The Role of Gut Microbiome on Glioblastoma Oncogenesis and Malignant Evolution
Abstract
1. Introduction
2. Overview of Gut Microbiome
3. Gut Microbiome in Cancers
4. The Role of Gut Microbiome in Glioma Development (Oncogenesis)
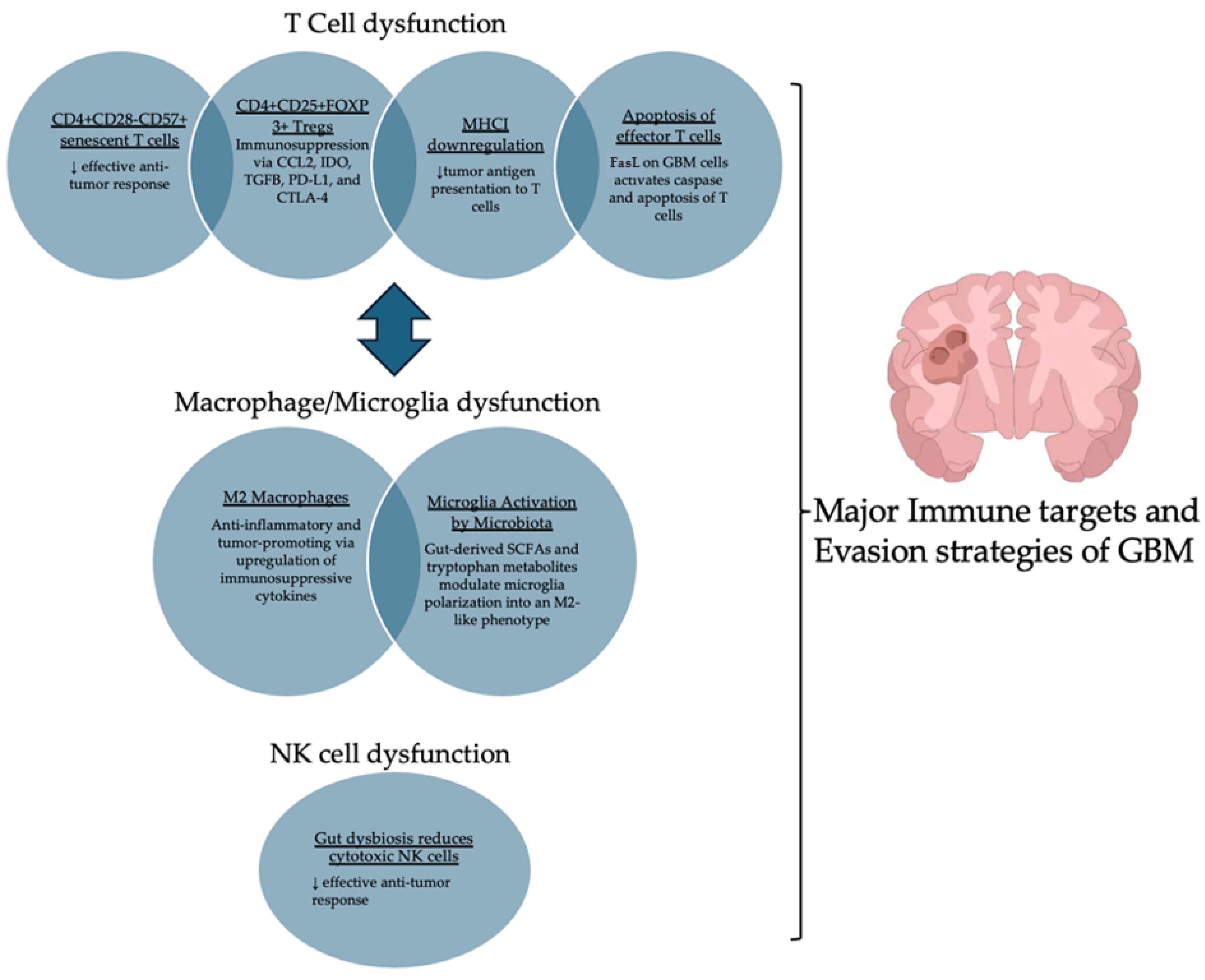
4.1. GBM’s Effects on T Cells: Inducing Immune Dysfunction
4.2. Microglia and M2 Macrophage Polarization and Tumor Growth
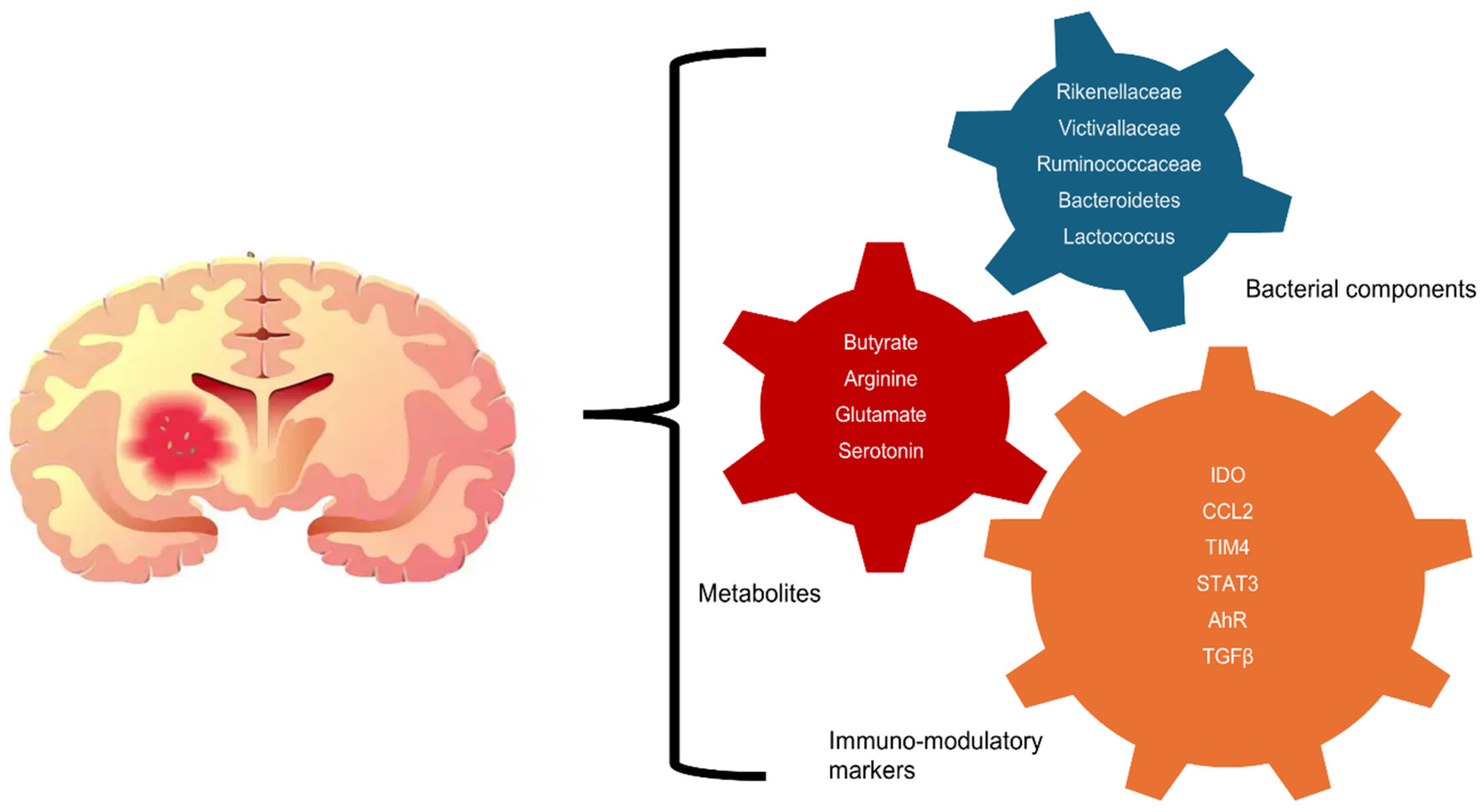
4.3. Gut Microbiota’s Role in Modulating Immunity and GBM Progression
5. Gut Microbiome Impacts Glioma Therapeutic Resistance and Progression
6. Insights into Modulating Gut Flora to Enhance Therapeutic Response in GBM
7. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee Sh, U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis Through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Chiariello, M.; Inzalaco, G.; Barone, V.; Gherardini, L. Overcoming challenges in glioblastoma treatment: Targeting infiltrating cancer cells and harnessing the tumor microenvironment. Front. Cell. Neurosci. 2023, 17, 1327621. [Google Scholar] [CrossRef]
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: Current state and future directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Broekman, M.L.; Maas, S.L.N.; Abels, E.R.; Mempel, T.R.; Krichevsky, A.M.; Breakefield, X.O. Multidimensional communication in the microenvirons of glioblastoma. Nat. Rev. Neurol. 2018, 14, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hambardzumyan, D. Immune Microenvironment in Glioblastoma Subtypes. Front. Immunol. 2018, 9, 1004. [Google Scholar] [CrossRef]
- Suganya, K.; Koo, B.S. Gut-Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; He, C.; Song, H. Investigating the causal impact of gut microbiota on glioblastoma: A bidirectional Mendelian randomization study. BMC Genom. 2023, 24, 784. [Google Scholar] [CrossRef]
- Green, G.B.H.; Cox-Holmes, A.N.; Potier, A.C.E.; Marlow, G.H.; McFarland, B.C. Modulation of the Immune Environment in Glioblastoma by the Gut Microbiota. Biomedicines 2024, 12, 2429. [Google Scholar] [CrossRef]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Dono, A.; Nickles, J.; Rodriguez-Armendariz, A.G.; McFarland, B.C.; Ajami, N.J.; Ballester, L.Y.; Wargo, J.A.; Esquenazi, Y. Glioma and the gut-brain axis: Opportunities and future perspectives. Neuro-Oncol. Adv. 2022, 4, vdac054. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Okada, H.; Kuhn, C.; Feillet, H.; Bach, J.F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: An update. Clin. Exp. Immunol. 