scholar] [CrossRef]
- Li, Z.; Ivanov, A.A.; Su, R. The OncoPPi network of cancer-focused protein–protein interactions to inform biological insights and therapeutic strategies. Nat. Commun. 2017, 8, 14356. [Google Scholar] [CrossRef]
- Cheng, S.-S.; Yang, G.-J.; Wang, W. The design and development of covalent protein-protein interaction inhibitors for cancer treatment. J. Hematol. Oncol. 2020, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Kar, G.; Gursoy, A.; Keskin, O. Human cancer protein-protein interaction network: A structural perspective. PLoS Comput. Biol. 2009, 5, e1000601. [Google Scholar] [CrossRef]
- Wang, X.; Ni, D.; Liu, Y. Rational Design of Peptide-Based Inhibitors Disrupting Protein-Protein Interactions. Front. Chem. 2021, 9, 682675. [Google Scholar] [CrossRef]
- Cagiada, M.; Bottaro, S.; Lindemose, S. Discovering functionally important sites in proteins. Nat. Commun. 2023, 14, 4175. [Google Scholar] [CrossRef]
- Milo, R. What is the total number of protein molecules per cell volume? A call to rethink some published values. Bioessays 2013, 35, 1050–1055. [Google Scholar] [CrossRef]
- Mabonga, L.; Kappo, A.P. Protein-protein interaction modulators: Advances, successes and remaining challenges. Biophys. Rev. 2019, 11, 559–581. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Lee, G.M.; Sijbesma, E. Modulating protein-protein interaction networks in protein homeostasis. Curr. Opin. Chem. Biol. 2019, 50, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.E.; Bayly, A.R.; Abell, C. Small molecules, big targets: Drug discovery faces the protein–protein interaction challenge. Nat. Rev. Drug Discov. 2016, 15, 533–550. [Google Scholar] [CrossRef]
- Luck, K.; Kim, D.K.; Lambourne, L. A reference map of the human binary protein interactome. Nature 2020, 580, 402–408. [Google Scholar] [CrossRef]
- Lu, H.; Zhou, Q.; He, J. Recent advances in the development of protein–protein interactions modulators: Mechanisms and clinical trials. Signal Transduct. Target. Ther. 2020, 5, 213. [Google Scholar] [CrossRef]
- Burke, D.F.; Bryant, P.; Barrio-Hernandez, I. Towards a structurally resolved human protein interaction network. Nat. Struct. Mol. Biol. 2023, 30, 216–225. [Google Scholar] [CrossRef]
- Devkota, P.; Wuchty, S. Controllability analysis of molecular pathways points to proteins that control the entire interaction network. Sci. Rep. 2020, 10, 2943. [Google Scholar] [CrossRef] [PubMed]
- Milroy, L.-G.; Grossmann, T.N.; Hennig, S. Modulators of Protein–Protein Interactions. Chem. Rev. 2014, 114, 4695–4748. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Bergner, A.; Schwede, T. Modelling three-dimensional protein structures for applications in drug design. Drug Discov. Today 2014, 19, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Śledź, P.; Caflisch, A. Protein structure-based drug design: From docking to molecular dynamics. Curr. Opin. Struct. Biol. 2018, 48, 93–102. [Google Scholar] [CrossRef]
- Arkin, M.R.; Wells, J.A. Small-molecule inhibitors of protein–protein interactions: Progressing towards the dream. Nat. Rev. Drug Discov. 2004, 3, 301–317. [Google Scholar] [CrossRef]
- Smith, M.C.; Gestwicki, J.E. Features of protein–protein interactions that translate into potent inhibitors: Topology, surface area and affinity. Expert Rev. Mol. Med. 2012, 14, e16. [Google Scholar] [CrossRef]
- Buchwald, P. Small-molecule protein-protein interaction inhibitors: Therapeutic potential in light of molecular size, chemical space, and ligand binding efficiency considerations. IUBMB Life 2010, 62, 724–731. [Google Scholar] [CrossRef]
- Chène, P. Drugs Targeting Protein–Protein Interactions. ChemMedChem 2006, 1, 400–411. [Google Scholar] [CrossRef]
- Ni, D.; Lu, S.; Zhang, J. Emerging roles of allosteric modulators in the regulation of protein-protein interactions (PPIs): A new paradigm for PPI drug discovery. Med. Res. Rev. 2019, 39, 2314–2342. [Google Scholar] [CrossRef]
- Conte, L.L.; Chothia, C.; Janin, J. The atomic structure of protein-protein recognition sites. J. Mol. Biol. 1999, 285, 2177–2198. [Google Scholar] [CrossRef]
- Ran, X.; Gestwicki, J.E. Inhibitors of protein-protein interactions (PPIs): An analysis of scaffold choices and buried surface area. Curr. Opin. Chem. Biol. 2018, 44, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.A.; Khuri, F.R.; Fu, H. Targeting protein-protein interactions as an anticancer strategy. Trends Pharmacol. Sci. 2013, 34, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Bogan, A.A.; Thorn, K.S. Anatomy of hot spots in protein interfaces. J. Mol. Biol. 1998, 280, 1–9. [Google Scholar] [CrossRef]
