Nidogen-1, a Player in KMT2A-Rearranged Pediatric Acute Myeloid Leukemia
Abstract
1. Introduction
2. Results
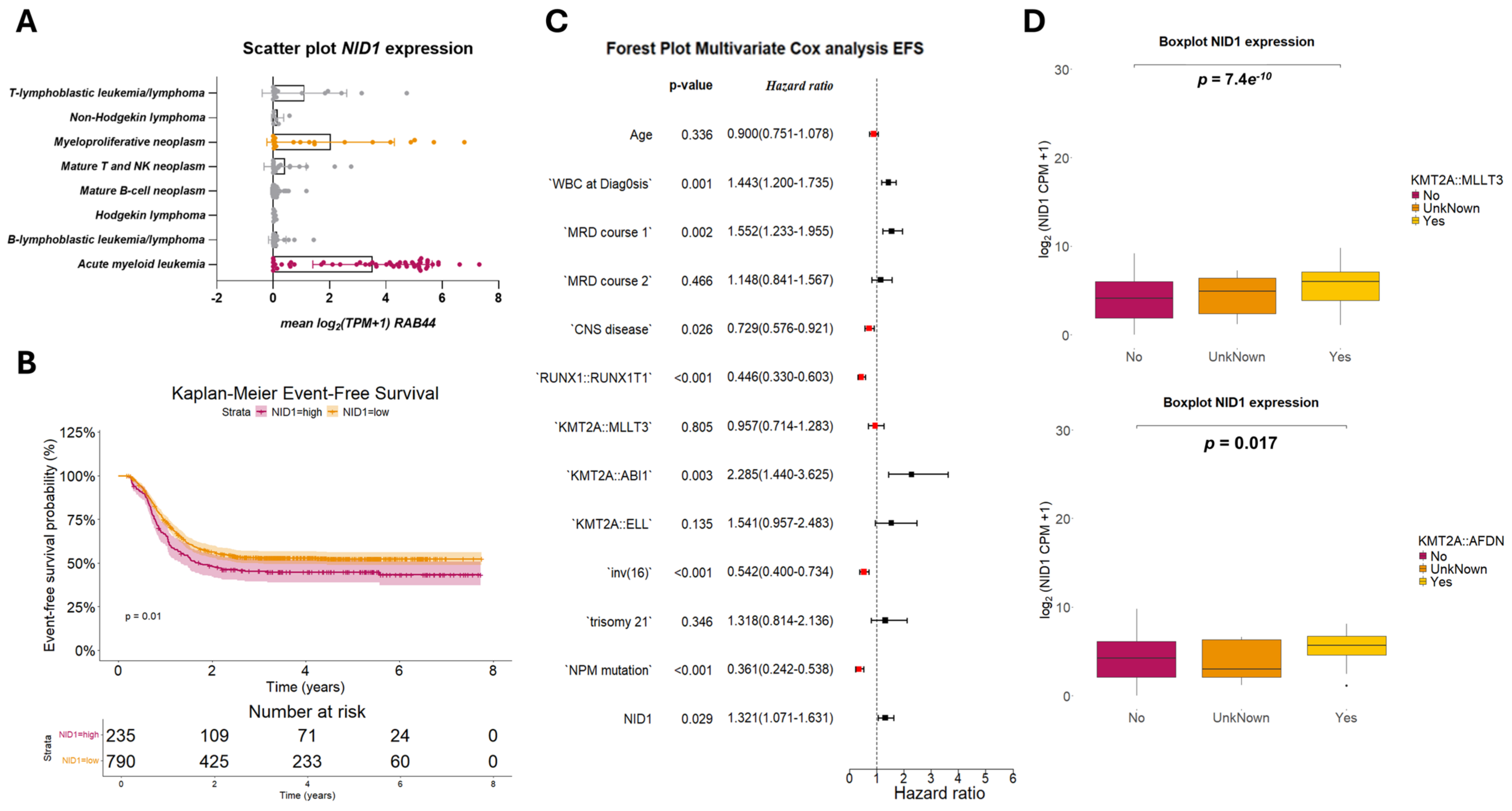
2.1. NID1 Is Expressed in the Leukemic Stem Cells and Leukemic Blasts of pedAML Patients
2.2. NID1 Expression Is Associated with KMT2A-Rearrangements and Worse Event-Free Survival
2.3. NID1 Modulation Alters Sensitivity Towards HSP90
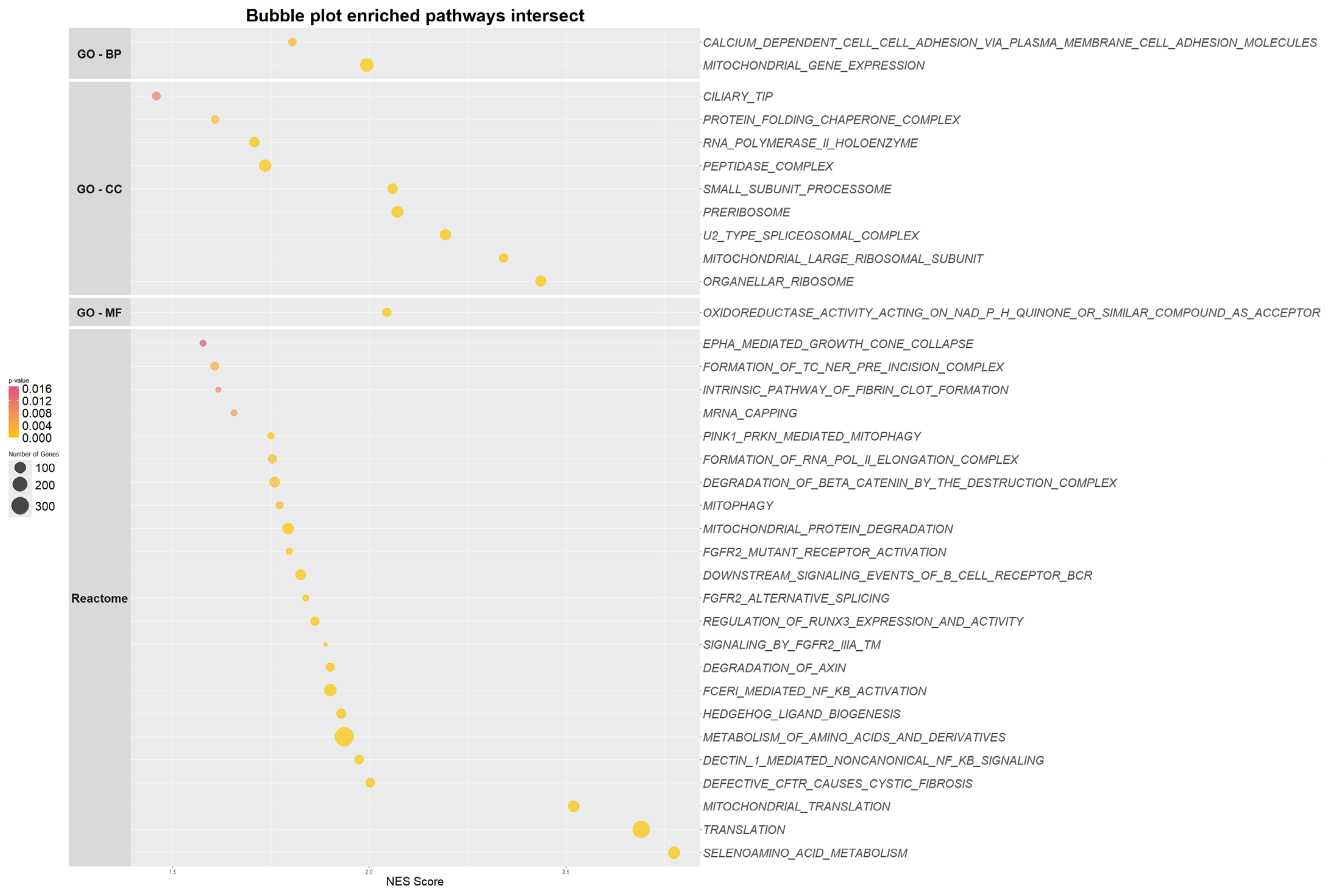
2.4. NID1 Modulation Impacts Genes Enriched in Metabolic Pathways in the Leukemic Stem Cell
3. Discussion
4. Materials and Methods
4.1. Patients and Controls
4.2. Micro-Array Analysis
4.3. Cell Culture
4.4. Public Expression Data
4.5. RNA Isolation, cDNA Synthesis and qPCR
4.6. Western Blot Analysis
4.7. Lentivirus Generation and Transduction
4.8. RNA Sequencing and Differential Expression Analysis
4.9. Drug Response Profiling
4.10. Chemosensitivity Assay
4.11. Data Processing and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | Acute myeloid leukemia |
| LSCJ | Leukemic stem cell |
| HSC | Hematopoietic stem cell |
| EFS | Event-free survival |
| pedAML | Pediatric acute myeloid leukemia |
| OS | Overall survival |
| L-Blast | Leukemic blast |
| NID1 | Nidogen-1 |
| NID1 | Nidogen-2 |
| BM | Bone marrow |
| PB | Peripheral blood |
| C-blast | Control blast |
| TARGET | Therapeutically Applicable Research To Generate Effective Treatments |
| GSEA | Gene set enrichment analysis |
| DRP | Drug response profiling |
| DMSO | Dimethyl sulfoxide |
| IC50 | Inhibitory concentration 50 |
| AUC | Area under the curve |
| CPM | Counts per million |
| HIF-1α | Hypoxia-inducible factor 1 α |
| HSP90 | Heat shock protein 90 |
| TRAP1 | Tumor necrosis factor receptor-associated protein 1 |
| ROS | Reactive oxygen species |
References
- De Moerloose, B.; Reedijk, A.; de Bock, G.H.; Lammens, T.; de Haas, V.; Denys, B.; Dedeken, L.; van den Heuvel-Eibrink, M.M.; Te Loo, M.; Uyttebroeck, A.; et al. Response-Guided Chemotherapy for Pediatric Acute Myeloid Leukemia without Hematopoietic Stem Cell Transplantation in First Complete Remission: Results from Protocol DB AML-01. Pediatr. Blood Cancer 2019, 66, e27605. [Google Scholar] [CrossRef] [PubMed]
