The Diagnostic and Predictive Potential of miR-328 in Atrial Fibrillation: Insights from a Spontaneously Hypertensive Rat Model
Abstract
1. Introduction
2. Results
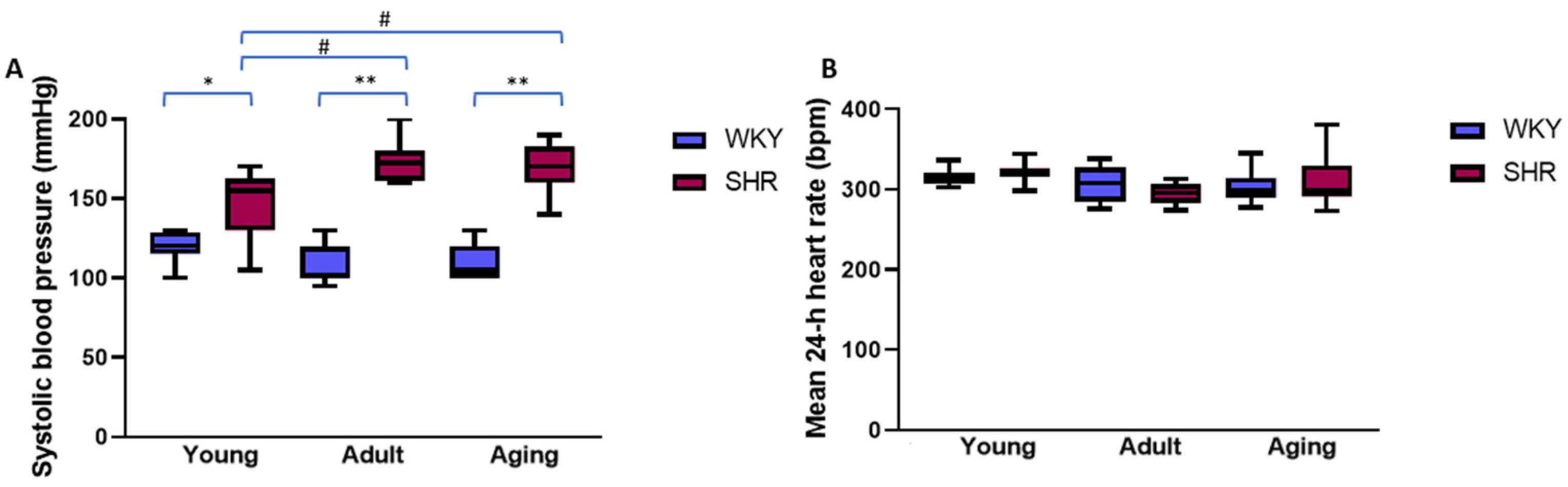
2.1. Systolic Blood Pressure and Mean 24 h Heart Rate in Young, Adult, and Aging Spontaneously Hypertensive and Control Rats
2.2. Spontaneous Atrial Arrhythmic Burden in Normotensive and Hypertensive Rats
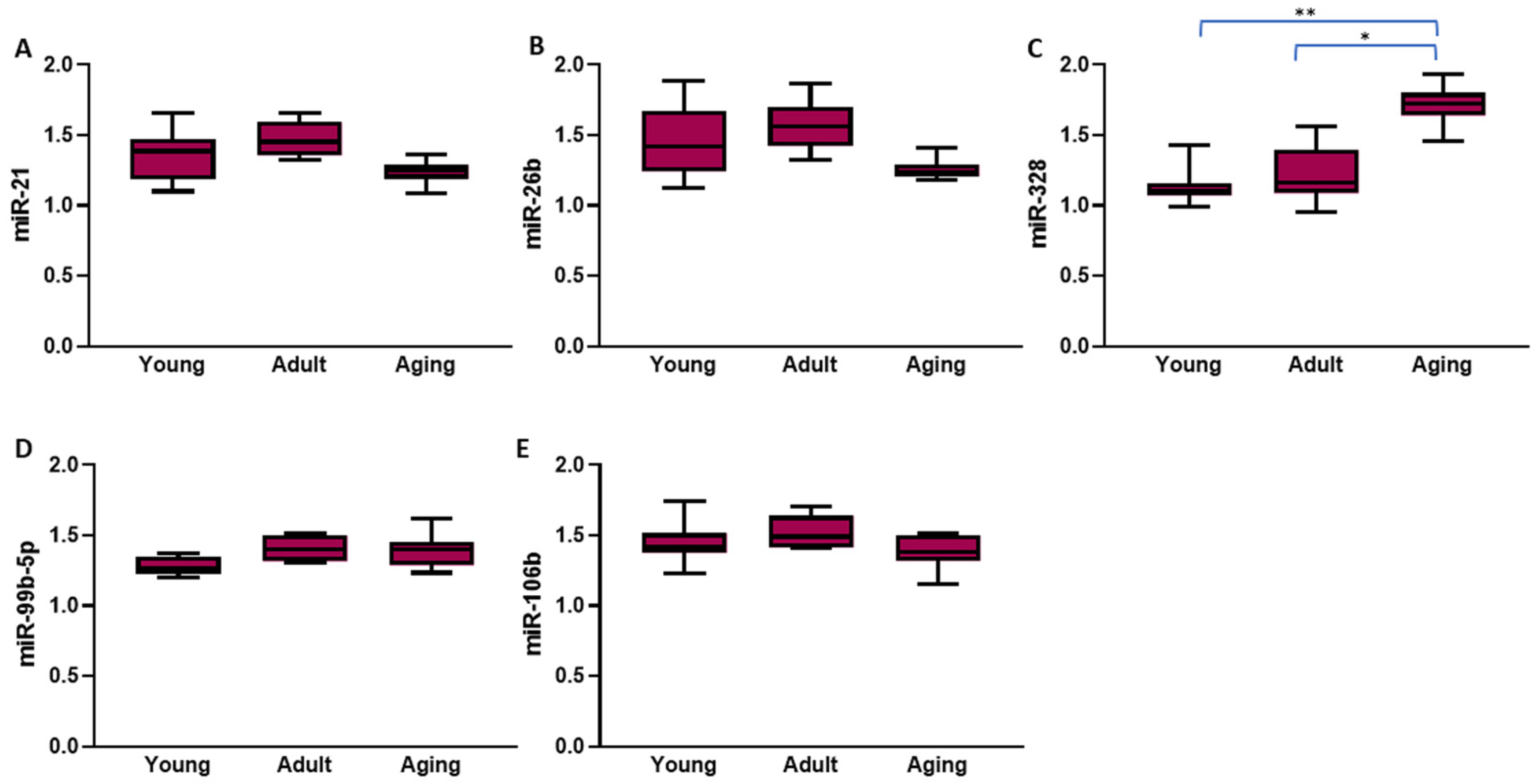
2.3. Left Atrial and Circulating Expression of Atrial Fibrillation-Related microRNAs in Normotensive and Hypertensive Rats
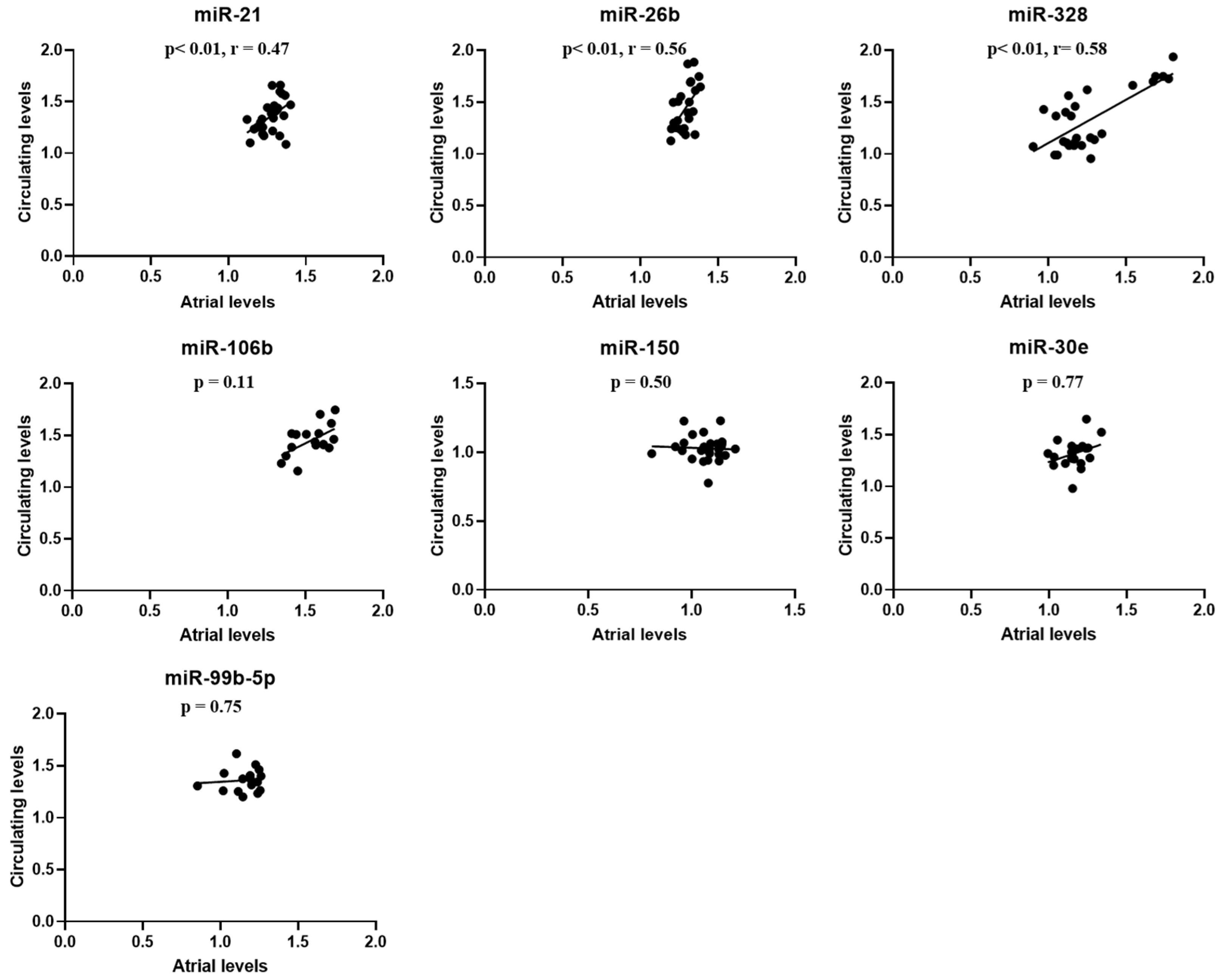
2.4. Atrial-Circulating microRNA Correspondence and Relationship Between miRNAs Levels and Atrial Fibrillation Burden
3. Discussion
3.1. Aging and Hypertension Create the Optimal Environment for Spontaneous Atrial Fibrillation Occurrence in Rats
3.2. Left Atrial and Circulating Atrial Fibrillation-Related microRNA Changes in Hypertensive Rats
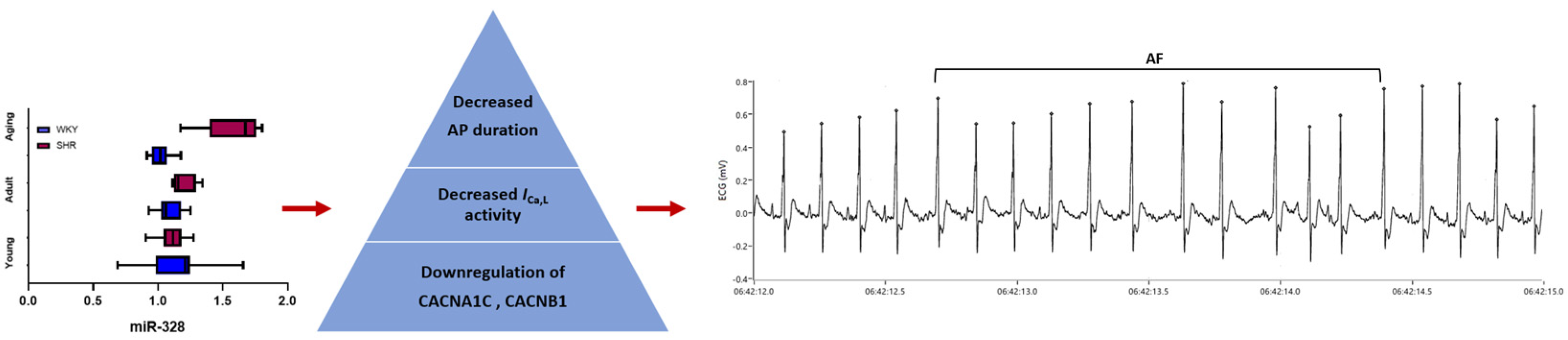
3.3. MicroRNA-328—A Promising Biomarker for Atrial Fibrillation Diagnosis and Prediction
3.4. Potential Limitations
4. Materials and Methods
4.1. Studied Animals
4.2. Radiotelemetry ECG Monitoring
4.3. Blood Pressure Measurement
4.4. MicroRNA Analysis
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| miR | microRNA |
| miRNAs | microRNAs |
| PACs | premature atrial contractions |
| PDGF-B | platelet-derived growth factor subunit B |
| SHR | spontaneously hypertensive rat |
| WKY | Wistar Kyoto |
References
- Kornej, J.; Börschel, C.S.; Börschel, C.S.; Benjamin, E.J.; Benjamin, E.J.; Schnabel, R.B.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century: Novel Methods and New Insights. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wu, X.Y.; Long, D.Y.; Jiang, C.X.; Sang, C.H.; Tang, R.B.; Li, S.N.; Wang, W.; Guo, X.Y.; Ning, M.; et al. Asymptomatic Atrial Fibrillation among Hospitalized Patients: Clinical Correlates and in-Hospital Outcomes in Improving Care for Cardiovascular Disease in China-Atrial Fibrillation. Europace 2023, 25, euad272. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, R.; Wu, J.; Hogg, D.; Raveendra, K.; Nakao, Y.M.; Nakao, K.; Arbel, R.; Haim, M.; Zahger, D.; Parry, J.; et al. Prediction of Short-Term Atrial Fibrillation Risk Using Primary Care Electronic Health Records. Heart 2023, 109, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Segan, L.; Canovas, R.; Nanayakkara, S.; Chieng, D.; Prabhu, S.; Voskoboinik, A.; Sugumar, H.; Ling, L.H.; Lee, G.; Morton, J.; et al. New-Onset Atrial Fibrillation Prediction: The HARMS2-AF Risk Score. Eur. Heart J. 2023, 44, 3443–3452. [Google Scholar] [CrossRef]
