Potential Mechanisms Underlying the Minimal Impact of Cry1Ab1 Protein on Myzus persicae
Abstract
1. Introduction
2. Results
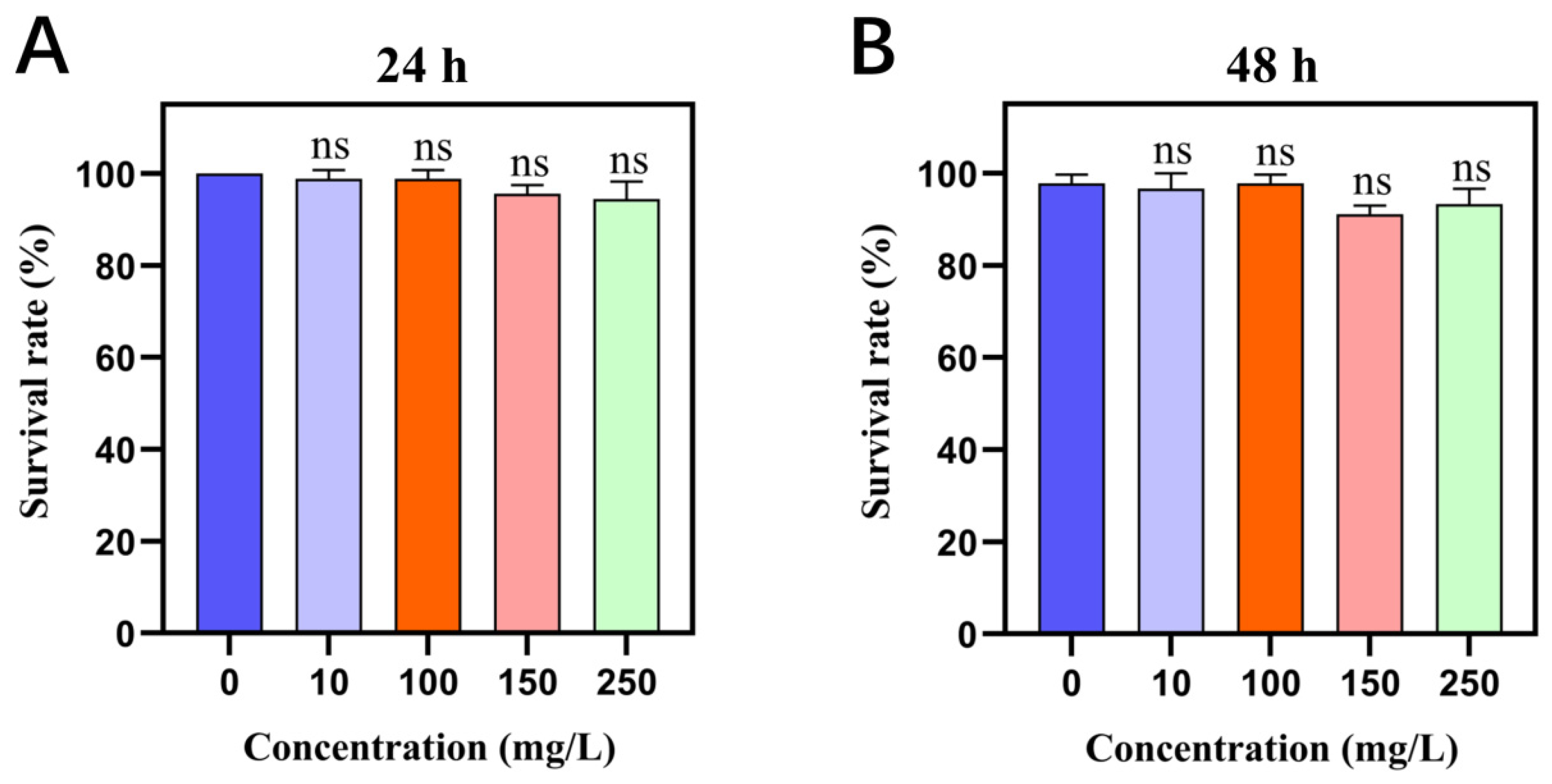