- Rasche, M.; Zimmermann, M.; Steidel, E.; Alonzo, T.; Aplenc, R.; Bourquin, J.-P.; Boztug, H.; Cooper, T.; Gamis, A.S.; Gerbing, R.B.; et al. Survival Following Relapse in Children with Acute Myeloid Leukemia: A Report from AML-BFM and COG. Cancers 2021, 13, 2336. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, S.; Yones, S.A.; Mayrhofer, M.; Norgren, N.; Skaftason, A.; Sun, J.; Smolinska, K.; Komorowski, J.; Herlin, M.K.; Sundström, C.; et al. Genomic Characterization of Relapsed Acute Myeloid Leukemia Reveals Novel Putative Therapeutic Targets. Blood Adv. 2021, 5, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Marchand, T.; Pinho, S. Leukemic Stem Cells: From Leukemic Niche Biology to Treatment Opportunities. Front. Immunol. 2021, 12, 775128. [Google Scholar] [CrossRef]
- Hansen, Q.; Bachas, C.; Smit, L.; Cloos, J. Characteristics of Leukemic Stem Cells in Acute Leukemia and Potential Targeted Therapies for Their Specific Eradication. Cancer Drug Resist. 2022, 5, 344–367. [Google Scholar] [CrossRef]
- Depreter, B.; Weening, K.E.; Vandepoele, K.; Essand, M.; De Moerloose, B.; Themeli, M.; Cloos, J.; Hanekamp, D.; Moors, I.; D’hont, I.; et al. TARP Is an Immunotherapeutic Target in Acute Myeloid Leukemia Expressed in the Leukemic Stem Cell Compartment. Haematologica 2020, 105, 1306–1316. [Google Scholar] [CrossRef]
- Majeti, R.; Becker, M.W.; Tian, Q.; Lee, T.-L.M.; Yan, X.; Liu, R.; Chiang, J.-H.; Hood, L.; Clarke, M.F.; Weissman, I.L. Dysregulated Gene Expression Networks in Human Acute Myelogenous Leukemia Stem Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3396–3401. [Google Scholar] [CrossRef]
- Patel, T.R.; Bernards, C.; Meier, M.; McEleney, K.; Winzor, D.J.; Koch, M.; Stetefeld, J. Structural Elucidation of Full-Length Nidogen and the Laminin–Nidogen Complex in Solution. Matrix Biol. 2014, 33, 60–67. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, S.; Pei, Y.A.; Pei, M. Nidogen: A Matrix Protein with Potential Roles in Musculoskeletal Tissue Regeneration. Genes Dis. 2021, 9, 598–609. [Google Scholar] [CrossRef]