- Balan, A.I.; Scridon, A. Atrial Fibrillation—An Orchestra of Classic and Modern Risk Factors. Acta Med. Marisiensis 2019, 65, 80–86. [Google Scholar] [CrossRef]
- O’Neal, W.T.; Alonso, A. The Appropriate Use of Risk Scores in the Prediction of Atrial Fibrillation. J. Thorac. Dis. 2016, 8, E1391–E1394. [Google Scholar] [CrossRef]
- van den Berg, N.W.E.; Kawasaki, M.; Berger, W.R.; Neefs, J.; Meulendijks, E.; Tijsen, A.J.; de Groot, J.R. MicroRNAs in Atrial Fibrillation: From Expression Signatures to Functional Implications. Cardiovasc Drugs Ther. 2017, 31, 345–365. [Google Scholar] [CrossRef]
- Ca, Y.; Cui, L. Identifying the Key MicroRNAs Implicated in Atrial Fibrillation. Anatol. J. Cardiol. 2021, 25, 429–436. [Google Scholar] [CrossRef]
- Balan, A.I.; Scridon, A. MicroRNAs in atrial fibrillation—Have we discovered the Holy Grail or opened a Pandora’s box? Front. Pharmacol. 2025, 16, 1535621. [Google Scholar] [CrossRef]
- Chiang, D.Y.; Kongchan, N.; Beavers, D.L.; Alsina, K.M.; Voigt, N.; Neilson, J.R.; Jakob, H.; Martin, J.F.; Dobrev, D.; Wehrens, X.H.; et al. Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release. Circ. Arrhythm. Electrophysiol. 2014, 7, 1214–1222. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, P.; Lin, Y.; Du, M.; Li, H.; Jiang, L.; Xu, H.; Sun, H.; Han, J.; Sun, Z.; et al. MiR-203 improves cardiac dysfunction by targeting PARP1-NAD+ axis in aging murine. Aging Cell 2024, 23, e14063. [Google Scholar] [CrossRef]
- Compagnucci, P.; Russo, A.D.; Mohanty, S.; Bergonti, M.; Torlapati, P.G.; Valeri, Y.; Gigante, C.; Conte, E.; Manfredi, R.; Giannoni, M.; et al. Catheter Ablation of Atrial Fibrillation in Patients With Psoriasis: A Multicenter Study. JAHA 2025, 14, e038882. [Google Scholar] [CrossRef]
- La Fazia, V.M.; Pierucci, N.; Mohanty, S.; Gianni, C.; Della Rocca, D.G.; Compagnucci, P.; MacDonald, B.; Mayedo, A.; Torlapati, P.G.; Bassiouny, M.; et al. Catheter ablation approach and outcome in HIV+ patients with recurrent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2023, 34, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Scridon, A.; Gallet, C.; Arisha, M.M.; Oréa, V.; Chapuis, B.; Li, N.; Tabib, A.; Christé, G.; Barrès, C.; Julien, C.; et al. Unprovoked Atrial Tachyarrhythmias in Aging Spontaneously Hypertensive Rats: The Role of the Autonomic Nervous System. Am. J. Physiol.—Heart. Cir. Physiol. 2012, 303, 386–392. [Google Scholar] [CrossRef]
