The Impact of Recreational Diving to a Depth of 40 m on Selected Intracellular DAMPs
Abstract
1. Introduction
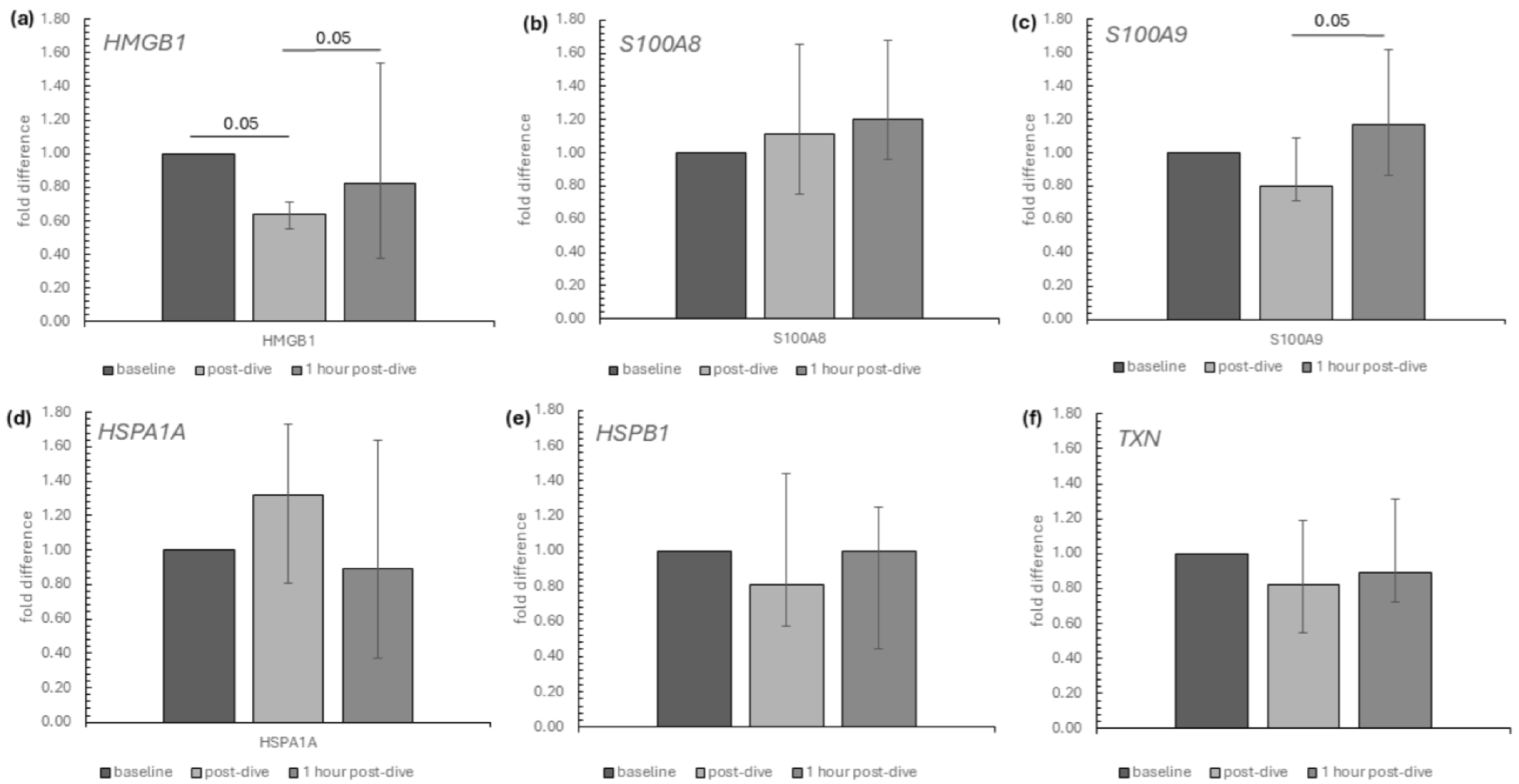
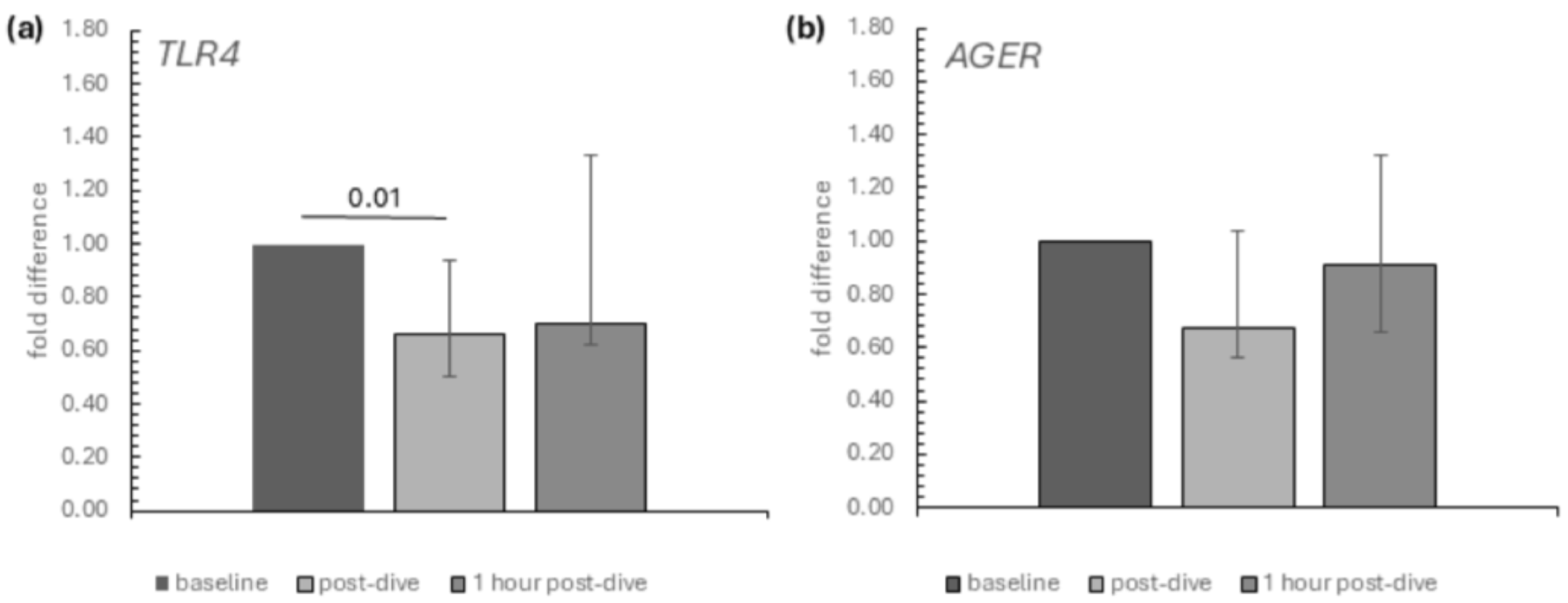
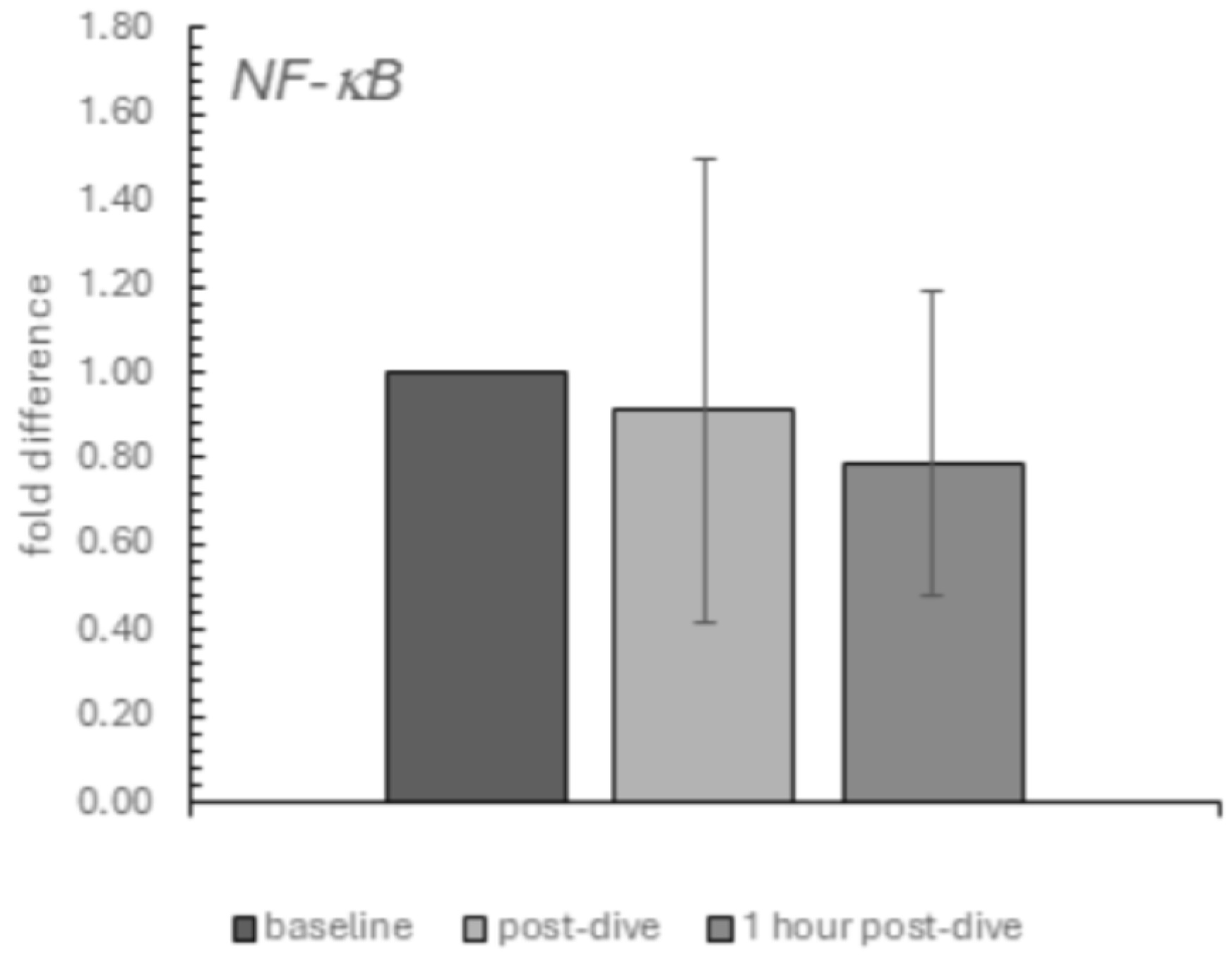
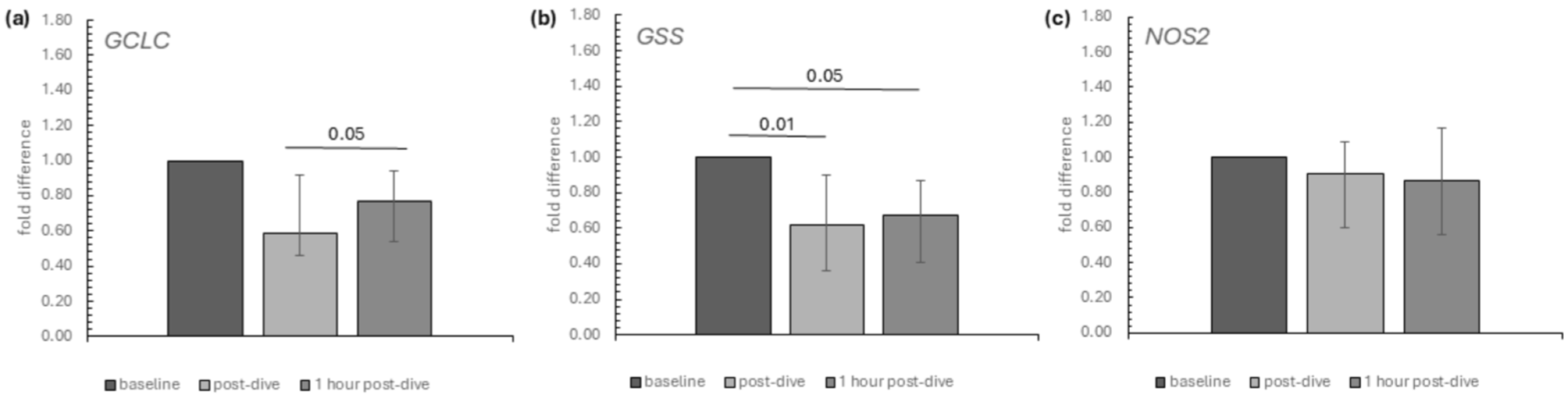
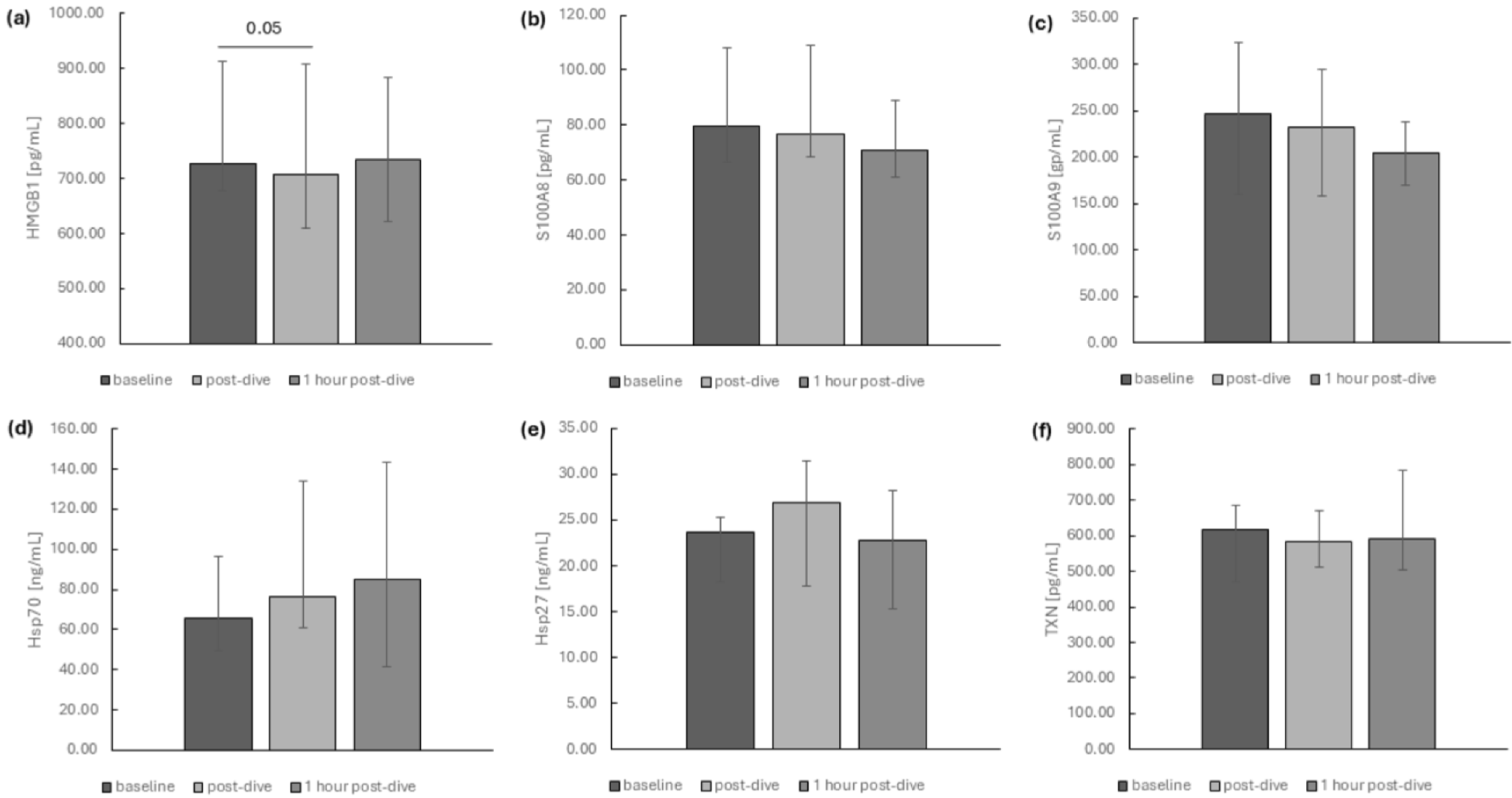
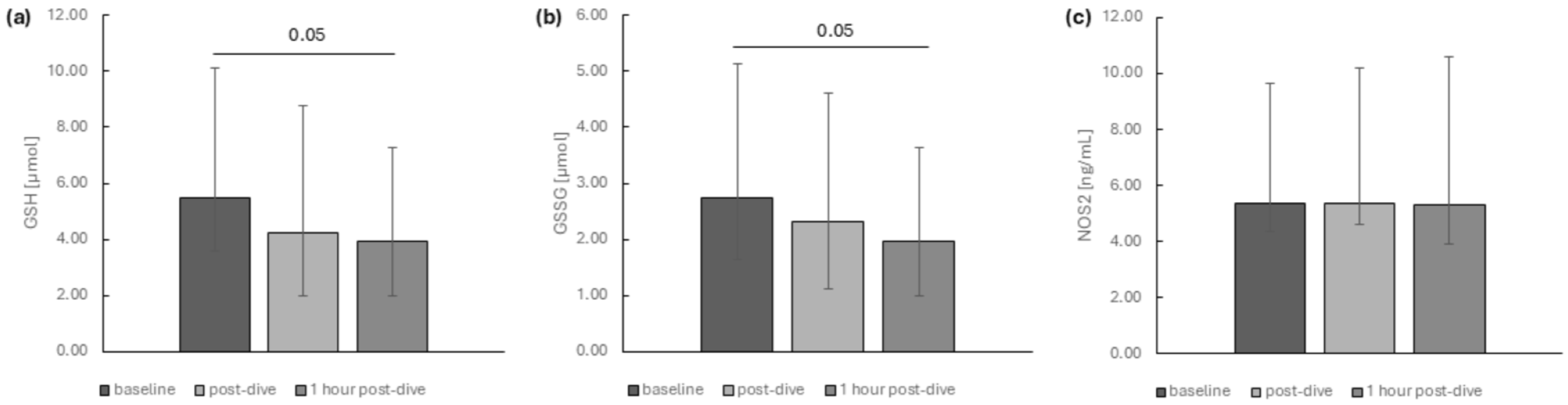
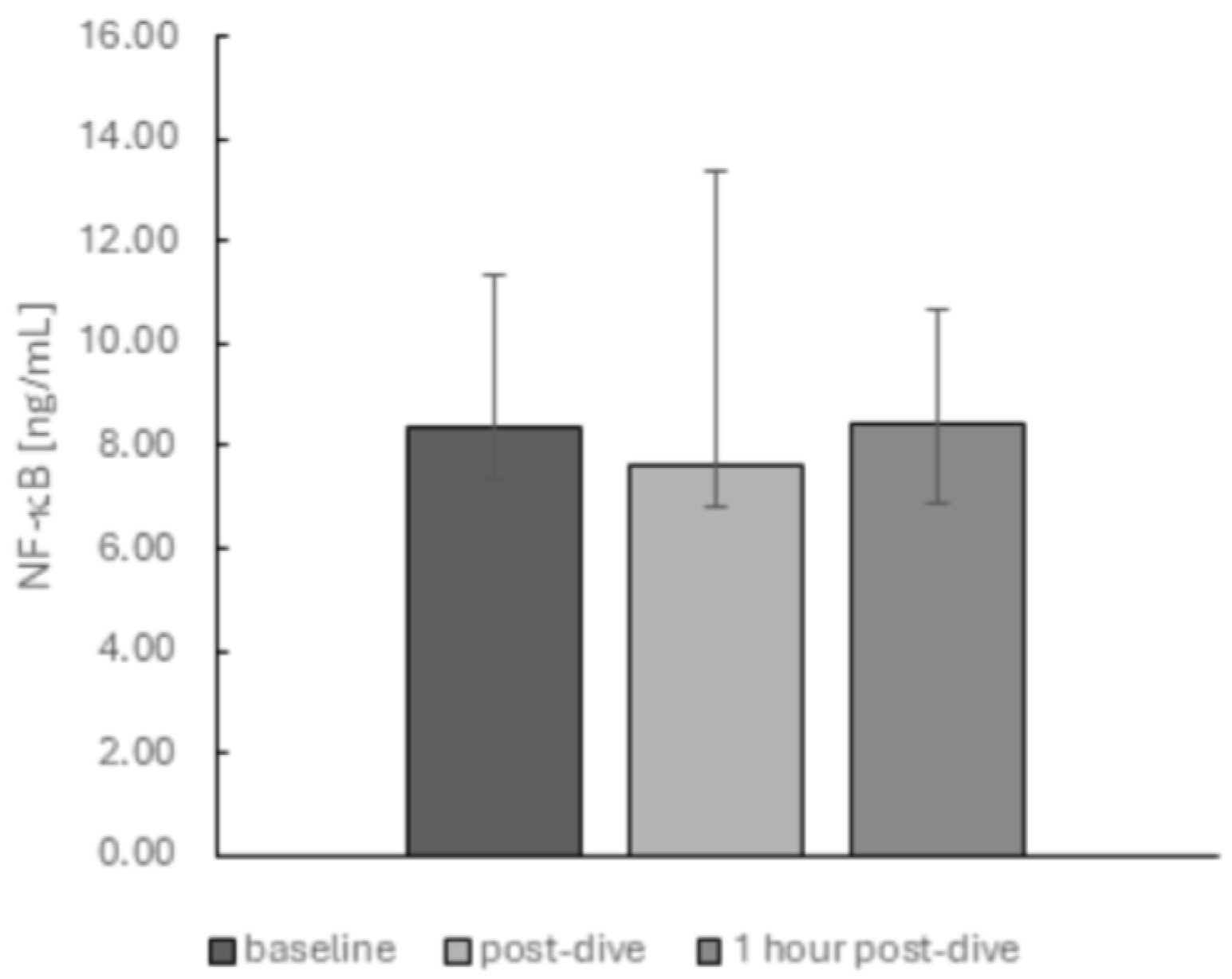
2. Results
3. Discussion
Study Limitations and Perspectives
4. Materials and Methods
4.1. Study Design and Participant Characteristics
4.2. Blood Sampling
4.3. ELISA
4.4. RNA Isolation and Reverse Transcription
4.5. Real-Time qPCR Protocol
