Exploring the Interaction of Tumor-Derived Exosomes and Mesenchymal Stem Cells in Tumor Biology
Abstract
1. Introduction
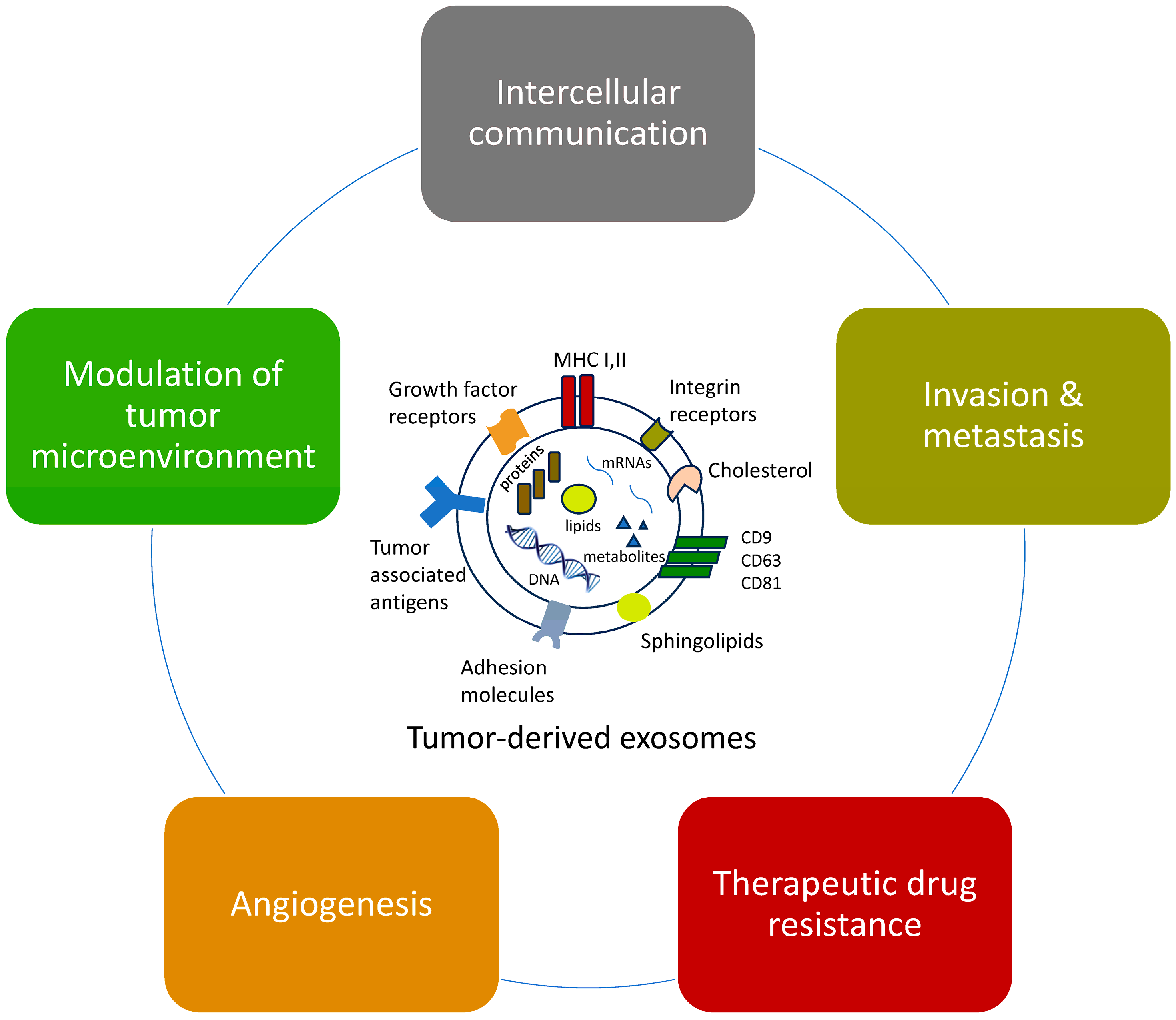
2. Functions of Tumor-Derived Exosomes (TDEs)
2.1. Effects on Intercellular Communication
2.2. Promotion of Metastasis
2.3. Effects on Axonogenesis
2.4. Effects on Angiogenesis
2.5. Effects on Therapeutic Drug Resistance
2.6. Modulation of the Tumor Microenvironment
3. TDEs Interact with Mesenchymal Cell Exosomes (MSC-exos) Within the Tumor Microenvironment
4. Clinical Applications of Tumor-Derived Exosomes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle Formation during Reticulocyte Maturation. Association of Plasma Membrane Activities with Released Vesicles (Exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b Control Different Steps of the Exosome Secretion Pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Morohashi, Y.; Yoshimura, S.; Manrique-Hoyos, N.; Jung, S.; Lauterbach, M.A.; Bakhti, M.; Grønborg, M.; Möbius, W.; Rhee, J.; et al. Regulation of Exosome Secretion by Rab35 and Its GTPase-Activating Proteins TBC1D10A–C. J. Cell Biol. 2010, 189, 223–232. [Google Scholar] [CrossRef]
- Lösche, W.; Scholz, T.; Temmler, U.; Oberle, V.; Claus, R.A. Platelet-Derived Microvesicles Transfer Tissue Factor to Monocytes but Not to Neutrophils. Platelets 2004, 15, 109–115. [Google Scholar] [CrossRef]
- Qu, Y.; Franchi, L.; Nunez, G.; Dubyak, G.R. Nonclassical IL-1β Secretion Stimulated by P2X7 Receptors Is Dependent on Inflammasome Activation and Correlated with Exosome Release in Murine Macrophages1. J. Immunol. 2007, 179, 1913–1925. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Kim, O.Y.; Gho, Y.S. Extracellular Vesicles as Emerging Intercellular Communicasomes. BMB Rep. 2014, 47, 531–539. [Google Scholar] [CrossRef]
- Yamashita, T.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Effect of Exosome Isolation Methods on Physicochemical Properties of Exosomes and Clearance of Exosomes from the Blood Circulation. Eur. J. Pharm. Biopharm. 2016, 98, 1–8. [Google Scholar] [CrossRef]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of Exosomal Proteins, RNA and Lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef]
- Haqqani, A.S.; Delaney, C.E.; Tremblay, T.-L.; Sodja, C.; Sandhu, J.K.; Stanimirovic, D.B. Method for Isolation and Molecular Characterization of Extracellular Microvesicles Released from Brain Endothelial Cells. Fluids Barriers CNS 2013, 10, 4. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Sedger, L.M. Intercellular Vesicular Transfer by Exosomes, Microparticles and Oncosomes—Implications for Cancer Biology and Treatments. Front. Oncol. 2019, 9, 125. [Google Scholar] [CrossRef]
