PRMT7-Mediated PTEN Activation Enhances Bone Regeneration in Female Mice
Abstract
1. Introduction
2. Results
2.1. Prmt7 CKO Mice Exhibit Impaired Bone and Dental Structures
2.2. PRMT7 Regulates Osteogenic Differentiation in a Methyltransferase Activity-Dependent Manner
2.3. PRMT7 Activates PTEN in Female Mice
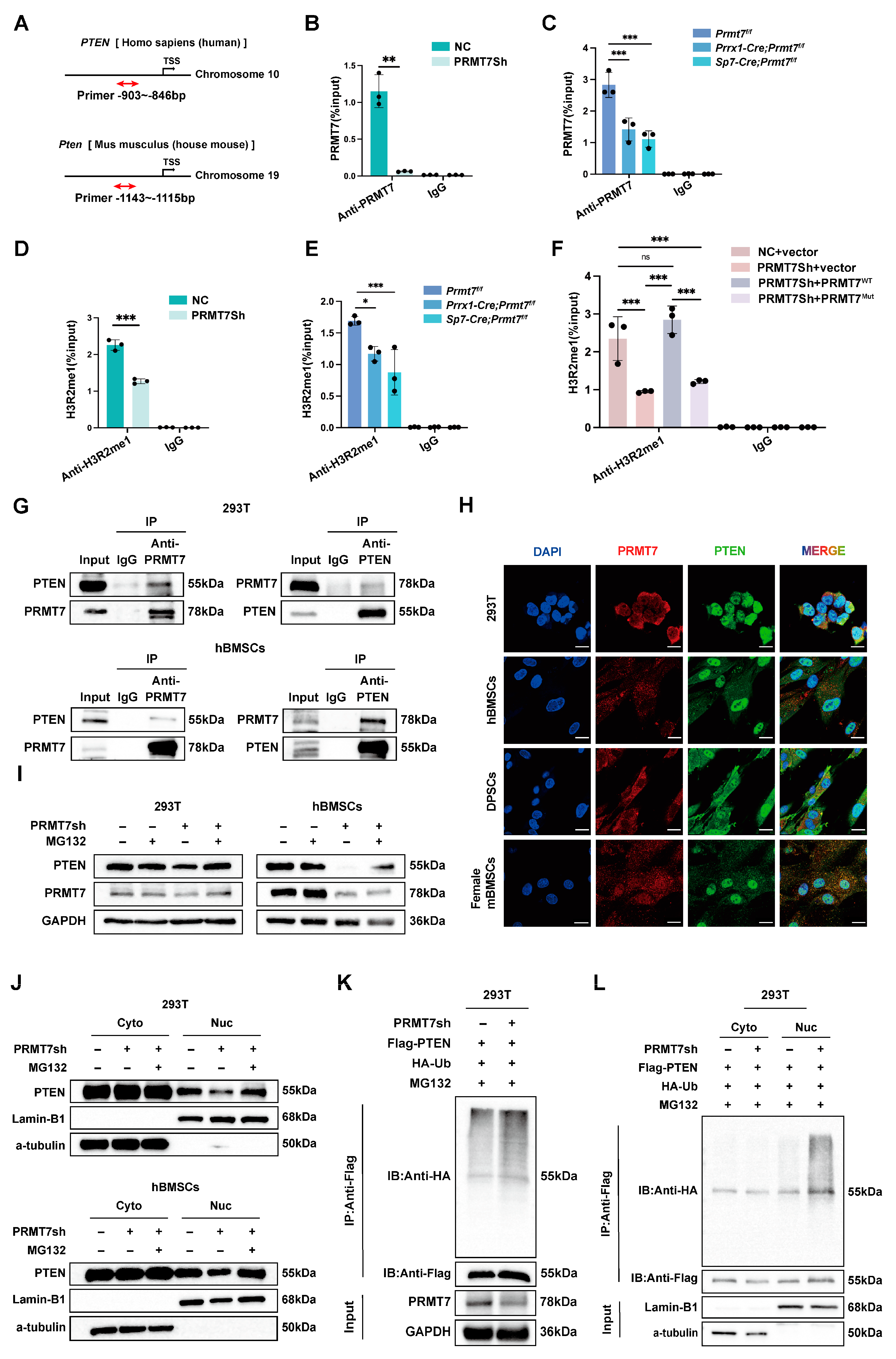
2.4. PRMT7 Regulates PTEN Stability and Transcriptional Activation
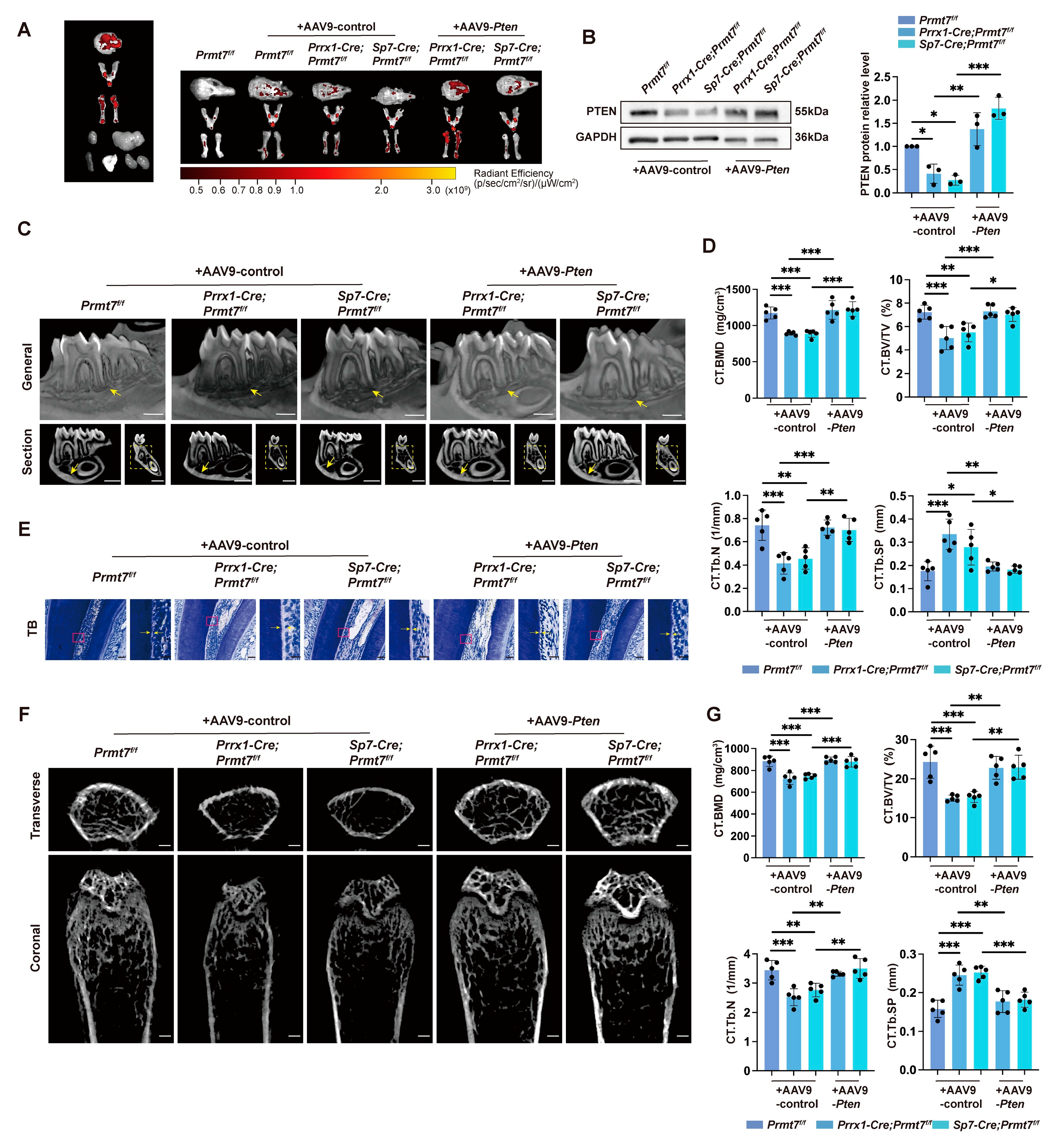
2.5. PTEN Rescued Bone Loss in Prmt7 CKO Mice
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Defect Models
4.3. Micro-CT and Quantitative Analysis
4.4. Whole-Body Skeleton Staining
4.5. Histological Staining
4.6. Immunofluorescence
4.7. Cell Extraction, Culture, and In Vitro Assays
4.8. Lentiviral Transduction and Plasmid, siRNA Transfection
4.9. Protein Extraction and Western Blot
4.10. RNA Extraction and qRT-PCR
4.11. RNA Sequencing and Analysis
4.12. ChIP-qPCR
4.13. Immunoprecipitation and In Vivo Ubiquitination Assay
4.14. Co-Localization Assay
4.15. Intravenous Injection and In Vivo Imaging
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montecino, M.; Carrasco, M.E.; Nardocci, G. Epigenetic Control of Osteogenic Lineage Commitment. Front. Cell Dev. Biol. 2020, 8, 611197. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Y.-H.; Li, L.-Y.; Lang, J.; Yeh, S.-P.; Shi, B.; Yang, C.-C.; Yang, J.-Y.; Lin, C.-Y.; Lai, C.-C.; et al. CDK1-Dependent Phosphorylation of EZH2 Suppresses Methylation of H3K27 and Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells. Nat. Cell Biol. 2011, 13, 87–94. [Google Scholar] [CrossRef]
- Bradley, E.W.; Carpio, L.R.; van Wijnen, A.J.; McGee-Lawrence, M.E.; Westendorf, J.J. Histone Deacetylases in Bone Development and Skeletal Disorders. Physiol. Rev. 2015, 95, 1359–1381. [Google Scholar] [CrossRef] [PubMed]
- Guccione, E.; Richard, S. The Regulation, Functions and Clinical Relevance of Arginine Methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Jang, A.-R.; Kim, D.; Park, M.-J.; Lim, S.-K.; Kim, M.-S.; Park, J.-H. PRMT1 Mediates RANKL-Induced Osteoclastogenesis and Contributes to Bone Loss in Ovariectomized Mice. Exp. Mol. Med. 2018, 50, 111. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Li, Z.; Du, Y.; Liu, X.; Lv, L.; Zhang, X.; Liu, Y.; Zhang, P.; Zhou, Y. Asymmetrical Methyltransferase PRMT3 Regulates Human Mesenchymal Stem Cell Osteogenesis via miR-3648. Cell Death Dis. 2019, 10, 581. [Google Scholar] [CrossRef]