- Scridon, A.; Tabib, A.; Barrès, C.; Julien, C.; Chevalier, P. Left Atrial Endocardial Fibrosis and Intra-Atrial Thrombosis -Landmarks of Left Atrial Remodeling in Rats with Spontaneous Atrial Tachyarrhythmias. Rom. J. Morphol. Embryol. 2013, 54, 405–411. [Google Scholar]
- Sayin, H.; Scridon, A.; Oréa, V.; Chapuis, B.; Chevalier, P.; Barrès, C.; Julien, C. Pyridostigmine Enhances Atrial Tachyarrhythmias in Aging Spontaneously Hypertensive Rats. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1084–1091. [Google Scholar] [CrossRef]
- Scridon, A.; Fouilloux-Meugnier, E.; Loizon, E.; Rome, S.; Julien, C.; Barrès, C.; Chevalier, P. Long-Standing Arterial Hypertension Is Associated with Pitx2 down-Regulation in a Rat Model of Spontaneous Atrial Tachyarrhythmias. Europace 2014, 17, 160–165. [Google Scholar] [CrossRef]
- Doñate Puertas, R.; Meugnier, E.; Romestaing, C.; Rey, C.; Morel, E.; Lachuer, J.; Gadot, N.; Scridon, A.; Julien, C.; Tronc, F.; et al. Atrial Fibrillation Is Associated with Hypermethylation in Human Left Atrium, and Treatment with Decitabine Reduces Atrial Tachyarrhythmias in Spontaneously Hypertensive Rats. Transl. Res. 2017, 184, 57–67.e5. [Google Scholar] [CrossRef]
- Farinha, J.M.; Gupta, D.; Lip, G.Y.H. Frequent Premature Atrial Contractions as a Signalling Marker of Atrial Cardiomyopathy, Incident Atrial Fibrillation, and Stroke. Cardiovasc. Res. 2023, 119, 429–439. [Google Scholar] [CrossRef]
- Menezes Junior, A.d.S.; Ferreira, L.C.; Barbosa, L.J.V.; Silva, D.D.M.E.; Saddi, V.A.; Silva, A.M.T.C. Circulating MicroRNAs as Specific Biomarkers in Atrial Fibrillation: A Meta-Analysis. Non-Coding RNA 2023, 9, 13. [Google Scholar] [CrossRef]
- Cooley, N.; Cowley, M.J.; Lin, R.C.Y.; Marasco, S.; Wong, C.; Kaye, D.M.; Dart, A.M.; Woodcock, E.A. Influence of Atrial Fibrillation on MicroRNA Expression Profiles in Left and Right Atria from Patients with Valvular Heart Disease. Physiol. Genom. 2012, 44, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Dawson, K.; Wakili, R.; Ördög, B.; Clauss, S.; Chen, Y.; Iwasaki, Y.; Voigt, N.; Qi, X.Y.; Sinner, M.F.; Dobrev, D.; et al. MicroRNA29: A Mechanistic Contributor and Potential Biomarker in Atrial Fibrillation. Circulation 2013, 127, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Shi, R.; Yu, X.; Sun, C.; Zang, W.; Tian, H. Identification of Atrial Fibrillation-Associated MicroRNAs in Left and Right Atria of Rheumatic Mitral Valve Disease Patients. Genes. Genet. Syst. 2019, 94, 181. [Google Scholar] [CrossRef]