- Di Vizio, D.; Kim, J.; Hager, M.H.; Morello, M.; Yang, W.; Lafargue, C.J.; True, L.D.; Rubin, M.A.; Adam, R.M.; Beroukhim, R.; et al. Oncosome Formation in Prostate Cancer: Association with a Region of Frequent Chromosomal Deletion in Metastatic Disease. Cancer Res. 2009, 69, 5601–5609. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; D’Asti, E.; Magnus, N.; Al-Nedawi, K.; Meehan, B.; Rak, J. Microvesicles as Mediators of Intercellular Communication in Cancer—The Emerging Science of Cellular ‘Debris’. Semin. Immunopathol. 2011, 33, 455–467. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Kerbel, R.S.; Allison, A.C.; Rak, J. Endothelial Expression of Autocrine VEGF upon the Uptake of Tumor-Derived Microvesicles Containing Oncogenic EGFR. Proc. Natl. Acad. Sci. USA 2009, 106, 3794–3799. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; You, S.; Spinelli, C.; Morley, S.; Zandian, M.; Aspuria, P.-J.; Cavallini, L.; Ciardiello, C.; Sobreiro, M.R.; Morello, M.; et al. Large Oncosomes Contain Distinct Protein Cargo and Represent a Separate Functional Class of Tumor-Derived Extracellular Vesicles. Oncotarget 2015, 6, 11327–11341. [Google Scholar] [CrossRef]
- Meehan, B.; Rak, J.; Di Vizio, D. Oncosomes—Large and Small: What Are They, Where They Came From? J. Extracell. Vesicles 2016, 5, 33109. [Google Scholar] [CrossRef]
- Ghayad, S.E.; Rammal, G.; Ghamloush, F.; Basma, H.; Nasr, R.; Diab-Assaf, M.; Chelala, C.; Saab, R. Exosomes Derived from Embryonal and Alveolar Rhabdomyosarcoma Carry Differential miRNA Cargo and Promote Invasion of Recipient Fibroblasts. Sci. Rep. 2016, 6, 37088. [Google Scholar] [CrossRef]
- Ghamloush, F.; Ghayad, S.E.; Rammal, G.; Fahs, A.; Ayoub, A.J.; Merabi, Z.; Harajly, M.; Zalzali, H.; Saab, R. The PAX3-FOXO1 Oncogene Alters Exosome miRNA Content and Leads to Paracrine Effects Mediated by Exosomal miR-486. Sci. Rep. 2019, 9, 14242. [Google Scholar] [CrossRef]
- Casadei, L.; Calore, F.; Creighton, C.J.; Guescini, M.; Batte, K.; Iwenofu, O.H.; Zewdu, A.; Braggio, D.A.; Bill, K.L.; Fadda, P.; et al. Exosome-Derived miR-25-3p and miR-92a-3p Stimulate Liposarcoma Progression. Cancer Res. 2017, 77, 3846–3856. [Google Scholar] [CrossRef]
- Morita, T.; Fujiwara, T.; Yoshida, A.; Uotani, K.; Kiyono, M.; Yokoo, S.; Hasei, J.; Kunisada, T.; Ozaki, T. Clinical Relevance and Functional Significance of Cell-Free microRNA-1260b Expression Profiles in Infiltrative Myxofibrosarcoma. Sci. Rep. 2020, 10, 9414. [Google Scholar] [CrossRef] [PubMed]
- Coulouarn, C.; Factor, V.M.; Thorgeirsson, S.S. Transforming Growth Factor-β Gene Expression Signature in Mouse Hepatocytes Predicts Clinical Outcome in Human Cancer. Hepatology 2008, 47, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, G.; Avnet, S.; Grisendi, G.; Salerno, M.; Granchi, D.; Dominici, M.; Kusuzaki, K.; Baldini, N. Role of Mesenchymal Stem Cells in Osteosarcoma and Metabolic Reprogramming of Tumor Cells. Oncotarget 2014, 5, 7575–7588. [Google Scholar] [CrossRef] [PubMed]
- Urciuoli, E.; Giorda, E.; Scarsella, M.; Petrini, S.; Peruzzi, B. Osteosarcoma-derived Extracellular Vesicles Induce a Tumor-like Phenotype in Normal Recipient Cells. J. Cell. Physiol. 2018, 233, 6158–6172. [Google Scholar] [CrossRef]
- Raimondi, L.; De Luca, A.; Gallo, A.; Costa, V.; Russelli, G.; Cuscino, N.; Manno, M.; Raccosta, S.; Carina, V.; Bellavia, D.; et al. Osteosarcoma Cell-Derived Exosomes Affect Tumor Microenvironment by Specific Packaging of microRNAs. Carcinogenesis 2020, 41, 666–677. [Google Scholar] [CrossRef]
- Ye, H.; Hu, X.; Wen, Y.; Tu, C.; Hornicek, F.; Duan, Z.; Min, L. Exosomes in the Tumor Microenvironment of Sarcoma: From Biological Functions to Clinical Applications. J. Nanobiotechnol. 2022, 20, 403. [Google Scholar] [CrossRef]
