Gut Microbiota Modulation in IBD: From the Old Paradigm to Revolutionary Tools
Abstract
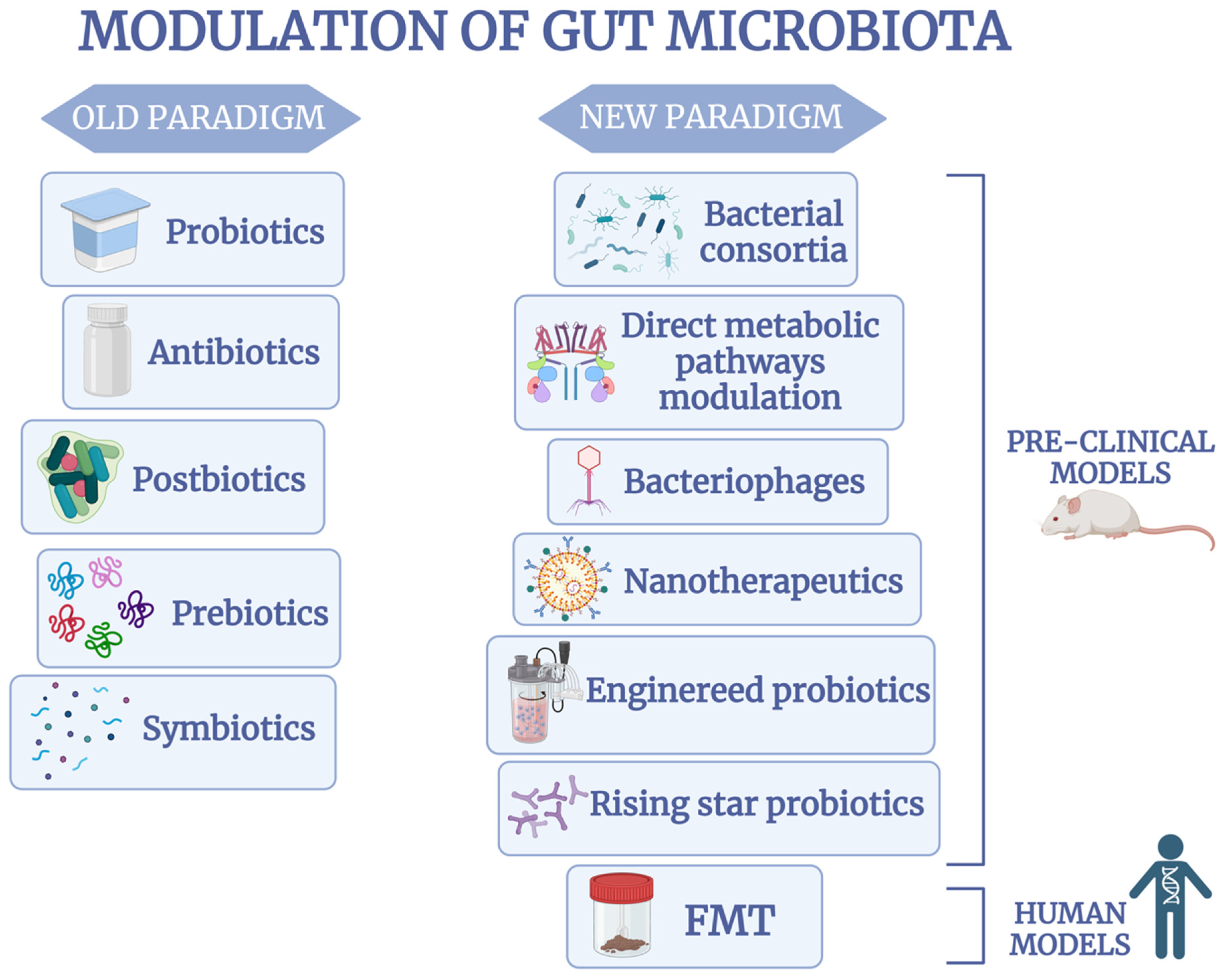
1. Introduction
2. Changes in Gut Microbiota Are Associated with IBD
3. The Old Paradigm of Gut Microbiota Modulation in IBD
3.1. Antibiotics
3.2. Probiotics
3.3. Prebiotics
3.4. Postbiotics
3.5. Symbiotics
4. Revolutionizing Microbiota Therapeutics in IBD: The New Paradigm
4.1. Fecal Microbiota Transplantation
4.2. Rising Star Probiotics: Faecalibacterium Prausnitzii
4.3. Rising Star Probiotics: Akkermansia Muciniphila
4.4. Bacterial Consortia
4.5. Bacteriophages
4.6. Engineered Probiotics
4.7. Direct Metabolic Pathways Modulation and Nanotherapeutics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dorofeyev, A.E.; Vasilenko, I.V.; Rassokhina, O.A.; Kondratiuk, R.B. Mucosal barrier in ulcerative colitis and Crohn’s disease. Gastroenterol. Res. Pract. 2013, 2013, 431231. [Google Scholar] [CrossRef] [PubMed]
- Carrière, J.; Darfeuille-Michaud, A.; Nguyen, H.T.T. Infectious etiopathogenesis of Crohn’s disease. World J. Gastroenterol. 2014, 20, 12102–12117. [Google Scholar] [CrossRef]
- Knox, N.C.; Forbes, J.D.; Peterson, C.L.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiome in Inflammatory Bowel Disease: Lessons Learned from Other Immune-Mediated Inflammatory Diseases. Am. J. Gastroenterol. 2019, 114, 1051–1070. [Google Scholar] [CrossRef]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Toor, D.; Wsson, M.K.; Kumar, P.; Karthikeyan, G.; Kaushik, N.K.; Goel, C.; Singh, S.; Kumar, A.; Prakash, H. Dysbiosis Disrupts Gut Immune Homeostasis and Promotes Gastric Diseases. Int. J. Mol. Sci. 2019, 20, 2432. [Google Scholar] [CrossRef] [PubMed]
- Casén, C.; Vebø, H.C.; Sekelja, M.; Hegge, F.T.; Karlsson, M.K.; Ciemniejewska, E.; Dzankovic, S.; Frøyland, C.; Nestestog, R.; Engstrand, L.; et al. Deviations in human gut microbiota: A novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment. Pharmacol. Ther. 2015, 42, 71–83. [Google Scholar] [CrossRef]
- Alshehri, D.; Saadah, O.; Mosli, M.; Edris, S.; Alhindi, R.; Bahieldin, A. Dysbiosis of gut microbiota in inflammatory bowel disease: Current therapies and potential for microbiota-modulating therapeutic approaches. Bosn. J. Basic Med. Sci. 2021, 21, 270–283. [Google Scholar] [CrossRef]
- Garrett, W.S.; Gordon, J.I.; Glimcher, L.H. Homeostasis and inflammation in the intestine. Cell 2010, 140, 859–870. [Google Scholar] [CrossRef]
- Hedin, C.R.; McCarthy, N.E.; Louis, P.; Farquharson, F.M.; McCartney, S.; Taylor, K.; Prescott, N.J.; Murrells, T.; Stagg, A.J.; Whelan, K.; et al. Altered intestinal microbiota and blood T cell phenotype are shared by patients with Crohn’s disease and their unaffected siblings. Gut 2014, 63, 1578–1586. [Google Scholar] [CrossRef]
- Willing, B.P.; Dicksved, J.; Halfvarson, J.; Andersson, A.F.; Lucio, M.; Zheng, Z.; Järnerot, G.; Tysk, C.; Jansson, J.K.; Engstrand, L. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 2010, 139, 1844–1854.e1. [Google Scholar] [CrossRef]
- Ashton, J.J.; Seaby, E.G.; Beattie, R.M.; Ennis, S. NOD2 in Crohn’s Disease-Unfinished Business. J. Crohn’s Colitis 2023, 17, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Yang, Z.; Chen, B.; Zhong, X. The multifaceted role of CARD9 in inflammatory bowel disease. J. Cell. Mol. Med. 2020, 24, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, S.; Conway, K.L.; Lassen, K.G.; Jijon, H.B.; Pan, H.; Chun, E.; Michaud, M.; Lang, J.K.; Gallini Comeau, C.A.; Dreyfuss, J.M.; et al. The Crohn’s disease polymorphism, ATG16L1 T300A, alters the gut microbiota and enhances the local Th1/Th17 response. eLfie 2019, 8, e39982. [Google Scholar] [CrossRef]
- Frank, D.N.; Robertson, C.E.; Hamm, C.M.; Kpadeh, Z.; Zhang, T.; Chen, H.; Zhu, W.; Sartor, R.B.; Boedeker, E.C.; Harpaz, N.; et al. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Knights, D.; Silverberg, M.S.; Weersma, R.K.; Gevers, D.; Dijkstra, G.; Huang, H.; Tyler, A.D.; van Sommeren, S.; Imhann, F.; Stempak, J.M.; et al. Complex host genetics influence the microbiome in inflammatory bowel disease. Genome Med. 2014, 6, 107. [Google Scholar] [CrossRef]
- Hu, S.; Vich Vila, A.; Gacesa, R.; Collij, V.; Stevens, C.; Fu, J.M.; Wong, I.; Talkowski, M.E.; Rivas, M.A.; Imhann, F.; et al. Whole Exome Sequencing Analyses Reveal Gene–Microbiota Interactions in the Context of IBD. Gut 2021, 70, 285–296. [Google Scholar] [CrossRef]
- Imhann, F.; Vich Vila, A.; Bonder, M.J.; Fu, J.; Gevers, D.; Visschedijk, M.C.; Spekhorst, L.M.; Alberts, R.; Franke, L.; van Dullemen, H.M.; et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 2018, 67, 108–119. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Biedermann, L.; Brülisauer, K.; Zeitz, J.; Frei, P.; Scharl, M.; Vavricka, S.R.; Fried, M.; Loessner, M.J.; Rogler, G.; Schuppler, M. Smoking cessation alters intestinal microbiota: Insights from quantitative investigations on human fecal samples using FISH. Inflamm. Bowel Dis. 2014, 20, 1496–1501. [Google Scholar] [CrossRef]
- Allais, L.; Kerckhof, F.M.; Verschuere, S.; Bracke, K.R.; De Smet, R.; Laukens, D.; Van den Abbeele, P.; De Vos, M.; Boon, N.; Brusselle, G.G.; et al. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ. Microbiol. 2016, 18, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Adolph, T.E.; Zhang, J. Diet fuelling inflammatory bowel diseases: Preclinical and clinical concepts. Gut 2022, 71, 2574–2586. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef]
- Cabral, D.J.; Wurster, J.I.; Korry, B.J.; Penumutchu, S.; Belenky, P. Consumption of a Western-style diet modulates the response of the murine gut microbiome to ciprofloxacin. mSystems 2020, 5, e00317–20. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Pan, R.; He, Y.; Yuan, J.; Zhao, S.; Ma, M.; Chai, Z.; Ji, X.; Hu, X.; He, C.; Zhou, D.; et al. The Role of Antibiotic Exposure as Risk Factor for IBD Epidemic: An Updated Meta-Analysis. J. Gastroenterol. Hepatol. 2024, 39, 2561–2571. [Google Scholar] [CrossRef]
- Mårild, K.; Lerchova, T.; Östensson, M.; Imberg, H.; Størdal, K.; Ludvigsson, J. Early-Life Infections, Antibiotics and Later Risk of Childhood and Early Adult-Onset Inflammatory Bowel Disease: Pooled Analysis of Two Scandinavian Birth Cohorts. Aliment. Pharmacol. Ther. 2024, 61, 323–334. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Xiong, R.G.; Li, J.; Cheng, J.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Gan, R.Y.; Li, H.B. The Role of Gut Microbiota in Anxiety, Depression, and Other Mental Disorders as Well as the Protective Effects of Dietary Components. Nutrients 2023, 15, 3258. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, B.; Duan, Z.; Xia, Z.; Ding, Y.; Chen, T.; Liu, H.; Wang, B.; Yang, B.; Wang, X.; et al. Depression and anxiety in patients with active ulcerative colitis: Crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes 2021, 13, 1987779. [Google Scholar] [CrossRef]
- Humbel, F.; Rieder, J.H.; Franc, Y.; Juillerat, P.; Scharl, M.; Misselwitz, B.; Schreiner, P.; Begré, S.; Rogler, G.; von Känel, R.; et al. Association of alterations in intestinal microbiota with impaired psychological function in patients with inflammatory bowel diseases in remission. Clin. Gastroenterol. Hepatol. 2020, 18, 2019–2029.e11. [Google Scholar] [CrossRef]
- Lo Presti, A.; Zorzi, F.; Del Chierico, F.; Altomare, A.; Cocca, S.; Avola, A.; De Biasio, F.; Russo, A.; Cella, E.; Reddel, S.; et al. Fecal and mucosal microbiota profiling in irritable bowel syndrome and inflammatory bowel disease. Front. Microbiol. 2019, 10, 1655. [Google Scholar] [CrossRef]
- Gkouskou, K.K.; Deligianni, C.; Tsatsanis, C.; Eliopoulos, A.G. The gut microbiota in mouse models of inflammatory bowel disease. Front. Cell. Infect. Microbiol. 2014, 4, 28. [Google Scholar] [CrossRef]