2.1. Cry1Ab1 Protein Rarely Kills M. persicae
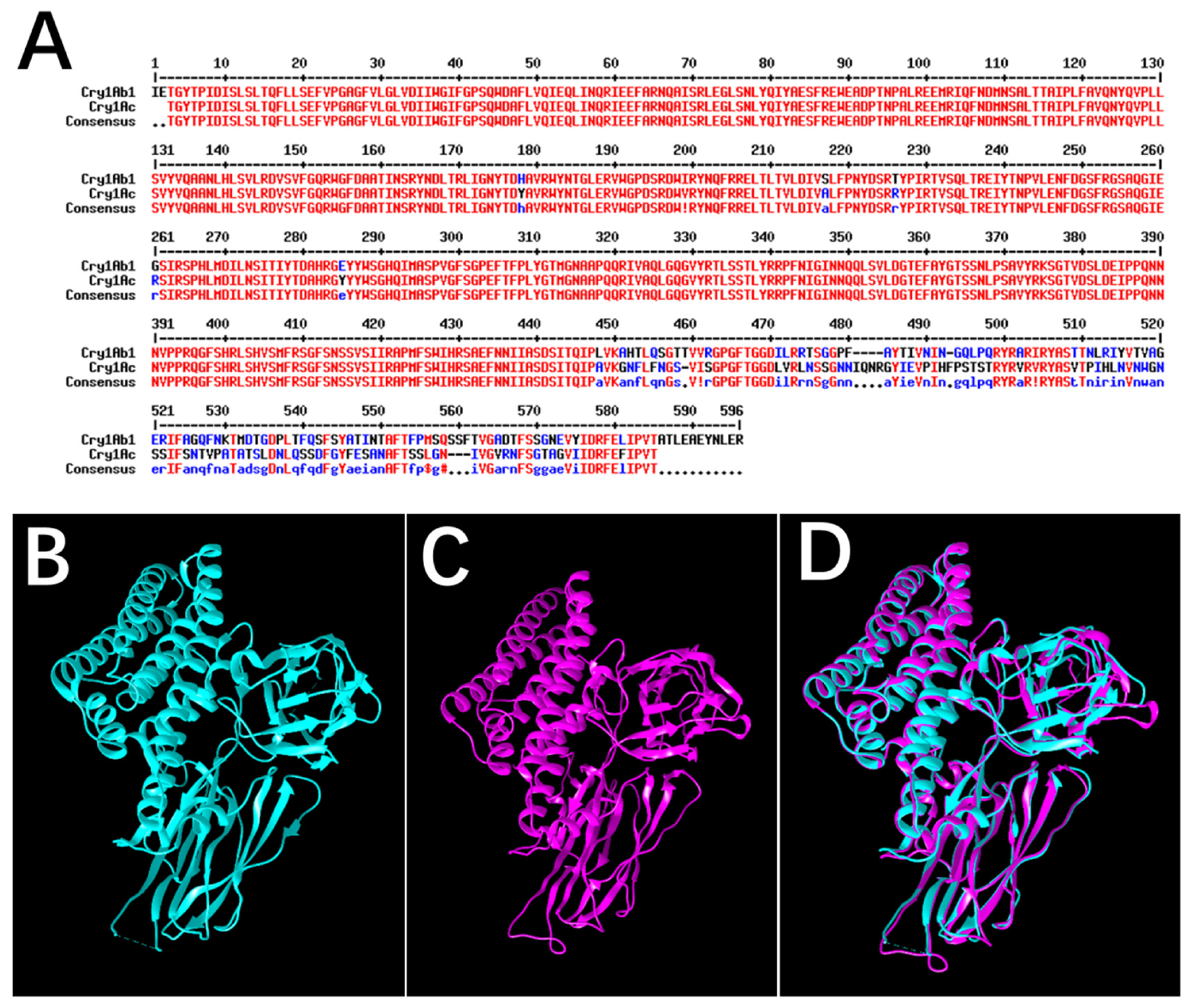
2.2. Identification of Cry1Ab1-Binding Proteins