- Papadopoulos, K.S.; Piperi, C.; Korkolopoulou, P. Clinical Applications of Adipose-Derived Stem Cell (ADSC) Exosomes in Tissue Regeneration. Int. J. Mol. Sci. 2024, 25, 5916. [Google Scholar] [CrossRef]
- Abd Elmageed, Z.Y.; Yang, Y.; Thomas, R.; Ranjan, M.; Mondal, D.; Moroz, K.; Fang, Z.; Rezk, B.M.; Moparty, K.; Sikka, S.C.; et al. Neoplastic Reprogramming of Patient-Derived Adipose Stem Cells by Prostate Cancer Cell-Associated Exosomes. Stem Cells 2014, 32, 983–997. [Google Scholar] [CrossRef]
- Cho, J.A.; Park, H.; Lim, E.H.; Lee, K.W. Exosomes from Breast Cancer Cells Can Convert Adipose Tissue-Derived Mesenchymal Stem Cells into Myofibroblast-like Cells. Int. J. Oncol. 2012, 40, 130–138. [Google Scholar] [CrossRef]
- Cho, J.A.; Park, H.; Lim, E.H.; Kim, K.H.; Choi, J.S.; Lee, J.H.; Shin, J.W.; Lee, K.W. Exosomes from Ovarian Cancer Cells Induce Adipose Tissue-Derived Mesenchymal Stem Cells to Acquire the Physical and Functional Characteristics of Tumor-Supporting Myofibroblasts. Gynecol. Oncol. 2011, 123, 379–386. [Google Scholar] [CrossRef]
- Madeo, M.; Colbert, P.L.; Vermeer, D.W.; Lucido, C.T.; Cain, J.T.; Vichaya, E.G.; Grossberg, A.J.; Muirhead, D.; Rickel, A.P.; Hong, Z.; et al. Cancer Exosomes Induce Tumor Innervation. Nat. Commun. 2018, 9, 4284. [Google Scholar] [CrossRef] [PubMed]
- Visconti, L.; Nelissen, K.; Deckx, L.; Akker, M.V.D.; Adriaensen, W.; Daniels, L.; Matheï, C.; Linsen, L.; Hellings, N.; Stinissen, P.; et al. Prognostic Value of Circulating Cytokines on Overall Survival and Disease-Free Survival in Cancer Patients. Biomark. Med. 2014, 8, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, I.; Rana, S.; Baumann, A.; McAlear, J.; Hellwig, A.; Trendelenburg, M.; Lochnit, G.; Preissner, K.T.; Zöller, M. Cell Surface Tetraspanin Tspan8 Contributes to Molecular Pathways of Exosome-Induced Endothelial Cell Activation. Cancer Res. 2010, 70, 1668–1678. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular Transfer of the Oncogenic Receptor EGFRvIII by Microvesicles Derived from Tumour Cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef]
- Park, J.E.; Tan, H.S.; Datta, A.; Lai, R.C.; Zhang, H.; Meng, W.; Lim, S.K.; Sze, S.K. Hypoxic Tumor Cell Modulates Its Microenvironment to Enhance Angiogenic and Metastatic Potential by Secretion of Proteins and Exosomes. Mol. Cell Proteom. 2010, 9, 1085–1099. [Google Scholar] [CrossRef]
- King, H.W.; Michael, M.Z.; Gleadle, J.M. Hypoxic Enhancement of Exosome Release by Breast Cancer Cells. BMC Cancer 2012, 12, 421. [Google Scholar] [CrossRef]
- Tang, D.G. Understanding Cancer Stem Cell Heterogeneity and Plasticity. Cell Res. 2012, 22, 457–472. [Google Scholar] [CrossRef]
- Shackleton, M.; Quintana, E.; Fearon, E.R.; Morrison, S.J. Heterogeneity in Cancer: Cancer Stem Cells versus Clonal Evolution. Cell 2009, 138, 822–829. [Google Scholar] [CrossRef]
- Feng, B.-H.; Liu, A.-G.; Gu, W.-G.; Deng, L.; Cheng, X.-G.; Tong, T.-J.; Zhang, H.-Z. CD133+ Subpopulation of the HT1080 Human Fibrosarcoma Cell Line Exhibits Cancer Stem-like Characteristics. Oncol. Rep. 2013, 30, 815–823. [Google Scholar] [CrossRef]
