Molecular Mechanism of Microgravity-Induced Intestinal Flora Dysbiosis on the Abnormalities of Liver and Brain Metabolism
Abstract
1. Introduction
2. Results
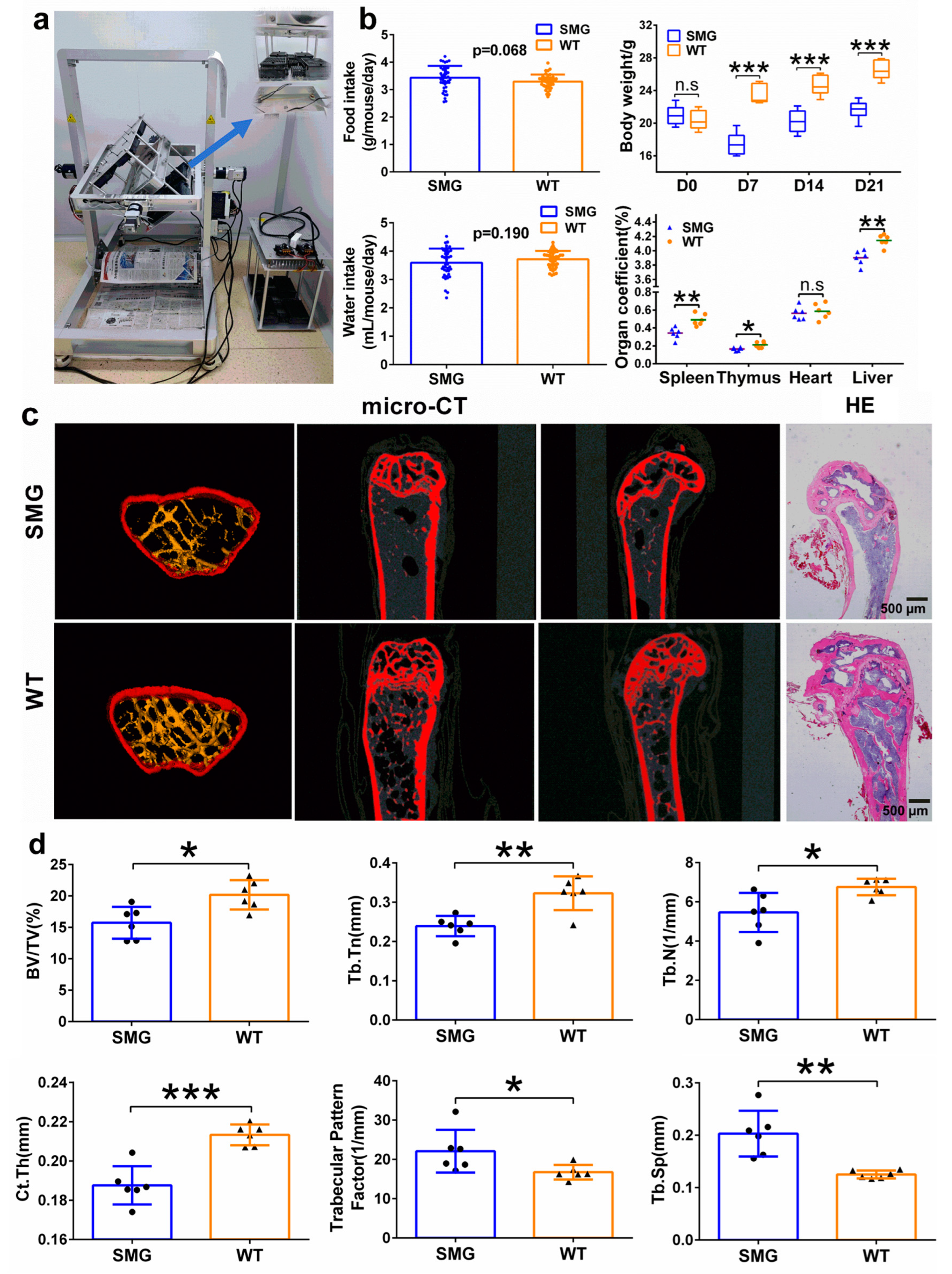
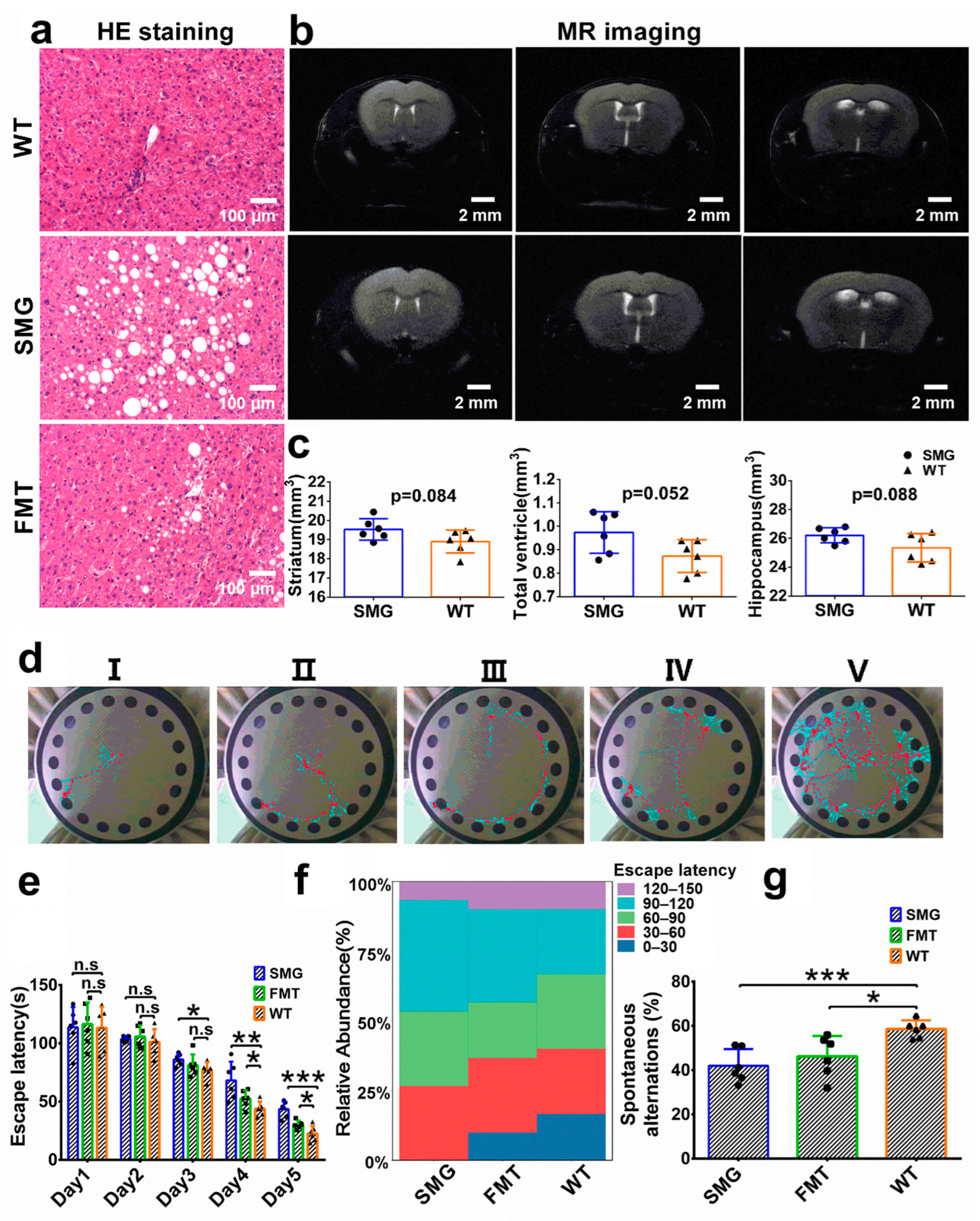
2.1. Establishment of Microgravity Mouse Model Based on 3D-Clinostat
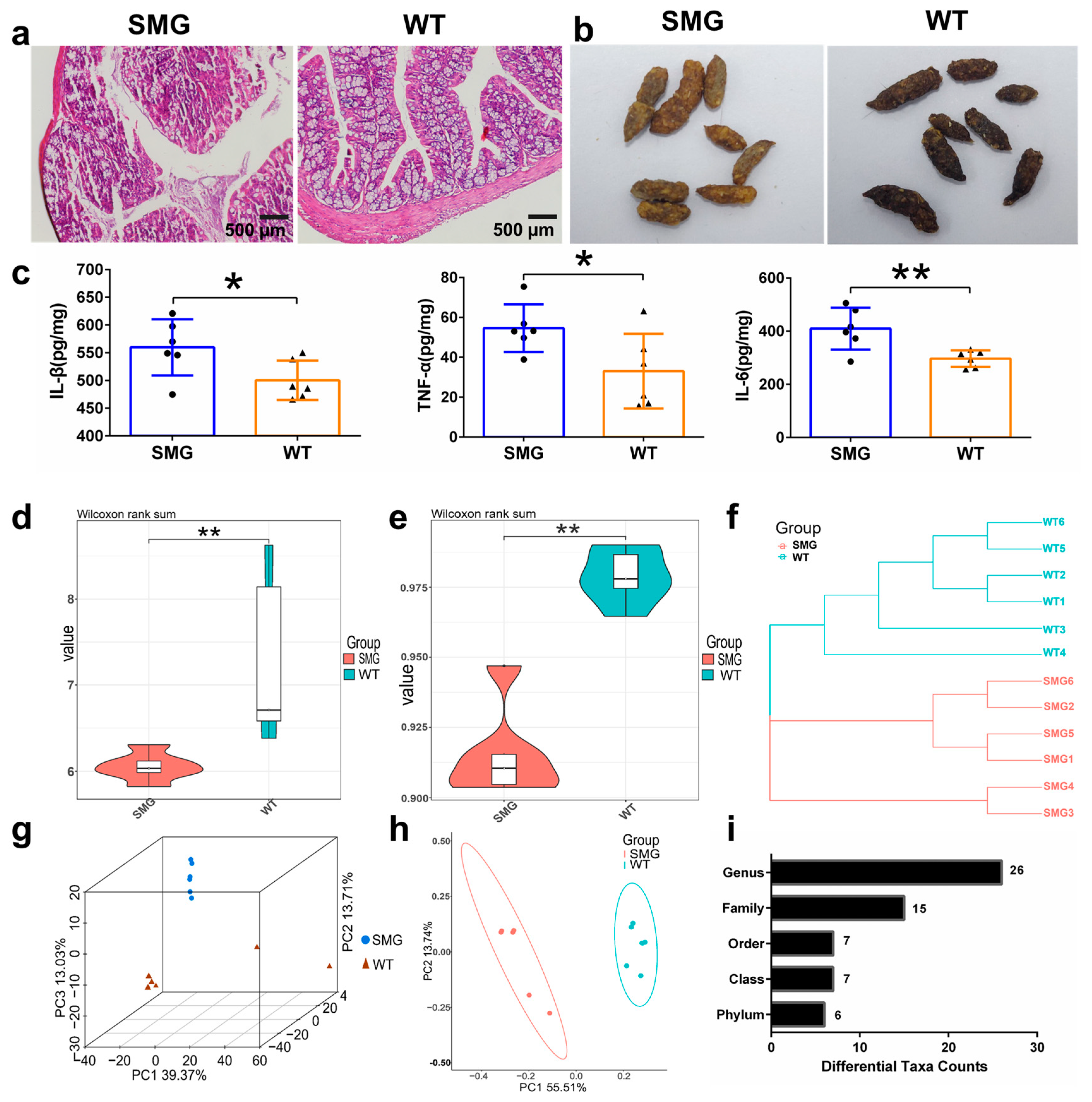
2.2. Microgravity-Induced Imbalance in Mouse Gut Microbiota
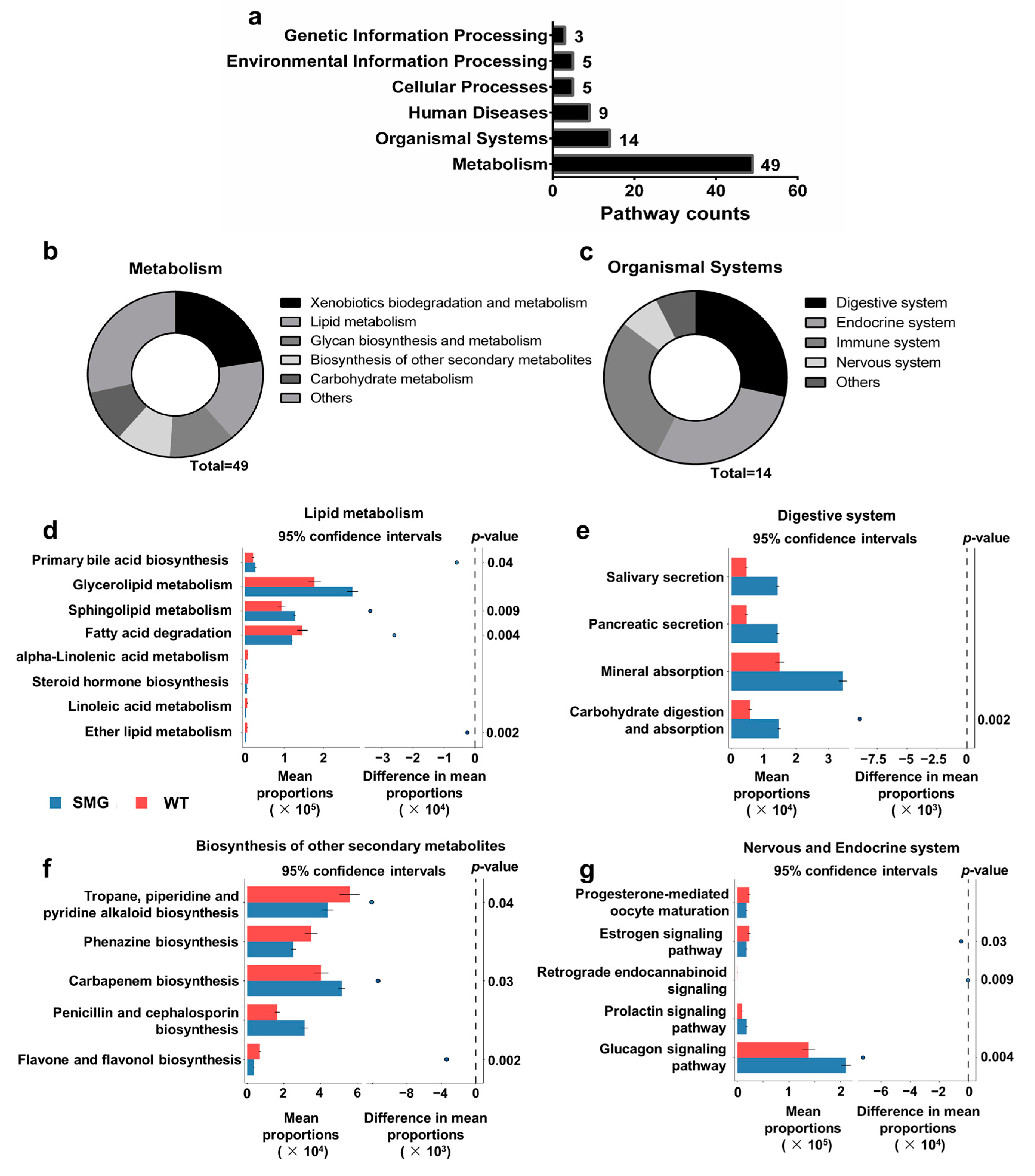
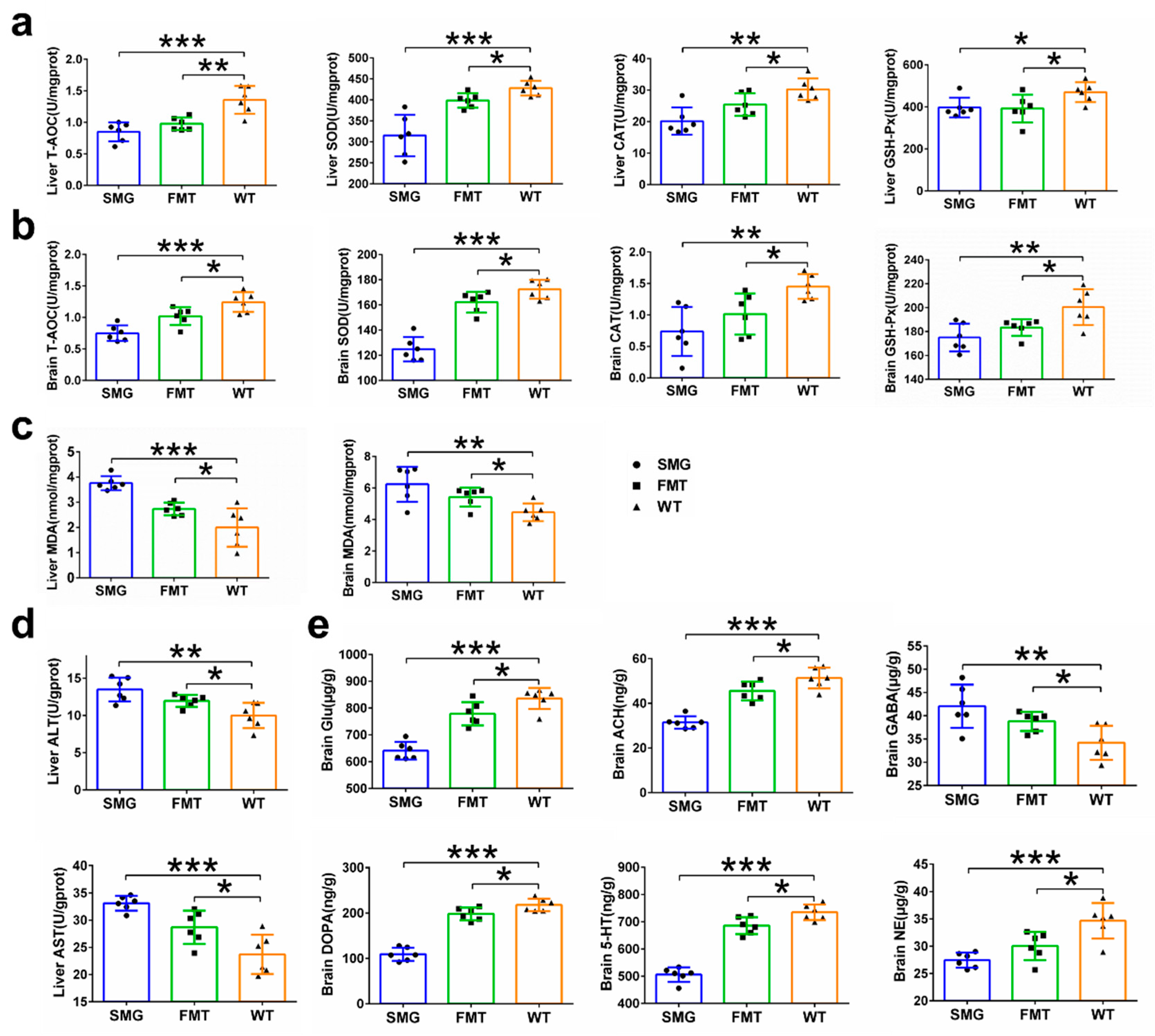
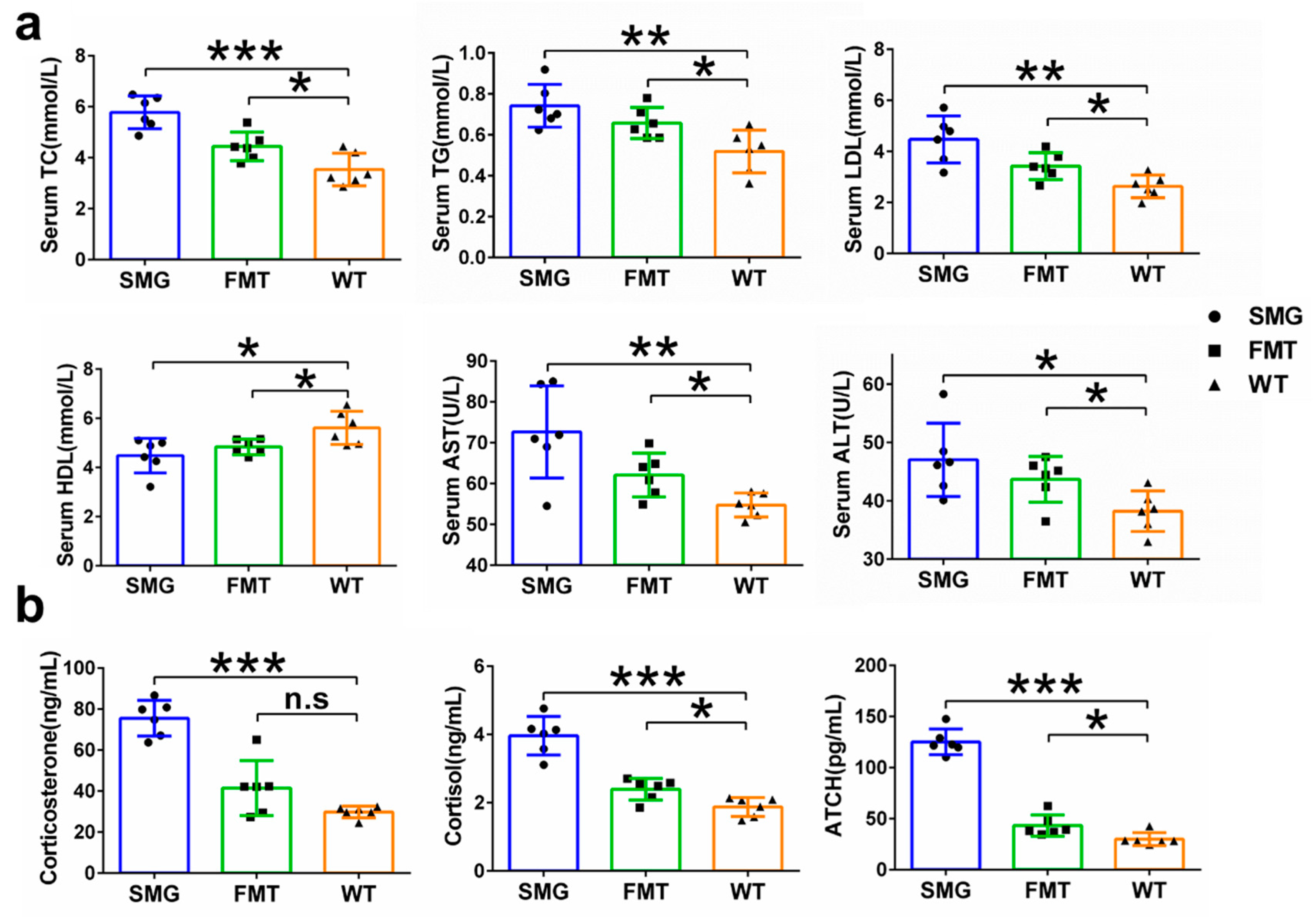
2.3. Microgravity-Induced Dysbiosis Leads to Metabolic Imbalance in Liver and Brain
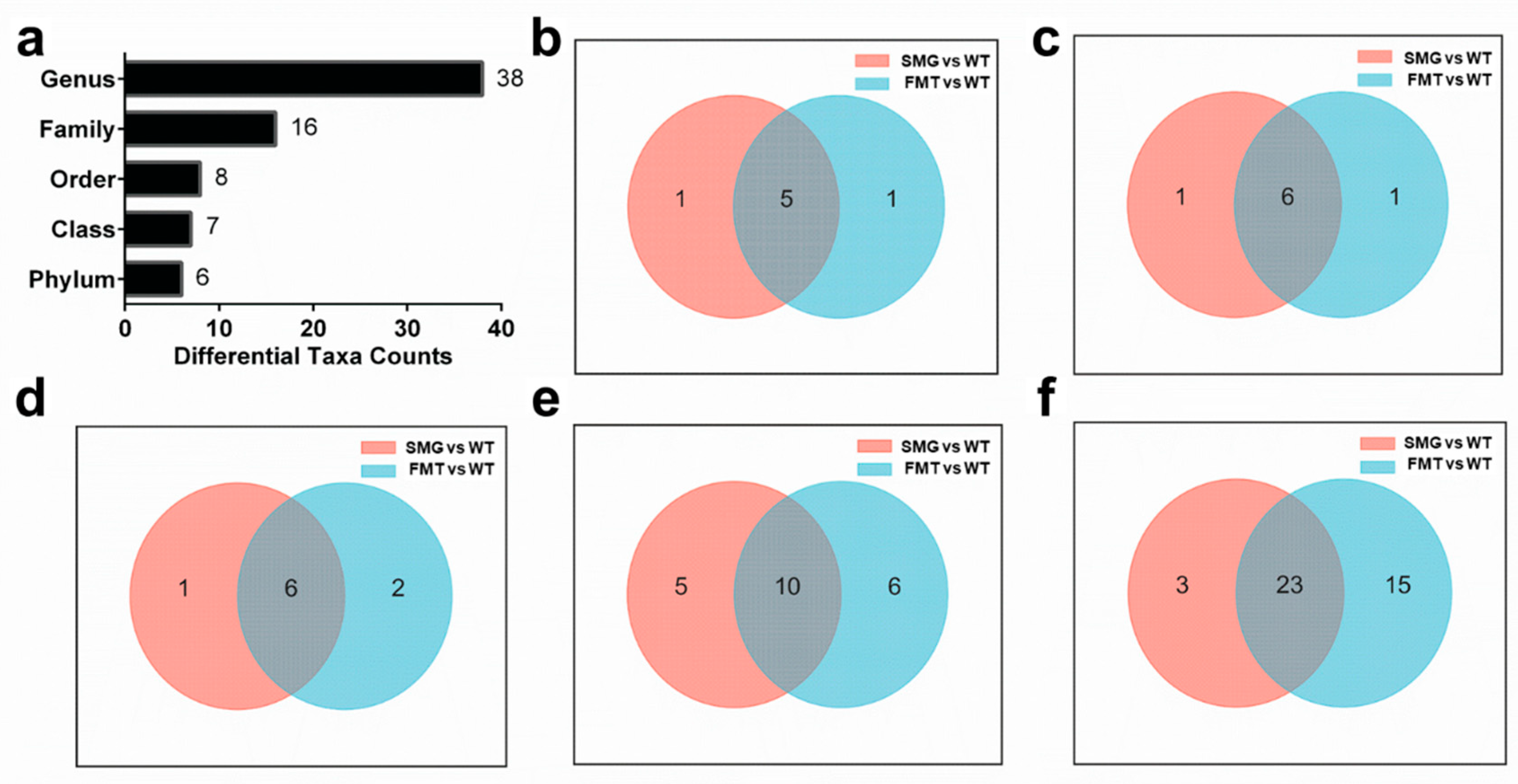
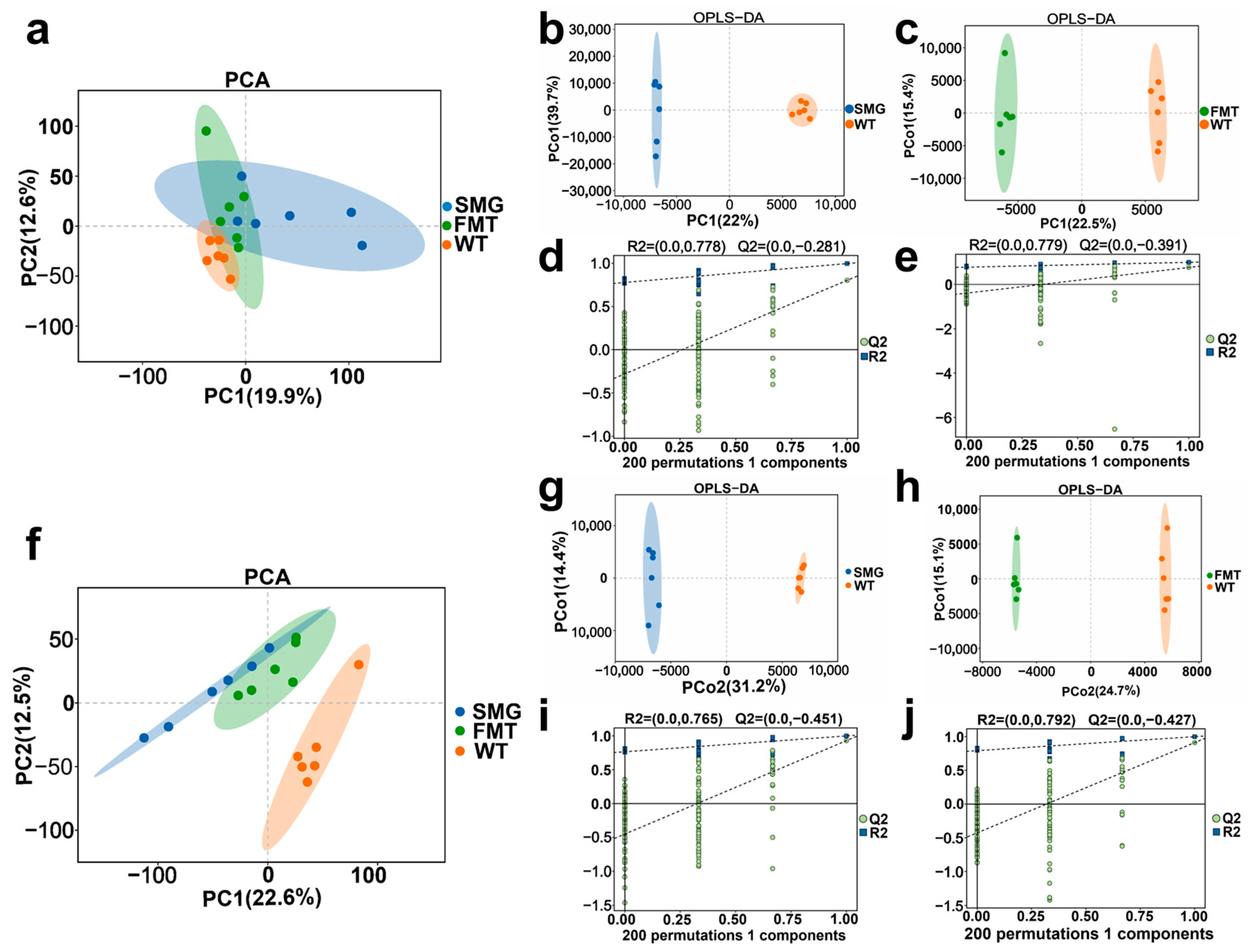
2.4. Replicability of Gut Microbiota in Mice Under Microgravity
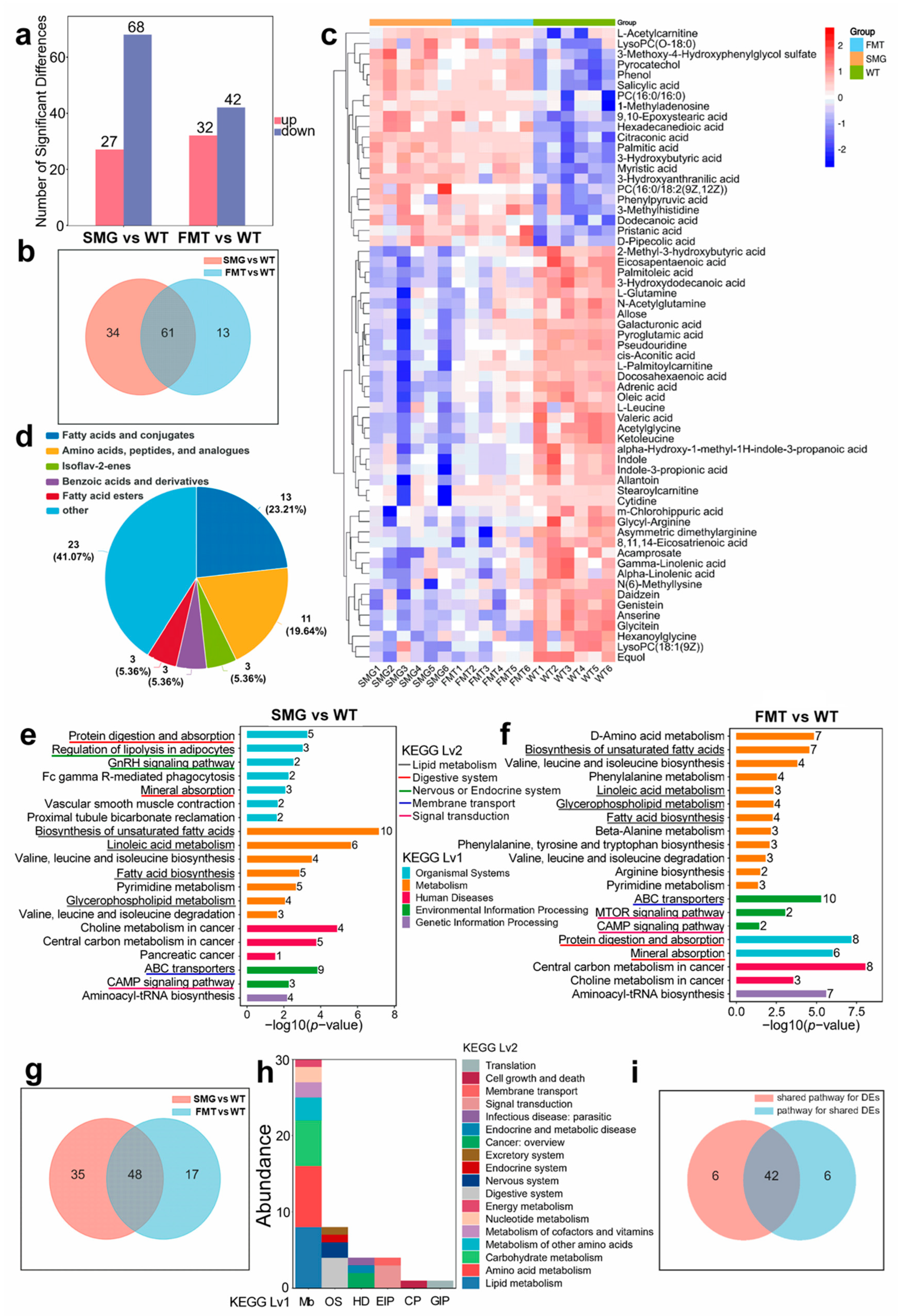
2.5. Microgravity Induces an Imbalance of Serum Metabolites in the Body
2.6. Microgravity-Induced Disruption of Serum Metabolites by the Gut Microbiota Leading to Hepatic and Cerebral Metabolic Disorders
2.7. Hepatic and Cerebral Metabolic Disorders Induced by Microgravity
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Structure and Parameters of the 3D-Clinostat
4.3. Animals Experimental Design
4.4. Micro-CT of Bone Tissue
4.5. Brain Magnetic Resonance Imaging (MRI)
4.6. Behavioral Experiments (Barnes Maze and Y-Maze)
4.7. Hematoxylin-Eosin (HE) Staining
4.8. Enzyme-Linked Immunosorbent Assays (ELISA)
4.9. Detection of Lipid Levels, Liver Function, and Antioxidant Capacity in Liver and Brain
4.10. Detection of Neurotransmitters and Corticosteroids
4.11. DNA Extraction and 16S rDNA Sequencing
4.12. Serum Untargeted Metabolomics Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sisovas, L.; Ceponis, A.; Borodinas, S. The Challenges of Piezoelectric Actuators and Motors Application in a Space Environment. Actuators 2024, 13, 312. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, X.L.; Yang, C.F.; Su, P.H.; Yin, C.; Qian, A.R. The Impact of Oxidative Stress on the Bone System in Response to the Space Special Environment. Int. J. Mol. Sci. 2017, 18, 2132. [Google Scholar] [CrossRef] [PubMed]
- Drago-Ferrante, R.; Di Fiore, R.; Karouia, F.; Subbannayya, Y.; Das, S.; Mathyk, B.A.; Arif, S.; Guevara-Cerdán, A.P.; Seylani, A.; Galsinh, A.S.; et al. Extraterrestrial Gynecology: Could Spaceflight Increase the Risk of Developing Cancer in Female Astronauts? An Updated Review. Int. J. Mol. Sci. 2022, 23, 7465. [Google Scholar] [CrossRef]
- Yuan, M.Q.; Liu, H.Z.; Zhou, S.H.; Zhou, X.; Huang, Y.E.; Hou, F.; Jiang, W. Integrative Analysis of Regulatory Module Reveals Associations of Microgravity with Dysfunctions of Multi-body Systems and Tumorigenesis. Int. J. Mol. Sci. 2020, 21, 7585. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar]
- Moser, D.; Sun, S.J.; Li, N.; Biere, K.; Hoerl, M.; Matzel, S.; Feuerecker, M.; Buchheim, J.I.; Strewe, C.; Thiel, C.S.; et al. Cells’ Flow and Immune Cell Priming under alternating g-forces in Parabolic Flight. Sci. Rep. 2019, 9, 11276. [Google Scholar]