- Halperin, I.; Wolfson, H.; Nussinov, R. Protein-Protein Interactions: Coupling of Structurally Conserved Residues and of Hot Spots across Interfaces. Implications for Docking. Structure 2004, 12, 1027–1038. [Google Scholar] [CrossRef]
- Geppert, T.; Hoy, B.; Wessler, S. Context-based identification of protein-protein interfaces and “hot-spot” residues. Chem. Biol. 2011, 18, 344–353. [Google Scholar] [CrossRef]
- Moreira, I.S.; Fernandes, P.A.; Ramos, M.J. Hot spots—A review of the protein-protein interface determinant amino-acid residues. Proteins 2007, 68, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Revennaugh, B.; Rusnak, L. The OncoPPi Portal: An integrative resource to explore and prioritize protein-protein interactions for cancer target discovery. Bioinformatics 2018, 34, 1183–1191. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Ting, L.; Bruckner, R.J. The BioPlex Network: A Systematic Exploration of the Human Interactome. Cell 2015, 162, 425–440. [Google Scholar] [CrossRef]
- Vo, T.V.; Das, J.; Meyer, M.J. A Proteome-wide Fission Yeast Interactome Reveals Network Evolution Principles from Yeasts to Human. Cell 2016, 164, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Sharifi Tabar, M.; Francis, H.; Yeo, D. Mapping oncogenic protein interactions for precision medicine. Int. J. Cancer 2022, 151, 7–19. [Google Scholar] [CrossRef]
- Oughtred, R.; Rust, J.; Chang, C. The BioGRID database: A comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Bruckner, R.J.; Navarrete-Perea, J. Dual proteome-scale networks reveal cell-specific remodeling of the human interactome. Cell 2021, 184, 3022–3040.e28. [Google Scholar] [CrossRef] [PubMed]
- Kerrien, S.; Aranda, B.; Breuza, L. The IntAct molecular interaction database in 2012. Nucleic Acids Res. 2012, 40, D841–D846. [Google Scholar] [CrossRef] [PubMed]
- Chatr-aryamontri, A.; Ceol, A.; Palazzi, L.M. MINT: The Molecular INTeraction database. Nucleic Acids Res. 2007, 35, D572–D574. [Google Scholar] [CrossRef] [PubMed]
- Alanis-Lobato, G.; Andrade-Navarro, M.A.; Schaefer, M.H. HIPPIE v2.0: Enhancing meaningfulness and reliability of protein-protein interaction networks. Nucleic Acids Res. 2017, 45, D408–D414. [Google Scholar] [CrossRef]
- Du, Y.; Cai, M.; Xing, X. PINA 3.0: Mining cancer interactome. Nucleic Acids Res. 2021, 49, D1351–D1357. [Google Scholar] [CrossRef]
- Wen, B.; Zhao, L.; Wang, Y. Nanobodies targeting the interaction interface of programmed death receptor 1 (PD-1)/PD-1 ligand 1 (PD-1/PD-L1). Prep. Biochem. Biotechnol. 2019, 50, 252–259. [Google Scholar] [CrossRef]
- Xie, X.; Yu, T.; Li, X. Recent advances in targeting the “undruggable” proteins: From drug discovery to clinical trials. Signal Transduct. Target. Ther. 2023, 8, 335. [Google Scholar] [CrossRef]
- Deeks, E.D. Venetoclax: First Global Approval. Drugs 2016, 76, 979–987. [Google Scholar] [CrossRef]
- Langer, C.J. Epidermal growth factor receptor inhibition in mutation-positive non-small-cell lung cancer: Is afatinib better or simply newer? J. Clin. Oncol. 2013, 31, 3303–3306. [Google Scholar] [PubMed]
- Blair, H.A. Sotorasib: First Approval. Drugs 2021, 81, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J. New horizons in therapeutic antibody discovery: Opportunities and challenges versus small-molecule therapeutics. J. Biomol. Screen. 2015, 20, 437–453. [Google Scholar] [CrossRef]
- Fischer, G.; Rossmann, M.; Hyvönen, M. Alternative modulation of protein–protein interactions by small molecules. Curr. Opin. Biotechnol. 2015, 35, 78–85. [Google Scholar] [CrossRef]
- Poduval, P.; Parsekar, S.; Meena, S.N. Chapter 10—Small molecules vs. biologics. In New Horizons in Natural Compound Research; Academic Press: Cambridge, MA, USA, 2023; pp. 179–199. [Google Scholar]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Cunningham, A.D.; Qvit, N.; Mochly-Rosen, D. Peptides and peptidomimetics as regulators of protein-protein interactions. Curr. Opin. Struct. Biol. 2017, 44, 59–66. [Google Scholar] [CrossRef]
- Di, L. Strategic Approaches to Optimizing Peptide ADME Properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef]