- Rosen, C.E.; Palm, N.W. Navigating the Microbiota Seas: Triangulation Finds a Way Forward. Cell Host Microbe 2018, 23, 1–3. [Google Scholar] [CrossRef]
- Orel, R.; Trop, T.K. Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 11505–11524. [Google Scholar] [CrossRef]
- Mullish, B.H.; Tohumcu, E.; Porcari, S.; Fiorani, M.; Di Tommaso, N.; Gasbarrini, A.; Cammarota, G.; Ponziani, F.R.; Ianiro, G. The role of faecal microbiota transplantation in chronic noncommunicable disorders. J. Autoimmun. 2023, 141, 103034. [Google Scholar] [CrossRef]
- Airola, C.; Severino, A.; Porcari, S.; Fusco, W.; Mullish, B.H.; Gasbarrini, A.; Cammarota, G.; Ponziani, F.R.; Ianiro, G. Future Modulation of Gut Microbiota: From Eubiotics to FMT, Engineered Bacteria, and Phage Therapy. Antibiotics 2023, 12, 868. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Hoffmann, C.; Dollive, S.; Grunberg, S.; Chen, J.; Li, H.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Archaea and fungi of the human gut microbiome: Correlations with diet and bacterial residents. PLoS ONE 2013, 8, e66019. [Google Scholar] [CrossRef]
- Tlaskalová-Hogenová, H.; Stěpánková, R.; Kozáková, H.; Hudcovic, T.; Vannucci, L.; Tučková, L.; Rossmann, P.; Hrnčíř, T.; Kverka, M.; Zákostelská, Z.; et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cell. Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef]
- Koboziev, I.; Webb, C.; Furr, K.L.; Grisham, M.B. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Radic Biol. Med. 2014, 68, 122–133. [Google Scholar] [CrossRef]
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M.; Mancini, C.; Cicerone, C.; Corazziari, E.; Pantanella, F.; et al. Eubiosis and dysbiosis: The two sides of the microbiota. New Microbiol. 2016, 39, 1–12. [Google Scholar]
- Talapko, J.; Včev, A.; Meštrović, T.; Pustijanac, E.; Jukić, M.; Škrlec, I. Homeostasis and Dysbiosis of the Intestinal Microbiota: Comparing Hallmarks of a Healthy State with Changes in Inflammatory Bowel Disease. Microorganisms 2022, 10, 2405. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut microbiota-host interaction: Role in health and disease. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Fava, F.; Danese, S. Intestinal microbiota in inflammatory bowel disease: Friend of foe? World J. Gastroenterol. 2011, 17, 557–566. [Google Scholar] [CrossRef]
- Rigottier-Gois, L. Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. ISME J. 2013, 7, 1256–1261. [Google Scholar] [CrossRef]
- Nenkov, M.; Shi, Y.; Ma, Y.; Gaßler, N.; Chen, Y. Targeting Farnesoid X Receptor in Tumor and the Tumor Microenvironment: Implication for Therapy. Int. J. Mol. Sci. 2023, 25, 6. [Google Scholar] [CrossRef]
- Fu, Y.; Lyu, J.; Wang, S. The role of intestinal microbes on intestinal barrier function and host immunity from a metabolite perspective. Front. Immunol. 2023, 14, 1277102. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.I.H.; Morgan, X.C. Searching for a Consensus Among Inflammatory Bowel Disease Studies: A Systematic Meta-Analysis. Inflamm. Bowel Dis. 2023, 29, 125–139. [Google Scholar] [CrossRef]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Mah, C.; Jayawardana, T.; Leong, G.; Koentgen, S.; Lemberg, D.; Connor, S.J.; Rokkas, T.; Grimm, M.C.; Leach, S.T.; Hold, G.L. Assessing the Relationship between the Gut Microbiota and Inflammatory Bowel Disease Therapeutics: A Systematic Review. Pathogens 2023, 12, 262. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Maukonen, J.; Kolho, K.L.; Paasela, M.; Honkanen, J.; Klemetti, P.; Vaarala, O.; Saarela, M. Altered Fecal Microbiota in Paediatric Inflammatory Bowel Disease. J. Crohn’s Colitis 2015, 9, 1088–1095. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, H.; Xu, J.; Guo, X.; Zhao, H.; Chen, Y.; Zhou, Y.; Nie, Y.F. prausnitzii and its supernatant increase SCFAs-producing bacteria to restore gut dysbiosis in TNBS-induced colitis. AMB Express 2021, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ji, X.; Lu, G.; Zhang, F. The potential of Akkermansia muciniphila in inflammatory bowel disease. Appl. Microbiol. Biotechnol. 2021, 105, 5785–5794. [Google Scholar] [CrossRef]
- Faden, H. The Role of Faecalibacterium, Roseburia, and Butyrate in Inflammatory Bowel Disease. Dig. Dis. 2022, 40, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Xu, B.; Wang, X.; Zhang, Y.; Wang, H.; Kong, X.; Zhu, H.; Wu, K. The biodiversity and composition of the dominant fecal microbiota in patients with inflammatory bowel disease. Diagn. Microbiol. Infect. Dis. 2013, 75, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, H.; Kataoka, K.; Ishikawa, H.; Ikata, K.; Arimochi, H.; Iwasaki, T.; Ohnishi, Y.; Kuwahara, T.; Yasutomo, K. Reduced diversity and imbalance of fecal microbiota in patients with ulcerative colitis. Dig. Dis. Sci. 2012, 57, 2955–2964. [Google Scholar] [CrossRef]
- Li, J.; Butcher, J.; Mack, D.; Stintzi, A. Functional impacts of the intestinal microbiome in the pathogenesis of inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 139–153. [Google Scholar] [CrossRef]
- Mondot, S.; Kang, S.; Furet, J.P.; Aguirre de Carcer, D.; McSweeney, C.; Morrison, M.; Marteau, P.; Doré, J.; Leclerc, M. Highlighting new phylogenetic specificities of Crohn’s disease microbiota. Inflamm. Bowel Dis. 2011, 17, 185–192. [Google Scholar] [CrossRef]
- Sartor, R.B.; Wu, G.D. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology 2017, 152, 327–339.e4. [Google Scholar] [CrossRef]
- Lo Sasso, G.; Khachatryan, L.; Kondylis, A.; Battey, J.N.D.; Sierro, N.; Danilova, N.A.; Grigoryeva, T.V.; Markelova, M.I.; Khusnutdinova, D.R.; Laikov, A.V.; et al. Inflammatory Bowel Disease-Associated Changes in the Gut: Focus on Kazan Patients. Inflamm. Bowel Dis. 2021, 27, 418–433. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, H.; Chen, S.; He, J.; Zhou, Y.; Nie, Y. Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2021, 36, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, H.; Kaschitzki, A.; Alberts, C.; Bodammer, P.; Bannert, K.; Köller, T.; Warnke, P.; Kreikemeyer, B.; Lamprecht, G. Alterations in the mucosa-associated bacterial composition in Crohn’s disease: A pilot study. Int. J. Color. Dis. 2016, 31, 961–971. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef]
- Scanu, M.; Toto, F.; Petito, V.; Masi, L.; Fidaleo, M.; Puca, P.; Baldelli, V.; Reddel, S.; Vernocchi, P.; Pani, G.; et al. An integrative multi-omic analysis defines gut microbiota, mycobiota, and metabolic fingerprints in ulcerative colitis patients. Front. Cell Infect. Microbiol. 2024, 14, 1366192. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.J.; Ullman, T.A.; Ford, A.C.; Abreu, M.T.; Abadir, A.; Marshall, J.K.; Talley, N.J.; Moayyedi, P. Antibiotic therapy in inflammatory bowel disease: A systematic review and meta-analysis. Am. J. Gastroenterol. 2011, 106, 661–673. [Google Scholar] [CrossRef]
- Wang, S.L.; Wang, Z.R.; Yang, C.Q. Meta-analysis of broad-spectrum antibiotic therapy in patients with active inflammatory bowel disease. Exp. Ther. Med. 2012, 4, 1051–1056. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO guidelines on therapeutics in Crohn’s disease: Medical treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Su, J.W.; Ma, J.J.; Zhang, H.J. Use of antibiotics in patients with Crohn’s disease: A systematic review and meta-analysis. J. Dig. Dis. 2015, 16, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, B.P.; Moss, A.C. Prevention of post-operative recurrence of Crohn’s disease. World J. Gastroenterol. 2014, 20, 1147–1154. [Google Scholar] [CrossRef]
- Guslandi, M. Rifaximin in the treatment of inflammatory bowel disease. World J. Gastroenterol. 2011, 17, 4643–4646. [Google Scholar] [CrossRef]