- Prasad, B.; Grimm, D.; Strauch, S.M.; Erzinger, G.S.; Corydon, T.J.; Lebert, M.; Magnusson, N.E.; Infanger, M.; Richter, P.; Krüger, M. Influence of Microgravity on Apoptosis in Cells, Tissues, and Other Systems In Vivo and In Vitro. Int. J. Mol. Sci. 2020, 21, 9373. [Google Scholar] [CrossRef]
- ElGindi, M.; Sapudom, J.; Ibrahim, I.H.; Al-Sayegh, M.; Chen, W.Q.; Garcia-Sabaté, A.; Teo, J.C.M. May the Force Be with You (Or Not): The Immune System under Microgravity. Cells 2021, 10, 1941. [Google Scholar] [CrossRef]
- Li, Z.; An, M.; Zhang, Q.; Du, Z.C. Research on Microgravity Measurement Technology of Manned Spacecraft. In Proceedings of the 34th Chinese Control and Decision Conference (CCDC), Hefei, China, 15–17 August 2022; pp. 422–424. [Google Scholar]
- Turner, N.D.; Braby, L.A.; Ford, J.; Lupton, J.R. Opportunities for nutritional amelioration of radiation-induced cellular damage. Nutrition 2002, 18, 904–912. [Google Scholar]
- Porterfield, D.M. The biophysical limitations in physiological transport and exchange in plants grown in microgravity. J. Plant Growth Regul. 2002, 21, 177–190. [Google Scholar]
- Li, Y.T.; Zhang, X.; Xu, Z.; Chu, X.X.; Hu, Z.Q.; Ye, Z.Y.; Li, C.Q.; Wang, Z.B.; Zeng, B.; Pan, J.Y.; et al. Simulated weightlessness procedure, head-down bed rest has reversible effects on the metabolism of rhesus macaque. Mol. Brain 2024, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, N.; Khandani, A.; Camirand, A.; Harrison, R.E. Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone 2011, 49, 965–974. [Google Scholar] [CrossRef]
- Vinken, M. Hepatology in space: Effects of spaceflight and simulated microgravity on the liver. Liver Int. 2022, 42, 2599–2606. [Google Scholar] [CrossRef]
- Bradbury, P.; Wu, H.; Choi, J.U.; Rowan, A.E.; Zhang, H.Y.; Poole, K.; Lauko, J.; Chou, J. Modeling the Impact of Microgravity at the Cellular Level: Implications for Human Disease. Front. Cell Dev. Biol. 2020, 8, 96. [Google Scholar] [CrossRef]
- da Silveira, W.A.; Fazelinia, H.; Rosenthal, S.B.; Laiakis, E.C.; Kim, M.S.; Meydan, C.; Kidane, Y.; Rathi, K.S.; Smith, S.M.; Stear, B.; et al. Comprehensive Multi-omics Analysis Reveals Mitochondrial Stress as a Central Biological Hub for Spaceflight Impact. Cell 2020, 183, 1185–1201.e20. [Google Scholar] [CrossRef] [PubMed]
- Kast, J.; Yu, Y.; Seubert, C.N.; Wotring, V.E.; Derendorf, H. Drugs in space: Pharmacokinetics and pharmacodynamics in astronauts. Eur. J. Pharm. Sci. 2017, 109s, S2–S8. [Google Scholar] [CrossRef]
- Eyal, S. How do the pharmacokinetics of drugs change in astronauts in space? Expert. Opin. Drug Metab. Toxicol. 2020, 16, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.G.; Allen, P.L.; Birdsall, H.H. Effects of space flight on mouse liver versus kidney: Gene pathway analyses. Int. J. Mol. Sci. 2018, 19, 4106. [Google Scholar] [CrossRef]