- Rokavec, M.; Jaeckel, S.; Hermeking, H. Nidogen-1/NID1 Function and Regulation during Progression and Metastasis of Colorectal Cancer. Cancers 2023, 15, 5316. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Y.; Fan, X.; Zhang, C.; Wang, Y.; Zhang, L.; Zhang, H.; Wen, T.; Zhang, K.; Huo, X.; et al. NID1, a New Regulator of EMT Required for Metastasis and Chemoresistance of Ovarian Cancer Cells. Oncotarget 2017, 8, 33110–33121. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Tey, S.K.; Yeung, C.L.S.; Kwong, E.M.L.; Fung, Y.M.E.; Chung, C.Y.S.; Mak, L.-Y.; Wong, D.K.H.; Yuen, M.-F.; Ho, J.C.M.; et al. Nidogen 1-Enriched Extracellular Vesicles Facilitate Extrahepatic Metastasis of Liver Cancer by Activating Pulmonary Fibroblasts to Secrete Tumor Necrosis Factor Receptor 1. Adv. Sci. 2020, 7, 2002157. [Google Scholar] [CrossRef]
- Murphy, L.A.; Winters, A.C. Emerging and Future Targeted Therapies for Pediatric Acute Myeloid Leukemia: Targeting the Leukemia Stem Cells. Biomedicines 2023, 11, 3248. [Google Scholar] [CrossRef] [PubMed]
- Tierens, A.; Arad-Cohen, N.; Cheuk, D.; De Moerloose, B.; Fernandez Navarro, J.M.; Hasle, H.; Jahnukainen, K.; Juul-Dam, K.L.; Kaspers, G.; Kovalova, Z.; et al. Mitoxantrone Versus Liposomal Daunorubicin in Induction of Pediatric AML With Risk Stratification Based on Flow Cytometry Measurement of Residual Disease. J. Clin. Oncol. 2024, 42, 2174–2185. [Google Scholar] [CrossRef]
- Elsayed, A.H.; Rafiee, R.; Cao, X.; Raimondi, S.; Downing, J.R.; Ribeiro, R.; Fan, Y.; Gruber, T.A.; Baker, S.; Klco, J.; et al. A 6-Gene Leukemic Stem Cell Score Identifies High Risk Pediatric Acute Myeloid Leukemia. Leukemia 2020, 34, 735–745. [Google Scholar] [CrossRef]
- Ng, S.W.K.; Mitchell, A.; Kennedy, J.A.; Chen, W.C.; McLeod, J.; Ibrahimova, N.; Arruda, A.; Popescu, A.; Gupta, V.; Schimmer, A.D.; et al. A 17-Gene Stemness Score for Rapid Determination of Risk in Acute Leukaemia. Nature 2016, 540, 433–437. [Google Scholar] [CrossRef]
- Passegué, E.; Jamieson, C.H.M.; Ailles, L.E.; Weissman, I.L. Normal and Leukemic Hematopoiesis: Are Leukemias a Stem Cell Disorder or a Reacquisition of Stem Cell Characteristics? Proc. Natl. Acad. Sci. USA 2003, 100, 11842–11849. [Google Scholar] [CrossRef]
- Wesely, J.; Kotini, A.G.; Izzo, F.; Luo, H.; Yuan, H.; Sun, J.; Georgomanoli, M.; Zviran, A.; Deslauriers, A.G.; Dusaj, N.; et al. Acute Myeloid Leukemia iPSCs Reveal a Role for RUNX1 in the Maintenance of Human Leukemia Stem Cells. Cell Rep. 2020, 31, 107688. [Google Scholar] [CrossRef]