- Bar, N.; Avraham, Y.; Dubinsky, V.; Cohen, N.A.; Weiss, G.A.; Banon, L.; Tulchinsky, H.; Maharshak, N.; Gophna, U.; Dotan, I. Long-term Antibiotic Treatment in Pouchitis—Patterns of Use and Safety. Inflamm. Bowel Dis. 2022, 28, 1027–1033. [Google Scholar] [CrossRef]
- Hildebrand, H.; Malmborg, P.; Askling, J.; Ekbom, A.; Montgomery, S.M. Early-life exposures associated with antibiotic use and risk of subsequent Crohn’s disease. Scand. J. Gastroenterol. 2008, 43, 961–966. [Google Scholar] [CrossRef]
- Turner, A.M.; Li, L.; Monk, I.R.; Lee, J.Y.H.; Ingle, D.J.; Portelli, S.; Sherry, N.L.; Isles, N.; Seemann, T.; Sharkey, L.K.; et al. Rifaximin prophylaxis causes resistance to the last-resort antibiotic daptomycin. Nature 2024, 635, 969–977. [Google Scholar] [CrossRef]
- Scott, B.M.; Gutiérrez-Vázquez, C.; Sanmarco, L.M.; da Silva Pereira, J.A.; Li, Z.; Plasencia, A.; Hewson, P.; Cox, L.M.; O’Brien, M.; Chen, S.K.; et al. Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease. Nat. Med. 2021, 27, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Lorentz, A. Probiotics in the Treatment of Inflammatory Bowel Diseases in Adulthood: A Systematic Review. J. Gastrointest. Liver Dis. 2022, 31, 74–84. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Leszczyńska, K.; Górska, S. The effectiveness of probiotics in the treatment of inflammatory bowel disease (Ibd)—A critical review. Nutrients 2020, 12, 1973. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011, 27, 496–501. [Google Scholar] [CrossRef]
- Ojetti, V.; Saviano, A.; Brigida, M.; Petruzziello, C.; Caronna, M.; Gayani, G.; Franceschi, F. Randomized control trial on the efficacy of Limosilactobacillus reuteri ATCC PTA 4659 in reducing inflammatory markers in acute uncomplicated diverticulitis. Eur. J. Gastroenterol. Hepatol. 2022, 34, 496–502. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Li, M.; Li, Y.Y.; Li, L.X.; Zhai, W.Z.; Wang, P.; Yang, X.X.; Gu, X.; Song, L.J.; Li, Z.; et al. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. Sci. Rep. 2018, 8, 2964. [Google Scholar] [CrossRef]
- Bourreille, A.; Cadiot, G.; Le Dreau, G.; Laharie, D.; Beaugerie, L.; Dupas, J.L.; Marteau, P.; Rampal, P.; Moyse, D.; Saleh, A.; et al. Saccharomyces boulardii does not prevent relapse of Crohn’s disease. Clin. Gastroenterol. Hepatol. 2013, 11, 982–987. [Google Scholar] [CrossRef]
- Bjarnason, I.; Sission, G.; Hayee, B.H. A randomised, double-blind, placebo-controlled trial of a multi-strain probiotic in patients with asymptomatic ulcerative colitis and Crohn’s disease. Inflammopharmacology 2019, 27, 465–473. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, S.B.; Kim, S.; Kim, T.O.; Cha, J.M.; Im, J.P.; Choi, C.H.; Kim, E.S.; Seo, G.S.; Eun, C.S.; et al. Additive effect of probiotics (Mutaflor) on 5-aminosalicylic acid therapy in patients with ulcerative colitis. Korean J. Intern. Med. 2022, 37, 949–957. [Google Scholar] [CrossRef]
- Agraib, L.M.; Yamani, M.I.; Tayyem, R.; Abu-Sneineh, A.T.; Rayyan, Y.M. Probiotic supplementation induces remission and changes in the immunoglobulins and inflammatory response in active ulcerative colitis patients: A pilot, randomized, double-blind, placebo-controlled study. Clin. Nutr. ESPEN 2022, 51, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.M.; Moon, W.; Seo, K.I.; Jung, K.; Kim, J.H.; Kim, S.E.; Park, M.I.; Park, S.J. Therapeutic Potential of Escherichia coli Nissle 1917 in Clinically Remission-attained Ulcerative Colitis Patients: A Hospital-based Cohort Study. Korean J. Gastroenterol. 2021, 77, 12–21. [Google Scholar] [CrossRef]
- Yılmaz, İ.; Enver Dolar, M.; Özpınar, H. Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: A randomized controlled trial. Turk. J. Gastroenterol. 2018, 30, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Uemura, Y.; Kanai, T.; Kunisaki, R.; Suzuki, Y.; Yokoyama, K.; Yoshimura, N.; Hibi, T. Efficacy of Bifidobacterium breve Fermented Milk in Maintaining Remission of Ulcerative Colitis. Dig. Dis. Sci. 2018, 63, 1910–1919. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Yamada, A.; Furukawa, R.; Sono, K.; Osamura, A.; Nakamura, K.; Aoki, H.; Tsuda, Y.; Hosoe, N.; Takada, N.; et al. Effectiveness of probiotic therapy for the prevention of relapse in patients with inactive ulcerative colitis. World J. Gastroenterol. 2015, 21, 5985–5994. [Google Scholar] [CrossRef]
- Oliva, S.; Di Nardo, G.; Ferrari, F.; Mallardo, S.; Rossi, P.; Patrizi, G.; Cucchiara, S.; Stronati, L. Randomised clinical trial: The effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment. Pharmacol. Ther. 2012, 35, 327–334. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Papa, A.; Giglio, A.; Elisei, W.; Giorgetti, G.M.; Forti, G.; Morini, S.; Hassan, C.; Pistoia, M.A.; et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: A double-blind, randomized, placebo-controlled study. Am. J. Gastroenterol. 2010, 105, 2218–2227. [Google Scholar] [CrossRef]
- Miele, E.; Pascarella, F.; Giannetti, E.; Quaglietta, L.; Baldassano, R.N.; Staiano, A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am. J. Gastroenterol. 2009, 104, 437–443. [Google Scholar] [CrossRef]
- Zocco, M.A.; dal Verme, L.Z.; Cremonini, F.; Piscaglia, A.C.; Nista, E.C.; Candelli, M.; Novi, M.; Rigante, D.; Cazzato, I.A.; Ojetti, V.; et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 2006, 23, 1567–1574. [Google Scholar] [CrossRef]
- Kato, K.; Mizuno, S.; Umesaki, Y.; Ishii, Y.; Sugitani, M.; Imaoka, A.; Otsuka, M.; Hasunuma, O.; Kurihara, R.; Iwasaki, A.; et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment. Pharmacol. Ther. 2004, 20, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int. J. Mol. Sci. 2021, 22, 6729. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Faseleh Jahromi, M.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 2017, 206, 1–9. [Google Scholar] [CrossRef]
- Masanetz, S.; Preißinger, W.; Meyer, H.H.; Pfaffl, M.W. Effects of the prebiotics inulin and lactulose on intestinal immunology and hematology of preruminant calves. Animal 2011, 5, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Du, Y.; Li, Q.; Liu, L.; Zhao, L.; Gao, C.; Tang, Z.; Zhang, X.; Zhao, Y.; Yang, X. Fructo-oligosaccharides Alleviated Ulcerative Colitis via Gut Microbiota-Dependent Tryptophan Metabolism in Association with Aromatic Hydrocarbon Receptor Activation in Mice. J. Agric. Food Chem. 2024, 72, 27912–27922. [Google Scholar] [CrossRef]
- Capitán-Cañadas, F.; Ocón, B.; Aranda, C.J.; Anzola, A.; Suárez, M.D.; Zarzuelo, A.; de Medina, F.S.; Martínez-Augustin, O. Fructooligosaccharides exert intestinal anti-inflammatory activity in the CD4+ CD62L+ T cell transfer model of colitis in C57BL/6J mice. Eur. J. Nutr. 2016, 55, 1445–1454. [Google Scholar] [CrossRef]
- Lindsay, J.O.; Whelan, K.; Stagg, A.J.; Gobin, P.; Al-Hassi, H.O.; Rayment, N.; Kamm, M.A.; Knight, S.C.; Forbes, A. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut 2006, 55, 348–355. [Google Scholar] [CrossRef]
- Casellas, F.; Borruel, N.; Torrejón, A.; Varela, E.; Antolin, M.; Guarner, F.; Malagelada, J.R. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment. Pharmacol. Ther. 2007, 25, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, S.; Nakamura, M.; Honda, T.; Yamamura, T.; Maeda, K.; Sawada, T.; Ishikawa, E.; Yamamoto, K.; Furune, S.; Ishikawa, T.; et al. Efficacy of 1-kestose supplementation in patients with mild to moderate ulcerative colitis: A randomised, double-blind, placebo-controlled pilot study. Aliment. Pharmacol. Ther. 2023, 57, 1249–1257. [Google Scholar] [CrossRef]