- Blaber, E.A.; Pecaut, M.J.; Jonscher, K.R. Spaceflight activates autophagy programs and the proteasome in mouse liver. Int. J. Mol. Sci. 2017, 18, 2062. [Google Scholar] [CrossRef]
- Du, F.; Ding, Y.; Zou, J.; Li, Z.L.; Tian, J.J.; She, R.P.; Wang, D.S.; Wang, H.J.; Lv, D.Q.; Chang, L.L. Morphology and Molecular Mechanisms of Hepatic Injury in Rats under Simulated Weightlessness and the Protective Effects of Resistance Training. PLoS ONE 2015, 10, e0127047. [Google Scholar] [CrossRef]
- Abraham, S.; Klein, H.P.; Lin, C.Y.; Volkmann, C. The effects of space flight on some rat liver enzymes regulating carbohydrate and lipid metabolism. Adv. Space Res. 1981, 1, 199–217. [Google Scholar]
- Rabot, S.; Szylit, O.; Nugon-Baudon, L.; Meslin, J.C.; Vaissade, P.; Popot, F.; Viso, M. Variations in digestive physiology of rats after short duration flights aboard the US space shuttle. Dig. Dis. Sci. 2000, 45, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.H.; Wang, E.; Jones, D.P.; Hargrove, J.L. Hepatic function in rats after spaceflight: Effects on lipids, glycogen, and enzymes. Am. J. Physiol. 1987, 252, R222–R226. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Lin, C.Y.; Volkmann, C.M.; Klein, H.P. Biochemical changes in rat liver after 18.5 days of spaceflight. Proc. Soc. Exp. Biol. Med. 1983, 172, 334–339. [Google Scholar] [PubMed]
- Merrill, A.H.; Hoel, M.; Wang, E.; Mullins, R.E.; Hargrove, J.L.; Jones, D.P.; Popova, I.A. Altered carbohydrate, lipid, and xenobiotic metabolism by liver from rats flown on Cosmos 1887. FASEB J 1990, 4, 95–100. [Google Scholar]
- Yin, Y.S.; Liu, J.L.; Fan, Q.C.; Zhao, S.; Wu, X.R.; Wang, J.P.; Liu, Y.; Li, Y.Z.; Lu, W.H. Long-term spaceflight composite stress induces depression and cognitive impairment in astronauts-insights from neuroplasticity. Transl. Psychiatry 2023, 13, 342. [Google Scholar]
- van Praag, H.M. Can stress cause depression? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 891–907. [Google Scholar]
- Dunlop, B.W.; Nemeroff, C.B. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 2007, 64, 327–337. [Google Scholar]
- Sharma, A.N.; da Costa e Silva, B.F.; Soares, J.C.; Carvalho, A.F.; Quevedo, J. Role of trophic factors GDNF, IGF-1 and VEGF in major depressive disorder: A comprehensive review of human studies. J. Affect. Disord. 2016, 197, 9–20. [Google Scholar]
- De la Torre, G.G. Cognitive Neuroscience in Space. Life 2014, 4, 281–294. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wu, A.D.; Cai, J.J.; She, Z.G.; Li, H.L. The contribution of the gut-liver axis to the immune signaling pathway of NAFLD. Front. Immunol. 2022, 13, 968799. [Google Scholar] [CrossRef] [PubMed]
- Zeineddine, Y.; Friedman, M.A.; Buettmann, E.G.; Abraham, L.B.; Hoppock, G.A.; Donahue, H.J. Genetic diversity modulates the physical and transcriptomic response of skeletal muscle to simulated microgravity in male mice. npj Micrograv. 2023, 9, 86. [Google Scholar] [CrossRef]