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAM10 | A disintegrin and metalloproteinase domain-containing protein 10 |
| ADAM10/17 | A disintegrin and metalloproteinase domain-containing protein 10/17 |
| AGER | advanced glycosylation end-product-specific receptor |
| DAMP | damage-associated molecular pattern |
| esRAGE | endogenous secretory RAGE |
| GCLC | glutamate-cysteine ligase, catalytic subunit |
| GSH | reduced glutathione |
| GSSG | glutathione disulfide (oxidised glutathione) |
| GSS | glutathione synthetase |
| HBO | hyperbaric oxygen therapy |
| HIFs | hypoxia-inducible factors |
| HSPA1A (Hsp70) | heat shock protein family A member 1A |
| HSPB1 (Hsp27) | heat shock protein family B, (small) member 1 |
| HMGB1 | high-mobility group box protein 1 |
| IKK | IkappaB kinase |
| MAPK | mitogen-activated protein kinases |
| MMP-9 | matrix metalloproteinase-9 |
| NFκB | nuclear factor-κB |
| NOS2 | nitric oxide synthase 2 |
| PRR | pattern recognition receptors |
| RAGE | receptors for advanced glycation end products |
| RNS | reactive oxygen species |
| ROS | reactive nitrogen species |
| S100A8 | S100 calcium-binding protein A8 |
| S100A9 | S100 calcium-binding protein A9 |
| SCUBA | Self-Contained Breathing Apparatus |
| SIRT1 | sirtuin1 |
| SIRT3 | sirtuin3 |
| SOD | superoxide dismutase |
| TLR4 | Toll-like receptor 4 |
| TXN | thioredoxin |
References
- Moon, R.E.; Cherry, A.D.; Stolp, B.W.; Camporesi, E.M. Pulmonary gas exchange in diving. J. Appl. Physiol. 2009, 106, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Confédération Mondiale des Activités Subaquatiques (CMAS) (World Underwater Federation). International Diver Training Standards and Procedures Manual Chapter 1: Universal Standards and Procedures. 2012. Available online: https://archives.cmas.org/technique/general-documents (accessed on 3 February 2025).