- de la Mare, J.-A.; Sterrenberg, J.N.; Sukhthankar, M.G.; Chiwakata, M.T.; Beukes, D.R.; Blatch, G.L.; Edkins, A.L. Assessment of Potential Anti-Cancer Stem Cell Activity of Marine Algal Compounds Using an in Vitro Mammosphere Assay. Cancer Cell Int. 2013, 13, 39. [Google Scholar] [CrossRef]
- Seo, M.; Kim, S.M.; Woo, E.Y.; Han, K.-C.; Park, E.J.; Ko, S.; Choi, E.W.; Jang, M. Stemness-Attenuating miR-503-3p as a Paracrine Factor to Regulate Growth of Cancer Stem Cells. Stem Cells Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Choi, J.S.; Cho, Y.W. Reprogramming of Cancer Stem Cells into Non-Tumorigenic Cells Using Stem Cell Exosomes for Cancer Therapy. Biochem. Biophys. Res. Commun. 2019, 512, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH Is a Key Factor for Exosome Traffic in Tumor Cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Lee, K.-M.; An, J.-H.; Yang, S.-J.; Park, S.-M.; Lee, J.-H.; Chae, H.-K.; Song, W.-J.; Youn, H.-Y. Influence of Canine Macrophage-Derived Extracellular Vesicles on Apoptosis in Canine Melanoma and Osteosarcoma Cell Lines. Anticancer Res. 2021, 41, 719–730. [Google Scholar] [CrossRef]
- Liu, W.; Long, Q.; Zhang, W.; Zeng, D.; Hu, B.; Liu, S.; Chen, L. miRNA-221-3p Derived from M2-Polarized Tumor-Associated Macrophage Exosomes Aggravates the Growth and Metastasis of Osteosarcoma through SOCS3/JAK2/STAT3 Axis. Aging 2021, 13, 19760–19775. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, Y.; Wang, J.; Han, Y.; Ren, T.; Huang, Y.; Chen, C.; Huang, Q.; Wang, W.; Niu, J.; et al. Macrophages-Derived Exosomal lncRNA LIFR-AS1 Promotes Osteosarcoma Cell Progression via miR-29a/NFIA Axis. Cancer Cell Int. 2021, 21, 192. [Google Scholar] [CrossRef]
- Zhao, A.; Zhao, Z.; Liu, W.; Cui, X.; Wang, N.; Wang, Y.; Wang, Y.; Sun, L.; Xue, H.; Wu, L.; et al. Carcinoma-Associated Fibroblasts Promote the Proliferation and Metastasis of Osteosarcoma by Transferring Exosomal LncRNA SNHG17. Am. J. Transl. Res. 2021, 13, 10094–10111. [Google Scholar]
- Mazumdar, A.; Urdinez, J.; Boro, A.; Migliavacca, J.; Arlt, M.J.E.; Muff, R.; Fuchs, B.; Snedeker, J.G.; Gvozdenovic, A. Osteosarcoma-Derived Extracellular Vesicles Induce Lung Fibroblast Reprogramming. Int. J. Mol. Sci. 2020, 21, 5451. [Google Scholar] [CrossRef]
- Gu, H.; Ji, R.; Zhang, X.; Wang, M.; Zhu, W.; Qian, H.; Chen, Y.; Jiang, P.; Xu, W. Exosomes Derived from Human Mesenchymal Stem Cells Promote Gastric Cancer Cell Growth and Migration via the Activation of the Akt Pathway. Mol. Med. Rep. 2016, 14, 3452–3458. [Google Scholar] [CrossRef]
- Lin, R.; Wang, S.; Zhao, R.C. Exosomes from Human Adipose-Derived Mesenchymal Stem Cells Promote Migration through Wnt Signaling Pathway in a Breast Cancer Cell Model. Mol. Cell. Biochem. 2013, 383, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhu, Z.; Zhu, F.; Ding, F.; Wang, Y.; Wang, X.; Luo, X.; Yang, J.; Liu, F.; Sun, D. Impact of Human Adipose Tissue-Derived Stem Cells on Dermatofibrosarcoma Protuberans Cells in an Indirect Co-Culture: An in Vitro Study. Stem Cell Res. Ther. 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chu, Y.; Li, K.; Zhang, G.; Guo, Z.; Wu, X.; Qiu, C.; Li, Y.; Wan, X.; Sui, J.; et al. Exosomes Secreted by Adipose-Derived Mesenchymal Stem Cells Foster Metastasis and Osteosarcoma Proliferation by Increasing COLGALT2 Expression. Front. Cell Dev. Biol. 2020, 8, 353. [Google Scholar] [CrossRef]