- Jonscher, K.R.; Alfonso-Garcia, A.; Suhalim, J.L.; Orlicky, D.J.; Potma, E.O.; Ferguson, V.L.; Bouxsein, M.L.; Bateman, T.A.; Stodieck, L.S.; Levi, M. Spaceflight Activates Lipotoxic Pathways in Mouse Liver. PLoS ONE 2016, 11, e0152877. [Google Scholar]
- Kamiya, H.; Sasaki, S.; Ikeuchi, T.; Umemoto, Y.; Tatsura, H.; Hayashi, Y.; Kaneko, S.; Kohri, K. Effect of simulated microgravity on testosterone and sperm motility in mice. J. Androl. 2003, 24, 885–890. [Google Scholar] [CrossRef]
- Dai, X.F.; Ye, S.M.; Chen, X.; Jiang, T.; Huang, H.X.; Li, W.J.; Yu, H.Q.; Bao, J.H.; Chen, H. Rodent retinal microcirculation and visual electrophysiology following simulated microgravity. Exp. Eye Res. 2020, 194, 108023. [Google Scholar] [CrossRef]
- Chen, Z.H.; Zhang, Y.; Zhao, F.; Yin, C.; Yang, C.F.; Huai, Y.; Liang, S.J.; Liu, S.Y.; Xu, X.; Wu, Z.X. miR-138-5p negatively regulates osteoblast differentiation through inhibiting β-catenin under simulated microgravity in MC3T3-E1 cells. Acta Astronaut. 2021, 182, 240–250. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, J.Y.; Liu, H.T.; Wu, Q.; Chen, J.C.; Chen, G.Q. The mechanism of anti-osteoporosis effects of 3-hydroxybutyrate and derivatives under simulated microgravity. Biomaterials 2014, 35, 8273–8283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Wang, Q.S.; Wan, Z.M.; Li, J.Y.; Liu, L.; Zhang, X.Z. CKIP-1 knockout offsets osteoporosis induced by simulated microgravity. Prog. Biophys. Mol. Biol. 2016, 122, 140–148. [Google Scholar] [CrossRef]
- Sun, P.M.; Yang, J.Q.; Wang, B.; Ma, H.; Zhang, Y.; Guo, J.H.; Chen, X.P.; Zhao, J.W.; Sun, H.W.; Yang, J.W.; et al. The effects of combined environmental factors on the intestinal flora of mice based on ground simulation experiments. Sci. Rep. 2021, 11, 1137. [Google Scholar] [CrossRef]
- Shallis, R.M.; Terry, C.M.; Lim, S.H. Changes in intestinal microbiota and their effects on allogeneic stem cell transplantation. Am. J. Hematol. 2018, 93, 122–128. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb. Ecol. Health Dis. 2016, 27, 30971. [Google Scholar]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [PubMed]
- Ding, H.R.; Wang, J.L.; Ren, H.Z.; Shi, X.L. Lipometabolism and Glycometabolism in Liver Diseases. Biomed. Res. Int. 2018, 2018, 1287127. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.H.; Yamamoto, M.; Hernandez, C.M.; Khodadadi, H.; Baban, B.; Stranahan, A.M. Beige adipocytes mediate the neuroprotective and anti-inflammatory effects of subcutaneous fat in obese mice. Nat. Commun. 2021, 12, 4623. [Google Scholar] [CrossRef]
- Demontis, G.C.; Germani, M.M.; Caiani, E.G.; Barravecchia, I.; Passino, C.; Angeloni, D. Human Pathophysiological Adaptations to the Space Environment. Front. Physiol. 2017, 8, 547. [Google Scholar]