- Lv, X.; Lu, P.; Hu, Y.; Xu, T. Overexpression of MiR-29b-3p Inhibits Atrial Remodeling in Rats by Targeting PDGF-B Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 3763529. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.T.; Li, X.X.; Cheng, Q.J.; Wang, Y.H.; Wang, J.H.; Liu, C.L. MiR-30a Regulates the Atrial Fibrillation-Induced Myocardial Fibrosis by Targeting Snail 1. Int. J. Clin. Exp. Pathol. 2015, 8, 15527–15536. [Google Scholar] [PubMed]
- Chen, S.; Zhu, H.; Sun, J.; Zhu, L.; Qin, L.; Wan, J. Anti-inflammatory Effects of MiR-150 Are Associated with the Downregulation of STAT1 in Macrophages Following Lipopolysaccharide Treatment. Exp. Ther. Med. 2021, 22, 1049. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Jing, L. MiRNA-99a Alleviates Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated PC-12 Cells and Rats Post Spinal Cord Injury. Bioengineered 2022, 13, 4248–4259. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Q.; Zhang, W.; Zhen, Y.; Cheng, F.; Hua, G.; Lan, J.; Tu, C. MiR-203-3p Inhibits the Oxidative Stress, Inflammatory Responses and Apoptosis of Mice Podocytes Induced by High Glucose through Regulating Sema3A Expression. Open Life Sci. 2020, 15, 939–950. [Google Scholar] [CrossRef]
- Yu, Y.-h.; Zhang, Y.-h.; Ding, Y.-q.; Bi, X.-y.; Yuan, J.; Zhou, H.; Wang, P.-x.; Zhang, L.-l.; Ye, J.-t. MicroRNA-99b-3p Promotes Angiotensin II-Induced Cardiac Fibrosis in Mice by Targeting GSK-3β. Acta Pharmacol. Sin. 2021, 42, 715–725. [Google Scholar] [CrossRef]
- Shen, J.; Xing, W.; Gong, F.; Wang, W.; Yan, Y.; Zhang, Y.; Xie, C.; Fu, S. MiR-150-5p Retards the Progression of Myocardial Fibrosis by Targeting EGR1. Cell Cycle 2019, 18, 1335–1348. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Lin, Q.; Xu, Q. Up-Regulation of MicroRNA-203 Inhibits Myocardial Fibrosis and Oxidative Stress in Mice with Diabetic Cardiomyopathy through the Inhibition of PI3K/Akt Signaling Pathway via PIK3CA. Gene 2019, 715, 143995. [Google Scholar] [CrossRef] [PubMed]
- Ameri, A.; Ahmed, H.M.; Pecho, R.D.C.; Arabnozari, H.; Sarabadani, H.; Esbati, R.; Mirabdali, S.; Yazdani, O. Diverse activity of miR-150 in Tumor development: Shedding light on the potential mechanisms. Cancer Cell Int. 2023, 23, 261. [Google Scholar] [CrossRef]