- Valcheva, R.; Koleva, P.; Martínez, I.; Walter, J.; Gänzle, M.G.; Dieleman, L.A. Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. Gut Microbes 2019, 10, 334–357. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Feng, C.; Kwok, L.Y.; He, Q.; Sun, Z. Stronger gut microbiome modulatory effects by postbiotics than probiotics in a mouse colitis model. NPJ Sci. Food 2022, 6, 53. [Google Scholar] [CrossRef]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R.; et al. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol. Motil. 2020, 32, e13914. [Google Scholar] [CrossRef]
- Henn, M.R.; O’Brien, E.J.; Diao, L.; Feagan, B.G.; Sandborn, W.J.; Huttenhower, C.; Wortman, J.R.; McGovern, B.H.; Wang-Weigand, S.; Lichter, D.I.; et al. A Phase 1b Safety Study of SER-287, a Spore-Based Microbiome Therapeutic, for Active Mild to Moderate Ulcerative Colitis. Gastroenterology 2021, 160, 115–127.e30. [Google Scholar] [CrossRef] [PubMed]
- Ivanovska, T.P.; Mladenovska, K.; Zhivikj, Z.; Pavlova, M.J.; Gjurovski, I.; Ristoski, T.; Petrushevska-Tozi, L. Synbiotic loaded chitosan-Ca-alginate microparticles reduces inflammation in the TNBS model of rat colitis. Int. J. Pharm. 2017, 527, 126–134. [Google Scholar] [CrossRef]
- Wang, Y.N.; Meng, X.C.; Dong, Y.F.; Zhao, X.H.; Qian, J.M.; Wang, H.Y.; Li, J.N. Effects of probiotics and prebiotics on intestinal microbiota in mice with acute colitis based on 16S rRNA gene sequencing. Chin. Med. J. 2019, 132, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Furrie, E.; Macfarlane, S.; Kennedy, A.; Cummings, J.H.; Walsh, S.V.; O’neil, D.A.; Macfarlane, G.T. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: A randomised controlled pilot trial. Gut 2005, 54, 242–249. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, F.; Wang, W.; Sun, C.; Gao, D.; Ma, J.; Hussain, M.A.; Xu, C.; Jiang, Z.; Hou, J. Study of the alleviation effects of a combination of Lactobacillus rhamnosus and inulin on mice with colitis. Food Funct. 2020, 11, 3823–3837. [Google Scholar] [CrossRef]
- Yu, F.; Hu, X.; Ren, H.; Wang, X.; Shi, R.; Guo, J.; Chang, J.; Zhou, X.; Jin, Y.; Li, Y.; et al. Protective effect of synbiotic combination of Lactobacillus plantarum SC-5 and olive oil extract tyrosol in a murine model of ulcerative colitis. J. Transl. Med. 2024, 22, 308. [Google Scholar] [CrossRef]
- Quaranta, G.; Guarnaccia, A.; Fancello, G.; Agrillo, C.; Iannarelli, F.; Sanguinetti, M.; Masucci, L. Fecal Microbiota Transplantation and Other Gut Microbiota Manipulation Strategies. Microorganisms 2022, 10, 2424. [Google Scholar] [CrossRef]
- Varga, A.; Kocsis, B.; Sipos, D.; Kása, P.; Vigvári, S.; Pál, S.; Dembrovszky, F.; Farkas, K.; Péterfi, Z. How to Apply FMT More Effectively, Conveniently and Flexible—A Comparison of FMT Methods. Front. Cell. Infect. Microbiol. 2021, 11, 657320. [Google Scholar] [CrossRef] [PubMed]
- Verdier, C.; Denis, S.; Gasc, C.; Boucinha, L.; Uriot, O.; Delmas, D.; Dore, J.; Le Camus, C.; Schwintner, C.; Blanquet-Diot, S. An Oral FMT Capsule as Efficient as an Enema for Microbiota Reconstruction Following Disruption by Antibiotics, as Assessed in an In Vitro Human Gut Model. Microorganisms 2021, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Clemente, J.C.; Colombel, J.F. Can inflammatory bowel disease be permanently treated with short-term interventions on the microbiome? Expert Rev. Gastroenterol. Hepatol. 2015, 9, 781–795. [Google Scholar] [CrossRef]
- Deleu, S.; Caenepeel, C.; Vazquez Castellanos, J.F.; Arnauts, K.; Braekeleire, S.; Machiels, K.; Baert, F.; Mana, F.; Pouillon, L.; Hindryckx, P.; et al. DOP48 Faecal microbiota transplantation in active Ulcerative Colitis: Key lessons from a randomized controlled trial halted for futility. J. Crohn’s Colitis 2024, 18, 159–160. [Google Scholar] [CrossRef]
- Haifer, C.; Paramsothy, S.; Kaakoush, N.O.; Saikal, A.; Ghaly, S.; Yang, T.; Luu, L.D.W.; Borody, T.J.; Leong, R.W. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): A randomised, double-blind, placebo-controlled trial. Lancet. Gastroenterol. Hepatol. 2022, 7, 141–151. [Google Scholar] [CrossRef]
- Shabat, S.C.; Scaldaferri, F.; Zittan, E.; Hirsch, A.; Mentella, M.C.; Musca, T.; Cohen, N.A.; Ron, Y.; Fliss Isakov, N.; Pfeffer, J.; et al. Use of Faecal Transplantation with a Novel Diet for Mild to Moderate Active Ulcerative Colitis: The CRAFT UC Randomised Controlled Trial. J. Crohn’s Colitis 2022, 16, 369–378. [Google Scholar] [CrossRef]
- Crothers, J.W.; Chu, N.D.; Nguyen, L.T.T.; Phillips, M.; Collins, C.; Fortner, K.; Del Rio-Guerra, R.; Lavoie, B.; Callas, P.; Velez, M.; et al. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: Results of a single-center, prospective, randomized pilot study. BMC Gastroenterol. 2021, 21, 281. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients with Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; Leong, R.W.L.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [PubMed]
- Rossen, N.G.; Fuentes, S.; van der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.; Duflou, A.; Löwenberg, M.; van den Brink, G.R.; Mathus-Vliegen, E.M.; de Vos, W.M.; et al. Findings from a Randomized Controlled Trial of Fecal Transplantation for Patients with Ulcerative Colitis. Gastroenterology 2015, 149, 110–118.e4. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Laterza, L.; Petito, V.; Pecere, S.; Quaranta, G.; Del Chierico, F.; Puca, P.; Schiavoni, E.; Napolitano, D.; Poscia, A.; et al. Serial Fecal Microbiota Infusions via Colonoscopy for Active Ulcerative Colitis: A Feasibility, Safety, and Translational Monocentric Italian Study. Microorganisms 2023, 11, 2536. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Y.; Liu, Y.; Lin, L.; Lin, X.; Gong, W.; Xia, R.; He, J.; Sheng, J.; Cai, H.; et al. Efficacy and safety of fecal microbiota transplantation in the treatment of ulcerative colitis: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 14494. [Google Scholar] [CrossRef]
- Zhang, J.T.; Zhang, N.; Dong, X.T.; Wang, X.R.; Ma, H.W.; Liu, Y.D.; Li, M.R. Efficacy and safety of fecal microbiota transplantation for treatment of ulcerative colitis: A post-consensus systematic review and meta-analysis. World J. Clin. Cases 2024, 12, 4691–4702. [Google Scholar] [CrossRef]
- Xiang, L.; Ding, X.; Li, Q.; Wu, X.; Dai, M.; Long, C.; He, Z.; Cui, B.; Zhang, F. Efficacy of faecal microbiota transplantation in Crohn’s disease: A new target treatment? Microb. Biotechnol. 2020, 13, 760–769. [Google Scholar] [CrossRef]
- Cheng, F.; Huang, Z.; Wei, W.; Li, Z. Fecal microbiota transplantation for Crohn’s disease: A systematic review and meta-analysis. Tech. Coloproctol. 2021, 25, 495–504. [Google Scholar] [CrossRef]
- Zhou, S.; Cui, Y.; Zhang, Y.; Zhao, T.; Cong, J. Fecal microbiota transplantation for induction of remission in Crohn’s disease: A systematic review and meta-analysis. Int. J. Color. Dis. 2023, 38, 62. [Google Scholar] [CrossRef]
- Fehily, S.R.; Basnayake, C.; Wright, E.K.; Kamm, M.A. Fecal microbiota transplantation therapy in Crohn’s disease: Systematic review. J. Gastroenterol. Hepatol. 2021, 36, 2672–2686. [Google Scholar] [CrossRef] [PubMed]
- Kahan, T.; Chandan, S.; Khan, S.R.; Deliwala, S.; Chang, S.; Axelrad, J.; Shaukat, A. Safety and Efficacy of Fecal Microbiota Transplant in Chronic Pouchitis—A Systematic Review with Meta-Analysis. Gastro Hep. Adv. 2023, 2, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Akingboye, A.; Mohamedahmed, A.Y.Y.; Peterknecht, E.; Bhattacharya, P.; El-Asrag, M.E.; Iqbal, T.H.; Quraishi, M.N.; Beggs, A.D. Faecal Microbiota Transplantation [FMT] in the Treatment of Chronic Refractory Pouchitis: A Systematic Review and Meta-analysis. J. Crohn’s Colitis 2024, 18, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Ezzatpour, S.; Mondragon Portocarrero, A.D.C.; Cardelle-Cobas, A.; Lamas, A.; López-Santamarina, A.; Miranda, J.M.; Aguilar, H.C. The Human Gut Virome and Its Relationship with Nontransmissible Chronic Diseases. Nutrients 2023, 15, 977. [Google Scholar] [CrossRef]