- Meyer, C.; Larghero, P.; Almeida Lopes, B.; Burmeister, T.; Gröger, D.; Sutton, R.; Venn, N.C.; Cazzaniga, G.; Corral Abascal, L.; Tsaur, G.; et al. The KMT2A Recombinome of Acute Leukemias in 2023. Leukemia 2023, 37, 988–1005. [Google Scholar] [CrossRef]
- Guarnera, L.; D’Addona, M.; Bravo-Perez, C.; Visconte, V. KMT2A Rearrangements in Leukemias: Molecular Aspects and Therapeutic Perspectives. Int. J. Mol. Sci. 2024, 25, 9023. [Google Scholar] [CrossRef]
- van Weelderen, R.E.; Harrison, C.J.; Klein, K.; Jiang, Y.; Abrahamsson, J.; Alonzo, T.; Aplenc, R.; Arad-Cohen, N.; Bart-Delabesse, E.; Buldini, B.; et al. Optimized Cytogenetic Risk-Group Stratification of KMT2A-Rearranged Pediatric Acute Myeloid Leukemia. Blood Adv. 2024, 8, 3200–3213. [Google Scholar] [CrossRef] [PubMed]
- Cabaud-Gibouin, V.; Durand, M.; Quéré, R.; Girodon, F.; Garrido, C.; Jego, G. Heat-Shock Proteins in Leukemia and Lymphoma: Multitargets for Innovative Therapeutic Approaches. Cancers 2023, 15, 984. [Google Scholar] [CrossRef] [PubMed]
- Magliulo, D.; Bernardi, R. Hypoxic Stress and Hypoxia-Inducible Factors in Leukemias. Front. Oncol. 2022, 12, 973978. [Google Scholar] [CrossRef]
- Velasco-Hernandez, T.; Trincado, J.L.; Vinyoles, M.; Closa, A.; Martínez-Moreno, A.; Gutiérrez-Agüera, F.; Molina, O.; Rodríguez-Cortez, V.C.; Ximeno-Parpal, P.; Fernández-Fuentes, N.; et al. Integrative Single-Cell Expression and Functional Studies Unravels a Sensitization to Cytarabine-Based Chemotherapy through HIF Pathway Inhibition in AML Leukemia Stem Cells. HemaSphere 2024, 8, e45. [Google Scholar] [CrossRef]
- Han, N.; Li, X.; Wang, Y.; Li, H.; Zhang, C.; Zhao, X.; Zhang, Z.; Ruan, M.; Zhang, C. HIF-1α Induced NID1 Expression Promotes Pulmonary Metastases via the PI3K-AKT Pathway in Salivary Gland Adenoid Cystic Carcinoma. Oral Oncol. 2022, 131, 105940. [Google Scholar] [CrossRef]
- Masgras, I.; Sanchez-Martin, C.; Colombo, G.; Rasola, A. The Chaperone TRAP1 As a Modulator of the Mitochondrial Adaptations in Cancer Cells. Front. Oncol. 2017, 7, 58. [Google Scholar] [CrossRef]
- Culp-Hill, R.; D’Alessandro, A.; Pietras, E.M. Extinguishing the Embers: Targeting AML Metabolism. Trends Mol. Med. 2021, 27, 332–344. [Google Scholar] [CrossRef]