- Sur, D.; Burz, C.; Sabarimurugan, S.; Irimie, A. Diagnostic and Prognostic Significance of MiR-150 in Colorectal Cancer: A Systematic Review and Meta-Analysis. J. Pers. Med. 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, Y.; Cai, J.; Cui, K.; Li, R.X.; Wang, H.; Shang, X.; Wei, D. MiR-30a-5p Suppresses Tumor Metastasis of Human Colorectal Cancer by Targeting ITGB3. Cell. Physiol. Biochem. 2016, 39, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Zhao, Y.; Feng, W.; Zhao, Z.; Liu, W.; Wang, N.; Xue, H.; Wu, L.; Cui, S.; Bai, R. MiR-30 Inhibits the Progression of Osteosarcoma by Targeting MTA1. J. Musculoskelet. Neuronal Interact. 2022, 22, 261–268. [Google Scholar]
- Liu, S.; Feng, P. MiR-203 Determines Poor Outcome and Suppresses Tumor Growth by Targeting TBK1 in Osteosarcoma. Cell. Physiol. Biochem. 2015, 37, 1956–1966. [Google Scholar] [CrossRef]
- Okugawa, Y.; Toiyama, Y.; Hur, K.; Yamamoto, A.; Yin, C.; Ide, S.; Kitajima, T.; Fujikawa, H.; Yasuda, H.; Koike, Y.; et al. Circulating MiR-203 Derived from Metastatic Tissues Promotes Myopenia in Colorectal Cancer Patients. J. Cachexia Sarcopenia Muscle 2019, 10, 536–548. [Google Scholar] [CrossRef]
- Huang, Y.H.; Yang, Y.L.; Wang, F.S. The Role of MiR-29a in the Regulation, Function, and Signaling of Liver Fibrosis. Int. J. Mol. Sci. 2018, 19, 1889. [Google Scholar] [CrossRef]
- Cushing, L.; Kuang, P.P.; Qian, J.; Shao, F.; Wu, J.; Little, F.; Thannickal, V.J.; Cardoso, W.V.; Lü, J. MiR-29 Is a Major Regulator of Genes Associated with Pulmonary Fibrosis. Am. J. Respir. Cell. Mol. Biol. 2011, 45, 287–294. [Google Scholar] [CrossRef]
- Ma, R.; Wang, M.; Gao, S.; Zhu, L.; Yu, L.; Hu, D.; Zhu, L.; Huang, W.; Zhang, W.; Deng, J.; et al. MiR-29a Promotes the Neurite Outgrowth of Rat Neural Stem Cells by Targeting Extracellular Matrix to Repair Brain Injury. Stem Cells Dev. 2020, 29, 599–614. [Google Scholar] [CrossRef]
- Messner, C.J.; Schmidt, S.; Özkul, D.; Gaiser, C.; Terracciano, L.; Krähenbühl, S.; Suter-Dick, L. Identification of Mir-199a-5p, Mir-214-3p and Mir-99b-5p as Fibrosis-Specific Extracellular Biomarkers and Promoters of HSC Activation. Int. J. Mol. Sci. 2021, 22, 9799. [Google Scholar] [CrossRef]
- Kang, J.; Lee, S.Y.; Lee, S.Y.; Kim, Y.J.; Park, J.Y.; Kwon, S.J.; Na, M.J.; Lee, E.J.; Jeon, H.S.; Son, J.W. MicroRNA-99b Acts as a Tumor Suppressor in Non-Small Cell Lung Cancer by Directly Targeting Fibroblast Growth Factor Receptor 3. Exp. Ther. Med. 2012, 3, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.N.; Zhang, C.; Li, Z.; Kong, L.C.; Wang, X.H.; Gu, Z.C.; Wang, J.L. MicroRNA Expression Signatures of Atrial Fibrillation: The Critical Systematic Review and Bioinformatics Analysis. Exp. Biol. Med. 2020, 245, 42–53. [Google Scholar] [CrossRef]