2010, 160, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Huang, L.; Wang, Y.; Huang, C.; Luo, Y.; Qin, X.; Zeng, J. Effect of different delivery modes on intestinal microbiota and immune function of neonates. Sci. Rep. 2024, 14, 17452. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e1412. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, H.M.; Yasin, R.; Mohammad, I.S.; Fan, Y.; Li, H.; Shahzad, M.; Xu, J. The gut-brain-axis: A positive relationship between gut microbial dysbiosis and glioblastoma brain tumour. Heliyon 2024, 10, e30494. [Google Scholar] [CrossRef] [PubMed]
- Rebersek, M. Gut microbiome and its role in colorectal cancer. BMC Cancer 2021, 21, 1325. [Google Scholar] [CrossRef]
- Long, Y.; Tang, L.; Zhou, Y.; Zhao, S.; Zhu, H. Causal relationship between gut microbiota and cancers: A two-sample Mendelian randomisation study. BMC Med. 2023, 21, 66. [Google Scholar] [CrossRef]
- Schettini, F.; Gattazzo, F.; Nucera, S.; Rubio Garcia, E.; López-Aladid, R.; Morelli, L.; Fontana, A.; Vigneri, P.; Casals-Pascual, C.; Iebba, V.; et al. Navigating the complex relationship between human gut microbiota and breast cancer: Physiopathological, prognostic and therapeutic implications. Cancer Treat. Rev. 2024, 130, 102816. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Mori, H.; Svegliati Baroni, G.; Marzioni, M.; Di Nicola, F.; Santori, P.; Maroni, L.; Abenavoli, L.; Scarpellini, E. Farnesoid X Receptor, Bile Acid Metabolism, and Gut Microbiota. Metabolites 2022, 12, 647. [Google Scholar] [CrossRef]
- Patrizz, A.; Dono, A.; Zorofchian, S.; Hines, G.; Takayasu, T.; Husein, N.; Otani, Y.; Arevalo, O.; Choi, H.A.; Savarraj, J.; et al. Glioma and temozolomide induced alterations in gut microbiome. Sci. Rep. 2020, 10, 21002. [Google Scholar] [CrossRef]
- Yust-Katz, S.; Gigi, E.; Rosenberg, D.; Kanner, A.A.; Laviv, Y.; Benouaich-Amiel, A.; Siegal, T.; Barda, A.L.; Straussman, R. TAMI-40. Tumor Microbiome and Glioblastoma (GBM). Neuro. Oncol. 2020, 22 (Suppl. S2), ii221–ii222. [Google Scholar] [CrossRef]
- Robinson, K.M.; Crabtree, J.; Mattick, J.S.A.; Anderson, K.E.; Dunning Hotopp, J.C. Distinguishing potential bacteria-tumor associations from contamination in a secondary data analysis of public cancer genome sequence data. Microbiome 2017, 5, 9. [Google Scholar] [CrossRef]
- Okada, M.; Saio, M.; Kito, Y.; Ohe, N.; Yano, H.; Yoshimura, S.; Iwama, T.; Takami, T. Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int. J. Oncol. 2009, 34, 1621–1627. [Google Scholar] [CrossRef]