- Kirkland, P.J.; Mathew, D.; Modi, P.; Cooper, J.S. Nitrogen Narcosis in Diving. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Brubakk, A.O.; Ross, J.A.; Thom, S.R. Saturation diving; physiology and pathophysiology. Compr. Physiol. 2014, 4, 1229–1272. [Google Scholar] [CrossRef] [PubMed]
- Min, H.J.; Kim, J.H.; Yoo, J.E.; Oh, J.H.; Kim, K.S.; Yoon, J.H.; Kim, C.H. ROS-dependent HMGB1 secretion upregulates IL-8 in upper airway epithelial cells under hypoxic condition. Mucosal Immunol. 2017, 10, 685–694. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Rizzato, A.; Della Noce, C.; Malacrida, S.; Montorsi, M.; Paganini, M.; Cancellara, P.; Bosco, G. Oxidative stress assessment in breath-hold diving. Eur. J. Appl. Physiol. 2019, 119, 2449–2456. [Google Scholar] [CrossRef]
- Thom, S.R.; Milovanova, T.N.; Bogush, M.; Yang, M.; Bhopale, V.M.; Pollock, N.W.; Ljubkovic, M.; Denoble, P.; Madden, D.; Lozo, M.; et al. Bubbles, microparticles, and neutrophil activation: Changes with exercise level and breathing gas during open-water SCUBA diving. J. Appl. Physiol. 2013, 114, 1396–1405. [Google Scholar] [CrossRef]
- Cialoni, D.; Brizzolari, A.; Samaja, M.; Pieri, M.; Marroni, A. Altered Venous Blood Nitric Oxide Levels at Depth and Related Bubble Formation During Scuba Diving. Front. Physiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Batle, J.M.; Tauler, P.; Cases, N.; Aguiló, A.; Tur, J.A.; Pons, A. Neutrophil tolerance to oxidative stress induced by hypoxia/reoxygenation. Free Radic. Res. 2004, 38, 1003–1009. [Google Scholar] [CrossRef]
- Sureda, A.; Batle, J.M.; Capó, X.; Martorell, M.; Córdova, A.; Tur, J.A.; Pons, A. Scuba diving induces nitric oxide synthesis and the expression of inflammatory and regulatory genes of the immune response in neutrophils. Physiol. Genom. 2014, 46, 647–654. [Google Scholar] [CrossRef]
- Vezzoli, A.; Mrakic-Sposta, S.; Brizzolari, A.; Balestra, C.; Camporesi, E.M.; Bosco, G. Oxy-Inflammation in Humans during Underwater Activities. Int. J. Mol. Sci. 2024, 25, 3060. [Google Scholar] [CrossRef]
- Salah, H.; El-Gazzar, R.M.; Abd El-Wahab, E.W.; Charl, F. Oxidative stress and adverse cardiovascular effects among professional divers in Egypt. J. Occup. Environ. Hyg. 2023, 20, 159–169. [Google Scholar] [CrossRef]
- Domoto, H.; Iwaya, K.; Ikomi, F.; Matsuo, H.; Tadano, Y.; Fujii, S.; Tachi, K.; Itoh, Y.; Sato, M.; Inoue, K.; et al. Up-Regulation of Antioxidant Proteins in the Plasma Proteome during Saturation Diving: Unique Coincidence under Hypobaric Hypoxia. PLoS ONE 2016, 11, e0163804. [Google Scholar] [CrossRef] [PubMed]
- Djurhuus, R.; Segadal, K.; Svardal, A.M. Glutathione in blood cells decreases without DNA breaks after a simulated saturation dive to 250 msw. Aviat. Space Environ. Med. 2006, 77, 597–604. [Google Scholar] [PubMed]
- Bosco, G.; Paganini, M.; Giacon, T.A.; Oppio, A.; Vezzoli, A.; Dellanoce, C.; Moro, T.; Paoli, A.; Zanotti, F.; Zavan, B.; et al. Oxidative Stress and Inflammation, MicroRNA, and Hemoglobin Variations after Administration of Oxygen at Different Pressures and Concentrations: A Randomized Trial. Int. J. Environ. Res. Public Health 2021, 18, 9755. [Google Scholar] [CrossRef] [PubMed]
- Eckenhoff, R.G.; Olstad, C.S.; Carrod, G. Human dose-response relationship for decompression and endogenous bubble formation. J. Appl. Physiol. 1990, 69, 914–918. [Google Scholar] [CrossRef]
- Rosén, A.; Gennser, M.; Oscarsson, N.; Kvarnström, A.; Sandström, G.; Seeman-Lodding, H.; Simrén, J.; Zetterberg, H. Protein tau concentration in blood increases after SCUBA diving: An observational study. Eur. J. Appl. Physiol. 2022, 122, 993–1005. [Google Scholar] [CrossRef]
- Theunissen, S.; Sponsiello, N.; Rozloznik, M.; Germonpré, P.; Guerrero, F.; Cialoni, D.; Balestra, C. Oxidative stress in breath-hold divers after repetitive dives. Diving Hyperb. Med. 2013, 43, 63–66. [Google Scholar]
- Thom, S.R. Hyperbaric oxygen: Its mechanisms and efficacy. Plast. Reconstr. Surg. 2011, 127, 131S–141S. [Google Scholar] [CrossRef]
- Morabito, C.; Bosco, G.; Pilla, R.; Corona, C.; Mancinelli, R.; Yang, Z.; Camporesi, E.M.; Fanò, G.; Mariggiò, M.A. Effect of pre-breathing oxygen at different depth on oxidative status and calcium concentration in lymphocytes of scuba divers. Acta Physiol. 2011, 202, 69–78. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Luo, Y.; Shen, J. Potential molecular targets of peroxynitrite in mediating blood-brain barrier damage and haemorrhagic transformation in acute ischaemic stroke with delayed tissue plasminogen activator treatment. Free Radic. Res. 2018, 52, 1220–1239. [Google Scholar] [CrossRef]
- Pisetsky, D.S.; Jiang, W. Role of Toll-like receptors in HMGB1 release from macrophages. Ann. N. Y. Acad. Sci. 2007, 1109, 58–65. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.S.; Sitapara, R.A.; Gore, A.; Phan, B.; Sharma, L.; Sampat, V.; Li, J.H.; Yang, H.; Chavan, S.S.; Wang, H.; et al. High mobility group box-1 mediates hyperoxia-induced impairment of Pseudomonas aeruginosa clearance and inflammatory lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2013, 48, 280–287. [Google Scholar] [CrossRef]
- Das, S.; Alhasson, F.; Dattaroy, D.; Pourhoseini, S.; Seth, R.K.; Nagarkatti, M.; Nagarkatti, P.S.; Michelotti, G.A.; Diehl, A.M.; Kalyanaraman, B.; et al. NADPH oxidase-derived peroxynitrite drives inflammation in mice and human nonalcoholic steatohepatitis via TLR4-lipid raft recruitment. Am. J. Pathol. 2015, 185, 1944–1957. [Google Scholar] [CrossRef]
- Loukili, N.; Rosenblatt-Velin, N.; Li, J.; Clerc, S.; Pacher, P.; Feihl, F.; Waeber, B.; Liaudet, L. Peroxynitrite induces HMGB1 release by cardiac cells in vitro and HMGB1 upregulation in the infarcted myocardium in vivo. Cardiovasc. Res. 2011, 89, 586–594. [Google Scholar] [CrossRef]
