Increased Preeclampsia Risk in GDM Pregnancies: The Role of SIRT1 rs12778366 Polymorphism and Telomere Length
Abstract
1. Introduction
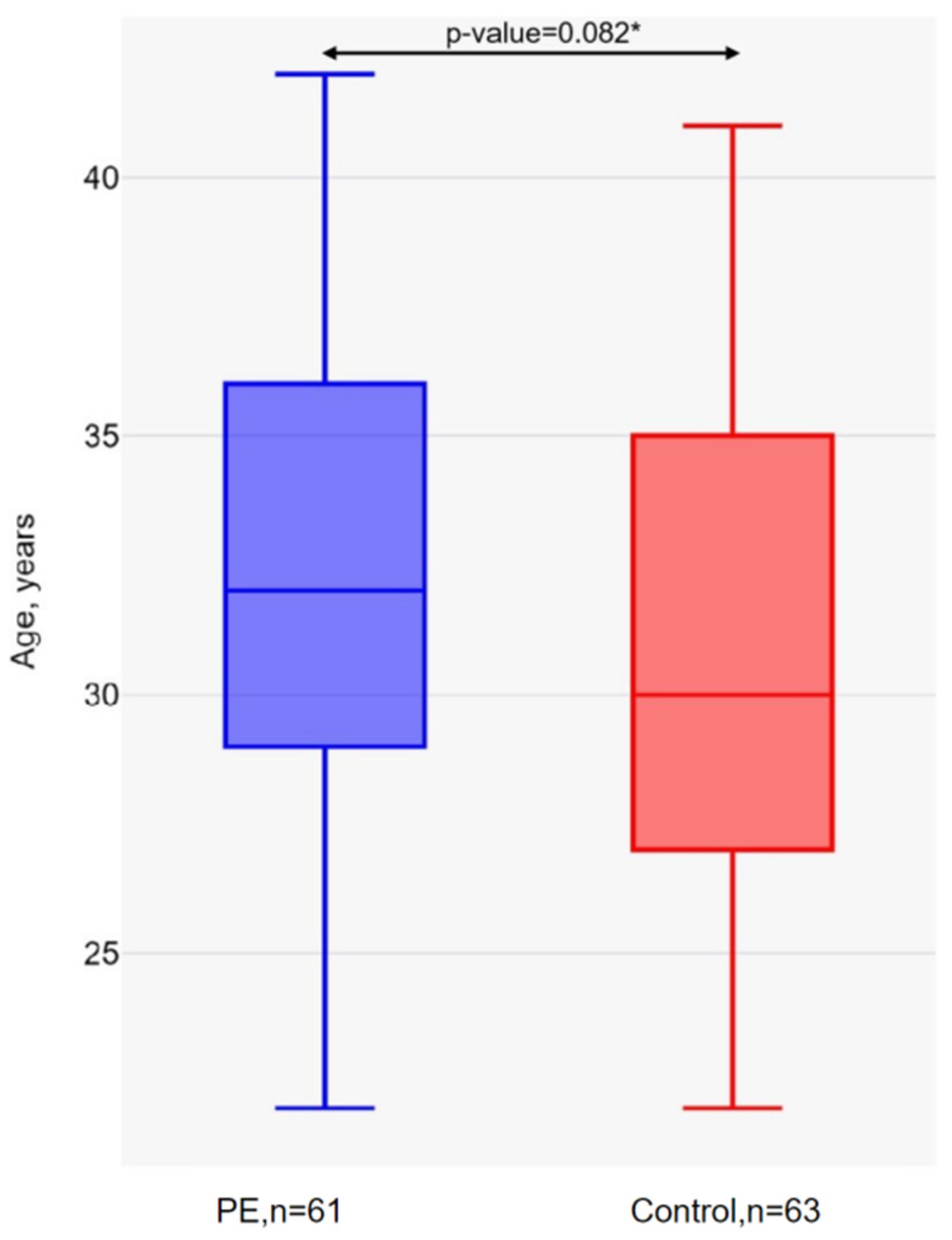
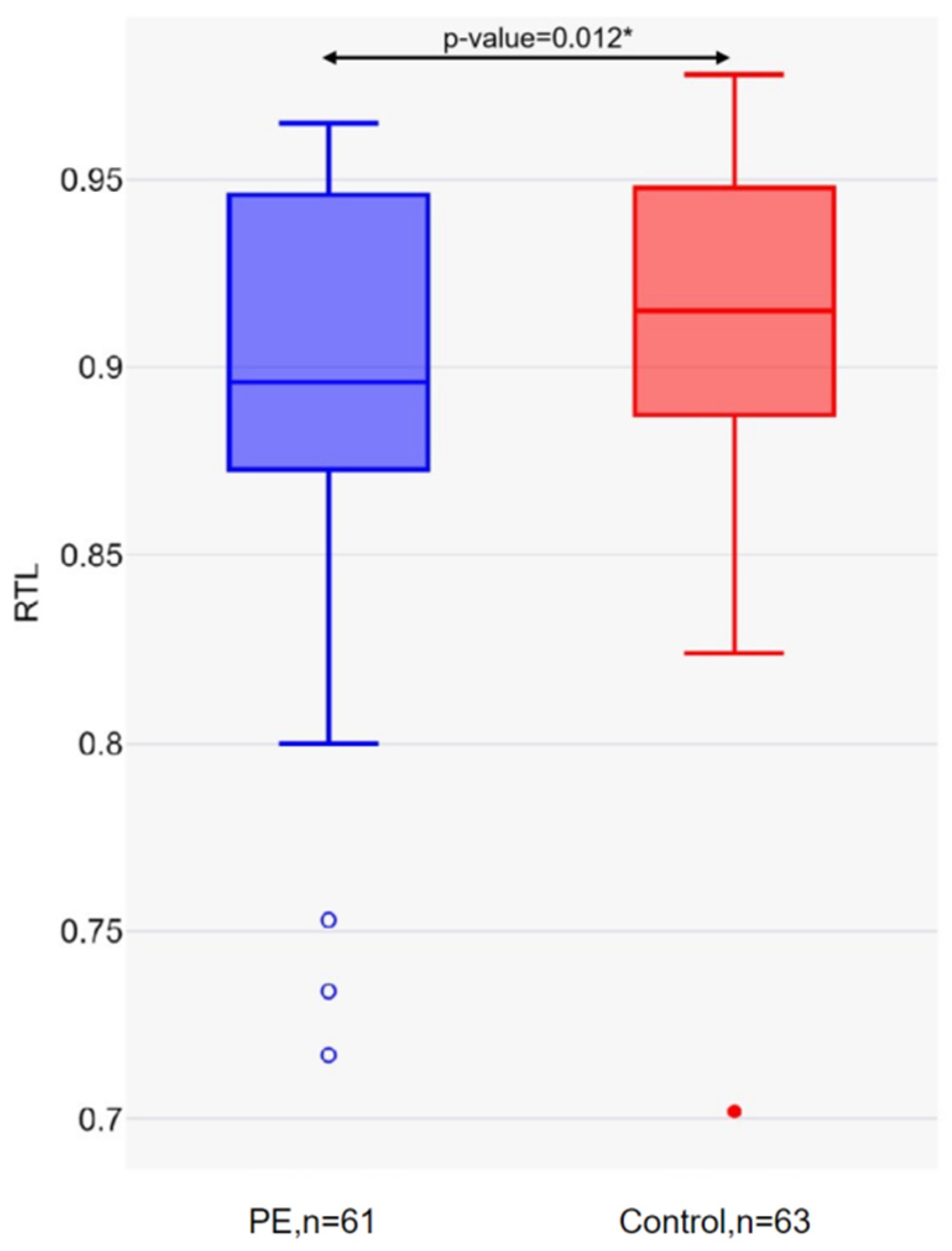
2. Results
3. Discussion
4. Materials and Methods
4.1. Genomic DNA Extraction
4.2. The Genotyping of rs12778366 and rs7895833 Polymorphisms in the SIRT1 Gene
4.3. Relative Telomere Length Assessment Using qPCR
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike information criterion |
| AMD | Age-related macular degeneration |
| CIs | Confidence interval |
| GDM | Gestational diabetes mellitus |
| HWE | Hardy–Weinberg equilibrium |
| IADPSG | International Association of the Diabetes and Pregnancy Study Groups |
| MAF | Minor allele frequency |
| OR | Odds ratio |
| PE | Preeclampsia |
| RLT | Relative telomere length |
| RT-PCR | Real-time polymerase chain reaction |
| SCG | Single copy gene |
| SNPs | Single nucleotide polymorphism |
| T2DM | Type 2 diabetes mellitus |
References
- Schneider, S.; Freerksen, N.; Röhrig, S.; Hoeft, B.; Maul, H. Gestational diabetes and preeclampsia--similar risk factor profiles? Early Hum. Dev. 2012, 88, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.L.; Mudd, L.M. Preeclampsia and diabetes. Curr. Diabetes Rep. 2015, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, I.; Haglund, B.; Hanson, U. Gestational diabetes and preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113, 12–16. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41 (Suppl. S1), S13–S27. [Google Scholar] [CrossRef]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef]
- Bilano, V.L.; Ota, E.; Ganchimeg, T.; Mori, R.; Souza, J.P. Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: A WHO secondary analysis. PLoS ONE 2014, 9, e91198. [Google Scholar] [CrossRef]
- Nerenberg, K.A.; Johnson, J.A.; Leung, B.; Savu, A.; Ryan, E.A.; Chik, C.L.; Kaul, P. Risks of gestational diabetes and preeclampsia over the last decade in a cohort of Alberta women. J. Obstet. Gynaecol. Can. 2013, 35, 986–994. [Google Scholar] [CrossRef]
- HAPO Study Cooperative Research Group. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with maternal body mass index. BJOG 2010, 117, 575–584. [Google Scholar] [CrossRef]
- Dedov, I.I.; Shestakova, M.V.; Suntsov Yu, I. Diabetes in Russia: Problems and solutions. In Diabetes Atlas; IDF: Brussels, Belgium; Moscow, Russia, 2008; pp. 3–6. (In Russian) [Google Scholar]
- Huet, J.; Beucher, G.; Rod, A.; Morello, R.; Dreyfus, M. Joint impact of gestational diabetes and obesity on perinatal outcomes. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 469–476. [Google Scholar] [CrossRef]