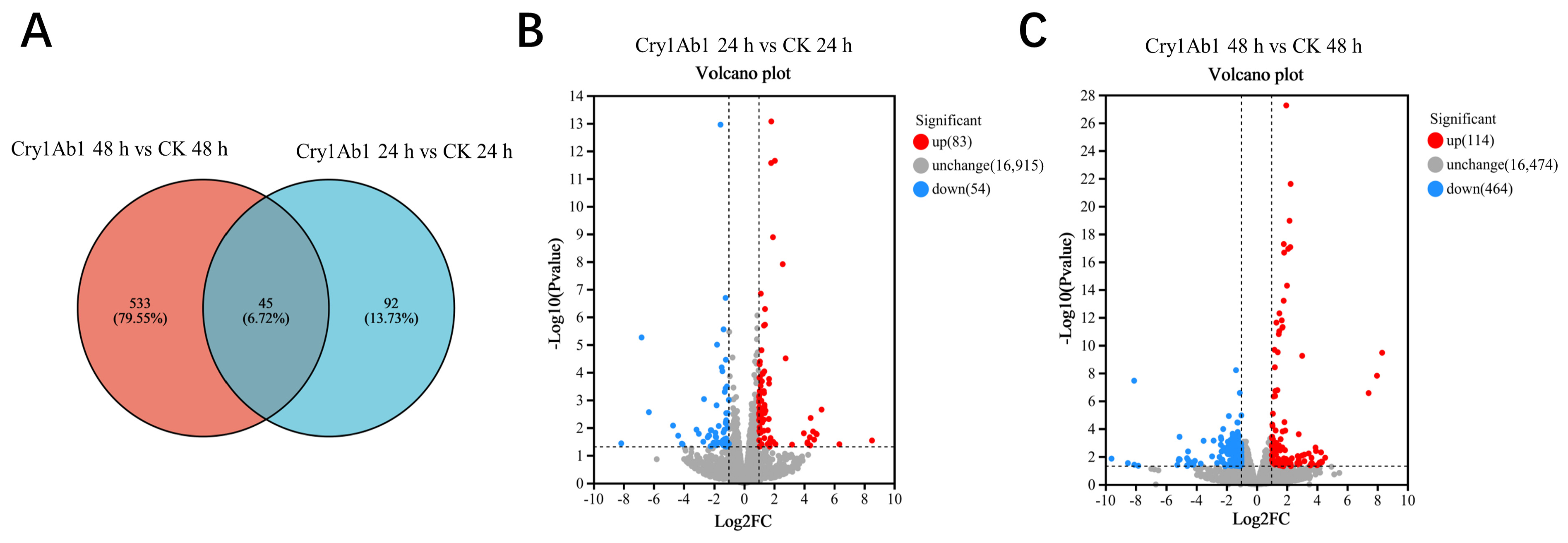
2.3. Transcriptome Sequencing and Assembly
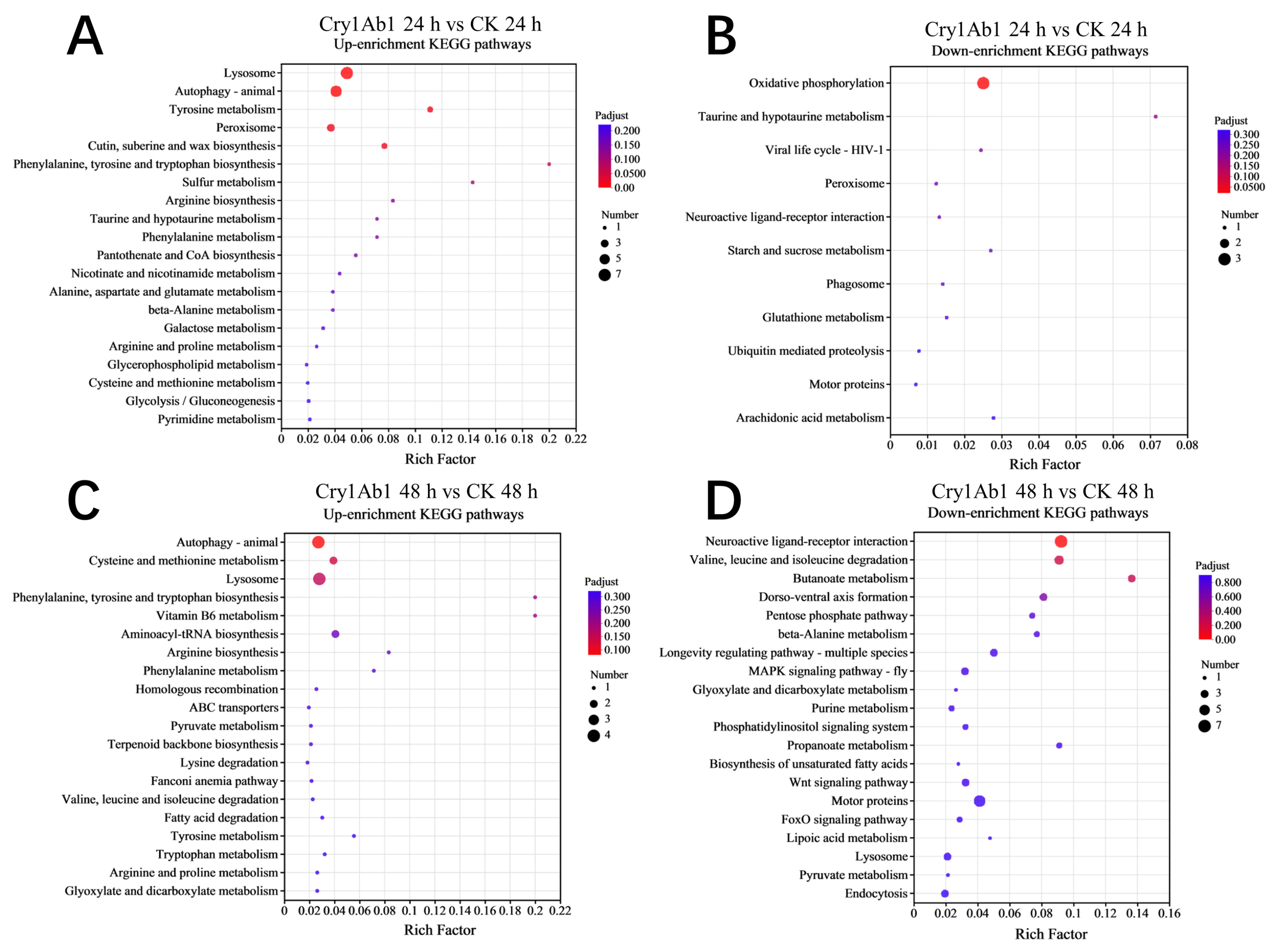