- Fan, J.; Liu, J.; Zhang, B.; Wang, X.; Wang, X.; Liang, J.; Li, Y.; Zhang, Y.; Zhang, C.; Yu, S.; et al. GPR65 contributes to constructing immunosuppressive microenvironment in glioma. Neurosurg. Rev. 2024, 47, 417. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Liu, D.; Zhu, H.; Shu, K. Microenvironmental regulation of tumor-associated neutrophils in malignant glioma: From mechanism to therapy. J. Neuroinflammation 2024, 21, 226. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.C.; Gabriely, G.; Rothhammer, V.; Mascanfroni, I.D.; Wheeler, M.A.; Chao, C.-C.; Gutiérrez-Vázquez, C.; Kenison, J.; Tjon, E.C.; Barroso, A.; et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 2019, 22, 729–740. [Google Scholar] [CrossRef]
- Fornara, O.; Odeberg, J.; Wolmer Solberg, N.; Tammik, C.; Skarman, P.; Peredo, I.; Stragliotto, G.; Rahbar, A.; Söderberg-Nauclér, C. Poor survival in glioblastoma patients is associated with early signs of immunosenescence in the CD4 T-cell compartment after surgery. Oncoimmunology 2015, 4, e1036211. [Google Scholar] [CrossRef]
- Focosi, D.; Bestagno, M.; Burrone, O.; Petrini, M. CD57+ T lymphocytes and functional immune deficiency. J. Leukoc. Biol. 2010, 87, 107–116. [Google Scholar] [CrossRef]
- Walker, D.G.; Chuah, T.; Rist, M.J.; Pender, M.P. T-cell apoptosis in human glioblastoma multiforme: Implications for immunotherapy. J. Neuroimmunol. 2006, 175, 59–68. [Google Scholar] [CrossRef][Green Version]
- Fecci, P.E.; Mitchell, D.A.; Whitesides, J.F.; Xie, W.; Friedman, A.H.; Archer, G.E.; Herndon, J.E., II; Bigner, D.D.; Dranoff, G.; Sampson, J.H. Increased Regulatory T-Cell Fraction Amidst a Diminished CD4 Compartment Explains Cellular Immune Defects in Patients with Malignant Glioma. Cancer Res. 2006, 66, 3294–3302. [Google Scholar] [CrossRef]
- Woroniecka, K.I.; Rhodin, K.E.; Chongsathidkiet, P.; Keith, K.A.; Fecci, P.E. T-cell Dysfunction in Glioblastoma: Applying a New Framework. Clin. Cancer Res. 2018, 24, 3792–3802. [Google Scholar] [CrossRef]
- Xu, L.; Xiao, H.; Xu, M.; Zhou, C.; Yi, L.; Liang, H. Glioma-derived T cell immunoglobulin- and mucin domain-containing molecule-4 (TIM4) contributes to tumor tolerance. J. Biol. Chem. 2011, 286, 36694–36699. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Henry, V.; Tiao, N.; Park, S.Y.; Martinez-Ledesma, J.; Dong, J.W.; Balasubramaniyan, V.; de Groot, J.F. Targeting intercellular adhesion molecule-1 prolongs survival in mice bearing bevacizumab-resistant glioblastoma. Oncotarget 2017, 8, 96970–96983. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Shin, E.-C.; Perosa, F.; Vacca, A.; Dammacco, F.; Racanelli, V. MHC Class I Antigen Processing and Presenting Machinery: Organization, Function, and Defects in Tumor Cells. JNCI J. Natl. Cancer Inst. 2013, 105, 1172–1187. [Google Scholar] [CrossRef]