- London, N.; Raveh, B.; Schueler-Furman, O. Druggable protein–protein interactions—From hot spots to hot segments. Curr. Opin. Chem. Biol. 2013, 17, 952–959. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.; Meibohm, B. Pharmacokinetics and Pharmacokinetic–Pharmacodynamic Correlations of Therapeutic Peptides. Clin. Pharmacokinet. 2013, 52, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Vadevoo, S.M.P.; Gurung, S.; Lee, H.-S. Peptides as multifunctional players in cancer therapy. Exp. Mol. Med. 2023, 55, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Huang, L.; Shen, B. Rational cyclization-based minimization of entropy penalty upon the binding of Nrf2-derived linear peptides to Keap1: A new strategy to improve therapeutic peptide activity against sepsis. Biophys. Chem. 2019, 244, 22–28. [Google Scholar] [CrossRef]
- Philippe, G.J.B.; Craik, D.J.; Henriques, S.T. Converting peptides into drugs targeting intracellular protein–protein interactions. Drug Discov. Today 2021, 26, 1521–1531. [Google Scholar] [CrossRef]
- Touti, F.; Gates, Z.P.; Bandyopadhyay, A. In-solution enrichment identifies peptide inhibitors of protein-protein interactions. Nat. Chem. Biol. 2019, 15, 410–418. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, Z.; Paul, P.K. Oral delivery of proteins and peptides: Challenges, status quo and future perspectives. Acta Pharm. Sin. B 2021, 11, 2416–2448. [Google Scholar] [CrossRef]
- Ricardo, M.G.; Ali, A.; Plewka, J. Multicomponent Peptide Stapling as a Diversity-Driven Tool for the Development of Inhibitors of Protein-Protein Interactions. Angew. Chem. Int. Ed. Engl. 2020, 59, 5235–5241. [Google Scholar] [CrossRef]
- Yang, P.Y.; Zou, H.; Lee, C. Stapled, Long-Acting Glucagon-like Peptide 2 Analog with Efficacy in Dextran Sodium Sulfate Induced Mouse Colitis Models. J. Med. Chem. 2018, 61, 3218–3223. [Google Scholar] [CrossRef]
- Dougherty, P.G.; Wen, J.; Pan, X. Enhancing the Cell Permeability of Stapled Peptides with a Cyclic Cell-Penetrating Peptide. J. Med. Chem. 2019, 62, 10098–10107. [Google Scholar] [CrossRef]
- He, J.; Ghosh, P.; Nitsche, C. Biocompatible strategies for peptide macrocyclisation. Chem. Sci. 2024, 15, 2300–2322. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dawber, R.S.; Zhang, P. Peptide-based inhibitors of protein-protein interactions: Biophysical, structural and cellular consequences of introducing a constraint. Chem. Sci. 2021, 12, 5977–5993. [Google Scholar] [CrossRef]
- Miller, S.E.; Thomson, P.F.; Arora, P.S. Synthesis of hydrogen-bond surrogate α-helices as inhibitors of protein-protein interactions. Curr. Protoc. Chem. Biol. 2014, 6, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Douse, C.H.; Maas, S.J.; Thomas, J.C. Crystal structures of stapled and hydrogen bond surrogate peptides targeting a fully buried protein-helix interaction. ACS Chem. Biol. 2014, 9, 2204–2209. [Google Scholar] [CrossRef]
- Chapman, R.N.; Dimartino, G.; Arora, P.S. A highly stable short alpha-helix constrained by a main-chain hydrogen-bond surrogate. J. Am. Chem. Soc. 2004, 126, 12252–12253. [Google Scholar] [CrossRef]
- Lamers, C. Overcoming the Shortcomings of Peptide-Based Therapeutics. Future Drug Discov. 2022, 4, FDD75. [Google Scholar] [CrossRef]
- Wójcik, P.; Berlicki, Ł. Peptide-based inhibitors of protein-protein interactions. Bioorg. Med. Chem. Lett. 2016, 26, 707–713. [Google Scholar] [CrossRef]
- Vernall, A.J.; Cassidy, P.; Alewood, P.F. A single alpha-helical turn stabilized by replacement of an internal hydrogen bond with a covalent ethylene bridge. Angew. Chem. Int. Ed. Engl. 2009, 48, 5675–5678. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Coric, P.; Zhu, K. Synthesis and characterization of water-soluble macrocyclic peptides stabilizing protein α-turn. Org. Biomol. Chem. 2018, 16, 459–471. [Google Scholar] [CrossRef]
- Mahon, A.B.; Arora, P.S. Design, synthesis and protein-targeting properties of thioether-linked hydrogen bond surrogate helices. Chem. Commun. 2012, 48, 1416–1418. [Google Scholar] [CrossRef]
- Pal, S. Impact of Hydrogen-Bond Surrogate Model on Helix Stabilization and Development of Protein-Protein Interaction Inhibitors. ChemistrySelect 2023, 8, e202204207. [Google Scholar] [CrossRef]