- Qu, Q.; Liu, L.; Cui, Y.; Chen, Y.; Wang, Y.; Wang, Y. Exosomes from Human Omental Adipose-Derived Mesenchymal Stem Cells Secreted into Ascites Promote Peritoneal Metastasis of Epithelial Ovarian Cancer. Cells 2022, 11, 3392. [Google Scholar] [CrossRef]
- Ko, S.-F.; Yip, H.-K.; Zhen, Y.-Y.; Lee, C.-C.; Lee, C.-C.; Huang, C.-C.; Ng, S.-H.; Lin, J.-W. Adipose-Derived Mesenchymal Stem Cell Exosomes Suppress Hepatocellular Carcinoma Growth in a Rat Model: Apparent Diffusion Coefficient, Natural Killer T-Cell Responses, and Histopathological Features. Stem Cells Int. 2015, 2015, 853506. [Google Scholar] [CrossRef]
- Ge, X.; Liu, W.; Zhao, W.; Feng, S.; Duan, A.; Ji, C.; Shen, K.; Liu, W.; Zhou, J.; Jiang, D.; et al. Exosomal Transfer of LCP1 Promotes Osteosarcoma Cell Tumorigenesis and Metastasis by Activating the JAK2/STAT3 Signaling Pathway. Mol. Ther. Nucleic Acids 2020, 21, 900–915. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, L.; Li, Y.; Zhang, X.; Gu, J.; Yan, Y.; Xu, X.; Wang, M.; Qian, H.; Xu, W. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Promote Tumor Growth in Vivo. Cancer Lett. 2012, 315, 28–37. [Google Scholar] [CrossRef]
- Wang, J.; Hendrix, A.; Hernot, S.; Lemaire, M.; De Bruyne, E.; Van Valckenborgh, E.; Lahoutte, T.; De Wever, O.; Vanderkerken, K.; Menu, E. Bone Marrow Stromal Cell–Derived Exosomes as Communicators in Drug Resistance in Multiple Myeloma Cells. Blood 2014, 124, 555–566. [Google Scholar] [CrossRef]
- Bruno, S.; Collino, F.; Deregibus, M.C.; Grange, C.; Tetta, C.; Camussi, G. Microvesicles Derived from Human Bone Marrow Mesenchymal Stem Cells Inhibit Tumor Growth. Stem Cells Dev. 2013, 22, 758–771. [Google Scholar] [CrossRef]
- Lee, J.-K.; Park, S.-R.; Jung, B.-K.; Jeon, Y.-K.; Lee, Y.-S.; Kim, M.-K.; Kim, Y.-G.; Jang, J.-Y.; Kim, C.-W. Exosomes Derived from Mesenchymal Stem Cells Suppress Angiogenesis by Down-Regulating VEGF Expression in Breast Cancer Cells. PLoS ONE 2013, 8, e84256. [Google Scholar] [CrossRef]
- Lin, L.Y.; Du, L.M.; Cao, K.; Huang, Y.; Yu, P.F.; Zhang, L.Y.; Li, F.Y.; Wang, Y.; Shi, Y.F. Tumour Cell-Derived Exosomes Endow Mesenchymal Stromal Cells with Tumour-Promotion Capabilities. Oncogene 2016, 35, 6038–6042. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Gopal, S.K.; Mathias, R.A.; Liu, L.; Sheng, J.; Zhu, H.-J.; Simpson, R.J. Emerging Roles of Exosomes during Epithelial–Mesenchymal Transition and Cancer Progression. Semin. Cell Dev. Biol. 2015, 40, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.H.; Jeong, H.E.; Seo, H.; Cho, S.; Kim, K.; Na, D.; Chung, S.; Park, J.; Choi, N.; Kang, J.Y. Cancer-Derived Exosomes Trigger Endothelial to Mesenchymal Transition Followed by the Induction of Cancer-Associated Fibroblasts. Acta Biomater. 2018, 76, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Dilsiz, N. A comprehensive review on recent advances in exosome isolation and characterization: Toward clinical applications. Transl. Oncol. 2024, 50, 102121. [Google Scholar] [CrossRef]