- Ng, R.W.; Dharmaratne, P.; Wong, S.; Hawkey, P.; Chan, P.; Ip, M. Revisiting the donor screening protocol of faecal microbiota transplantation (FMT): A systematic review. Gut 2024, 73, 1029–1031. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Kelly, C.R.; Mullish, B.H.; Allegretti, J.R.; Kassam, Z.; Putignani, L.; Fischer, M.; Keller, J.J.; Costello, S.P. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 2019, 68, 2111–2121. [Google Scholar] [CrossRef]
- Bénard, M.V.; de Bruijn, C.M.A.; Fenneman, A.C.; Wortelboer, K.; Zeevenhoven, J.; Rethans, B.; Herrema, H.J.; van Gool, T.; Nieuwdorp, M.; Benninga, M.A.; et al. Challenges and costs of donor screening for fecal microbiota transplantations. PLoS ONE 2022, 17, e0276323. [Google Scholar] [CrossRef]
- Breyner, N.M.; Michon, C.; de Sousa, C.S.; Vilas Boas, P.B.; Chain, F.; Azevedo, V.A.; Langella, P.; Chatel, J.M. Microbial anti-inflammatory molecule (MAM) from Faecalibacterium prausnitzii shows a protective effect on DNBS and DSS-induced colitis model in mice through inhibition of NF-κB pathway. Front. Microbiol. 2017, 8, 114. [Google Scholar] [CrossRef]
- Olsson, L.M.; Boulund, F.; Nilsson, S.; Khan, M.T.; Gummesson, A.; Fagerberg, L.; Engstrand, L.; Perkins, R.; Uhlén, M.; Bergström, G.; et al. Dynamics of the normal gut microbiota: A longitudinal one-year population study in Sweden. Cell Host Microbe 2022, 30, 726–739.e3. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Quévrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B.; et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Laval, L.; Martin, R.; Natividad, J.N.; Chain, F.; Miquel, S.; Desclée de Maredsous, C.; Capronnier, S.; Sokol, H.; Verdu, E.F.; van Hylckama Vlieg, J.E.; et al. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune Netw. 2020, 20, e15. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- 116 Martín, R.; Chain, F.; Miquel, S.; Lu, J.; Gratadoux, J.J.; Sokol, H.; Verdu, E.F.; Bercik, P.; Bermúdez-Humarán, L.G.; Langella, P. The commensal bacterium Faecalibacterium prausnitzii is protective in DNBS-induced chronic moderate and severe colitis models. Inflamm. Bowel Dis. 2014, 20, 417–430. [Google Scholar] [CrossRef]
- Jian, H.; Liu, Y.; Wang, X.; Dong, X.; Zou, X. Akkermansia muciniphila as a Next-Generation Probiotic in Modulating Human Metabolic Homeostasis and Disease Progression: A Role Mediated by Gut–Liver–Brain Axes? Int. J. Mol. Sci. 2023, 24, 3900. [Google Scholar] [CrossRef]
- Pellegrino, A.; Coppola, G.; Santopaolo, F.; Gasbarrini, A.; Ponziani, F.R. Role of Akkermansia in Human Diseases: From Causation to Therapeutic Properties. Nutrients 2023, 15, 1815. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Dunn, K.A.; Moore-Connors, J.; MacIntyre, B.; Stadnyk, A.W.; Thomas, N.A.; Noble, A.; Mahdi, G.; Rashid, M.; Otley, A.R.; Bielawski, J.P.; et al. Early changes in microbial community structure are associated with sustained remission after nutritional treatment of pediatric Crohn’s disease. Inflamm. Bowel Dis. 2016, 22, 2853–2862. [Google Scholar] [CrossRef]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef]
- Zheng, M.; Han, R.; Yuan, Y.; Xing, Y.; Zhang, W.; Sun, Z.; Liu, Y.; Li, J.; Mao, T. The role of Akkermansia muciniphila in inflammatory bowel disease: Current knowledge and perspectives. Inflamm. Bowel Dis. 2023, 13, 1089600. [Google Scholar] [CrossRef] [PubMed]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef]
- Kump, P.; Wurm, P.; Gröchenig, H.P.; Wenzl, H.; Petritsch, W.; Halwachs, B.; Wagner, M.; Stadlbauer, V.; Eherer, A.; Hoffmann, K.M.; et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 67–77. [Google Scholar] [CrossRef]
- 127Earley, H.; Lennon, G.; Balfe, Á.; Coffey, J.C.; Winter, D.C.; O’Connell, P.R. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci. Rep. 2019, 9, 15683. [Google Scholar] [CrossRef]
- Vigsnæs, L.K.; Brynskov, J.; Steenholdt, C.; Wilcks, A.; Licht, T.R. Gram-negative bacteria account for main differences between faecal microbiota from patients with ulcerative colitis and healthy controls. Benef. Microbes 2012, 3, 287–297. [Google Scholar] [CrossRef]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8 + T cells in mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef]
- Maria, H.S. Safety aspects of next generation probiotics. Curr. Opin. Food Sci. 2019, 30, 8–13. [Google Scholar] [CrossRef]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef] [PubMed]
- D’Agostin, M.; Squillaci, D.; Lazzerini, M.; Barbi, E.; Wijers, L.; Da Lozzo, P. Invasive Infections Associated with the Use of Probiotics in Children: A Systematic Review. Children 2021, 8, 924. [Google Scholar] [CrossRef]
- Tiwari, A.; Krisnawati, D.I.; Susilowati, E.; Mutalik, C.; Kuo, T.R. Next-Generation Probiotics and Chronic Diseases: A Review of Current Research and Future Direction. J. Agric. Food Chem. 2024, 72, 27679–27700. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; Naska, A.; et al. Guidance on the preparation and submission of an application for authorisation of a novel food in the context of Regulation (EU) 2015/22831. EFSA J. 2016, 19, e06555. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.J.; Yoon, J.Y.; Kemmitt, J.; Wright, C.; Schneider, K.; Sphabmixay, P.; Hernandez-Gordillo, V.; Holcomb, S.J.; Bhushan, B.; et al. Primary human colonic mucosal barrier crosstalk with super oxygen-sensitive Faecalibacterium prausnitzii in continuous culture. Med 2021, 2, 74–98.e9. [Google Scholar] [CrossRef]
- Bag, S.; Ghosh, T.S.; Das, B. Complete genome sequence of Faecalibacterium prausnitzii isolated from the gut of a healthy Indian adult. Genome Announc. 2017, 5, 10–1128. [Google Scholar] [CrossRef]
- Machado, D.; Barbosa, J.C.; Domingos, M.; Almeida, D.; Andrade, J.C.; Freitas, A.C.; Gomes, A.M. Revealing antimicrobial resistance profile of the novel probiotic candidate Faecalibacterium prausnitzii DSM 17677. Int. J. Food Microbiol. 2022, 363, 109501. [Google Scholar] [CrossRef]
- Hu, W.; Gao, W.; Liu, Z.; Fang, Z.; Zhao, J.; Zhang, H.; Lu, W.; Chen, W. Biodiversity and Physiological Characteristics of Novel Faecalibacterium prausnitzii Strains Isolated from Human Feces. Microorganisms 2022, 10, 297. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, L.; Hu, G.; Sun, Z.; Zhan, X.; Gao, M. Akkermansia muciniphila fermentation culture based on a novel bionic large intestine dynamic digestion model. Food Biosci. 2021, 43, 101260. [Google Scholar] [CrossRef]
- Dubourg, G.; Lagier, J.C.; Armougom, F.; Robert, C.; Audoly, G.; Papazian, L.; Raoult, D. High-level colonisation of the human gut by Verrucomicrobia following broad-spectrum antibiotic treatment. Int. J. Antimicrob. Agents 2013, 41, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Barbosa, J.C.; Almeida, D.; Andrade, J.C.; Freitas, A.C.; Gomes, A.M. Insights into the antimicrobial resistance profile of a next generation probiotic Akkermansia muciniphila DSM 22959. Int. J. Environ. Res. Public Health 2022, 19, 9152. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Tsilingiris, D.; Panagopoulos, F.; Christodoulatos, G.S.; Evangelopoulos, A.; Karampela, I.; Dalamaga, M. The role of next-generation probiotics in obesity and obesity-Associated disorders: Current knowledge and future perspectives. Int. J. Mol. Sci. 2023, 24, 6755. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- Zhang, S.; Merino, N.; Okamoto, A.; Gedalanga, P. Interkingdom microbial consortia mechanisms to guide biotechnological applications. Microb. Biotechnol. 2018, 11, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Walana, W.; Ye, Y.; Li, M.; Wang, J.; Wang, B.; Cheng, J.W.; Gordon, J.R.; Li, F. IL-8 antagonist, CXCL8(3-72)K11R/G31P coupled with probiotic exhibit variably enhanced therapeutic potential in ameliorating ulcerative colitis. Biomed. Pharmacother. 2018, 103, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Y.; Li, C.; Xie, Z.; Dai, L. Amelioration of Colitis by a Gut Bacterial Consortium Producing Anti-Inflammatory Secondary Bile Acids. Microbiol. Spectr. 2023, 11, e0333022. [Google Scholar] [CrossRef]