- Wang, D.; Liao, W.; Arora, P.S. Enhanced metabolic stability and protein-binding properties of artificial alpha helices derived from a hydrogen-bond surrogate: Application to Bcl-xL. Angew. Chem. Int. Ed. Engl. 2005, 44, 6525–6529. [Google Scholar] [CrossRef]
- Nag, S.; Zhang, X.; Srivenugopal, K.S. Targeting MDM2-p53 interaction for cancer therapy: Are we there yet? Curr. Med. Chem. 2014, 21, 553–574. [Google Scholar] [CrossRef] [PubMed]
- Henchey, L.K.; Porter, J.R.; Ghosh, I. High specificity in protein recognition by hydrogen-bond-surrogate α-helices: Selective inhibition of the p53/MDM2 complex. Chembiochem 2010, 11, 2104–2107. [Google Scholar] [CrossRef]
- Hillig, R.C.; Bader, B. Chapter Six—Targeting RAS oncogenesis with SOS1 inhibitors. In Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2022; pp. 169–203. [Google Scholar]
- Patgiri, A.; Yadav, K.K.; Arora, P.S. An orthosteric inhibitor of the Ras-Sos interaction. Nat. Chem. Biol. 2011, 7, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Obrecht, D.; Chevalier, E.; Moehle, K. β-Hairpin protein epitope mimetic technology in drug discovery. Drug Discov. Today Technol. 2012, 9, e63–e69. [Google Scholar] [CrossRef]
- Wen, J.; Liao, H.; Stachowski, K. Rational design of cell-permeable cyclic peptides containing a d-Pro-l-Pro motif. Bioorg. Med. Chem. 2020, 28, 115711. [Google Scholar] [CrossRef]
- Fasan, R.; Dias, R.L.; Moehle, K. Using a beta-hairpin to mimic an alpha-helix: Cyclic peptidomimetic inhibitors of the p53-HDM2 protein-protein interaction. Angew. Chem. Int. Ed. Engl. 2004, 43, 2109–2112. [Google Scholar] [CrossRef]
- Perry, S.R.; Hill, T.A.; de Araujo, D. Contiguous hydrophobic and charged surface patches in short helix-constrained peptides drive cell permeability. Org. Biomol. Chem. 2018, 16, 367–371. [Google Scholar] [CrossRef]
- Ganesh Kumar, M.; Mali, S.M.; Raja, K.M. Design of stable β-hairpin mimetics through backbone disulfide bonds. Org. Lett. 2015, 17, 230–233. [Google Scholar] [CrossRef]
- Makwana, K.M.; Mahalakshmi, R. Trp-Trp Cross-Linking: A Structure–Reactivity Relationship in the Formation and Design of Hyperstable Peptide β-Hairpin and α-Helix Scaffolds. Org. Lett. 2015, 17, 2498–2501. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.A. β-Hairpin Peptidomimetics: Design, Structures and Biological Activities. Acc. Chem. Res. 2008, 41, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Song, Y.; Su, Y. Effect of the hairpin structure of peptide inhibitors on the blockade of PD-1/PD-L1 axis. Biochem. Biophys. Res. Commun. 2020, 527, 453–457. [Google Scholar] [CrossRef]
- Ji, Y.; Majumder, S.; Millard, M. In Vivo Activation of the p53 Tumor Suppressor Pathway by an Engineered Cyclotide. J. Am. Chem. Soc. 2013, 135, 11623–11633. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, S.; Erdelyi, M. Solution-State Preorganization of Cyclic β-Hairpin Ligands Determines Binding Mechanism and Affinities for MDM2. J. Chem. Inf. Model. 2021, 61, 2353–2367. [Google Scholar] [CrossRef]
- Batalha, I.L.; Lychko, I.; Branco, R.J.F. β-Hairpins as peptidomimetics of human phosphoprotein-binding domains. Org. Biomol. Chem. 2019, 17, 3996–4004. [Google Scholar] [CrossRef]
- Walensky, L.D.; Bird, G.H. Hydrocarbon-Stapled Peptides: Principles, Practice, and Progress. J. Med. Chem. 2014, 57, 6275–6288. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhang, W.-D. Stapled Helical Peptides Bearing Different Anchoring Residues. Chem. Rev. 2020, 120, 10079–10144. [Google Scholar] [CrossRef]
- Guerlavais, V.; Sawyer, T.K. Chapter Twenty-One—Advancements in Stapled Peptide Drug Discovery & Development. In Annual Reports in Medicinal Chemistry; Academic Press: Cambridge, MA, USA, 2014; pp. 331–345. [Google Scholar]
- Unarta, I.C.; Xu, J.; Shang, Y. Entropy of stapled peptide inhibitors in free state is the major contributor to the improvement of binding affinity with the GK domain. RSC Chem. Biol. 2021, 2, 1274–1284. [Google Scholar] [CrossRef]
- Quartararo, J.S.; Wu, P.; Kritzer, J.A. Peptide bicycles that inhibit the Grb2 SH2 domain. ChemBioChem 2012, 13, 1490–1496. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Grossmann, T.N.; Verdine, G.L. Synthesis of all-hydrocarbon stapled α-helical peptides by ring-closing olefin metathesis. Nat. Protoc. 2011, 6, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.N.; Driver, R.W.; Beyer, R.L. Helix Nucleation by the Smallest Known α-Helix in Water. Angew. Chem. Int. Ed. Engl. 2016, 55, 8275–8279. [Google Scholar] [CrossRef] [PubMed]