- Tan, S.B.; Pei, W.W.; Huang, H.; Zhou, G.M.; Hu, W.T. Additive effects of simulated microgravity and ionizing radiation in cell death, induction of ROS and expression of RAC2 in human bronchial epithelial cells. npj Micrograv. 2020, 6, 34. [Google Scholar]
- Song, S.H.; Gao, Z.Y.; Lei, X.J.; Niu, Y.B.; Zhang, Y.; Li, C.Q.; Lu, Y.; Wang, Z.Z.; Shang, P. Total Flavonoids of Drynariae Rhizoma Prevent Bone Loss Induced by Hindlimb Unloading in Rats. Molecules 2017, 22, 1033. [Google Scholar] [CrossRef]
- Somody, L.; Fagette, S.; Blanc, S.; Frutoso, J.; Gharib, C.; Gauquelin-Koch, G. Regional blood flow in conscious rats after head-down suspension. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 78, 296–302. [Google Scholar] [CrossRef]
- Jang, T.Y.; Jung, A.Y.; Kim, Y.H. Effect of Long-Term Antiorthostatic Suspension in a Murine Model of Acute Lung Injury. Clin. Exp. Otorhinolaryngol. 2016, 9, 332–338. [Google Scholar]
- Chen, Y.J.; Xu, J.; Yang, C.; Zhang, H.Y.; Wu, F.; Chen, J.; Li, K.; Wang, H.L.; Li, Y.; Li, Y.H.; et al. Upregulation of miR-223 in the rat liver inhibits proliferation of hepatocytes under simulated microgravity. Exp. Mol. Med. 2017, 49, e348. [Google Scholar]
- Zibrín, M.; Cigánková, V.; Kocisová, J.; Tomajková, E.; Komorová, T.; Boda, K.; Weismann, P.; Dadasheva, O.A.; Guryeva, T.S. Effect of short-term space flight on structure of liver, lungs, bone and bone marrow of Japanese quail hatched on orbital station Mir. Acta Vet. Brno 2005, 74, 167–174. [Google Scholar] [CrossRef][Green Version]
- Beheshti, A.; Chakravarty, K.; Fogle, H.; Fazelinia, H.; da Silveira, W.A.; Boyko, V.; Polo, S.H.L.; Saravia-Butler, A.M.; Hardiman, G.; Taylor, D.; et al. Multi-omics analysis of multiple missions to space reveal a theme of lipid dysregulation in mouse liver. Sci. Rep. 2019, 9, 19195. [Google Scholar] [CrossRef] [PubMed]
- Zong, B.B.; Wang, Y.J.; Wang, J.Y.; Zhang, P.; Kan, G.H.; Li, M.Y.; Feng, J.; Wang, Y.F.; Chen, X.P.; Jin, R.; et al. Effects of long-term simulated microgravity on liver metabolism in rhesus macaques. FASEB J. 2022, 36, e22536. [Google Scholar] [CrossRef]
- Jiang, N.; Lv, J.W.; Zhang, Y.W.; Sun, X.R.; Yao, C.H.; Wang, Q.; He, Q.H.; Liu, X.M. Protective effects of ginsenosides Rg1 and Rb1 against cognitive impairment induced by simulated microgravity in rats. Front. Pharmacol. 2023, 14, 1167398. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.O.; Ruarte, M.B. Glutamic acid and histamine-sensitive neurons in the ventral hippocampus and the basolateral amygdala of the rat: Functional interaction on memory and learning processes. Behav. Brain Res. 2004, 152, 209–219. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Singer, W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol. Sci. 1990, 11, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Suhr, S.T.; Gage, F.H.; THAL, L.J.; FISHER, L.J. Essential role of neocortical acetylcholine in spatial memory. Nature 1995, 375, 484–487. [Google Scholar] [CrossRef]