- Moerloose, B.D. The Dutch-Belgian Pediatric Acute Myeloid Leukemia Protocol for Chil- Dren with Newly Diagnosed Acute Myeloid Leukemia (DB AML-01). Belg. J. Hematol. 2010, 1, 32–34. [Google Scholar]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Tsherniak, A.; Vazquez, F.; Montgomery, P.G.; Weir, B.A.; Kryukov, G.; Cowley, G.S.; Gill, S.; Harrington, W.F.; Pantel, S.; Krill-Burger, J.M.; et al. Defining a Cancer Dependency Map. Cell 2017, 170, 564–576.e16. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Pemovska, T.; Kontro, M.; Yadav, B.; Edgren, H.; Eldfors, S.; Szwajda, A.; Almusa, H.; Bespalov, M.M.; Ellonen, P.; Elonen, E.; et al. Individualized Systems Medicine Strategy to Tailor Treatments for Patients with Chemorefractory Acute Myeloid Leukemia. Cancer Discov. 2013, 3, 1416–1429. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Pemovska, T.; Szwajda, A.; Kulesskiy, E.; Kontro, M.; Karjalainen, R.; Majumder, M.M.; Malani, D.; Murumägi, A.; Knowles, J.; et al. Quantitative Scoring of Differential Drug Sensitivity for Individually Optimized Anticancer Therapies. Sci. Rep. 2014, 4, 5193. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanhooren, J.; Deneweth, L.; Pagliaro, L.; Ren, Z.; Giaimo, M.; Zamponi, R.; Roti, G.; Depreter, B.; Hofmans, M.; De Moerloose, B.; et al. Nidogen-1, a Player in KMT2A-Rearranged Pediatric Acute Myeloid Leukemia. Int. J. Mol. Sci. 2025, 26, 3011. https://doi.org/10.3390/ijms26073011
Vanhooren J, Deneweth L, Pagliaro L, Ren Z, Giaimo M, Zamponi R, Roti G, Depreter B, Hofmans M, De Moerloose B, et al. Nidogen-1, a Player in KMT2A-Rearranged Pediatric Acute Myeloid Leukemia. International Journal of Molecular Sciences. 2025; 26(7):3011. https://doi.org/10.3390/ijms26073011
Chicago/Turabian StyleVanhooren, Jolien, Larissa Deneweth, Luca Pagliaro, Zhiyao Ren, Mariateresa Giaimo, Rafaella Zamponi, Giovanni Roti, Barbara Depreter, Mattias Hofmans, Barbara De Moerloose, and et al. 2025. "Nidogen-1, a Player in KMT2A-Rearranged Pediatric Acute Myeloid Leukemia" International Journal of Molecular Sciences 26, no. 7: 3011. https://doi.org/10.3390/ijms26073011
APA StyleVanhooren, J., Deneweth, L., Pagliaro, L., Ren, Z., Giaimo, M., Zamponi, R., Roti, G., Depreter, B., Hofmans, M., De Moerloose, B., & Lammens, T. (2025). Nidogen-1, a Player in KMT2A-Rearranged Pediatric Acute Myeloid Leukemia. International Journal of Molecular Sciences, 26(7), 3011. https://doi.org/10.3390/ijms26073011