- Di Vizio, D.; Morello, M.; Dudley, A.C.; Schow, P.W.; Adam, R.M.; Morley, S.; Mulholland, D.; Rotinen, M.; Hager, M.H.; Insabato, L.; et al. Large Oncosomes in Human Prostate Cancer Tissues and in the Circulation of Mice with Metastatic Disease. Am. J. Pathol. 2012, 181, 1573–1584. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, X.; He, M.; Shang, Y.; Tetlow, A.L.; Godwin, A.K.; Zeng, Y. Ultrasensitive Detection of Circulating Exosomes with a 3D-Nanopatterned Microfluidic Chip. Nat. Biomed. Eng. 2019, 3, 438–451. [Google Scholar] [CrossRef]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.K.; Enderle, D.; Noerholm, M.; Breakefield, X.O.; Skog, J.K. Exosome-Based Liquid Biopsies in Cancer: Opportunities and Challenges. Ann. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef]
- Greening, D.W.; Ji, H.; Chen, M.; Robinson, B.W.S.; Dick, I.M.; Creaney, J.; Simpson, R.J. Secreted Primary Human Malignant Mesothelioma Exosome Signature Reflects Oncogenic Cargo. Sci. Rep. 2016, 6, 32643. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, Z.; Cheng, F.; Shao, Z. Exosomal lncRNA RAMP2-AS1 Derived from Chondrosarcoma Cells Promotes Angiogenesis Through miR-2355-5p/VEGFR2 Axis. OncoTargets Ther. 2020, 13, 3291–3301. [Google Scholar] [CrossRef]
- Gong, L.; Bao, Q.; Hu, C.; Wang, J.; Zhou, Q.; Wei, L.; Tong, L.; Zhang, W.; Shen, Y. Exosomal miR-675 from Metastatic Osteosarcoma Promotes Cell Migration and Invasion by Targeting CALN1. Biochem. Biophys. Res. Commun. 2018, 500, 170–176. [Google Scholar] [CrossRef]
- Osaki, M.; Takeshita, F.; Sugimoto, Y.; Kosaka, N.; Yamamoto, Y.; Yoshioka, Y.; Kobayashi, E.; Yamada, T.; Kawai, A.; Inoue, T.; et al. MicroRNA-143 Regulates Human Osteosarcoma Metastasis by Regulating Matrix Metalloprotease-13 Expression. Mol. Ther. 2011, 19, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and Delivery Efficiency of Unmodified Tumor-Derived Exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Shimbo, K.; Miyaki, S.; Ishitobi, H.; Kato, Y.; Kubo, T.; Shimose, S.; Ochi, M. Exosome-Formed Synthetic microRNA-143 Is Transferred to Osteosarcoma Cells and Inhibits Their Migration. Biochem. Biophys. Res. Commun. 2014, 445, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, Z.; Feng, G.; Wang, L.; Xie, J.; Jin, Y.; Wang, L.; Liu, S. Tumor Suppressing Role of Serum-Derived Exosomal microRNA-15a in Osteosarcoma Cells through the GATA Binding Protein 2/Murine Double Minute 2 Axis and the P53 Signaling Pathway. Bioengineered 2021, 12, 8378–8395. [Google Scholar] [CrossRef]
- Thway, K. Well-Differentiated Liposarcoma and Dedifferentiated Liposarcoma: An Updated Review. Semin. Diagn. Pathol. 2019, 36, 112–121. [Google Scholar] [CrossRef]
- Hou, H.; Sun, D.; Zhang, X. The Role of MDM2 Amplification and Overexpression in Therapeutic Resistance of Malignant Tumors. Cancer Cell Int. 2019, 19, 216. [Google Scholar] [CrossRef]