- Russian Clinical Guidelines (2021) Preeclampsia: Eclampsia: Edema, Proteinuria and Hypertensive Disorders During Pregnancy, Childbirth and the Postpartum Period; Russian Society of Obstetricians and Gynecologists and the Association of Obstetric Anesthesiologists and Intensivists: Moscow, Russia, 2021. (In Russian)
- Eiland, E.; Nzerue, C.; Faulkner Faulkner, M. Preeclampsia 2012. J. Pregnancy 2012, 2012, 586578. [Google Scholar]
- Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Harville, E.W.; Williams, M.A.; Qiu, C.F.; Mejia, J.; Risques, R.A. Telomere length, pre-eclampsia, and gestational diabetes. BMC Res. Notes 2010, 3, 113. [Google Scholar] [CrossRef] [PubMed]
- Scaife, P.J.; Simpson, A.; Kurlak, L.O.; Briggs, L.V.; Gardner, D.S.; Pipkin, F.B.; Jones, C.J.P.; Mistry, H.D. Increased Placental Cell Senescence and Oxidative Stress in Women with Pre-Eclampsia and Normotensive Post-Term Pregnancies. Int. J. Mol. Sci. 2021, 22, 7295. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.J.; Spicer, S.S. Ultrastructural features of cellular maturation and aging in human trophoblast. J. Ultrastruct. Res. 1973, 43, 133–149. [Google Scholar] [CrossRef]
- Burstein, R.; Frankel, S.; Soule, S.D.; Blumenthal, H.T. Aging of the placenta: Autoimmune theory of senescence. Am. J. Obstet. Gynecol. 1973, 116, 271–276. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Fogarty, N.M.E.; Jones, C.J.P.; Kingdom, J.; Burton, G.J. Evidence of oxidative stress-induced senescence in mature, post-mature and pathological human placentas. Placenta 2018, 68, 15–22. [Google Scholar] [CrossRef]
- Victorelli, S.; Passos, J.F. Telomeres and Cell Senescence—Size Matters Not. eBioMedicine 2017, 21, 14–20. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Armanios, M.; Blackburn, E.H. The telomere syndromes. Nat. Rev. Genet. 2012, 13, 693–704. [Google Scholar] [CrossRef]
- Nakashima, H.; Ozono, R.; Suyama, C.; Sueda, T.; Kambe, M.; Oshima, T. Telomere attrition in white blood cell correlating with cardiovascular damage. Hypertens. Res. 2004, 27, 319–325. [Google Scholar] [CrossRef]
- Hemminki, K.; Försti, A.; Lorenzo Bermejo, J. Single nucleotide polymorphisms (SNPs) are inherited from parents and they measure heritable events. J. Carcinog. 2005, 4, 2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Codd, V.; Mangino, M.; van der Harst, P.; Braund, P.S.; Kaiser, M.; Beveridge, A.J.; Rafelt, S.; Moore, J.; Nelson, C.; Soranzo, N.; et al. Common variants near TERC are associated with mean telomere length. Nat. Genet. 