- Flint, D.G.; Kumita, J.R.; Smart, O.S. Using an azobenzene cross-linker to either increase or decrease peptide helix content upon trans-to-cis photoisomerization. Chem. Biol. 2002, 9, 391–397. [Google Scholar] [CrossRef]
- Jo, H.; Meinhardt, N.; Wu, Y. Development of α-Helical Calpain Probes by Mimicking a Natural Protein–Protein Interaction. J. Am. Chem. Soc. 2012, 134, 17704–17713. [Google Scholar] [CrossRef]
- Cantel, S.; Le Chevalier Isaad, A.; Scrima, M. Synthesis and Conformational Analysis of a Cyclic Peptide Obtained via i to i+4 Intramolecular Side-Chain to Side-Chain Azide−Alkyne 1,3-Dipolar Cycloaddition. J. Org. Chem. 2008, 73, 5663–5674. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Li, R. Fine Tuning the Properties of Stapled Peptides by Stereogenic α-Amino Acid Bridges. Chemistry 2023, 29, e202203624. [Google Scholar] [CrossRef]
- Bernal, F.; Wade, M.; Godes, M. A stapled p53 helix overcomes HDMX-mediated suppression of p53. Cancer Cell 2010, 18, 411–422. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Fu, Y. Therapeutic stapled peptides: Efficacy and molecular targets. Pharmacol. Res. 2024, 203, 107137. [Google Scholar] [CrossRef]
- Li, X.; Tolbert, W.D.; Hu, H.G. Dithiocarbamate-inspired side chain stapling chemistry for peptide drug design. Chem. Sci. 2019, 10, 1522–1530. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Li, Y. Design of stapled peptide-based PROTACs for MDM2/MDMX atypical degradation and tumor suppression. Theranostics 2022, 12, 6665–6681. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Bai, H. Unleashing the potential of natural biological peptide Macropin: Hydrocarbon stapling for effective breast cancer treatment. Bioorg. Chem. 2023, 140, 106770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chou, D.H. A Thiol-Ene Coupling Approach to Native Peptide Stapling and Macrocyclization. Angew. Chem. Int. Ed. Engl. 2015, 54, 10931–10934. [Google Scholar] [CrossRef]
- Montgomery, J.E.; Donnelly, J.A.; Fanning, S.W. Versatile Peptide Macrocyclization with Diels–Alder Cycloadditions. J. Am. Chem. Soc. 2019, 141, 16374–16381. [Google Scholar] [CrossRef] [PubMed]
- Méndez, Y.; Vasco, A.V.; Ivey, G. Merging the Isonitrile-Tetrazine (4+1) Cycloaddition and the Ugi Four-Component Reaction into a Single Multicomponent Process. Angew. Chem. Int. Ed. Engl. 2023, 62, e202311186. [Google Scholar] [CrossRef]
- Guerlavais, V.; Sawyer, T.K.; Carvajal, L. Discovery of Sulanemadlin (ALRN-6924), the First Cell-Permeating, Stabilized α-Helical Peptide in Clinical Development. J. Med. Chem. 2023, 66, 9401–9417. [Google Scholar] [CrossRef]
- Walensky, L.D.; Kung, A.L.; Escher, I. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 2004, 305, 1466–1470. [Google Scholar] [CrossRef]
- Grossmann, T.N.; Yeh, J.T.; Bowman, B.R. Inhibition of oncogenic Wnt signaling through direct targeting of β-catenin. Proc. Natl. Acad. Sci. USA 2012, 109, 17942–17947. [Google Scholar] [CrossRef]
- Liao, H.; Li, X.; Zhao, L. A PROTAC peptide induces durable β-catenin degradation and suppresses Wnt-dependent intestinal cancer. Cell Discov. 2020, 6, 35. [Google Scholar] [CrossRef]
- Teng, Y.; Bahassan, A.; Dong, D. Targeting the WASF3-CYFIP1 Complex Using Stapled Peptides Suppresses Cancer Cell Invasion. Cancer Res. 2016, 76, 965–973. [Google Scholar] [CrossRef]
- Wu, S.; He, Y.; Qiu, X. Targeting the potent Beclin 1-UVRAG coiled-coil interaction with designed peptides enhances autophagy and endolysosomal trafficking. Proc. Natl. Acad. Sci. USA 2018, 115, E5669–E5678. [Google Scholar] [CrossRef]
- Yang, Q.; Qiu, X.; Zhang, X. Optimization of Beclin 1-Targeting Stapled Peptides by Staple Scanning Leads to Enhanced Antiproliferative Potency in Cancer Cells. J. Med. Chem. 2021, 64, 13475–13486. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Xu, B. Inspiration from the mirror: D-amino acid containing peptides in biomedical approaches. Biomol. Concepts 2016, 7, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.; Mackay, A.S.; Kambanis, L. Synthesis and applications of mirror-image proteins. Nat. Rev. Chem. 2023, 7, 383–404. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Xie, Z. D-Peptides as Recognition Molecules and Therapeutic Agents. Chem. Rec. 2016, 16, 1772–1786. [Google Scholar] [CrossRef]