- Jenike, A.E.; Halushka, M.K. MiR-21: A Non-specific Biomarker of All Maladies. Biomark. Res. 2021, 9, 18. [Google Scholar] [CrossRef]
- Wang, M.; Li, W.; Chang, G.Q.; Ye, C.S.; Ou, J.S.; Li, X.X.; Liu, Y.; Cheang, T.Y.; Huang, X.L.; Wang, S.M. MicroRNA-21 Regulates Vascular Smooth Muscle Cell Function via Targeting Tropomyosin 1 in Arteriosclerosis Obliterans of Lower Extremities. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2044–2053. [Google Scholar] [CrossRef]
- Oh, J.; Matkovich, S.J.; Riek, A.E.; Bindom, S.M.; Shao, J.S.; Head, R.D.; Barve, R.A.; Sands, M.S.; Carmeliet, G.; Osei-Owusu, P.; et al. Macrophage Secretion of MiR-106b-5p Causes Renin-Dependent Hypertension. Nat. Commun. 2020, 11, 4798. [Google Scholar] [CrossRef]
- Watanabe, K.; Narumi, T.; Watanabe, T.; Otaki, Y.; Takahashi, T.; Aono, T.; Goto, J.; Toshima, T.; Sugai, T.; Wanezaki, M.; et al. The Association between MicroRNA-21 and Hypertension-Induced Cardiac Remodeling. PLoS ONE 2020, 15, e0226053. [Google Scholar] [CrossRef]
- Olivieri, F.; Spazzafumo, L.; Santini, G.; Lazzarini, R.; Albertini, M.C.; Rippo, M.R.; Galeazzi, R.; Abbatecola, A.M.; Marcheselli, F.; Monti, D.; et al. Age-Related Differences in the Expression of Circulating MicroRNAs: MiR-21 as a New Circulating Marker of Inflammaging. Mech. Ageing Dev. 2012, 133, 675–685. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Wang, N.; Pan, Z.; Gao, X.; Zhang, F.; Zhang, Y.; Shan, H.; Luo, X.; Bai, Y.; et al. MicroRNA-328 Contributes to Adverse Electrical Remodeling in Atrial Fibrillation. Circulation 2010, 122, 2378–2387. [Google Scholar] [CrossRef]
- Huang, H.; Chen, H.; Liang, X.; Chen, X.; Chen, X.; Chen, C. Upregulated MiR-328-3p and Its High Risk in Atrial Fibrillation. Medicine 2022, 101, e28980. [Google Scholar] [CrossRef]
- Balan, A.I.; Pintilie, I.; Somkereki, C.; Perian, M.; Chinezu, L.; Banescu, C.; Serban, R.C.; Scridon, A. Role of Preexisting Proarrhythmic Atrial Remodeling in Post-Coronary Artery Bypass Grafting Atrial Fibrillation. Rev. Rom. Cardiol. 2021, 31, 597–607. [Google Scholar] [CrossRef]
- Halațiu, V.B.; Perian, M.; Balan, A.I.; Scridon, A. Transesophageal Atrial Burst Pacing for Atrial Fibrillation Induction in Rats. J. Vis. Exp. 2022, 180, e63567. [Google Scholar] [CrossRef]