2010, 42, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.; Reichwald, K.; Lechel, A.; Graf, M.; Kirschner, J.; Dorn, A.; Terzibasi, E.; Wellner, J.; Platzer, M.; Rudolph, K.L.; et al. Telomeres shorten while Tert expression increases during ageing of the short-lived fish Nothobranchius furzeri. Mech. Ageing Dev. 2009, 130, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Mangino, M.; Hwang, S.J.; Spector, T.D.; Hunt, S.C.; Kimura, M.; Fitzpatrick, A.L.; Christiansen, L.; Petersen, I.; Elbers, C.C.; Harris, T.; et al. Genome-wide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Hum. Mol. Genet. 2012, 21, 5385–5394. [Google Scholar] [CrossRef]
- Protsenko, E.; Rehkopf, D.; Prather, A.A.; Epel, E.; Lin, J. Are long telomeres better than short? Relative contributions of genetically predicted telomere length to neoplastic and non-neoplastic disease risk and population health burden. PLoS ONE 2020, 15, e0240185. [Google Scholar] [CrossRef]
- Kim, S.; Bi, X.; Czarny-Ratajczak, M.; Dai, J.; Welsh, D.A.; Myers, L.; Welsch, M.A.; Cherry, K.E.; Arnold, J.; Poon, L.W.; et al. Telomere maintenance genes SIRT1 and XRCC6 impact age-related decline in telomere length but only SIRT1 is associated with human longevity. Biogerontology 2012, 13, 119–131. [Google Scholar] [CrossRef]
- Osum, M.; Serakinci, N. Impact of circadian disruption on health; SIRT1 and Telomeres. DNA Repair. 2020, 96, 102993. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Lee, H.Y.; Min, K.J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef]
- McReynolds, M.R.; Chellappa, K.; Baur, J.A. Age-related NAD+ decline. Exp. Gerontol. 2020, 134, 110888. [Google Scholar] [CrossRef]
- Pieters, N.; Janssen, B.G.; Valeri, L.; Cox, B.; Cuypers, A.; Dewitte, H.; Plusquin, M.; Smeets, K.; Nawrot, T.S. Molecular responses in the telomere-mitochondrial axis of ageing in the elderly: A candidate gene approach. Mech. Ageing Dev. 2015, 145, 51–57. [Google Scholar] [CrossRef]
- Amano, H.; Chaudhury, A.; Rodriguez-Aguayo, C.; Lu, L.; Akhanov, V.; Catic, A.; Popov, Y.V.; Verdin, E.; Johnson, H.; Stossi, F.; et al. Telomere Dysfunction Induces Sirtuin Repression that Drives Telomere-Dependent Disease. Cell Metab. 2019, 29, 1274–1290.e9. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Mitton, A.; Lim, R.; Barker, G.; Riley, C.; Permezel, M. SIRT1 is a novel regulator of key pathways of human labor. Biol. Reprod. 2011, 84, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.S.; Zhao, H.; Hattis, S.; Kumar, S. Elevated Glucose and Insulin Levels Decrease DHA Transfer across Human Trophoblasts via SIRT1-Dependent Mechanism. Nutrients 2020, 12, 1271. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.; Eastwood, K.A.; Jerotic, D.; Todd, N.; Hoch, D.; McNally, R.; Obradovic, D.; Dugalic, S.; Hunter, A.J.; Holmes, V.A.; et al. FKBPL and SIRT-1 Are Downregulated by Diabetes in Pregnancy Impacting on Angiogenesis and Endothelial Function. Front. Endocrinol. 2021, 12, 650328. [Google Scholar] [CrossRef]
- Deihimi, T.; Niazi, A.; Ebrahimi, M.; Kajbaf, K.; Fanaee, S.; Bakhtiarizadeh, M.R.; Ebrahimie, E. Finding the undiscovered roles of genes: An approach using mutual ranking of coexpressed genes and promoter architecture-case study: Dual roles of thaumatin like proteins in biotic and abiotic stresses. Springerplus 2012, 1, 30. [Google Scholar] [CrossRef]
- Dmitrenko, O.P.; Karpova, N.S.; Abramova, O.I.; Nurbekov, M.K.; Arshinova, E.S. Association of Polymorphism rs12778366 of the SIRT1 Gene with the Risk of Age-Related Macular Degeneration. J. Ophthalmic. Clin. Res. 2022, 9, 097. [Google Scholar]
- Chen, Z.; Zhai, Y.; Zhang, W.; Teng, Y.; Yao, K. Single Nucleotide Polymorphisms of the Sirtuin 1 (SIRT1) Gene are Associated with Age-Related Macular Degeneration in Chinese Han Individuals: A Case-Control Pilot Study. Medicine 2015, 94, e2238. [Google Scholar] [CrossRef]
- Gambino, R.; Fanni, G.; Togliatto, G.; Ponzo, V.; Goitre, I.; Cassader, M.; Brizzi, M.F.; Bo, S. Rs12778366 single nucleotide polymorphism of Sirtuin 1 (SIRT1) and response to resveratrol supplementation in patients with type 2 diabetes mellitus. Acta Diabetol. 2019, 56, 963–966. [Google Scholar] [CrossRef]
- Zillikens, M.C.; van Meurs, J.B.; Rivadeneira, F.; Amin, N.; Hofman, A.; Oostra, B.A.; Sijbrands, E.J.; Witteman, J.C.; Pols, H.A.; van Duijn, C.M.; et al. SIRT1 genetic variation is related to BMI and risk of obesity. Diabetes 2009, 58, 2828–2834. [Google Scholar] [CrossRef]
- Botden, I.P.; Zillikens, M.C.; de Rooij, S.R.; Langendonk, J.G.; Danser, A.J.; Sijbrands, E.J.; Roseboom, T.J. Variants in the SIRT1 gene may affect diabetes risk in interaction with prenatal exposure to famine. Diabetes Care 2012, 35, 424–426. [Google Scholar] [CrossRef]
- Shimoyama, Y.; Suzuki, K.; Hamajima, N.; Niwa, T. Sirtuin 1 gene polymorphisms are associated with body fat and blood pressure in Japanese. Transl. Res. 2011, 157, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Dmitrenko, O.P.; Karpova, N.S.; Nurbekov, M.K. Association of Polymorphisms rs1801282 of the PPARG Gene, rs8192678 of the PPARGC1A Gene and rs7895833 of the SIRT1 Gene with the Risk of Preeclampsia in Pregnant Women with Gestational Diabetes in the Russian Population. J. Genet. Genom. Sci. 2022, 7, 2. [Google Scholar]