- Li, J.-Y.; Wang, T.-T.; Ma, L.; Zheng, L.-L. CARM1 Deficiency Inhibits Osteoblastic Differentiation of Bone Marrow Mesenchymal Stem Cells and Delays Osteogenesis in Mice. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119544. [Google Scholar] [CrossRef]
- Kota, S.K.; Roening, C.; Patel, N.; Kota, S.B.; Baron, R. PRMT5 Inhibition Promotes Osteogenic Differentiation of Mesenchymal Stromal Cells and Represses Basal Interferon Stimulated Gene Expression. Bone 2018, 117, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Song, C.; Wang, Y.; Lei, Z.; Xu, F.; Guan, H.; Chen, A.; Li, F. Inhibition of PRMT5 Suppresses Osteoclast Differentiation and Partially Protects against Ovariectomy-Induced Bone Loss through Downregulation of CXCL10 and RSAD2. Cell. Signal. 2017, 34, 55–65. [Google Scholar] [CrossRef]
- Li, Z.; Wang, P.; Li, J.; Xie, Z.; Cen, S.; Li, M.; Liu, W.; Ye, G.; Zheng, G.; Ma, M.; et al. The N6-Methyladenosine Demethylase ALKBH5 Negatively Regulates the Osteogenic Differentiation of Mesenchymal Stem Cells through PRMT6. Cell Death Dis. 2021, 12, 578. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Chen, M.; Pandolfi, P.P. The Functions and Regulation of the PTEN Tumour Suppressor: New Modes and Prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-C.; Lai, Y.-C.; Li, L.-H.; Liao, K.; Lai, H.-C.; Kao, S.-Y.; Wang, J.; Chuong, C.-M.; Hung, S.-C. Methylation and PTEN Activation in Dental Pulp Mesenchymal Stem Cells Promotes Osteogenesis and Reduces Oncogenesis. Nat. Commun. 2019, 10, 2226. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.; Morrison, S.J. Leptin Receptor-Expressing Mesenchymal Stromal Cells Represent the Main Source of Bone Formed by Adult Bone Marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Nowwarote, N.; Osathanon, T.; Fournier, B.P.J.; Theerapanon, T.; Yodsanga, S.; Kamolratanakul, P.; Porntaveetus, T.; Shotelersuk, V. PTEN Regulates Proliferation and Osteogenesis of Dental Pulp Cells and Adipogenesis of Human Adipose-derived Stem Cells. Oral Dis. 2023, 29, 735–746. [Google Scholar] [CrossRef]
- Fenton, T.R.; Nathanson, D.; Ponte de Albuquerque, C.; Kuga, D.; Iwanami, A.; Dang, J.; Yang, H.; Tanaka, K.; Oba-Shinjo, S.M.; Uno, M.; et al. Resistance to EGF Receptor Inhibitors in Glioblastoma Mediated by Phosphorylation of the PTEN Tumor Suppressor at Tyrosine 240. Proc. Natl. Acad. Sci. USA 2012, 109, 14164–14169. [Google Scholar] [CrossRef]