- Jacobs, J.F.; Idema, A.J.; Bol, K.F.; Nierkens, S.; Grauer, O.M.; Wesseling, P.; Grotenhuis, J.A.; Hoogerbrugge, P.M.; de Vries, I.J.; Adema, G.J. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol. 2009, 11, 394–402. [Google Scholar] [CrossRef]
- Bloch, O.; Crane, C.A.; Kaur, R.; Safaee, M.; Rutkowski, M.J.; Parsa, A.T. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin. Cancer Res. 2013, 19, 3165–3175. [Google Scholar] [CrossRef]
- Naghavian, R.; Faigle, W.; Oldrati, P.; Wang, J.; Toussaint, N.C.; Qiu, Y.; Medici, G.; Wacker, M.; Freudenmann, L.K.; Bonté, P.-E.; et al. Microbial peptides activate tumour-infiltrating lymphocytes in glioblastoma. Nature 2023, 617, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, T.; Zhao, J.; Wang, C.; Sun, H. Current understanding of the human microbiome in glioma. Front. Oncol. 2022, 12, 781741. [Google Scholar] [CrossRef]
- Dzutsev, A.; Badger, J.H.; Perez-Chanona, E.; Roy, S.; Salcedo, R.; Smith, C.K.; Trinchieri, G. Microbes and Cancer. Annu. Rev. Immunol. 2017, 35, 199–228. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Antonangeli, F.; Marrocco, F.; Porzia, A.; Lauro, C.; Santoni, A.; Limatola, C. Gut microbiota alterations affect glioma growth and innate immune cells involved in tumor immunosurveillance in mice. Eur. J. Immunol. 2020, 50, 705–711. [Google Scholar] [CrossRef]
- Teng, Y.; Mu, J.; Xu, F.; Zhang, X.; Sriwastva, M.K.; Liu, Q.M.; Li, X.; Lei, C.; Sundaram, K.; Hu, X.; et al. Gut bacterial isoamylamine promotes age-related cognitive dysfunction by promoting microglial cell death. Cell Host Microbe 2022, 30, 944–960.e8. [Google Scholar] [CrossRef]
- Ustjanzew, A.; Sencio, V.; Trottein, F.; Faber, J.; Sandhoff, R.; Paret, C. Interaction Between Bacteria and the Immune System for Cancer Immunotherapy: The α-GalCer Alliance. Int. J. Mol. Sci. 2022, 23, 5896. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.-W.; Kim, M.J.; Kim, J.; Lee, H.G.; Ryoo, S.-B.; Ku, J.-L.; Jeong, S.-Y.; Park, K.J.; Kim, D.; Kim, J.F.; et al. Enterotypical Prevotella and three novel bacterial biomarkers in preoperative stool predict the clinical outcome of colorectal cancer. Microbiome 2022, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Walker, A.J.; Card, T.; Bates, T.E.; Muir, K. Tricyclic antidepressants and the incidence of certain cancers: A study using the GPRD. Br. J. Cancer 2011, 104, 193–197. [Google Scholar] [CrossRef]
- Caragher, S.P.; Hall, R.R.; Ahsan, R.; Ahmed, A.U. Monoamines in glioblastoma: Complex biology with therapeutic potential. Neuro Oncol. 2018, 20, 1014–1025. [Google Scholar] [CrossRef]