- Liu, T.; Li, T.; Zheng, Y.; Xu, X.; Sun, R.; Zhan, S.; Guo, X.; Zhao, Z.; Zhu, W.; Feng, B.; et al. Evaluating Adipose-derived Stem Cell Exosomes as miRNA Drug Delivery Systems for the Treatment of Bladder Cancer. Cancer Med. 2022, 11, 3687–3699. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, C.; Chen, M.; Yang, T.; Wang, X.; Gao, Y.; Wang, L.; Wen, Y.; Chen, G.; Wang, X.; et al. Extracellular Vesicle-Mediated Delivery of miR-101 Inhibits Lung Metastasis in Osteosarcoma. Theranostics 2020, 10, 411–425. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, L.; Yang, P.; Lu, Y.; Lin, S.; Yuan, G. The Exosomal Transfer of Human Bone Marrow Mesenchymal Stem Cell-Derived miR-1913 Inhibits Osteosarcoma Progression by Targeting NRSN2. Am. J. Transl. Res. 2021, 13, 10178–10192. [Google Scholar]
- Xu, Z.; Zhou, X.; Wu, J.; Cui, X.; Wang, M.; Wang, X.; Gao, Z. Mesenchymal Stem Cell-Derived Exosomes Carrying microRNA-150 Suppresses the Proliferation and Migration of Osteosarcoma Cells via Targeting IGF2BP1. Transl. Cancer Res. TCR 2020, 9, 5323–5335. [Google Scholar] [CrossRef]
- Yuan, Z.; Kolluri, K.K.; Gowers, K.H.C.; Janes, S.M. TRAIL Delivery by MSC-derived Extracellular Vesicles Is an Effective Anticancer Therapy. J. Extracell. Vesicles 2017, 6, 1265291. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes Derived from miR-122-Modified Adipose Tissue-Derived MSCs Increase Chemosensitivity of Hepatocellular Carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Takahara, K.; Ii, M.; Inamoto, T.; Nakagawa, T.; Ibuki, N.; Yoshikawa, Y.; Tsujino, T.; Uchimoto, T.; Saito, K.; Takai, T.; et al. microRNA-145 Mediates the Inhibitory Effect of Adipose Tissue-Derived Stromal Cells on Prostate Cancer. Stem Cells Dev. 2016, 25, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Ectosomes | Exosomes | Large Oncosomes |
|---|---|---|---|
| Diameter range | 100–350 nm | 50–100 nm | 1–10 μm |
| Accumulation site | Plasma membrane | Intracellular MVBs | Plasma membrane |
| ESCRT complex utilization | Yes (Partially) | Yes | Yes (Partially) |
| Release procedure | Pinching off | Exocytosis | Pinching off |
| Release timing and amount | Early—high amount | Late—controlled | Early—high mount |
| Possible markers | TyA, C1q | CD63, CD61, CD81, CD9 | CK18, GAPDH, HSPA5 |
| Type of Cancer Cells | Exosomal miRNA | Target Cells | Mechanism of Action | Effects | References |
|---|---|---|---|---|---|
| RMS | miR-486-5p | CAFs, fibroblasts, myoblasts | Angiogenesis, migration, differentiation and proliferation of fibroblasts and cancer cells | Tumor invasion and increased metastatic capacity | [18,19] |
| LPS | miR-486-5p miR-92a-3p | TAMs | Increased IL-6 | Tumor proliferation and increased metastatic capacity | [20] |
| MFS | miR-1260b | Fibroblasts | Down-regulation of PCDH9 | Tumor proliferation | [21] |
| OS | EVs | MSCs | Effect on the tumor microenvironment TNF-α, IL-6, TGF-β, MMP-9 | Oncogenic potential in the absence of cancer cells | [23,24] |
| OS | miR-148a miR-21-5p | HUVECs | Effect on the tumor microenvironment | Induction of immortality in target cells | [25] |
| Prostate | miR-125b, miR-130b miR-155 | ADSCs | Expression of epithelial, neoplastic and angiogenic tumor markers | Mesenchymal-epithelial transition | [28] |
| Breast | - | ADSCs | Overexpression SDF-1, VEGF, CCL5, TGF-β, SMAD2 | Convert ADSCs to myofibroblasts | [29] |