- Xu, L.; Liu, B.; Huang, L.; Li, Z.; Cheng, Y.; Tian, Y.; Pan, G.; Li, H.; Xu, Y.; Wu, W.; et al. Probiotic Consortia and Their Metabolites Ameliorate the Symptoms of Inflammatory Bowel Diseases in a Colitis Mouse Model. Microbiol. Spectr. 2022, 10, e0065722. [Google Scholar] [CrossRef]
- van der Lelie, D.; Oka, A.; Taghavi, S.; Umeno, J.; Fan, T.J.; Merrell, K.E.; Watson, S.D.; Ouellette, L.; Liu, B.; Awoniyi, M.; et al. Rationally designed bacterial consortia to treat chronic immune-mediated colitis and restore intestinal homeostasis. Nat. Commun. 2021, 12, 3105. [Google Scholar] [CrossRef]
- Polonsky, O.; Meshner, S.; Eshar, S.; Ben-Shabat, S.K.; Tirosh, O.; Haber, E.; Ringel, Y. BMC322- a rationally-designed live bacterial consortium based on microbiome functional genomic analysis for treatment of ibd. Inflamm. Bowel Dis. 2021, 27, S38. [Google Scholar] [CrossRef]
- Guthrie, L.; Kelly, L. Bringing microbiome-drug interaction research into the clinic. EBioMedicine 2019, 44, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Deleu, S.; Puca, P.; Abreu, M.T.; Armuzzi, A.; Barbara, G.; Caprioli, F.; Chieng, S.; Costello, S.P.; Damiani, A.; et al. Guidance for Fecal Microbiota Transplantation Trials in Ulcerative Colitis: The Second ROME Consensus Conference. Inflamm. Bowel Dis. 2025, izaf013, advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, F.L.; Vlot, M.; de Jonge, P.A.; Dreesens, L.L.; Beaumont, H.J.E.; Lavigne, R.; Dutilh, B.E.; Brouns, S.J.J. Targeting mechanisms of tailed bacteriophages. Nat. Rev. Microbiol. 2018, 16, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Balcha, F.B.; Neja, S.A. CRISPR-Cas9 mediated phage therapy as an alternative to antibiotics. Anim. Dis. 2023, 3, 4. [Google Scholar] [CrossRef]
- Hodyra-Stefaniak, K.; Miernikiewicz, P.; Drapała, J.; Drab, M.; Jończyk-Matysiak, E.; Lecion, D.; Kaźmierczak, Z.; Beta, W.; Majewska, J.; Harhala, M.; et al. Mammalian Host-Versus-Phage immune response determines phage fate in vivo. Sci. Rep. 2015, 5, 14802. [Google Scholar] [CrossRef]
- Park, H.; Laffin, M.R.; Jovel, J.; Millan, B.; Hyun, J.E.; Hotte, N.; Kao, D.; Madsen, K.L. The success of fecal microbial transplantation in Clostridium difficile infection correlates with bacteriophage relative abundance in the donor: A retrospective cohort study. Gut Microbes 2019, 10, 676–687. [Google Scholar] [CrossRef]
- Machuca, P.; Daille, L.; Vinés, E.; Berrocal, L.; Bittner, M. Isolation of a novel bacteriophage specific for the periodontal pathogen Fusobacterium nucleatum. Appl. Environ. Microbiol. 2010, 76, 7243–7250. [Google Scholar] [CrossRef]
- Qv, L.; Mao, S.; Li, Y.; Zhang, J.; Li, L. Roles of Gut Bacteriophages in the Pathogenesis and Treatment of Inflammatory Bowel Disease. Front. Cell. Infect. Microbiol. 2021, 11, 755650. [Google Scholar] [CrossRef]
- Federici, S.; Kredo-Russo, S.; Valdés-Mas, R.; Kviatcovsky, D.; Weinstock, E.; Matiuhin, Y.; Silberberg, Y.; Atarashi, K.; Furuichi, M.; Oka, A.; et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 2022, 185, 2879–2898.e24. [Google Scholar] [CrossRef]
- Speck, P.; Smithyman, A. Safety and efficacy of phage therapy via the intravenous route. FEMS Microbiol. Lett. 2016, 363, fnv242. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef]
- Hyman, P.; Abedon, S.T. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 2010, 70, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.J.; Hyman, P. Phage choice, isolation, and preparation for phage therapy. Curr. Pharm. Biotechnol. 2010, 11, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Carascal, M.B.; Dela Cruz-Papa, D.M.; Remenyi, R.; Cruz, M.C.B.; Destura, R.V. Phage Revolution Against Multidrug-Resistant Clinical Pathogens in Southeast Asia. Front. Microbiol. 2022, 13, 820572. [Google Scholar] [CrossRef] [PubMed]
- Tsonos, J.; Vandenheuvel, D.; Briers, Y.; De Greve, H.; Hernalsteens, J.P.; Lavigne, R. Hurdles in bacteriophage therapy: Deconstructing the parameters. Vet. Microbiol. 2014, 171, 460–469. [Google Scholar] [CrossRef]
- Lin, J.; Du, F.; Long, M.; Li, P. Limitations of Phage Therapy and Corresponding Optimization Strategies: A Review. Molecules 2022, 27, 1857. [Google Scholar] [CrossRef]
- Federici, S.; Kviatcovsky, D.; Valdés-Mas, R.; Elinav, E. Microbiome-phage interactions in inflammatory bowel disease. Clin. Microbiol. Infect. 2023, 29, 682–688. [Google Scholar] [CrossRef]
- Tetz, G.V.; Ruggles, K.V.; Zhou, H.; Heguy, A.; Tsirigos, A.; Tetz, V. Bacteriophages as potential new mammalian pathogens. Sci. Rep. 2017, 7, 7043. [Google Scholar] [CrossRef]
- Champagne-Jorgensen, K.; Luong, T.; Darby, T.; Roach, D.R. Immunogenicity of bacteriophages. Trends Microbiol. 2023, 31, 1058–1071. [Google Scholar] [CrossRef]
- Pesce, M.; Seguella, L.; Del Re, A.; Lu, J.; Palenca, I.; Corpetti, C.; Rurgo, S.; Sanseverino, W.; Sarnelli, G.; Esposito, G. Next-Generation Probiotics for Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 5466. [Google Scholar] [CrossRef]
- Aggarwal, N.; Breedon, A.M.E.; Davis, C.M.; Hwang, I.Y.; Chang, M.W. Engineering probiotics for therapeutic applications: Recent examples and translational outlook. Curr. Opin. Biotechnol. 2020, 65, 171–179. [Google Scholar] [CrossRef]
- Kan, A.; Gelfat, I.; Emani, S.; Praveschotinunt, P.; Joshi, N.S. Plasmid Vectors for in Vivo Selection-Free Use with the Probiotic E. coli Nissle 1917. ACS Synth. Biol. 2021, 10, 94–106. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, L.; Zuo, W.; Zheng, Y.; Zhang, P.; Sun, Y.; Wang, Y.; Du, G.; Kang, Z. Minimizing endogenous cryptic plasmids to construct antibiotic-free expression systems for Escherichia coli Nissle 1917. Synth. Syst. Biotechnol. 2024, 9, 165–175. [Google Scholar] [CrossRef]
- Cook, D.P.; Gysemans, C.; Mathieu, C. Lactococcus lactis As a Versatile Vehicle for Tolerogenic Immunotherapy. Front. Immunol. 2018, 8, 1961. [Google Scholar] [CrossRef] [PubMed]
- Berlec, A.; Škrlec, K.; Kocjan, J.; Olenic, M.; Štrukelj, B. Single plasmid systems for inducible dual protein expression and for CRISPR-Cas9/CRISPRi gene regulation in lactic acid bacterium Lactococcus lactis. Sci. Rep. 2018, 8, 1009. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liao, Y.; Yang, R.; Zhu, Z.; Zhang, L.; Wu, Z.; Sun, X. An engineered probiotic secreting Sj16 ameliorates colitis via Ruminococcaceae/butyrate/retinoic acid axis. Bioeng. Transl. Med. 2021, 6, e10219. [Google Scholar] [CrossRef]
- Cui, M.; Pang, G.; Zhang, T.; Sun, T.; Zhang, L.; Kang, R.; Xue, X.; Pan, H.; Yang, C.; Zhang, X.; et al. Optotheranostic Nanosystem with Phone Visual Diagnosis and Optogenetic Microbial Therapy for Ulcerative Colitis At-Home Care. ACS Nano 2021, 15, 7040–7052. [Google Scholar] [CrossRef]
- Esposito, G.; Pesce, M.; Seguella, L.; Lu, J.; Corpetti, C.; Del Re, A.; De Palma, F.D.E.; Esposito, G.; Sanseverino, W.; Sarnelli, G. Engineered Lactobacillus paracasei Producing Palmitoylethanolamide (PEA) Prevents Colitis in Mice. Int. J. Mol. Sci. 2021, 22, 2945. [Google Scholar] [CrossRef]
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut 2014, 63, 1300–1312. [Google Scholar] [CrossRef]
- Kim, W.K.; Jang, Y.J.; Seo, B.; Han, D.H.; Park, S.; Ko, G.P. Administration of Lactobacillus paracasei strains improves immunomodulation and changes the composition of gut microbiota leading to improvement of colitis in mice. J. Funct. Foods 2019, 5, 565–575. [Google Scholar] [CrossRef]