- Hou, X.; Du, H.; Deng, Y.; Wang, H.; Liu, J.; Qiao, J.; Liu, W.; Shu, X.; Sun, B.; Liu, Y. Gut microbiota mediated the individualized efficacy of Temozolomide via immunomodulation in glioma. J. Transl. Med. 2023, 21, 198. [Google Scholar] [CrossRef]
- Dono, A.; Patrizz, A.; McCormack, R.M.; Putluri, N.; Ganesh, B.P.; Kaur, B.; McCullough, L.D.; Ballester, L.Y.; Esquenazi, Y. Glioma induced alterations in fecal short-chain fatty acids and neurotransmitters. CNS Oncol. 2020, 9, CNS57. [Google Scholar] [CrossRef]
- Desland, F.A.; Hormigo, A. The CNS and the Brain Tumor Microenvironment: Implications for Glioblastoma Immunotherapy. Int. J. Mol. Sci. 2020, 21, 7358. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Yan, J.; Li, B.; Luo, C. Gut microbiota’s role in glioblastoma risk, with a focus on the mediating role of metabolites. Front. Neurol. 2024, 15, 1386885. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Tomaszewski, W.; Sanchez-Perez, L.; Gajewski, T.F.; Sampson, J.H. Brain Tumor Microenvironment and Host State: Implications for Immunotherapy. Clin. Cancer Res. 2019, 25, 4202–4210. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Maus, M.V.; June, C.H. Immunotherapy for Brain Tumors. J. Clin. Oncol. 2017, 35, 2450–2456. [Google Scholar] [CrossRef]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Dees, K.J.; Koo, H.; Humphreys, J.F.; Hakim, J.A.; Crossman, D.K.; Crowley, M.R.; Nabors, L.B.; Benveniste, E.N.; Morrow, C.D.; McFarland, B.C. Human gut microbial communities dictate efficacy of anti-PD-1 therapy in a humanized microbiome mouse model of glioma. Neuro-Oncol. Adv. 2021, 3, vdab023. [Google Scholar] [CrossRef]
- Keane, L.; Cryan, J.F.; Gleeson, J.P. Exploiting the gut microbiome for brain tumour treatment. Trends Mol. Med. 2024, 31, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, F.; Gao, Y.; Kang, S.; Li, J.; Guo, J. Microbiome in radiotherapy: An emerging approach to enhance treatment efficacy and reduce tissue injury. Mol. Med. 2024, 30, 105. [Google Scholar] [CrossRef]
- Park, M.; Kwon, J.; Shin, H.J.; Moon, S.M.; Kim, S.B.; Shin, U.S.; Han, Y.H.; Kim, Y. Butyrate enhances the efficacy of radiotherapy via FOXO3A in colorectal cancer patient-derived organoids. Int. J. Oncol. 2020, 57, 1307–1318. [Google Scholar] [CrossRef]
- Yang, S.; Fu, Z.Z.; Zhang, Y.Q.; Fu, B.H.; Dong, L. The G to A transformation of rs4702 polymorphism in 3’UTR of FURIN reduced the risk of radiotherapy-induced cognitive impairment in glioma patients. J. Cell Mol. Med. 2022, 26, 684–692. [Google Scholar] [CrossRef]