- Meng, Z.; Jia, L.-F.; Gan, Y.-H. PTEN Activation through K163 Acetylation by Inhibiting HDAC6 Contributes to Tumour Inhibition. Oncogene 2016, 35, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Dang, Y.; Zhang, W.; Zhao, X.; Zhang, C.; Hou, Z.; Jin, Y.; McNutt, M.A.; Marks, A.R.; Yin, Y. PTEN Arginine Methylation by PRMT6 Suppresses PI3K-AKT Signaling and Modulates Pre-mRNA Splicing. Proc. Natl. Acad. Sci. USA 2019, 116, 6868–6877. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Chen, M.; Lee, J.D.; Zhang, J.; Lin, S.-Y.; Fu, T.-M.; Chen, H.; Ishikawa, T.; Chiang, S.-Y.; Katon, J.; et al. Reactivation of PTEN Tumor Suppressor for Cancer Treatment through Inhibition of a MYC-WWP1 Inhibitory Pathway. Science 2019, 364, eaau0159. [Google Scholar] [CrossRef]
- Li, W.-J.; He, Y.-H.; Yang, J.-J.; Hu, G.-S.; Lin, Y.-A.; Ran, T.; Peng, B.-L.; Xie, B.-L.; Huang, M.-F.; Gao, X.; et al. Profiling PRMT Methylome Reveals Roles of hnRNPA1 Arginine Methylation in RNA Splicing and Cell Growth. Nat. Commun. 2021, 12, 1946. [Google Scholar] [CrossRef]
- Günes Günsel, G.; Conlon, T.M.; Jeridi, A.; Kim, R.; Ertüz, Z.; Lang, N.J.; Ansari, M.; Novikova, M.; Jiang, D.; Strunz, M.; et al. The Arginine Methyltransferase PRMT7 Promotes Extravasation of Monocytes Resulting in Tissue Injury in COPD. Nat. Commun. 2022, 13, 1303. [Google Scholar] [CrossRef]
- Kirmizis, A.; Santos-Rosa, H.; Penkett, C.J.; Singer, M.A.; Green, R.D.; Kouzarides, T. Distinct Transcriptional Outputs Associated with Mono- and Di-Methylated Histone H3 Arginine 2. Nat. Struct. Mol. Biol. 2009, 16, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Maity, R.; Whitelegge, J.P.; Hadjikyriacou, A.; Li, Z.; Zurita-Lopez, C.; Al-Hadid, Q.; Clark, A.T.; Bedford, M.T.; Masson, J.-Y.; et al. Mammalian Protein Arginine Methyltransferase 7 (PRMT7) Specifically Targets RXR Sites in Lysine- and Arginine-Rich Regions. J. Biol. Chem. 2013, 288, 37010–37025. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.T.; Clarke, S.G. Protein Arginine Methylation in Mammals: Who, What, and Why. Mol. Cell 2009, 33, 1–13. [Google Scholar] [CrossRef]