- Liang, G.; Yang, Z.; Zhang, R. Supramolecular hydrogel of a D-amino acid dipeptide for controlled drug release in vivo. Langmuir 2009, 25, 8419–8422. [Google Scholar] [CrossRef]
- Pallerla, S.; Naik, H.; Singh, S. Design of cyclic and d-amino acids containing peptidomimetics for inhibition of protein-protein interactions of HER2-HER3. J. Pept. Sci. 2018, 24, e3066. [Google Scholar] [CrossRef]
- Chen, X.; Fan, Z.; Chen, Y. Retro-inverso carbohydrate mimetic peptides with annexin1-binding selectivity, are stable in vivo, and target tumor vasculature. PLoS ONE 2013, 8, e80390. [Google Scholar] [CrossRef]
- Zhan, C.; Zhao, L.; Wei, X. An ultrahigh affinity d-peptide antagonist Of MDM2. J. Med. Chem. 2012, 55, 6237–6241. [Google Scholar] [CrossRef]
- Lander, A.J.; Jin, Y.; Luk, L.Y.P. D-Peptide and D-Protein Technology: Recent Advances, Challenges, and Opportunities. ChemBioChem 2023, 24, e202200537. [Google Scholar] [CrossRef]
- Li, C.; Pazgier, M.; Li, J. Limitations of peptide retro-inverso isomerization in molecular mimicry. J. Biol. Chem. 2010, 285, 19572–19581. [Google Scholar] [CrossRef]
- Li, C.; Zhan, C.; Zhao, L. Functional consequences of retro-inverso isomerization of a miniature protein inhibitor of the p53–MDM2 interaction. Bioorg. Med. Chem. 2013, 21, 4045–4050. [Google Scholar] [CrossRef]
- Schumacher, T.N.M.; Mayr, L.M.; Minor, D.L. Identification of d-Peptide Ligands Through Mirror-Image Phage Display. Science 1996, 271, 1854–1857. [Google Scholar] [CrossRef]
- Liu, M.; Li, C.; Pazgier, M. D-peptide inhibitors of the p53–MDM2 interaction for targeted molecular therapy of malignant neoplasms. Proc. Natl. Acad. Sci. USA 2010, 107, 14321–14326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zuo, C.; Li, W. A Novel d-Peptide Identified by Mirror-Image Phage Display Blocks TIGIT/PVR for Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2020, 59, 15114–15118. [Google Scholar] [CrossRef] [PubMed]
- Uppalapati, M.; Lee, D.J.; Mandal, K. A Potent d-Protein Antagonist of VEGF-A is Nonimmunogenic, Metabolically Stable, and Longer-Circulating in Vivo. ACS Chem. Biol. 2016, 11, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pazgier, M.; Li, C. A left-handed solution to peptide inhibition of the p53-MDM2 interaction. Angew. Chem. Int. Ed. Engl. 2010, 49, 3649–3652. [Google Scholar] [CrossRef]
- Chang, H.N.; Liu, B.Y.; Qi, Y.K.; Zhou, Y.; Chen, Y.P.; Pan, K.M.; Li, W.W.; Zhou, X.M.; Ma, W.W.; Fu, C.Y.; et al. Blocking of the PD-1/PD-L1 Interaction by a D-Peptide Antagonist for Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2015, 54, 11760–11764. [Google Scholar] [CrossRef]
- Mandal, K.; Uppalapati, M.; Ault-Riché, D. Chemical synthesis and X-ray structure of a heterochiral {D-protein antagonist plus vascular endothelial growth factor} protein complex by racemic crystallography. Proc. Natl. Acad. Sci. USA 2012, 109, 14779–14784. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, Y.; Asangani, I.A. Development of peptidomimetic inhibitors of the ERG gene fusion product in prostate cancer. Cancer Cell 2017, 31, 532–548. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, Y.; Yang, M. Highly Potent, Selective, Biostable, and Cell-Permeable Cyclic d-Peptide for Dual-Targeting Therapy of Lung Cancer. J. Am. Chem. Soc. 2022, 144, 7117–7128. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Tan, Y. An NRP1/MDM2-Targeted D-Peptide Supramolecular Nanomedicine for High-Efficacy and Low-Toxic Liver Cancer Therapy. Adv. Healthc. Mater. 2021, 10, 2002197. [Google Scholar] [CrossRef]
- Ramaswamy, K.; Forbes, L.; Minuesa, G. Peptidomimetic blockade of MYB in acute myeloid leukemia. Nat. Commun. 2018, 9, 110. [Google Scholar] [CrossRef]
- Cabrele, C.; Martinek, T.A.; Reiser, O. Peptides Containing β-Amino Acid Patterns: Challenges and Successes in Medicinal Chemistry. J. Med. Chem. 2014, 57, 9718–9739. [Google Scholar] [CrossRef]
- Horne, W.S. Peptide and peptoid foldamers in medicinal chemistry. Expert Opin. Drug Discov. 2011, 6, 1247–1262. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.M.; Wirz, B.; Schweitzer, A. ADME Investigations of Unnatural Peptides: Distribution of a 14C-Labeled β 3-Octaarginine in Rats. Chem. Biodivers. 2007, 4, 1413–1437. [Google Scholar] [CrossRef]
- Seebach, D.; Beck, A.K.; Bierbaum, D.J. The world of beta- and gamma-peptides comprised of homologated proteinogenic amino acids and other components. Chem. Biodivers. 2004, 1, 1111–1239. [Google Scholar] [CrossRef] [PubMed]