- Tonneau, M.; Elkrief, A.; Pasquier, D.; Paz Del Socorro, T.; Chamaillard, M.; Bahig, H.; Routy, B. The role of the gut microbiome on radiation therapy efficacy and gastrointestinal complications: A systematic review. Radiother. Oncol. 2021, 156, 1–9. [Google Scholar] [CrossRef]
- Majc, B.; Novak, M.; Kopitar-Jerala, N.; Jewett, A.; Breznik, B. Immunotherapy of Glioblastoma: Current Strategies and Challenges in Tumor Model Development. Cells 2021, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zuo, M.; Zhou, Q.; Wang, Y. Oncolytic virotherapy in cancer treatment: Challenges and optimization prospects. Front. Immunol. 2023, 14, 1308890. [Google Scholar] [CrossRef]
- Luksik, A.S.; Yazigi, E.; Shah, P.; Jackson, C.M. CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. Cancers 2023, 15, 1414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hong, Y.; Wu, T.; Ben, E.; Li, S.; Hu, L.; Xie, T. Role of gut microbiota in regulating immune checkpoint inhibitor therapy for glioblastoma. Front. Immunol. 2024, 15, 1401967. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef] [PubMed]
- Montalban-Arques, A.; Katkeviciute, E.; Busenhart, P.; Bircher, A.; Wirbel, J.; Zeller, G.; Morsy, Y.; Borsig, L.; Glaus Garzon, J.F.; Müller, A.; et al. Commensal Clostridiales strains mediate effective anti-cancer immune response against solid tumors. Cell Host Microbe 2021, 29, 1573–1588.e7. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Xia, Q. Role of gut microbiome in cancer immunotherapy: From predictive biomarker to therapeutic target. Exp. Hematol. Oncol. 2023, 12, 84. [Google Scholar] [CrossRef]
- Jiang, H.; Zeng, W.; Zhang, X.; Pei, Y.; Zhang, H.; Li, Y. The role of gut microbiota in patients with benign and malignant brain tumors: A pilot study. Bioengineered 2022, 13, 7847–7859. [Google Scholar] [CrossRef]
- Valerio, J.; Borro, M.; Proietti, E.; Pisciotta, L.; Olarinde, I.O.; Fernandez Gomez, M.; Alvarez Pinzon, A.M. Systematic Review and Clinical Insights: The Role of the Ketogenic Diet in Managing Glioblastoma in Cancer Neuroscience. J. Pers. Med. 2024, 14, 929. [Google Scholar] [CrossRef]
- Windemuth, S.; Ali, A.; Molotkov, A.; Hahn, J.; Danino, T.; Leong, K.; Mintz, A. SYST-31 Probiotic Delivery to Orthotopic Glioblastoma Multiforme Models as an Immunotherapy. Neuro-Oncol. Adv. 2023, 5 (Suppl. S3), iii33–iii34. [Google Scholar] [CrossRef]