- Micheau, J.; Marighetto, A. Acetylcholine and memory: A long, complex and chaotic but still living relationship. Behav. Brain Res. 2011, 221, 424–429. [Google Scholar] [CrossRef]
- Seyedabadi, M.; Fakhfouri, G.; Ramezani, V.; Mehr, S.E.; Rahimian, R. The role of serotonin in memory: Interactions with neuro-transmitters and downstream signaling. Exp. Brain Res. 2014, 232, 723–738. [Google Scholar] [CrossRef]
- Roozendaal, B.; Castello, N.A.; Vedana, G.; Barsegyan, A.; McGaugh, J.L. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol. Learn. Mem. 2008, 90, 576–579. [Google Scholar]
- Stuchlik, A.; Rehakova, L.; Telensky, P.; Vales, K. Morris water maze learning in Long-Evans rats is differentially affected by blockade of D1-like and D2-like dopamine receptors. Neurosci. Lett. 2007, 422, 169–174. [Google Scholar]
- Perez-Garcia, G.; Meneses, A. Memory time-course: mRNA 5-HT1A and 5-HT7 receptors. Behav. Brain Res. 2009, 202, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Rojas, P.S.; Fiedler, J.L. What Do We Really Know About 5-HT(1A) Receptor Signaling in Neuronal Cells? Front. Cell. Neurosci. 2016, 10, 272. [Google Scholar]
- Nobili, A.; Latagliata, E.C.; Viscomi, M.T.; Cavallucci, V.; Cutuli, D.; Giacovazzo, G.; Krashia, P.; Rizzo, F.R.; Marino, R.; Federici, M.; et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 2017, 8, 14727. [Google Scholar]
- Goldman-Rakic, P.S. The cortical dopamine system: Role in memory and cognition. Adv. Pharmacol. 1998, 42, 707–711. [Google Scholar]
- Hooper, L.V.; Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar]
- Yamashita, T.; Emoto, T.; Sasaki, N.; Hirata, K. Gut Microbiota and Coronary Artery Disease. Int. Heart J. 2016, 57, 663–671. [Google Scholar]
- Sirisinha, S. The potential impact of gut microbiota on your health:Current status and future challenges. Asian Pac. J. Allergy Immunol. 2016, 34, 249–264. [Google Scholar] [PubMed]
- Zhang, D.; Frenette, P.S. Cross talk between neutrophils and the microbiota. Blood 2019, 133, 2168–2177. [Google Scholar]
- Diamant, M.; Blaak, E.E.; de Vos, W.M. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes. Rev. 2011, 12, 272–281. [Google Scholar]
- Kou, Y.H.; Li, J.Y.; Zhu, Y.Y.; Liu, J.; Ren, R.Z.; Jiang, Y.Q.; Wang, Y.Y.; Qiu, C.; Zhou, J.Y.; Yang, Z.H.; et al. Human Amniotic Epithelial Stem Cells Promote Colonic Recovery in Experimental Colitis via Exosomal MiR-23a-TNFR1-NF-κB Signaling. Adv. Sci. 2024, 11, 2401429. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.K.; Kim, S.W.; Lee, S.T. Efficient stool collection methods for evaluating the diarrhea score in mouse diarrhea models. In Vivo 2021, 35, 2115–2125. [Google Scholar] [CrossRef]
- Salavatifar, M.; Ahmadi, S.M.; Todorov, S.D.; Khosravi-Darani, K.; Tripathy, A. Impact of Microgravity on Virulence, Antibiotic Resistance and Gene Expression in Beneficial and Pathogenic Microorganisms. Mini Rev. Med. Chem. 2023, 23, 1608–1622. [Google Scholar] [CrossRef]