- Andriantsoanirina, V.; Allano, S.; Butel, M.J.; Aires, J. Tolerance of Bifidobacterium human isolates to bile, acid and oxygen. Anaerobe 2013, 21, 39–42. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Zhang, Q.; Xu, Z.; Wang, J.; Sun, H. Oral engineered Bifidobacterium longum expressing rhMnSOD to suppress experimental colitis. Int. Immunopharmacol. 2018, 57, 25–32. [Google Scholar] [CrossRef]
- Wei, X.; Yan, X.; Chen, X.; Yang, Z.; Li, H.; Zou, D.; He, X.; Wang, S.; Cui, Q.; Liu, W.; et al. Proteomic analysis of the interaction of Bifidobacterium longum NCC2705 with the intestine cells Caco-2 and identification of plasminogen receptors. J. Proteom. 2014, 108, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Avila-Calderón, E.D.; Ruiz-Palma, M.D.S.; Aguilera-Arreola, M.G.; Velázquez-Guadarrama, N.; Ruiz, E.A.; Gomez-Lunar, Z.; Witonsky, S.; Contreras-Rodríguez, A. Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis. Front. Microbiol. 2021, 12, 557902. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.; Fonseca, S.; Miquel-Clopés, A.; Cross, K.; Kok, K.S.; Wegmann, U.; Gil-Cordoso, K.; Bentley, E.G.; Al Katy, S.H.M.; Coombes, J.L.; et al. Bioengineering commensal bacteria-derived outer membrane vesicles for delivery of biologics to the gastrointestinal and respiratory tract. J. Extracell. Vesicles 2019, 8, 1632100. [Google Scholar] [CrossRef]
- Fábrega, M.J.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Algieri, F.; Badía, J.; Giménez, R.; Gálvez, J.; Baldomà, L. Intestinal anti-inflammatory effects of outer membrane vesicles from Escherichia coli Nissle 1917 in DSS-experimental colitis in mice. Front. Microbiol. 2017, 8, 1274. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Lan, X.; Xu, X.; Zhang, X.; Li, X.; Zhao, Y.; Li, G.; Du, C.; Lu, S.; et al. Oral Escherichia coli expressing IL-35 meliorates experimental colitis in mice. J. Transl. Med. 2018, 16, 71. [Google Scholar] [CrossRef]
- Hanson, M.L.; Hixon, J.A.; Li, W.; Felber, B.K.; Anver, M.R.; Stewart, C.A.; Janelsins, B.M.; Datta, S.K.; Shen, W.; McLean, M.H.; et al. Oral delivery of il-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology 2014, 146, 210–221.e13. [Google Scholar] [CrossRef]
- Xia, J.Y.; Hepler, C.; Tran, P.; Waldeck, N.J.; Bass, J.; Prindle, A. Engineered calprotectin-sensing probiotics for IBD surveillance in humans. Proc. Natl. Acad. Sci. USA 2023, 120, e2221121120. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Murali, S.K.; Mansell, T.J. Next generation probiotics: Engineering live biotherapeutics. Biotechnol. Adv. 2024, 72, 108336. [Google Scholar] [CrossRef]
- Gulliver, E.L.; Young, R.B.; Chonwerawong, M.; D’Adamo, G.L.; Thomason, T.; Widdop, J.T.; Rutten, E.L.; Rossetto Marcelino, V.; Bryant, R.V.; Costello, S.P.; et al. Review article: The future of microbiome-based therapeutics. Aliment. Pharmacol. Ther. 2022, 56, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Pols, T.; Sikkema, H.R.; Gaastra, B.F.; Frallicciardi, J.; Śmigiel, W.M.; Singh, S.; Poolman, B. A synthetic metabolic network for physicochemical homeostasis. Nat. Commun. 2019, 10, 4239. [Google Scholar] [CrossRef] [PubMed]
- Schiering, C.; Wincent, E.; Metidji, A.; Iseppon, A.; Li, Y.; Potocnik, A.J.; Omenetti, S.; Henderson, C.J.; Wolf, C.R.; Nebert, D.W.; et al. Feedback control of AHR signalling regulates intestinal immunity. Nature 2017, 542, 242–245. [Google Scholar] [CrossRef]
- McAleer, J.P.; Fan, J.; Roar, B.; Primerano, D.A.; Denvir, J. Cytokine Regulation in Human CD4 T Cells by the Aryl Hydrocarbon Receptor and Gq-Coupled Receptors. Sci. Rep. 2018, 8, 10954. [Google Scholar] [CrossRef]
- Baricza, E.; Tamási, V.; Marton, N.; Buzás, E.I.; Nagy, G. The emerging role of aryl hydrocarbon receptor in the activation and differentiation of Th17 cells. Cell. Mol. Life Sci. 2016, 73, 95–117. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Nikolaus, S.; Schulte, B.; Al-Massad, N.; Thieme, F.; Schulte, D.M.; Bethge, J.; Rehman, A.; Tran, F.; Aden, K.; Häsler, R.; et al. Increased Tryptophan Metabolism Is Associated With Activity of Inflammatory Bowel Diseases. Gastroenterology 2017, 153, 1504–1516.e2. [Google Scholar] [CrossRef]
- Liu, J.Z.; van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015, 47, 979–986. [Google Scholar] [CrossRef]
- Alharbi, R.S.; Shaik, N.A.; Almahdi, H.; ElSokary, H.A.; Jamalalail, B.A.; Mosli, M.H.; Alsufyani, H.A.; Al-Aama, J.Y.; Elango, R.; Saadah, O.I.; et al. Genetic association study of NOD2 and IL23R amino acid substitution polymorphisms in Saudi Inflammatory Bowel Disease patients. J. King Saud Univ.-Sci. 2022, 34, 101726. [Google Scholar] [CrossRef]
- Michaudel, C.; Danne, C.; Agus, A.; Magniez, A.; Aucouturier, A.; Spatz, M.; Lefevre, A.; Kirchgesner, J.; Rolhion, N.; Wang, Y.; et al. Rewiring the altered tryptophan metabolism as a novel therapeutic strategy in inflammatory bowel diseases. Gut 2023, 72, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.P.; Wu, J.; Quan, W.; Zhou, Y.; Hong, H.; Niu, G.Y.; Li, T.; Huang, S.B.; Qiao, C.M.; Zhao, W.J.; et al. DSS-induced colitis activates the kynurenine pathway in serum and brain by affecting IDO-1 and gut microbiota. Front. Immunol. 2023, 13, 1089200. [Google Scholar] [CrossRef]
- Yang, S.; Li, W.; Bai, X.; Di Nunzio, G.; Fan, L.; Zhao, Y.; Ren, L.; Zhao, R.; Bian, S.; Liu, M.; et al. Ginseng-derived nanoparticles alleviate inflammatory bowel disease via the TLR4/MAPK and p62/Nrf2/Keap1 pathways. J. Nanobiotechnol. 2024, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, Z.; Wu, J.; He, Y.; Lu, G.; Zhang, D.; Zhao, Y.; Wu, R.; Lv, Y.; Cai, K.; et al. An Orally-Administered Nanotherapeutics with Carbon Monoxide Supplying for Inflammatory Bowel Disease Therapy by Scavenging Oxidative Stress and Restoring Gut Immune Homeostasis. ACS Nano 2023, 17, 21116–21133. [Google Scholar] [CrossRef] [PubMed]
- Min, D.K.; Kim, Y.E.; Kim, M.K.; Choi, S.W.; Park, N.; Kim, J. Orally Administrated Inflamed Colon-Targeted Nanotherapeutics for Inflammatory Bowel Disease Treatment by Oxidative Stress Level Modulation in Colitis. ACS Nano 2023, 17, 24404–24416. [Google Scholar] [CrossRef]
- Barani, M.; Rahdar, A.; Sargazi, S.; Amiri, M.S.; Sharma, P.; Bhalla, N. Nanotechnology for inflammatory bowel disease management: Detection, imaging and treatment. Sens. Bio-Sens. Res. 2021, 32, 100417. [Google Scholar] [CrossRef]
| Probiotic Tested | Reference | Disease Activity (UC vs. CD) | Trial Design | Outcomes |
|---|---|---|---|---|
| L. rhamnosus NCIMB 30174, L. plantarum NCIMB 30173, L. acidophilus NCIMB 30175 and E. faecium NCIMB 30176 | Bjarnason et al., 2019 [90] | Quiescent UC and CD | Single center, randomized, double-blind, placebo-controlled trial | No significant differences in IBD-QoL scores; reduced intestinal inflammation in UC patients |
| E. coli Nissle 1917 | Park et al., 2022 [91] | Mild to moderate active UC | Multicenter, double-blind, randomized, placebo-controlled study | Prevents exacerbations of IBD-QoL scores; achieves clinical response and endoscopic remission |
| L. paracasei (A234), L. gasseri (A237), L. rhamnosus (A119), L. acidophilus (A118), L. plantarum (A138), L. casei (A179), L. reuteri (A113), L. lactis (A328), B. animalis subsp lactis (A026), B. breve (A055), B. longum subsp. longum (A027), B. bifidum (A058), B. longum subsp. infantis (A041) species. | Agraib et al., 2022 [92] | Mild to moderate UC | Randomized, double-blind, placebo-controlled, parallel-arms, multicenter study | Induces clinical (partial Mayo score) and biochemical remission in UC patients |
| E. coli Nissle 1917 | Oh et al., 2021 [93] | Quiescent UC | Uncontrolled, observational, retrospective study | Additional administration of E. coli Nissle 1917 may improve UC symptoms |