- Gellman, S.H. Foldamers: A manifesto. Acc. Chem. Res. 1998, 31, 173–180. [Google Scholar]
- Martinek, T.A.; Fülöp, F. Peptidic foldamers: Ramping up diversity. Chem. Soc. Rev. 2012, 41, 687–702. [Google Scholar] [CrossRef]
- Fülöp, F.; Martinek, T.A.; Tóth, G.K. Application of alicyclic β-amino acids in peptide chemistry. Chem. Soc. Rev. 2006, 35, 323–334. [Google Scholar] [CrossRef]
- Bautista, A.D.; Appelbaum, J.S.; Craig, C.J. Bridged beta(3)-peptide inhibitors of p53-hDM2 complexation: Correlation between affinity and cell permeability. J. Am. Chem. Soc. 2010, 132, 2904–2906. [Google Scholar] [CrossRef]
- Harker, E.A.; Daniels, D.S.; Guarracino, D.A. β-Peptides with improved affinity for hDM2 and hDMX. Bioorg. Med. Chem. 2009, 17, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Peterson-Kaufman, K.J.; Haase, H.S.; Boersma, M.D. Residue-Based Preorganization of BH3-Derived α/β-Peptides: Modulating Affinity, Selectivity and Proteolytic Susceptibility in α-Helix Mimics. ACS Chem. Biol. 2015, 10, 1667–1675. [Google Scholar] [CrossRef]
- Haase, H.S.; Peterson-Kaufman, K.J.; Lan Levengood, S.K.; Checco, J.W.; Murphy, W.L.; Gellman, S.H. Extending Foldamer Design beyond α-Helix Mimicry: α/β-Peptide Inhibitors of Vascular Endothelial Growth Factor Signaling. J. Am. Chem. Soc. 2012, 134, 7652–7655. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.D.; Xuan, S.; Molchanova, N. Chapter Eight—Submonomer synthesis of sequence defined peptoids with diverse side-chains. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2021; pp. 241–270. [Google Scholar]
- Zhu, J.; Chen, S.; Liu, Z. Recent advances in anticancer peptoids. Bioorg. Chem. 2023, 139, 106686. [Google Scholar] [CrossRef]
- Rosales, A.M.; Murnen, H.K.; Kline, S.R. Determination of the persistence length of helical and non-helical polypeptoids in solution. Soft Matter 2012, 8, 3673–3680. [Google Scholar] [CrossRef]
- Huang, W.; Seo, J.; Willingham, S.B. Learning from host-defense peptides: Cationic, amphipathic peptoids with potent anticancer activity. PLoS ONE 2014, 9, e90397. [Google Scholar] [CrossRef]
- Clapperton, A.M.; Babi, J.; Tran, H. A Field Guide to Optimizing Peptoid Synthesis. ACS Polym. Au 2022, 2, 417–429. [Google Scholar] [CrossRef]
- Abdildinova, A.; Kurth, M.J.; Gong, Y.D. Solid-Phase Synthesis of Peptidomimetics with Peptide Backbone Modifications. Asian J. Org. Chem. 2021, 10, 2300–2317. [Google Scholar] [CrossRef]
- Brekker, M.A.; Sartawi, T.; Sawatzky, T.M. A peptoid-based inhibitor of protein arginine methyltransferase 1 (PRMT1) induces apoptosis and autophagy in cancer cells. J. Biol. Chem. 2022, 298, 102205. [Google Scholar] [CrossRef]
- Schneider, J.A.; Craven, T.W.; Kasper, A.C. Design of Peptoid-peptide Macrocycles to Inhibit the β-catenin TCF Interaction in Prostate Cancer. Nat. Commun. 2018, 9, 4396. [Google Scholar] [CrossRef]
- Zhang, H.; Udugamasooriya, D.G. Optimization of a cell surface vimentin binding peptoid to extract antagonist effect on lung cancer cells. Bioorg. Chem. 2022, 129, 106113. [Google Scholar] [CrossRef] [PubMed]
| Peptide | Approval Year | Molar Mass (g/mol) | Target | Half-Life | Administration | Therapeutic Application |
|---|---|---|---|---|---|---|
| Exenatide [17] | 2005 | 4186.63 | GLP-1 | 2.4 h | SC | Type 2 diabetes mellitus |
| Pramlintide [18] | 2005 | 3949.44 | Calcitonin receptor | 48 min | SC | Type 1 and 2 diabetes mellitus |
| Anidulafungin [19] | 2006 | 1140.254 | beta-1,3-D-glucan synthase | 40–50 h | IV, O | Fungal infections: Candida infections, Aspergillus infections, and esophageal candidiasis |
| Lanreotide [20] | 2007 | 1096.33 | SSTR | 22 days | SC | Neuroendocrine tumours and acromegaly |
| Degarelix [21] | 2008 | 1632.29 | GNRH receptor | 41.5–70.2 days | SC | Prostate cancer |
| Icatibant [22] | 2008 | 1304.54 | Bradykinin B2 receptor | 1–2 h | SC | Hereditary Angioedema |
| Liraglutide [23] | 2009 | 3751.262 | GLP-1 | 13 h | SC | Type 2 Diabetes Mellitus |
| Mifamurtide [24] | 2009 | 1237.518 | NOD2 | 18 h | IV | High-grade resectable non-metastatic osteosarcoma |
| Romidepsin [25] | 2009 | 540.69 | Histone deacetylase | 3 h | IV | Cutaneous T-cell lymphoma (CTCL) or/and peripheral T-cell lymphoma (PTCL) |