- Balan, I.A.; Grigoraș, T.; Matei, C.; Grigoraș, A.; Halațiu, V.B.; Șerban, R.C.; Scridon, A. Effect of Isoflurane Anesthesia on the Heart Rate and Blood Pressure Response to Autonomic Nervous System Stimulation and Inhibition in Rats. Acta Marisiensis 2020, 66, 92–96. [Google Scholar] [CrossRef]


| MicroRNAs | Young | p-Value | Adult | p-Value | Aging | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| WKY (n = 16) | SHR (n = 13) | WKY (n = 12) | SHR (n = 8) | WKY (n = 11) | SHR (n = 10) | ||||
| miR-100-5p | 1.27 [1.19–1.35] | 1.22 [1.19–1.28] | 0.83 | 1.26 [1.25–1.31] | 1.26 [1.22–1.28] | 0.52 | 1.25 [1.21–1.27] | 1.24 [1.19–1.28] | 0.64 |
| miR-106b | 1.59 [1.39–1.64] | 1.56 [1.41–1.67] | 0.94 | 1.55 [1.50–1.56] | 1.59 [1.51–1.61] | 0.28 | 1.55 [1.54–1.61] | 1.47 [1.41–1.63] | 0.19 |
| miR-150 | 1.13 [0.99–1.17- | 1.07 [1.01–1.14] | 0.73 | 1.14 [1.11–1.16] | 1.08 [1.05–1.13] | 0.03 | 1.16 [1.14–1.17] | 1.07 [0.95–1.10] | <0.01 |
| miR-203 | 1.74 [1.61–1.86] | 1.70 [1.58–1.78] | 0.56 | 1.62 [1.56–1.68] | 1.82 [1.76–1.91] | <0.01 | 1.59 [1.54–1.68] | 1.75 [1.71–1.81] | <0.01 |
| miR-21 | 1.30 [1.10–1.42] | 1.28 [1.21–1.31] | 0.77 | 1.30 [1.27–1.32] | 1.30 [1.26–1.33] | 0.58 | 1.26 [1.25–1.29] | 1.23 [1.20–1.34] | 0.52 |
| miR-26b | 1.28 [1.26–1.37] | 1.27 [1.22–1.33] | 0.58 | 1.27 [1.23–1.34] | 1.31 [1.25–1.32] | 0.47 | 1.24 [1.21–1.30] | 1.27 [1.23–1.31] | 0.65 |
| miR-29a | 1.55 [1.22–1.59] | 1.41 [1.29–1.60] | 0.85 | 1.54 [1.50–1.58] | 1.54 [1.47–1.57] | >0.99 | 1.59 [1.51–1.62] | 1.40 [1.30–1.60] | 0.04 |
| miR-30e | 1.25 [1.06–1.29] | 1.18 [1.11–1.24] | 0.8 | 1.07 [0.98–1.11] | 1.20 [1.17–1.21] | <0.01 | 1.27 [1.26–1.29] | 1.14 [1.03–1.17] | <0.001 |
| miR-328 | 1.20 [0.98–1.24] | 1.11 [1.04–1.17] | 0.64 | 1.05 [1.02–1.17] | 1.15 [1.12–1.29] | 0.01 | 1.01 [0.95–1.06] | 1.67 [1.39–1.75] | <0.0001 |
| miR-9-5p | 1.55 [1.45–1.67] | 1.51 [1.46–1.57] | 0.39 | 1.50 [1.48–1.55] | 1.56 [1.52–1.58] | 0.13 | 1.50 [1.45–1.54] | 1.48 [1.41–1.56] | 0.74 |
| miR-99-5p | 1.28 [1.06–1.33] | 1.17 [1.11–1.27] | 0.42 | 1.27 [1.25–1.28] | 1.23 [1.17–1.25] | 0.01 | 1.26 [1.22–1.28] | 1.19 [1.02–1.21] | <0.01 |
| MicroRNAs | Young | p-Value | Adult | p-Value | Aging | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| WKY (n = 16) | SHR (n = 13) | WKY (n = 12) | SHR (n = 8) | WKY (n = 11) | SHR (n = 10) | ||||
| miR-106b | 1.37 [1.29–1.56] | 1.42 [1.37–1.51] | 0.57 | 1.26 [1.14–1.32] | 1.49 [1.41–1.63] | <0.01 | 1.22 [1.12–1.33] | 1.38 [1.31–1.5] | 0.01 |