- Frevert, C.W.; Felgenhauer, J.; Wygrecka, M.; Nastase, M.V.; Schaefer, L. Danger-associated molecular patterns derived from the extracellular matrix provide temporal control of innate immunity. J. Histochem. Cytochem. 2018, 66, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.S.; Sohn, D.H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Yang, D.; Han, Z.; Oppenheim, J.J. Alarmins and immunity. Immunol. Rev. 2017, 280, 41–56. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining chronic inflammation in aging and age-related diseases: Proposal of the senoinflammation concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Francaux, M. Toll-like receptor signalling induced by endurance exercise. Appl. Physiol. Nutr. Metab. 2009, 34, 454–458. [Google Scholar] [CrossRef]
- Zbinden-Foncea, H.; Raymackers, J.M.; Deldicque, L.; Renard, P.; Francaux, M. TLR2 and TLR4 activate p38 MAPK and JNK during endurance exercise in skeletal muscle. Med. Sci. Sports Exerc. 2012, 44, 1463–1472. [Google Scholar] [CrossRef]
- Chandrashekaran, V.; Seth, R.K.; Dattaroy, D.; Alhasson, F.; Ziolenka, J.; Carson, J.; Berger, F.G.; Kalyanaraman, B.; Diehl, A.M.; Chatterjee, S. HMGB1-RAGE pathway drives peroxynitrite signaling-induced IBD-like inflammation in murine nonalcoholic fatty liver disease. Redox Biol. 2017, 13, 8–19. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Golenbock, D.; Bowie, A.G. The history of Toll-like receptors—redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Pei, L.; Zhang, Q.; Han, W.; Jiang, S.; Lin, Y.; Dong, B.; Cui, L.; Li, M. Ox-LDL induces endothelial cell apoptosis and macrophage migration by regulating caveolin-1 phosphorylation. J. Cell Physiol. 2018, 233, 6683–6692. [Google Scholar] [CrossRef]
- Hiramoto, S.; Tsubota, M.; Yamaguchi, K.; Okazaki, K.; Sakaegi, A.; Toriyama, Y.; Tanaka, J.; Sekiguchi, F.; Ishikura, H.; Wake, H.; et al. Cystitis-related bladder pain involves ATP-dependent HMGB1 release from macrophages and its downstream H2S/Cav3.2 signaling in mice. Cells 2020, 9, 1748. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Jiang, M.; Wang, H.J. Effect of NF-κB inhibitor on high mobility group protein B1 expression in a COPD rat model. Mol. Med. Rep. 2013, 7, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Vezzoli, M.; Castellani, P.; Corna, G.; Castiglioni, A.; Bosurgi, L.; Monno, A.; Brunelli, S.; Manfredi, A.A.; Rubartelli, A.; Rovere-Querini, P. High-mobility group box 1 release and redox regulation accompany regeneration and remodeling of skeletal muscle. Antioxid. Redox Signal. 2011, 15, 2161–2174. [Google Scholar] [CrossRef]
- Goh, J.; Behringer, M. Exercise alarms the immune system: A HMGB1 perspective. Cytokine 2018, 110, 222–225. [Google Scholar] [CrossRef]
- Kazama, H.; Ricci, J.E.; Herndon, J.M.; Hoppe, G.; Green, D.R.; Ferguson, T.A. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 2008, 29, 21–32. [Google Scholar] [CrossRef]
- Frank, M.G.; Weber, M.D.; Watkins, L.R.; Maier, S.F. Stress sounds the alarmin: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav. Immun. 2015, 48, 1–7. [Google Scholar] [CrossRef]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef]
- Rovere-Querini, P.; Capobianco, A.; Scaffidi, P.; Valentinis, B.; Catalanotti, F.; Giazzon, M.; Dumitriu, I.E.; Müller, S.; Iannacone, M.; Traversari, C.; et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004, 5, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Heizmann, C.W.; Ackermann, G.E.; Galichet, A. Pathologies involving the S100 proteins and RAGE. In Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2007; Volume 45, pp. 93–138. [Google Scholar] [CrossRef]
- Eftedal, I.; Ljubkovic, M.; Flatberg, A.; Jørgensen, A.; Brubakk, A.O.; Dujic, Z. Acute and potentially persistent effects of scuba diving on the blood transcriptome of experienced divers. Physiol. Genomics 2013, 45, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Feldman, N.; Rotter-Maskowitz, A.; Okun, E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res. Rev. 2015, 24, 29–39. [Google Scholar] [CrossRef]
- Pirnie, R.; Gillespie, K.P.; Mesaros, C.; Blair, I.A. Reappraisal of oxidized HMGB1 as a mediator and biomarker. Future Sci. OA 2023, 8, FSO828. [Google Scholar] [CrossRef]
- Weber, D.J.; Allette, Y.M.; Wilkes, D.S.; White, F.A. The HMGB1-RAGE Inflammatory Pathway: Implications for Brain Injury-Induced Pulmonary Dysfunction. Antioxid. Redox Signal. 2015, 23, 1316–1328. [Google Scholar] [CrossRef]
- Lotfi, R.; Herzog, G.I.; DeMarco, R.A.; Beer-Stolz, D.; Lee, J.J.; Rubartelli, A.; Schrezenmeier, H.; Lotze, M.T. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J. Immunol. 2009, 183, 5023–5031. [Google Scholar] [CrossRef]
- Davalos, A.R.; Kawahara, M.; Malhotra, G.K.; Schaum, N.; Huang, J.; Ved, U.; Beausejour, C.M.; Coppe, J.P.; Rodier, F.; Campisi, J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J. Cell Biol. 2013, 201, 613–629. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, L.; Huang, X.; Guo, F. Effects of nebulized N-acetylcysteine on the expression of HMGB1 and RAGE in rats with hyperoxia-induced lung injury. J. Cell Physiol. 2019, 234, 10547–10553. [Google Scholar] [CrossRef]
- Lin, S.S.; Yuan, L.J.; Niu, C.C.; Tu, Y.K.; Yang, C.Y.; Ueng, S.W.N. Hyperbaric oxygen inhibits the HMGB1/RAGE signaling pathway by upregulating Mir-107 expression in human osteoarthritic chondrocytes. Osteoarthr. Cartil. 2019, 27, 1372–1381. [Google Scholar] [CrossRef]
- Gore, A.; Muralidhar, M.; Espey, M.G.; Degenhardt, K.; Mantell, L.L. Hyperoxia sensing: From molecular mechanisms to significance in disease. J. Immunotoxicol. 2010, 7, 239–254. [Google Scholar] [CrossRef]