- Migliori, V.; Müller, J.; Phalke, S.; Low, D.; Bezzi, M.; Mok, W.C.; Sahu, S.K.; Gunaratne, J.; Capasso, P.; Bassi, C.; et al. Symmetric Dimethylation of H3R2 Is a Newly Identified Histone Mark That Supports Euchromatin Maintenance. Nat. Struct. Mol. Biol. 2012, 19, 136–144. [Google Scholar] [CrossRef]
- Feng, Y.; Hadjikyriacou, A.; Clarke, S.G. Substrate Specificity of Human Protein Arginine Methyltransferase 7 (PRMT7): The Importance of Acidic Residues in the Double E Loop. J. Biol. Chem. 2014, 289, 32604–32616. [Google Scholar] [CrossRef]
- Jain, K.; Jin, C.Y.; Clarke, S.G. Epigenetic Control via Allosteric Regulation of Mammalian Protein Arginine Methyltransferases. Proc. Natl. Acad. Sci. USA 2017, 114, 10101–10106. [Google Scholar] [CrossRef]
- Kirmizis, A.; Santos-Rosa, H.; Penkett, C.J.; Singer, M.A.; Vermeulen, M.; Mann, M.; Bähler, J.; Green, R.D.; Kouzarides, T. Arginine Methylation at Histone H3R2 Controls Deposition of H3K4 Trimethylation. Nature 2007, 449, 928–932. [Google Scholar] [CrossRef]
- Guccione, E.; Bassi, C.; Casadio, F.; Martinato, F.; Cesaroni, M.; Schuchlautz, H.; Lüscher, B.; Amati, B. Methylation of Histone H3R2 by PRMT6 and H3K4 by an MLL Complex Are Mutually Exclusive. Nature 2007, 449, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Hyllus, D.; Stein, C.; Schnabel, K.; Schiltz, E.; Imhof, A.; Dou, Y.; Hsieh, J.; Bauer, U.-M. PRMT6-Mediated Methylation of R2 in Histone H3 Antagonizes H3 K4 Trimethylation. Genes Dev. 2007, 21, 3369–3380. [Google Scholar] [CrossRef]
- Banasavadi-Siddegowda, Y.K.; Russell, L.; Frair, E.; Karkhanis, V.A.; Relation, T.; Yoo, J.Y.; Zhang, J.; Sif, S.; Imitola, J.; Baiocchi, R.; et al. PRMT5-PTEN Molecular Pathway Regulates Senescence and Self-Renewal of Primary Glioblastoma Neurosphere Cells. Oncogene 2017, 36, 263–274. [Google Scholar] [CrossRef]
- Lindsay, Y.; McCoull, D.; Davidson, L.; Leslie, N.R.; Fairservice, A.; Gray, A.; Lucocq, J.; Downes, C.P. Localization of Agonist-Sensitive PtdIns(3,4,5)P3 Reveals a Nuclear Pool That Is Insensitive to PTEN Expression. J. Cell Sci. 2006, 119, 5160–5168. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.H.; Balajee, A.S.; Wang, J.; Wu, H.; Eng, C.; Pandolfi, P.P.; Yin, Y. Essential Role for Nuclear PTEN in Maintaining Chromosomal Integrity. Cell 2007, 128, 157–170. [Google Scholar] [CrossRef]
- Song, M.S.; Carracedo, A.; Salmena, L.; Song, S.J.; Egia, A.; Malumbres, M.; Pandolfi, P.P. Nuclear PTEN Regulates the APC-CDH1 Tumor Suppressive Complex in a Phosphatase-Independent Manner. Cell 2011, 144, 187–199. [Google Scholar] [CrossRef]