| Kefir (L. pentosus, L. brevis, L. plantarum, L. fermentum, L. kefiri, and L. lindneri) | Yilmaz et al., 2019 [94] | Active UC and CD | single-center, prospective, open-label randomized controlled trial | Kefir consumption may modulate gut microbiota and enhance short-term quality of life |
| B. breve strain Yakult, L. Acidophilus | Matsuoka et al., 2018 [95] | Quiescent UC | Multicenter, randomized, placebo-controlled, double-blind parallel-group study | No effect on relapse timing |
| S. faecalis T-110, C. butyricum TO-A, B. mesentericus TO-A | Yoshimatsu et al., 2015 [96] | Quiescent UC | Randomized, double-blind, placebo-controlled study | Supports clinical remission mantainance |
| L. reuteri ATCC55730 | Oliva et al., 2012 [97] | Mild to moderate active distal UC (children) | Prospective randomized, placebo-controlled study | Reduces Mayo score and histological scores; modulates mucosal cytokine expression |
| S. thermophilus BT01, B. breve BB02, B. longum * BL03, B. infantis * BI04, L. acidophilus BA05, L. plantarum BP06, L. paracasei BP07, L. delbrueckii subs | Tursi et al., 2010 [98] | Relapsing mild-moderate UC | Multicenter, double-blind, randomized placebo-controlled, parallel study | Decreases UCDAI scores; improves rectal bleeding; may reinduce remission after 8 weeks of treatment |
| L. acidophilus, L. plantarum, L. casei, L. delbruecki subspecies bulgaricus, B. breve, B. longum, B. infantis, S. salivarius subspecies thermophilus | Miele et al., 2009 [99] | Active UC (children) | Randomized, placebo-controlled, double-blind study | Maintains clinical and endoscopic remission |
| Lactobacillus GG | Zocco et al., 2006 [100] | Quiescent UC | Single center, prospective, open-label randomized trial | Maintains remission compared to mesalazine; delays UC relapse |
| B. breve strain Yakult, B. bifidum strain Yakult, L. acidophillus strain | Kato et al., 2004 [101] | Mild to moderate active UC | randomized placebo-controlled clinical trial | Reduces clinical activity index; significantly improves post-treatment endoscopic activity index and histological score |
| Way of Administration | Reference | Disease Activity | Trial Design | Results Summary |
|---|---|---|---|---|
| FMT by colonoscopy | Deleu et al., 2024 [127] | Moderate to severe UC | Multi-centric, double-blind, sham-controlled randomized trial | Failure to achieve steroid-free clinical remission at week 8 |
| Lyophilized oral FMT | Haifer. et al., 2022 [128] | Mild to moderate UC | Double-blind, randomized, placebo-controlled trial | Induction of clinical remission with endoscopic remission or response at week 8; maintenance of clinical, endoscopic, and histological remission at week 56 |
| FMT by colonoscopy and enemas | Shabat et al., 2022 [129] | Moderate to severe active UC | Single, blinded, randomized, controlled trial | UC exclusion diet (UCED) leads to higher clinical remission and mucosal healing than single donor FMT, with or without diet |
| Encapsuled oral FMT | Crothers et al., 2021 [130] | Mild to moderate UC | single center, double-blinded, placebo-controlled, randomized control trial | Prolonged durability of FMT-induced changes in gut bacterial community structure; association between MAIT cell cytokine production and clinical response |
| FMT by colonoscopy and enemas | Costello et al., 2019 [131] | Mild to moderate UC | Double blind, randomized, clinical trial | A 1-week treatment with anaerobically prepared donor FMT results in higher remission rates at week 8 compared to autologous FMT |
| FMT by colonoscopy and enemas | Paramsothy et al., 2017 [132] | Mild to moderate UC | Multicenter, double-blind, randomized, placebo-controlled trial | Induction of clinical remission and endoscopic improvement |
| FMT via enema | Moayyedi et al., 2015 [133] | Mild to severe UC | Double-blind randomized controlled trial | Induction of remission in a significantly higher percentage of patients with active UC; greater microbial diversity |
| FMT via nasoduodenal tube | Rossen et al., 2015 [134] | Mild to moderate UC | Single-center, double-blind, placebo-controlled, randomized, proof-of-concept phase 2 trial | No significant difference in clinical and endoscopic remission between patients receiving FMT from healthy donors and those receiving autologous FMT |
| Bacterial Consortia | Reference | Trial Design | Results Summary |
|---|---|---|---|
| BAC (bile acid consortium, made up of Clostridium AP sp000509125, Bacteroides ovatus, and Eubacterium limosum) | Zhou et al., 2023 [187] | DSS-induced colitis mice model | Increases secondary bile acids (Ursodeoxycholic acid (UDCA) and Lithocholic acid (LCA)) in vitro; exerts protective effects against colitis (reduces weight loss, increases colon length, strengthens the intestinal barrier) |
| Lactobacillus reuteri, Lactobacillus gasseri, Lactobacillus acidophilus (Lactobacillus spp.), and Bifidobacterium lactis (Bifidobacterium spp.) | Xu et al., 2022 [188] | DSS-induced colitis mice model | Alleviates disease phenotype; restores the composition and structure of the gut microbiota |
| GUT 103 (strains of genera Bacteroides, Akkermansia, Clostridium, Faecalibacterium) and GUT108 (strains of Clostridium, Intestinimonas, Bitterella, Barneseilla) | Van der Leile et al., 2021 [189] | Immune-mediated colitis in germ free mice | GUT-103 and GUT-108: Correct the dysbiotic microbiome environment; activate IL-10-producing immune cells; reduce inflammatory responses; restore bacterial metabolic profiles to levels observed in healthy individuals’ stool samples |
| BMC332 | Polonsky et al., 2021 [190] | DSS-induced colitis in mice | Exhibits anti-inflammatory properties; maintains intestinal barrier integrity |
| Engineered Probiotic | Reference | Trial Design | Results Summary |
|---|---|---|---|
| E. coli | Wang et al., 2021 [216] | DSS-induced colitis in mice |
|
| E. coli | Cui et al., 2021 [217] | DSS-treated colitis in mice |
|
| L. paracasei (KBL382,384,385) | Kim et al., 2019 [220] | DSS-induced colitis in mice |
|
| B. longum | Liu et al., 2016 [222] | DSS-induced colitis in mice |
|
| B. longum | Wei et al., 2016 [223] | DSS-induced colitis in mice |
|
| OMV of Bacteroides thetaiotaomicron (Bt) | Carvalho et al., 2019 [225] | DSS-induced colitis in mice |
|
| OMV from E. coli Nissle 1917 | Fabrega et al., 2017 [226] | DSS-induced colitis in mice |
|
| E. coli | Zhang et al., 2018 [227] | DSS-induced experimental colitis in mice |
|
| L. lactis | Hanson et al., 2014 [228] | DSS-induced colitis in mice |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murgiano, M.; Bartocci, B.; Puca, P.; di Vincenzo, F.; Del Gaudio, A.; Papa, A.; Cammarota, G.; Gasbarrini, A.; Scaldaferri, F.; Lopetuso, L.R. Gut Microbiota Modulation in IBD: From the Old Paradigm to Revolutionary Tools. Int. J. Mol. Sci. 2025, 26, 3059. https://doi.org/10.3390/ijms26073059
Murgiano M, Bartocci B, Puca P, di Vincenzo F, Del Gaudio A, Papa A, Cammarota G, Gasbarrini A, Scaldaferri F, Lopetuso LR. Gut Microbiota Modulation in IBD: From the Old Paradigm to Revolutionary Tools. International Journal of Molecular Sciences. 2025; 26(7):3059. https://doi.org/10.3390/ijms26073059
Chicago/Turabian StyleMurgiano, Marco, Bianca Bartocci, Pierluigi Puca, Federica di Vincenzo, Angelo Del Gaudio, Alfredo Papa, Giovanni Cammarota, Antonio Gasbarrini, Franco Scaldaferri, and Loris Riccardo Lopetuso. 2025. "Gut Microbiota Modulation in IBD: From the Old Paradigm to Revolutionary Tools" International Journal of Molecular Sciences 26, no. 7: 3059. https://doi.org/10.3390/ijms26073059
APA StyleMurgiano, M., Bartocci, B., Puca, P., di Vincenzo, F., Del Gaudio, A., Papa, A., Cammarota, G., Gasbarrini, A., Scaldaferri, F., & Lopetuso, L. R. (2025). Gut Microbiota Modulation in IBD: From the Old Paradigm to Revolutionary Tools. International Journal of Molecular Sciences, 26(7), 3059. https://doi.org/10.3390/ijms26073059