- Fatahi, A.; Soleimani, N.; Afrough, P. Anticancer Activity of Kefir on Glioblastoma Cancer Cell as a New Treatment. Int. J. Food Sci. 2021, 2021, 8180742. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Fan, H.; Han, M.; Xie, J.; Du, J.; Peng, F. Bifidobacterium lactis combined with Lactobacillus plantarum inhibit glioma growth in mice through modulating PI3K/AKT pathway and gut microbiota. Front. Microbiol. 2022, 13, 986837. [Google Scholar] [CrossRef]
- Colardo, M.; Segatto, M.; Di Bartolomeo, S. Targeting RTK-PI3K-mTOR Axis in Gliomas: An Update. Int. J. Mol. Sci. 2021, 22, 4899. [Google Scholar] [CrossRef]
- Fan, Y.; Su, Q.; Chen, J.; Wang, Y.; He, S. Gut Microbiome Alterations Affect Glioma Development and Foxp3 Expression in Tumor Microenvironment in Mice. Front. Oncol. 2022, 12, 836953. [Google Scholar] [CrossRef]
- Routy, B.; Lenehan, J.G.; Miller, W.H.; Jamal, R.; Messaoudene, M.; Daisley, B.A.; Hes, C.; Al, K.F.; Martinez-Gili, L.; Punčochář, M.; et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: A phase I trial. Nat. Med. 2023, 29, 2121–2132. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Perez-Gracia, J.L.; Schalper, K.A.; Fusco, J.P.; Gonzalez, A.; Rodriguez-Ruiz, M.E.; Oñate, C.; Perez, G.; Alfaro, C.; Martín-Algarra, S.; et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann. Oncol. 2017, 28, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, F.F.; Schmitz, M.; Beissert, S.; Meier, F. Anti-PD-1 and Novel Combinations in the Treatment of Melanoma—An Update. J. Clin. Med. 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ou, Z.; Huang, X.; Wang, J.; Li, Q.; Wen, M.; Zheng, L. Microbiota and glioma: A new perspective from association to clinical translation. Gut Microbes 2024, 16, 2394166. [Google Scholar] [CrossRef]
- Kim, H.; Roh, H.S.; Kim, J.E.; Park, S.D.; Park, W.H.; Moon, J.-Y. Compound K attenuates stromal cell-derived growth factor 1 (SDF-1)-induced migration of C6 glioma cells. Nutr. Res. Pract. 2016, 10, 259–264. [Google Scholar]
- Aljarrah, D.; Chalour, N.; Zorgani, A.; Nissan, T.; Pranjol, M.Z.I. Exploring the gut microbiota and its potential as a biomarker in gliomas. Biomed. Pharmacother. 2024, 173, 116420. [Google Scholar] [CrossRef]
- Meléndez-Vázquez, N.M.; Gomez-Manzano, C.; Godoy-Vitorino, F. Oncolytic Virotherapies and Adjuvant Gut Microbiome Therapeutics to Enhance Efficacy Against Malignant Gliomas. Viruses 2024, 16, 1775. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Vázquez, N.M.; Nguyen, T.T.; Fan, X.; López-Rivas, A.R.; Fueyo, J.; Gomez-Manzano, C.; Godoy-Vitorino, F. Gut microbiota composition is associated with the efficacy of Delta-24-RGDOX in malignant gliomas. Mol. Ther. Oncol. 2024, 32, 200787. [Google Scholar] [CrossRef] [PubMed]
| Factor | Mechanism | Effect on GBM | Implication for Therapy | References |