- Hosking, L.; Lumsden, S.; Lewis, K.; Yeo, A.; McCarthy, L.; Bansal, A.; Riley, J.; Purvis, I.; Xu, C.-F. Detection of genotyping errors by Hardy-Weinberg equilibrium testing. Eur. J. Hum. Genet. 2004, 12, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Salanti, G.; Amountza, G.; Ntzani, E.E.; Ioannidis, J.P. Hardy-Weinberg equilibrium in genetic association studies: An empirical evaluation of reporting, deviations, and power. Eur. J. Hum. Genet. 2005, 13, 840–848. [Google Scholar] [CrossRef]
- Powe, C.E.; Levine, R.J.; Karumanchi, S.A. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011, 123, 2856–2869. [Google Scholar] [CrossRef]
- Brennan, L.J.; Morton, J.S.; Davidge, S.T. Vascular dysfunction in preeclampsia. Microcirculation 2014, 21, 4–14. [Google Scholar] [CrossRef]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2020, 10, 1568. [Google Scholar] [CrossRef]
- Guimarães, M.F.; Brandão, A.H.; Rezende, C.A.; Cabral, A.C.V.; Brum, A.P.; Leite, H.V.; Capuruço, C.A.B. Assessment of endothelial function in pregnant women with preeclampsia and gestational diabetes mellitus by flow-mediated dilation of brachial artery. Arch. Gynecol. Obstet. 2014, 290, 441–447. [Google Scholar] [CrossRef]
- Pănuş, C.; Moţa, M.; Vladu, D.; Vanghelie, L.; Răducanu, C.L. The endothelial dysfunction in diabetes mellitus. Rom. J. Intern. Med. 2003, 41, 27–33. [Google Scholar]
- Kul, Ş.; Güvenç, T.S.; Baycan, Ö.F.; Çelik, F.B.; Çalışkan, Z.; Güvenç, R.Ç.; Çiftçi, F.C.; Caliskan, M. Combined past preeclampsia and gestational diabetes is associated with a very high frequency of coronary microvascular dysfunction. Microvasc. Res. 2021, 134, 104104. [Google Scholar] [CrossRef]
- Yeh, J.K.; Wang, C.Y. Telomeres and Telomerase in Cardiovascular Diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Tellechea, M.L.; Pirola, C.J. The impact of hypertension on leukocyte telomere length: A systematic review and meta-analysis of human studies. J. Hum. Hypertens. 2017, 31, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Biron-Shental, T.; Sukenik-Halevy, R.; Sharon, Y.; Goldberg-Bittman, L.; Kidron, D.; Fejgin, M.D.; Amiel, A. Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am. J. Obstet. Gynecol. 2010, 202, 381.e1–381.e7. [Google Scholar] [CrossRef] [PubMed]
- Polettini, J.; da Silva, M.G. Telomere-Related Disorders in Fetal Membranes Associated with Birth and Adverse Pregnancy Outcomes. Front. Physiol. 2020, 11, 561771. [Google Scholar] [CrossRef]