- Wohlrab, P.; Johann Danhofer, M.; Schaubmayr, W.; Tiboldi, A.; Krenn, K.; Markstaller, K.; Ullrich, R.; Ulrich Klein, K.; Tretter, V. Oxygen conditions oscillating between hypoxia and hyperoxia induce different effects in the pulmonary endothelium compared to constant oxygen conditions. Physiol. Rep. 2021, 9, e14590. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Fang, F.; Xu, F. Hyperoxia induces inflammation and regulates cytokine production in alveolar epithelium through TLR2/4-NF-κB-dependent mechanism. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1399–1410. [Google Scholar]
- Singh, H.; Agrawal, D.K. Therapeutic Potential of Targeting the HMGB1/RAGE Axis in Inflammatory Diseases. Molecules 2022, 27, 7311. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, P.; Du, M.; Chen, K.; Chen, A.; Wang, Y.; Cao, F.; Deng, S.; Xu, Y. Hyperoxia-induced immature brain injury through the TLR4 signaling pathway in newborn mice. Brain Res. 2015, 1610, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.O.R.; He, Y.; Bertagnolli, M.; Mai-Vo, T.A.; Fernandes, R.O.; Boudreau, F.; Cloutier, A.; Luu, T.M.; Nuyt, A.M. TLR (Toll-Like Receptor) 4 antagonism prevents left ventricular hypertrophy and dysfunction caused by neonatal hyperoxia exposure in rats. Hypertension. 2019, 74, 843–853. [Google Scholar] [CrossRef]
- Zhong, H.; Li, X.; Zhou, S.; Jiang, P.; Liu, X.; Ouyang, M.; Nie, Y.; Chen, X.; Zhang, L.; Liu, Y.; et al. Interplay between RAGE and TLR4 regulates HMGB1-induced inflammation by promoting cell surface expression of RAGE and TLR4. J. Immunol. 2020, 205, 767–775. [Google Scholar] [CrossRef]
- Lua, J.; Ekanayake, K.; Fangman, M.; Doré, S. Potential role of soluble Toll-like receptors 2 and 4 as therapeutic agents in stroke and brain hemorrhage. Int. J. Mol. Sci. 2021, 22, 9977. [Google Scholar] [CrossRef]
- Erusalimsky, J.D. The use of the soluble receptor for advanced glycation-end products (sRAGE) as a potential biomarker of disease risk and adverse outcomes. Redox Biol. 2021, 42, 101958. [Google Scholar] [CrossRef]
- Zunt, S.L.; Burton, L.V.; Goldblatt, L.I.; Dobbins, E.E.; Srinivasan, M. Soluble forms of Toll-like receptor 4 are present in human saliva and modulate tumour necrosis factor-alpha secretion by macrophage-like cells. Clin Exp Immunol. 2009, 156, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.I.; Lippman, M.E. Targeting RAGE signaling in inflammatory disease. Annu. Rev. Med. 2018, 69, 349–364. [Google Scholar] [CrossRef]
- Liew, F.Y.; Xu, D.; Brint, E.K.; O’Neill, L.A. Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005, 5, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Vazzana, N.; Bucciarelli, L.G.; Davì, G. Soluble forms of RAGE in human diseases: Clinical and therapeutical implications. Curr. Med. Chem. 2009, 16, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Vazzana, N.; Santilli, F.; Cuccurullo, C.; Davì, G. Soluble forms of RAGE in internal medicine. Intern. Emerg. Med. 2009, 4, 389–401. [Google Scholar] [CrossRef]
- Ten Oever, J.; Kox, M.; van de Veerdonk, F.L.; Mothapo, K.M.; Slavcovici, A.; Jansen, T.L.; Tweehuysen, L.; Giamarellos-Bourboulis, E.J.; Schneeberger, P.M.; Wever, P.C.; et al. The discriminative capacity of soluble Toll-like receptor (sTLR)2 and sTLR4 in inflammatory diseases. BMC Immunol. 2014, 15, 55. [Google Scholar] [CrossRef]
- Iwami, K.I.; Matsuguchi, T.; Masuda, A.; Kikuchi, T.; Musikacharoen, T.; Yoshikai, Y. Cutting edge: Naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J. Immunol. 2000, 165, 6682–6686. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Zhou, Y.; Wang, G.; Gao, C.; Su, Q. Hyperbaric oxygen alleviates experimental (spinal cord) injury by downregulating HMGB1/NF-κB expression. Spine 2013, 38, E1641–E1648. [Google Scholar] [CrossRef]
- Kang, N.; Hai, Y.; Yang, J.; Liang, F.; Gao, C.J. Hyperbaric oxygen intervention reduces secondary spinal cord injury in rats via regulation of HMGB1/TLR4/NF-κB signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 1141–1153. [Google Scholar]
- Sun, L.; Zhao, L.; Li, P.; Liu, X.; Liang, F.; Jiang, Y.; Kang, N.; Gao, C.; Yang, J. Effect of hyperbaric oxygen therapy on HMGB1/NF-κB expression and prognosis of acute spinal cord injury: A randomized clinical trial. Neurosci. Lett. 2019, 692, 47–52. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Gao, C.; Li, Z.; Yang, J.; Liu, X.; Liang, F. Effects of hyperbaric oxygen therapy on RAGE and MCP-1 expression in rats with spinal cord injury. Mol. Med. Rep. 2016, 14, 5619–5625. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.C.; Xu, S.N.; Huang, Z.S.; Jiang, G.W.; Deng, P.C.; Zhang, Y.M. Hyperbaric oxygen via mediating SIRT1-induced deacetylation of HMGB1 improved reperfusion injury. Eur. J. Neurosci. 2021, 54, 7318–7331. [Google Scholar] [CrossRef]
- Perović, A.; Sobočanec, S.; Dabelić, S.; Balog, T.; Dumić, J. Effect of scuba diving on the oxidant/antioxidant status, SIRT1 and SIRT3 expression in recreational divers after a winter nondive period. Free Radic. Res. 2018, 52, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, P.; Zhang, J.C.; Zhang, Q.; Yao, S.L. Proinflammatory effects of S100A8/A9 via TLR4 and RAGE signaling pathways in BV-2 microglial cells. Int. J. Mol. Med. 2017, 40, 31–38. [Google Scholar] [CrossRef]
- Vincent, A.M.; Perrone, L.; Sullivan, K.A.; Backus, C.; Sastry, A.M.; Lastoskie, C.; Feldman, E.L. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology 2007, 148, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Role of S100 proteins in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118677. [Google Scholar] [CrossRef]
- Wu, R.W.; Lian, W.S.; Kuo, C.W.; Chen, Y.S.; Ko, J.Y.; Wang, F.S. S100 calcium binding protein A9 represses angiogenic activity and aggravates osteonecrosis of the femoral head. Int. J. Mol. Sci. 2019, 20, 5786. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, C.; Zou, J.; Zhang, L. Hyperbaric oxygen therapy for the treatment of bone-related diseases. Int. J. Mol. Sci. 2025, 26, 1067. [Google Scholar] [CrossRef]