|---|---|---|---|---|
| Microbiota Diversity | ↓ Diversity in GBM patients, shift toward pro-inflammatory taxa | Promotes immunosuppressive tumor microenvironment (TME), favors MDSCs and Tregs | Microbiome-targeted interventions may enhance response to immunotherapy | [7,55,75] |
| SCFA Production (Butyrate, Propionate, Acetate) | Modulates Treg and Th1/Th17 balance, increases antigen presentation by DCs | Enhances anti-tumor immunity, reduces chronic inflammation | Could enhance TMZ/radiotherapy efficacy by improving immune activation | [4,67,68] |
| Tryptophan Metabolism (Kynurenine Pathway Shift) | ↑ IDO1-mediated kynurenine production ↑ Treg, ↓ cytotoxic CD8+ T cells | Suppresses anti-tumor immune responses, promotes T cell exhaustion | IDO1 inhibition could improve response to checkpoint inhibitors | [39,55,62] |
| Microbiota-Mediated BBB Modulation | Alters P-glycoprotein (P-gp) and tight junction expression, affecting drug penetration | Impacts CNS drug bioavailability and immune cell infiltration | Targeting microbiota to regulate BBB permeability could improve chemotherapy efficacy | [3,72,77] |
| FMT Studies (Mouse Models) | Restores gut microbiome balance, improves gut--immune crosstalk | Enhances response to immune checkpoint blockade (ICB) | Potential adjunct for GBM immunotherapy | [65,75] |
| Probiotic Intervention (Lactobacillus, Bifidobacterium) | Modifies dendritic cell function, increases IL-12 and IFN-γ | Boosts anti-tumor immunity but effects in GBM remain unclear | May support immune checkpoint therapy but requires trials | [80,81,89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, Z.S.; Wu, Q.; Jacome, M.A.; Chen, J.; Etame, A.B. The Role of Gut Microbiome on Glioblastoma Oncogenesis and Malignant Evolution. Int. J. Mol. Sci. 2025, 26, 2935. https://doi.org/10.3390/ijms26072935
Mohamed ZS, Wu Q, Jacome MA, Chen J, Etame AB. The Role of Gut Microbiome on Glioblastoma Oncogenesis and Malignant Evolution. International Journal of Molecular Sciences. 2025; 26(7):2935. https://doi.org/10.3390/ijms26072935
Chicago/Turabian StyleMohamed, Zaynab Sidi, Qiong Wu, Maria A. Jacome, Jianan Chen, and Arnold B. Etame. 2025. "The Role of Gut Microbiome on Glioblastoma Oncogenesis and Malignant Evolution" International Journal of Molecular Sciences 26, no. 7: 2935. https://doi.org/10.3390/ijms26072935
APA StyleMohamed, Z. S., Wu, Q., Jacome, M. A., Chen, J., & Etame, A. B. (2025). The Role of Gut Microbiome on Glioblastoma Oncogenesis and Malignant Evolution. International Journal of Molecular Sciences, 26(7), 2935. https://doi.org/10.3390/ijms26072935