- Yang, X.; Benny, P.A.; Cervera-Marzal, E.; Wu, B.; Lassiter, C.B.; Astern, J.; Garmire, L.X. Placental telomere length shortening is not associated with severe preeclampsia but the gestational age. Aging 2022, 15, 353–370. [Google Scholar] [CrossRef]
- Sukenik-Halevy, R.; Amiel, A.; Kidron, D.; Liberman, M.; Ganor-Paz, Y.; Biron-Shental, T. Telomere homeostasis in trophoblasts and in cord blood cells from pregnancies complicated with preeclampsia. Am. J. Obstet. Gynecol. 2016, 214, 283.e1–283.e7. [Google Scholar] [CrossRef]
- Lekva, T.; Roland, M.C.P.; Estensen, M.E.; Norwitz, E.R.; Tilburgs, T.; Henriksen, T.; Bollerslev, J.; Normann, K.R.; Magnus, P.; Olstad, O.K.; et al. Dysregulated non-coding telomerase RNA component and associated exonuclease XRN1 in leucocytes from women developing preeclampsia-possible link to enhanced senescence. Sci. Rep. 2021, 11, 19735, Erratum in Sci. Rep. 2021, 11, 22572. [Google Scholar] [CrossRef]
- Zhang, R.; Du, J.; Xiao, Z.; Jiang, Y.; Jin, L.; Weng, Q. Association between the peripartum maternal and fetal telomere lengths and mitochondrial DNA copy numbers and preeclampsia: A prospective case-control study. BMC Pregnancy Childbirth 2022, 22, 483. [Google Scholar] [CrossRef]
- Abu-Awwad, S.-A.; Craina, M.; Gluhovschi, A.; Ciordas, P.D.; Marian, C.; Boscu, L.; Bernad, E.; Iurciuc, M.; Abu-Awwad, A.; Iurciuc, S.; et al. Linking Pregnancy and Long-Term Health: The Impact of Cardiovascular Risk on Telomere Shortening in Pregnant Women. Medicina 2023, 59, 1012. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Y.; Jiang, M.; Wang, F.; Gong, G. Effect of Autophagy Regulated by Sirt1/FoxO1 Pathway on the Release of Factors Promoting Thrombosis from Vascular Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4132. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, H.; Zhang, B.; Zhang, E.; Wu, Y. Tumor Necrosis Factor-alpha (TNF-α) Enhances miR-155-Mediated Endothelial Senescence by Targeting Sirtuin1 (SIRT1). Med Sci Monit. 2019, 25, 8820–8835. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chao, L.; Chao, J. Kallistatin attenuates endothelial senescence by modulating Let-7g-mediated miR-34a-SIRT1-eNOS pathway. J. Cell Mol. Med. 2018, 22, 4387–4398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, D.; Zhang, N.; Yuan, T.; Tao, H. MiR-217 promotes endothelial cell senescence through the SIRT1/p53 signaling pathway. J. Mol. Histol. 2021, 52, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Orimo, M.; Minamino, T.; Miyauchi, H.; Tateno, K.; Okada, S.; Moriya, J.; Komuro, I. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 889–894. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Figarska, S.M.; Vonk, J.M.; Boezen, H.M. SIRT1 polymorphism, long-term survival and glucose tolerance in the general population. PLoS ONE 2013, 8, e58636. [Google Scholar] [CrossRef]