- Wang, X.; Trotman, L.; Koppie, T.; Alimonti, A.; Gao, Z.; Wang, J.; Erdjument-Bromage, H.; Tempst, P.; Cordon-Cardo, C.; Pandolfi, P.P.; et al. NEDD4-1 Is a Proto-Oncogenic Ubiquitin Ligase for PTEN. Cell 2007, 128, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Maddika, S.; Kavela, S.; Rani, N.; Palicharla, V.R.; Pokorny, J.L.; Sarkaria, J.N.; Chen, J. WWP2 Is an E3 Ubiquitin Ligase for PTEN. Nat. Cell Biol. 2011, 13, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.-K.; Zhang, N.; Xia, L.; Zhang, C.; Dong, S.-S.; Li, Z.-M.; Ji, Y.; Zheng, M.-H.; Sun, J.; Chen, G.-Q.; et al. FBXO22 Degrades Nuclear PTEN to Promote Tumorigenesis. Nat. Commun. 2020, 11, 1720. [Google Scholar] [CrossRef]
- Trotman, L.C.; Wang, X.; Alimonti, A.; Chen, Z.; Teruya-Feldstein, J.; Yang, H.; Pavletich, N.P.; Carver, B.S.; Cordon-Cardo, C.; Erdjument-Bromage, H.; et al. Ubiquitination Regulates PTEN Nuclear Import and Tumor Suppression. Cell 2007, 128, 141–156. [Google Scholar] [CrossRef]
- Yang, X.; Schadt, E.E.; Wang, S.; Wang, H.; Arnold, A.P.; Ingram-Drake, L.; Drake, T.A.; Lusis, A.J. Tissue-Specific Expression and Regulation of Sexually Dimorphic Genes in Mice. Genome Res. 2006, 16, 995–1004. [Google Scholar] [CrossRef]
- Melé, M.; Ferreira, P.G.; Reverter, F.; DeLuca, D.S.; Monlong, J.; Sammeth, M.; Young, T.R.; Goldmann, J.M.; Pervouchine, D.D.; Sullivan, T.J.; et al. Human Genomics. The Human Transcriptome across Tissues and Individuals. Science 2015, 348, 660–665. [Google Scholar] [CrossRef]
- Naqvi, S.; Godfrey, A.K.; Hughes, J.F.; Goodheart, M.L.; Mitchell, R.N.; Page, D.C. Conservation, Acquisition, and Functional Impact of Sex-Biased Gene Expression in Mammals. Science 2019, 365, eaaw7317. [Google Scholar] [CrossRef]
- Zhang, L.; Reidy, S.P.; Nicholson, T.E.; Lee, H.-J.; Majdalawieh, A.; Webber, C.; Stewart, B.R.; Dolphin, P.; Ro, H.-S. The Role of AEBP1 in Sex-Specific Diet-Induced Obesity. Mol. Med. 2005, 11, 39–47. [Google Scholar] [CrossRef]
- Metcalfe, M.; Steward, O. PTEN Deletion in Spinal Pathways via Retrograde Transduction with AAV-RG Enhances Forelimb Motor Recovery after Cervical Spinal Cord Injury; Sex Differences and Late-Onset Pathophysiologies. Exp. Neurol. 2023, 370, 114551. [Google Scholar] [CrossRef] [PubMed]