- Barupal, D.K.; Haldiya, P.K.; Wohlgemuth, G.; Kind, T.; Kothari, S.L.; Pinkerton, K.E.; Fiehn, O. MetaMapp: Mapping and Visualizing Metabolomic Data by Integrating Information from Biochemical Pathways and Chemical and Mass Spectral Similarity. BMC Bioinf. 2012, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Stork, C.A.; Sayoc-Becerra, A.; Marchelletta, R.R.; Prisk, G.K.; McCole, D.F. A simulated microgravity environment causes a sustained defect in epithelial barrier function. Sci. Rep. 2019, 9, 17531. [Google Scholar] [CrossRef]
- Nie, H.Y.; Ge, J.; Huang, G.X.; Liu, K.G.; Yue, Y.; Li, H.; Lin, H.G.; Zhang, T.; Yan, H.F.; Xu, B.X.; et al. New insights into the intestinal barrier through “gut-organ” axes and a glimpse of the microgravity’s effects on intestinal barrier. Front. Physiol. 2024, 15, 1465649. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.R.; Liu, L.W.; Zhang, G.Z.; Dong, J.X.; Ren, Z.G.; Li, Z.Q. The pathogenesis of gut microbiota in hepatic encephalopathy by the gut-liver-brain axis. Biosci. Rep. 2023, 43, BSR20222524. [Google Scholar] [CrossRef]
- Fava, M.; De Dominicis, N.; Forte, G.; Bari, M.; Leuti, A.; Maccarrone, M. Cellular and Molecular Effects of Microgravity on the Immune System: A Focus on Bioactive Lipids. Biomolecules 2024, 14, 446. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Li, B.; Qiang, Y.; Yuan, T.; Tan, X.T.; Wang, Z.H.; Liu, Z.G.; Liu, X.B. Lycopene attenuates western-diet-induced cognitive deficits via improving glycolipid metabolism dysfunction and inflammatory responses in gut–liver–brain axis. Int. J. Obes. 2019, 43, 1735–1746. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Revel, J.S.; Choi, J.; Miranda, C.L.; Lak, P.; Kioussi, C.; Bobe, G.; Gombart, A.F.; Raber, J.; Maier, C.S.; et al. Targeting the Liver-Brain axis with hop-derived flavonoids improves lipid metabolism and cognitive performance in mice. Mol. Nutr. Food Res. 2020, 64, 2000341. [Google Scholar] [CrossRef]
- Yin, Y.S.; Zhu, Y.B.; Liu, J.L.; Fan, Q.C.; Wu, X.R.; Zhao, S.; Wang, J.P.; Liu, Y.; Li, Y.Z.; Lu, W.H. Long-term spaceflight composite stress induces depressive behaviors in model rats through disrupting hippocampus synaptic plasticity. CNS Neurosci. Ther. 2024, 30, e14438. [Google Scholar] [CrossRef] [PubMed]
- Tseilikman, V.E.; Tseilikman, O.B.; Shevyrin, V.A.; Yegorov, O.N.; Epitashvili, A.A.; Aristov, M.R.; Karpenko, M.N.; Lipatov, I.A.; Pashkov, A.A.; Shamshurin, M.V.; et al. Unraveling the Liver-Brain Axis: Resveratrol’s Modulation of Key Enzymes in Stress-Related Anxiety. Biomedicines 2024, 12, 2063. [Google Scholar] [CrossRef] [PubMed]
- Song, C.C.; Kang, T.S.; Gao, K.; Shi, X.D.; Zhang, M.; Zhao, L.L.; Zhou, L.; Guo, J.G. Preparation for mice spaceflight: Indications for training C57BL/6J mice to adapt to microgravity effect with three-dimensional clinostat on the ground. Heliyon 2023, 9, e19355. [Google Scholar] [PubMed]