- Huopio, H.; Cederberg, H.; Vangipurapu, J.; Hakkarainen, H.; Pääkkönen, M.; Kuulasmaa, T.; Heinonen, S.; Laakso, M. Association of risk variants for type 2 diabetes and hyperglycemia with gestational diabetes. Eur. J. Endocrinol. 2013, 169, 291–297. [Google Scholar] [CrossRef]
- Franzago, M.; Fraticelli, F.; Marchetti, D.; Celentano, C.; Liberati, M.; Stuppia, L.; Vitacolonna, E. Nutrigenetic variants and cardio-metabolic risk in women with or without gestational diabetes. Diabetes Res. Clin. Pract. 2018, 137, 64–71. [Google Scholar] [CrossRef]
- Han, J.; Wei, M.; Wang, Q.; Li, X.; Zhu, C.; Mao, Y.; Wei, L.; Sun, Y.; Jia, W. Association of Genetic Variants of SIRT1 with Type 2 Diabetes Mellitus. Gene Exp. 2015, 16, 177–185. [Google Scholar] [CrossRef]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; de Leiva, A.; Hod, M.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Dedov, I.I.; Krasnopolskiy, V.I.; Sukhikh, G.T. Russian national consensus statement on gestational diabetes: Diagnostics, treatment and postnatal care. Diabetes Mellit. 2012, 15, 4–10. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef] [PubMed]
- González, J.R.; Armengol, L.; Solé, X.; Guinó, E.; Mercader, J.M.; Estivill, X.; Moreno, V. SNPassoc: An R package to perform whole genome association studies. Bioinformatics 2007, 23, 644–645. [Google Scholar] [CrossRef] [PubMed]
| SNP | Allele Frequencies | Genotype Distribution | p-Value | |
|---|---|---|---|---|
| rs12778366 | 0.96 T | 0.04 C | 58/5/0 | 1 |
| rs7895833 | 0.64 A | 0.36 G | 22/37/4 | 0.05 |
| SNP | Model of Inheritance | Genotypes | PE, n = 61 | Control, n = 63 | OR (95% of CI) 1 | p-Value 2 | AIC |
|---|---|---|---|---|---|---|---|
| rs12778366 | Codominant | TT | 44 (72) | 58 (92) | 1.00 | 0.003 | 167.1 |
| TC | 17 (28) | 5 (8) | 4.48 (1.54–13.08) | ||||
| log-additive | 0, 1, 2 | 61 (49) | 63 (51) | 4.48 (1.54–13.08) | 0.003 | 167.1 | |
| rs7895833 | Codominant | AA | 27 (44) | 22 (35) | 1.00 | 0.27 | 175.2 |
| AG | 33 (54) | 37 (59) | 0.73 (0.35–1.51) | ||||
| GG | 1 (2) | 4 (6) | 0.20 (0.02–1.96) | ||||
| Dominant | AA | 27 (44) | 22 (35) | 1.00 | 0.29 | 174.7 | |
| AG + GG | 34 (56) | 41 (65) | 0.68 (0.33–1.39) | ||||
| Recessive | AA + AG | 60 (98) | 59 (94) | 1.00 | 0.17 | 174.0 | |
| GG | 1 (2) | 4 (6) | 0.25 (0.03–2.26) | ||||
| Overdominant | AA + GG | 28 (46) | 26 (41) | 1.00 | 0.6 | 175.6 | |