- Piotrowicz, G.; Kot, J.; Babicki, A.; Banaszkiewicz, P.; Piotrowicz, A.; Rzeszutek, M.; Rudnik, A.; Zientara, P.; Adamska-Mieruszewska, J.; Rydzewska, G. The effects of hyperbaric treatment on perianal fistula activity in patients with Crohn’s disease. Gastroenterol. Rev. 2024, 19, 321–332. [Google Scholar] [CrossRef]
- Goh, J.; Hofmann, P.; Aw, N.H. Concurrent high-intensity aerobic and resistance exercise modulates systemic release of alarmins (HMGB1, S100A8/A9, HSP70) and inflammatory biomarkers in healthy young men: A pilot study. Transl. Med. Commun. 2020, 5, 4. [Google Scholar] [CrossRef]
- Bilopavlovic, N.; Marinovic, J.; Ljubkovic, M.; Obad, A.; Zanchi, J.; Pollock, N.W.; Denoble, P.; Dujic, Z. Effect of repetitive SCUBA diving on humoral markers of endothelial and central nervous system integrity. Eur. J. Appl. Physiol. 2013, 113, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.P.; Linér, M.H.; Jönsson, H. Increased serum levels of the brain damage marker S100B after apnea in trained breath-hold divers: A study including respiratory and cardiovascular observations. J. Appl. Physiol. 2009, 107, 809–815. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Shan, P.; Hunt, C.R.; Pandita, T.K.; Lee, P.J. A protective Hsp70-TLR4 pathway in lethal oxidant lung injury. J. Immunol. 2013, 191, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Wu, Y.; Huang, X.; Wang, W.; Ang, B.; Cao, X.; Wan, T. Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J. Biol. Chem. 2011, 286, 30393–30400. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Tukaj, S. Heat Shock Protein 70 as a double agent acting inside and outside the cell: Insights into autoimmunity. Int. J. Mol. Sci. 2020, 21, 5298. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.; Yi, H.J.; Wang, Y.W.; Zhou, Q.; Ariyadewa, D.K.; Xu, W.G. Benefits of hyperbaric oxygen pretreatment for decompression sickness in Bama pigs. J. Exp. Biol. 2018, 221, jeb171066. [Google Scholar] [CrossRef]
- Szyller, J.; Kozakiewicz, M.; Siermontowski, P.; Kaczerska, D. Oxidative stress, HSP70/HSP90 and eNOS/iNOS serum levels in professional divers during hyperbaric exposition. Antioxidants 2022, 11, 1008. [Google Scholar] [CrossRef]
- Thom, S.R. Oxidative stress is fundamental to hyperbaric oxygen therapy. J. Appl. Physiol. 2009, 106, 988–995. [Google Scholar] [CrossRef]
- Welsh, S.J.; Bellamy, W.T.; Briehl, M.M.; Powis, G. The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res. 2002, 62, 5089–5095. [Google Scholar]
- Gröger, M.; Oter, S.; Simkova, V.; Bolten, M.; Koch, A.; Warninghoff, V.; Georgieff, M.; Muth, C.M.; Speit, G.; Radermacher, P. DNA damage after long-term repetitive hyperbaric oxygen exposure. J. Appl. Physiol. 2009, 106, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Djurhuus, R.; Nossum, V.; Lundsett, N.; Hovin, W.; Svardal, A.M.; Havnes, M.B.; Fismen, L.; Hjelde, A.; Brubakk, A.O. Simulated diving after heat stress potentiates the induction of heat shock protein 70 and elevates glutathione in human endothelial cells. Cell Stress Chaperones 2010, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, S.J.; Lyn, N.; Florentino, A.; Li, A.; Davies, K.J.A.; Forman, H.J. Down regulation of glutathione and glutamate cysteine ligase in the inflammatory response of macrophages. Free Radic. Biol. Med. 2020, 158, 53–59. [Google Scholar] [CrossRef]
- Modun, D.; Krnic, M.; Vukovic, J.; Kokic, V.; Kukoc-Modun, L.; Tsikas, D.; Dujic, Z. Plasma nitrite concentration decreases after hyperoxia-induced oxidative stress in healthy humans. Clin. Physiol. Funct. Imaging 2012, 32, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Ferrer, M.D.; Batle, J.M.; Tauler, P.; Tur, J.A.; Pons, A. Scuba diving increases erythrocyte and plasma antioxidant defenses and spares NO without oxidative damage. Med. Sci. Sports Exerc. 2009, 41, 1271–1276. [Google Scholar] [CrossRef]
- Angermüller, S.; Islinger, M.; Völkl, A. Peroxisomes and reactive oxygen species, a lasting challenge. Histochem. Cell Biol. 2009, 131, 459–463. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Yuan, X.; Wang, T.; Luo, S.; Lei, H.; Xia, Y. Requirement of heat shock protein 70 for inducible nitric oxide synthase induction. Cell Signal. 2013, 25, 1310–1317. [Google Scholar] [CrossRef]
- Wisløff, U.; Richardson, R.S.; Brubakk, A.O. NOS inhibition increases bubble formation and reduces survival in sedentary but not exercised rats. J. Physiol. 2003, 546, 577–582. [Google Scholar] [CrossRef]

| Recreational Diver Group N = 21 | |
|---|---|
| age [years] | 50.0 (48.00−61.00) |
| height [cm] | 1.80 (1.76−1.81) |
| weight [kg] | 89.5 (85.0−92.5) |
| BMI [kg/m2] | 27.8 (26.5−28.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowakowska, A.; Marchelek-Myśliwiec, M.; Skórka-Majewicz, M.; Żwierełło, W.; Grzeszczak, K.; Gutowska, I. The Impact of Recreational Diving to a Depth of 40 m on Selected Intracellular DAMPs. Int. J. Mol. Sci. 2025, 26, 3061. https://doi.org/10.3390/ijms26073061
Nowakowska A, Marchelek-Myśliwiec M, Skórka-Majewicz M, Żwierełło W, Grzeszczak K, Gutowska I. The Impact of Recreational Diving to a Depth of 40 m on Selected Intracellular DAMPs. International Journal of Molecular Sciences. 2025; 26(7):3061. https://doi.org/10.3390/ijms26073061
Chicago/Turabian StyleNowakowska, Anna, Małgorzata Marchelek-Myśliwiec, Marta Skórka-Majewicz, Wojciech Żwierełło, Konrad Grzeszczak, and Izabela Gutowska. 2025. "The Impact of Recreational Diving to a Depth of 40 m on Selected Intracellular DAMPs" International Journal of Molecular Sciences 26, no. 7: 3061. https://doi.org/10.3390/ijms26073061
APA StyleNowakowska, A., Marchelek-Myśliwiec, M., Skórka-Majewicz, M., Żwierełło, W., Grzeszczak, K., & Gutowska, I. (2025). The Impact of Recreational Diving to a Depth of 40 m on Selected Intracellular DAMPs. International Journal of Molecular Sciences, 26(7), 3061. https://doi.org/10.3390/ijms26073061