- Hoedt, E.C.; Hueston, C.M.; Cash, N.; Bongers, R.S.; Keane, J.M.; van Limpt, K.; Ben Amor, K.; Knol, J.; MacSharry, J.; van Sinderen, D. A synbiotic mixture of selected oligosaccharides and bifidobacteria assists murine gut microbiota restoration following antibiotic challenge. Microbiome 2023, 11, 168. [Google Scholar]
- Ren, B.; Wang, L.; Shi, L.; Jin, X.; Liu, Y.; Liu, R.H.; Yin, F.; Cadenas, E.; Dai, X.S.; Liu, Z.G.; et al. Methionine restriction alleviates age-associated cognitive decline via fibroblast growth factor 21. Redox Biol. 2021, 41, 101940. [Google Scholar]
- Liu, X.N.; Li, X.; Xia, B.; Jin, X.; Zou, Q.H.; Zeng, Z.H.; Zhao, W.Y.; Yan, S.K.; Li, L.; Yuan, S.F.; et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. 2021, 33, 923–938.e6. [Google Scholar]
- Wu, R.; Li, N.; Wang, X.; Wang, S.; Tan, J.; Wang, R.; Zheng, W. Mouse model of Graves’ orbitopathy induced by the immunization with TSHR A and IGF-1R α subunit gene. J. Endocrinol. Investig. 2024, 47, 2507–2519. [Google Scholar]
- Şensoy, E. Comparison of the effect of Sunset yellow on the stomach and small intestine of developmental period of mice. Heliyon 2024, 10, e31998. [Google Scholar]
- Lu, Z.G.; Zhang, T.L.; Shen, J.; Xiao, Z.B.; Hu, J.; Niu, Y.W.; Yu, D.; Chen, L.; Zhang, X. Effects of Fragrance-Loaded Mesoporous Silica Nanocolumns on Central Nervous System. J. Biomed. Nanotechnol. 2018, 14, 1578–1589. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Guo, J.; Yu, W.; Zeng, D.; Song, C.; Zhou, L.; Anatolyevna, N.L.; Baranenko, D.; Xiao, D.; Zhou, Y.; et al. Molecular Mechanism of Microgravity-Induced Intestinal Flora Dysbiosis on the Abnormalities of Liver and Brain Metabolism. Int. J. Mol. Sci. 2025, 26, 3094. https://doi.org/10.3390/ijms26073094
Xiong Y, Guo J, Yu W, Zeng D, Song C, Zhou L, Anatolyevna NL, Baranenko D, Xiao D, Zhou Y, et al. Molecular Mechanism of Microgravity-Induced Intestinal Flora Dysbiosis on the Abnormalities of Liver and Brain Metabolism. International Journal of Molecular Sciences. 2025; 26(7):3094. https://doi.org/10.3390/ijms26073094
Chicago/Turabian StyleXiong, Yi, Jianguo Guo, Wenchen Yu, Deyong Zeng, Chenchen Song, Li Zhou, Nadtochii Liudmila Anatolyevna, Denis Baranenko, Dan Xiao, Yingyu Zhou, and et al. 2025. "Molecular Mechanism of Microgravity-Induced Intestinal Flora Dysbiosis on the Abnormalities of Liver and Brain Metabolism" International Journal of Molecular Sciences 26, no. 7: 3094. https://doi.org/10.3390/ijms26073094
APA StyleXiong, Y., Guo, J., Yu, W., Zeng, D., Song, C., Zhou, L., Anatolyevna, N. L., Baranenko, D., Xiao, D., Zhou, Y., & Lu, W. (2025). Molecular Mechanism of Microgravity-Induced Intestinal Flora Dysbiosis on the Abnormalities of Liver and Brain Metabolism. International Journal of Molecular Sciences, 26(7), 3094. https://doi.org/10.3390/ijms26073094