| AG | 33 (54) | 37 (59) | 0.83 (0.41–1.69) | ||||
| log-additive | 0, 1, 2 | 61 (49) | 63 (51) | 0.63 (0.33–1.20) | 0.16 | 173.9 |
| Parameter | PE, n = 61 | Control, n = 63 | p-Value * |
|---|---|---|---|
| Long telomeres, n (%) | 21 (34.43) | 33 (52.38) | 0.044 |
| Short telomeres, n (%) | 40 (65.57) | 30 (47.62) |
| SNP | Genotypes and Alleles | Short Telomere Ratio | p-Value * | |
|---|---|---|---|---|
| PE, n (%) | Control, n (%) | |||
| rs12778366 | TT | 29 (72.5) | 30 (93.8) | 0.019 |
| TC | 11 (27.5) | 2 (6.2) | ||
| T | 69 (86.3) | 62 (96.9) | 0.027 | |
| C | 11 (13.7) | 2 (3.1) | ||
| rs7895833 | AA | 15 (37.5) | 8 (25.0) | 0.528 |
| AG | 24 (60.0) | 23 (71.9) | ||
| GG | 1 (2.3) | 1 (3.1) | ||
| A | 54 (67.5) | 37 (57.8) | 0.476 | |
| G | 26 (32.5) | 23 (42.2) | ||
| SNP | Oligonucleotide Type | Sequences (5′ to 3′) |
|---|---|---|
| rs12778366 | Forward primer Reverse primer Taq-Man probe for T allele Taq-Man probe for C allele | CCCCACGCAACCAAAGAT ATCGCTAAGGTCCTATCTACA FAM-TGGTCACCACTATTCATTTCTGAA-BHQ1 HEX-TGGTCACCACTGTTCATTTCTGAA-BHQ1 |
| rs7895833 | Forward Revers Taq-Man probe for A allele Taq-Man probe for G allele | TTCTGAAGTAATGAGGTGG AGGAGACTCTGCCAGAAAT FAM-CCTACAGGAAATCAACGTAA-BHQ1 HEX-CCTACAGGAAGTCAACGTAA-BHQ1 |
| Primer Name | Sequences (5′ to 3′) |
|---|---|
| telg | ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT |
| telc | TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA |
| Hbgu | CGGCGGCGGGCGGCGCGGGCTGGGCGGcttcatccacgttcaccttg |
| Hbgd | GCCCGGCCCGCCGCGCCCGTCCCGCCGgaggagaagtctgccgtt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrenko, O.; Karpova, N.; Nurbekov, M. Increased Preeclampsia Risk in GDM Pregnancies: The Role of SIRT1 rs12778366 Polymorphism and Telomere Length. Int. J. Mol. Sci. 2025, 26, 2967. https://doi.org/10.3390/ijms26072967
Dmitrenko O, Karpova N, Nurbekov M. Increased Preeclampsia Risk in GDM Pregnancies: The Role of SIRT1 rs12778366 Polymorphism and Telomere Length. International Journal of Molecular Sciences. 2025; 26(7):2967. https://doi.org/10.3390/ijms26072967
Chicago/Turabian StyleDmitrenko, Olga, Nataliia Karpova, and Malik Nurbekov. 2025. "Increased Preeclampsia Risk in GDM Pregnancies: The Role of SIRT1 rs12778366 Polymorphism and Telomere Length" International Journal of Molecular Sciences 26, no. 7: 2967. https://doi.org/10.3390/ijms26072967
APA StyleDmitrenko, O., Karpova, N., & Nurbekov, M. (2025). Increased Preeclampsia Risk in GDM Pregnancies: The Role of SIRT1 rs12778366 Polymorphism and Telomere Length. International Journal of Molecular Sciences, 26(7), 2967. https://doi.org/10.3390/ijms26072967