| miR-150 | 1.03 [0.89–1.09] | 1.03 [0.97–1.07] | 0.47 | 0.96 [0.74–1.06] | 1.02 [0.99–1.06] | 0.06 | 0.84 [0.78–0.99] | 1.03 [0.97–1.06] | 0.09 |
| miR-21 | 1.31 [1.23–1.40] | 1.38 [1.18–1.46] | 0.57 | 1.33 [1.18–1.44] | 1.45 [1.35–1.59] | 0.02 | 1.20 [1.15–1.32] | 1.25 [1.19–1.28] | 0.78 |
| miR-26b | 1.32 [1.27–1.51] | 1.41 [1.24–1.67] | 0.44 | 1.35 [1.27–1.63] | 1.55 [1.42–1.69] | 0.10 | 1.29 [1.16–1.48] | 1.24 [1.20–1.29] | 0.27 |
| miR-30e | 1.27 [1.18–1.33] | 1.34 [1.23–1.48] | 0.21 | 1.20 [1.07–1.35] | 1.31 [1.27–1.38] | 0.07 | 1.18 [1.05–1.29] | 1.31 [1.18–1.40] | 0.09 |
| miR-328 | 1.17 [1.04–1.25] | 1.11 [1.07–1.15] | 0.34 | 1.19 [0.95–1.29] | 1.16 [1.08–1.39] | 0.40 | 1.10 [1.05–1.33] | 1.72 [1.64–1.80] | <0.0001 |
| miR-99-5p | 1.29 [1.04–1.41] | 1.26 [1.22–1.34] | >0.99 | 1.19 [1.08–1.41] | 1.40 [1.31–1.49] | 0.19 | 0.99 [0.93–1.25] | 1.40 [1.28–1.45] | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balan, A.I.; Halaţiu, V.B.; Comșulea, E.; Mutu, C.C.; Cozac, D.A.; Aspru, I.; Păcurar, D.; Bănescu, C.; Perian, M.; Scridon, A. The Diagnostic and Predictive Potential of miR-328 in Atrial Fibrillation: Insights from a Spontaneously Hypertensive Rat Model. Int. J. Mol. Sci. 2025, 26, 3049. https://doi.org/10.3390/ijms26073049
Balan AI, Halaţiu VB, Comșulea E, Mutu CC, Cozac DA, Aspru I, Păcurar D, Bănescu C, Perian M, Scridon A. The Diagnostic and Predictive Potential of miR-328 in Atrial Fibrillation: Insights from a Spontaneously Hypertensive Rat Model. International Journal of Molecular Sciences. 2025; 26(7):3049. https://doi.org/10.3390/ijms26073049
Chicago/Turabian StyleBalan, Alkora Ioana, Vasile Bogdan Halaţiu, Emilian Comșulea, Cosmin Constantin Mutu, Dan Alexandru Cozac, Ioana Aspru, Delia Păcurar, Claudia Bănescu, Marcel Perian, and Alina Scridon. 2025. "The Diagnostic and Predictive Potential of miR-328 in Atrial Fibrillation: Insights from a Spontaneously Hypertensive Rat Model" International Journal of Molecular Sciences 26, no. 7: 3049. https://doi.org/10.3390/ijms26073049
APA StyleBalan, A. I., Halaţiu, V. B., Comșulea, E., Mutu, C. C., Cozac, D. A., Aspru, I., Păcurar, D., Bănescu, C., Perian, M., & Scridon, A. (2025). The Diagnostic and Predictive Potential of miR-328 in Atrial Fibrillation: Insights from a Spontaneously Hypertensive Rat Model. International Journal of Molecular Sciences, 26(7), 3049. https://doi.org/10.3390/ijms26073049