| Ovaries | - | ADSCs | Overexpression SDF-1, TGF-β, SMAD2 | Convert ADSCs to myofibroblasts | [30] |
| Origin of Exosomes | Target Cells | Mechanism of Action | Effects | Reference |
|---|---|---|---|---|
| ADSC-exos | CSCs | Suppression of spheroid formation of CSCs by miR-503-3p | Tumor suppression | [40] |
| ADSC-exos | CSCs | Increased expression of APLP, RUNX2, and BGLAP | Reprogramming of CSCs into non-tumorigenic cells | [41] |
| ADSC-exos | Breast cancer | Induction of the Wnt/b-catenin signaling pathway | Tumor progression | [51] |
| ADSC-exos | DFSP | Overexpression of VEGF, HGF, and bFGF, PDGFRB, COL1A1 | Tumor progression | [52] |
| ADSC-exos | OS | Vimentin and MMP 2/9 overexpression, EMT induction | Tumor progression | [53] |
| ADSC-exos | Ovarian cancer | FOXM1, Cyclin F, KIF20A, and MAPK | Tumor progression | [54] |
| ADSC-exos | Hepatocellular carcinoma | Enhancement of NK T-cell activity | Tumor suppression | [55] |
| BMSC-exos | OS | LCP1, JAK2/STAT3 pathway, miR-135a-5p suppression | Tumor progression | [56] |
| BMSC-exos | Stomach cancer | Activation of the ERK 1/2 pathway | Tumor progression | [57] |
| BMSC-exos | Multiple myeloma | p53, p38 and Akt pathways | Tumor progression | [58] |
| BMSC-exos | Hepatocellular carcinoma, ovarian cancer, Kaposi’s sarcoma | DIRAS3, RBL2, RBL1, CDKN2B, CDKN1A, CCNE1, SKP2, CCND2, CUL3, GAPDH | Tumor suppression | [59] |
| MSC-exos | Breast cancer | miR-16 cargoes of exosomes inhibit VEGF | Tumor suppression | [60] |
| ADSC-exos | Bladder cancer | Tissue invasion and tumor growth suppression via miR-138-5p | Tumor suppression | [77] |
| ADSC-EVs | OS | They limit the development of pulmonary metastases through miR-101 | Tumor suppression | [78] |
| ADSC-exos | OS | Negative regulation in NRSN2 by miR-1913 | Tumor suppression | [79] |
| ADSC-exos | OS | Negative regulation of IGF2BP1 by miR-150 | Tumor suppression | [80] |
| ADSC-exos | Hepatocellular cancer | Chemosensitivity of hepatocellular tumors to sorafenib via miR-122 | Tumor suppression | [81] |
| ADSC-exos | Prostate cancer | Enhancement of apoptosis through Bcl-xL inhibition via miR-145 | Tumor suppression | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, K.S.; Korkolopoulou, P.; Piperi, C. Exploring the Interaction of Tumor-Derived Exosomes and Mesenchymal Stem Cells in Tumor Biology. Int. J. Mol. Sci. 2025, 26, 3095. https://doi.org/10.3390/ijms26073095
Papadopoulos KS, Korkolopoulou P, Piperi C. Exploring the Interaction of Tumor-Derived Exosomes and Mesenchymal Stem Cells in Tumor Biology. International Journal of Molecular Sciences. 2025; 26(7):3095. https://doi.org/10.3390/ijms26073095
Chicago/Turabian StylePapadopoulos, Konstantinos S., Penelope Korkolopoulou, and Christina Piperi. 2025. "Exploring the Interaction of Tumor-Derived Exosomes and Mesenchymal Stem Cells in Tumor Biology" International Journal of Molecular Sciences 26, no. 7: 3095. https://doi.org/10.3390/ijms26073095
APA StylePapadopoulos, K. S., Korkolopoulou, P., & Piperi, C. (2025). Exploring the Interaction of Tumor-Derived Exosomes and Mesenchymal Stem Cells in Tumor Biology. International Journal of Molecular Sciences, 26(7), 3095. https://doi.org/10.3390/ijms26073095