| Telavancin [26] | 2009 | 1755.65 | Peptidoglycan | 8 ± 1.5 h | IV | Complicated skin and skin structure infections (cSSSI) caused by gram-positive bacteria like methicillin-susceptible or -resistant Staphylococcus aureus |
| Tesamorelin [27] | 2010 | 5005.76 | GnRH receptors | 38 min | SC | Reduction of lipodystrophy in HIV-infected patients |
| Telaprevir [28] | 2011 | 679.863 | NS3/4A viral protease | 4 h | O | Chronic Hepatitis C genotype 1 infection in adults |
| Teduglutide [29] | 2012 | 3752.13 | GLP-2 | 1.3 h | SC | Short bowel syndrome (SBS) |
| Linaclotide [30] | 2012 | 1526.73 | GC-C receptor | O | Irritable bowel syndrome (IBS) | |
| Pasireotide [31] | 2012 | 1047.227 | SSTR | 12 h | SC, IM | Cushing’s disease |
| Carfilzomib [32] | 2012 | 719.924 | Proteosome | ≤1 h | IV | Multiple myeloma |
| Lixisenatide [33] | 2013 | 4858.56 | GLP-1 | 3 h | SC | Type 2 Diabetes mellitus |
| Afamelanotide [34] | 2014 | 1646.874 | MCR | 30 min | SC | Prevention of phototoxicity in adult patients with erythropoietic protoporphyria (EPP) |
| Dalbavancin [35] | 2014 | 1816.7 | Peptidoglycan | 346 h | IV | Adult patients with acute bacterial skin and skin structure infections (ABSSSI) |
| Oritavancin [36] | 2014 | 1793.12 | Peptidoglycan | 245 h | IV | Skin infections |
| Ombitasvir [37] | 2014 | 894.12 | NS5A | 21–25 h | O | Genotype 4 chronic hepatitis C virus (HCV) infection |
| Etelcalcetide [38] | 2016 | 1048.26 | CaSR | 3–4 days | IV | Secondary hyperparathyroidism (HPT) |
| Abaloparatide [39] | 2017 | 3960.657 | PTH1R | 1.7 h | SC | Osteoporosis |
| Plecanatide [40] | 2017 | 1681.89 | GC-C receptor | O | Chronic idiopathic constipation (CIC) | |
| Angiotensin II [41] | 2017 | 1046.197 | ATR | <1 min | IV | Sepsis and septic Shock |
| Semaglutide [42] | 2017 | 4113.641 | GLP-1 | 168 h | SC, O | Type 2 diabetes mellitus |
| Lutetium (Lu)177-Dotatate [43] | 2018 | 1609.55 | SSTR | 71 h | IV | Somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors |
| Bremelanotide [44] | 2019 | 1025.182 | MCR | 2.7 h | SC | Hypoactive sexual desire disorder |
| Setmelanotide [45] | 2020 | 1117.3 | MCR | 11 h | SC | Chronic weight management of obesity |
| Difelikefalin [46] | 2021 | 679.863 | kappa opioid receptor | 23–31 h | IV | Moderate-to-severe pruritus associated with chronic kidney disease |
| Voclosporin [47] | 2021 | 1214.646 | Calcineurin | 30 h | O | Lupus nephritis |
| Odevixibat [48] | 2021 | 740.93 | IBAT | 2.36 h | O | Pruritus in patients with progressive familial intrahepatic cholestasis. |
| Vosoritide [49] | 2021 | 4102.78 | NPR-B | 21–27.9 min | SC | Pediatric patients with achondroplasia |
| Tirzepatide [50] | 2022 | 4813.53 | GLP-1 | 5 days | SC | Type 2 diabetes mellitus |
| Trofinetide [51] | 2023 | 315.14 | IGF-1R | 1.5 h | O | Rett syndrome |
| Motixafortide [52] | 2023 | 2159.6 | CXCR4 | 2 h | SC | Multiple myeloma |
| Nirmatrelvir [53] | 2023 | 499.5 | Mpro | 6.05 h | O | Mild-to-moderate COVID-19 |
| Zilucoplan [54] | 2023 | 3562.23 | Complement protein C5 | 172 h | SC | Myasthenia gravis |
| Danicopan [55] | 2024 | 580.4 | Complement factor D | 7.9 h | O | Extravascular hemolysis in patients that have paroxysmal nocturnal hemoglobinuria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, A.L.; Cavaleiro, C.T.; Castanho, M.A.R.B.; Neves, V.; Cavaco, M. The Potential of Peptide-Based Inhibitors in Disrupting Protein–Protein Interactions for Targeted Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 3117. https://doi.org/10.3390/ijms26073117
Afonso AL, Cavaleiro CT, Castanho MARB, Neves V, Cavaco M. The Potential of Peptide-Based Inhibitors in Disrupting Protein–Protein Interactions for Targeted Cancer Therapy. International Journal of Molecular Sciences. 2025; 26(7):3117. https://doi.org/10.3390/ijms26073117
Chicago/Turabian StyleAfonso, Alexandra L., Catarina T. Cavaleiro, Miguel A. R. B. Castanho, Vera Neves, and Marco Cavaco. 2025. "The Potential of Peptide-Based Inhibitors in Disrupting Protein–Protein Interactions for Targeted Cancer Therapy" International Journal of Molecular Sciences 26, no. 7: 3117. https://doi.org/10.3390/ijms26073117
APA StyleAfonso, A. L., Cavaleiro, C. T., Castanho, M. A. R. B., Neves, V., & Cavaco, M. (2025). The Potential of Peptide-Based Inhibitors in Disrupting Protein–Protein Interactions for Targeted Cancer Therapy. International Journal of Molecular Sciences, 26(7), 3117. https://doi.org/10.3390/ijms26073117