- Antico-Arciuch, V.G.; Dima, M.; Liao, X.-H.; Refetoff, S.; Di Cristofano, A. Cross-Talk between PI3K and Estrogen in the Mouse Thyroid Predisposes to the Development of Follicular Carcinomas with a Higher Incidence in Females. Oncogene 2010, 29, 5678–5686. [Google Scholar] [CrossRef] [PubMed]
- Anezaki, Y.; Ohshima, S.; Ishii, H.; Kinoshita, N.; Dohmen, T.; Kataoka, E.; Sato, W.; Iizuka, M.; Goto, T.; Sasaki, J.; et al. Sex Difference in the Liver of Hepatocyte-Specific Pten-Deficient Mice: A Model of Nonalcoholic Steatohepatitis. Hepatol. Res. 2009, 39, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, M.; Liu, W.; Chen, H.; Cai, T.; Xiong, H.; Sheng, X.; Liu, S.; Peng, J.; Wang, F.; et al. Estrogen Affects the Negative Feedback Loop of PTENP1-miR200c to Inhibit PTEN Expression in the Development of Endometrioid Endometrial Carcinoma. Cell Death Dis. 2018, 10, 4. [Google Scholar] [CrossRef]
- Vilgelm, A.; Lian, Z.; Wang, H.; Beauparlant, S.L.; Klein-Szanto, A.; Ellenson, L.H.; Di Cristofano, A. Akt-Mediated Phosphorylation and Activation of Estrogen Receptor Alpha Is Required for Endometrial Neoplastic Transformation in Pten+/- Mice. Cancer Res. 2006, 66, 3375–3380. [Google Scholar] [CrossRef]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qing, J.; Li, Y.; Gao, X.; Lu, D.; Wang, Y.; Gu, L.; Zhang, H.; Li, Z.; Wang, X.; et al. PRMT7-Mediated PTEN Activation Enhances Bone Regeneration in Female Mice. Int. J. Mol. Sci. 2025, 26, 2981. https://doi.org/10.3390/ijms26072981
Zhang Y, Qing J, Li Y, Gao X, Lu D, Wang Y, Gu L, Zhang H, Li Z, Wang X, et al. PRMT7-Mediated PTEN Activation Enhances Bone Regeneration in Female Mice. International Journal of Molecular Sciences. 2025; 26(7):2981. https://doi.org/10.3390/ijms26072981
Chicago/Turabian StyleZhang, Yingfei, Jia Qing, Yang Li, Xin Gao, Dazhuang Lu, Yiyang Wang, Lanxin Gu, Hui Zhang, Zechuan Li, Xu Wang, and et al. 2025. "PRMT7-Mediated PTEN Activation Enhances Bone Regeneration in Female Mice" International Journal of Molecular Sciences 26, no. 7: 2981. https://doi.org/10.3390/ijms26072981
APA StyleZhang, Y., Qing, J., Li, Y., Gao, X., Lu, D., Wang, Y., Gu, L., Zhang, H., Li, Z., Wang, X., Zhou, Y., & Zhang, P. (2025). PRMT7-Mediated PTEN Activation Enhances Bone Regeneration in Female Mice. International Journal of Molecular Sciences, 26(7), 2981. https://doi.org/10.3390/ijms26072981