2.4. Functional Analysis of DEGs
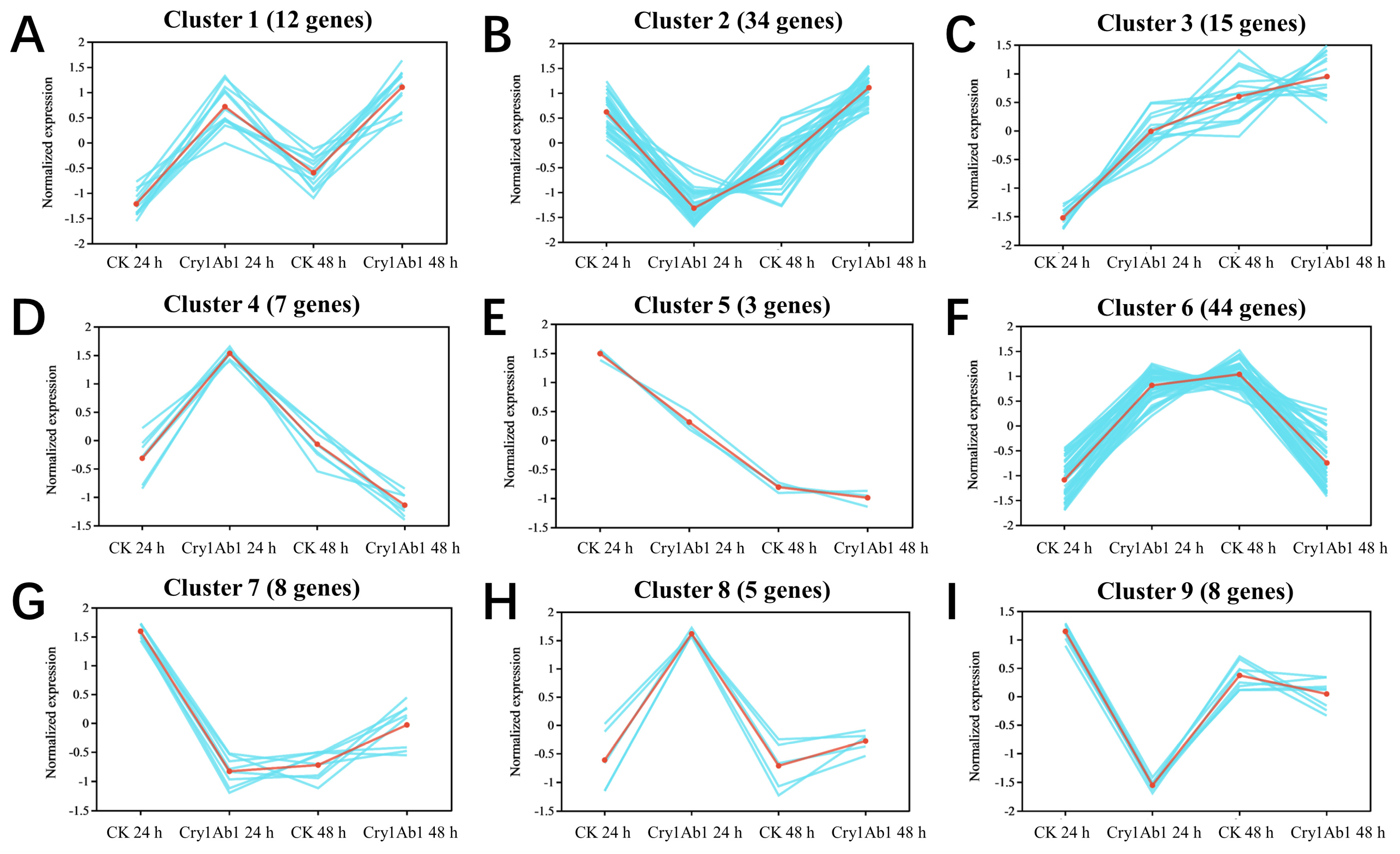
2.5. Cluster Analysis of DEGs
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
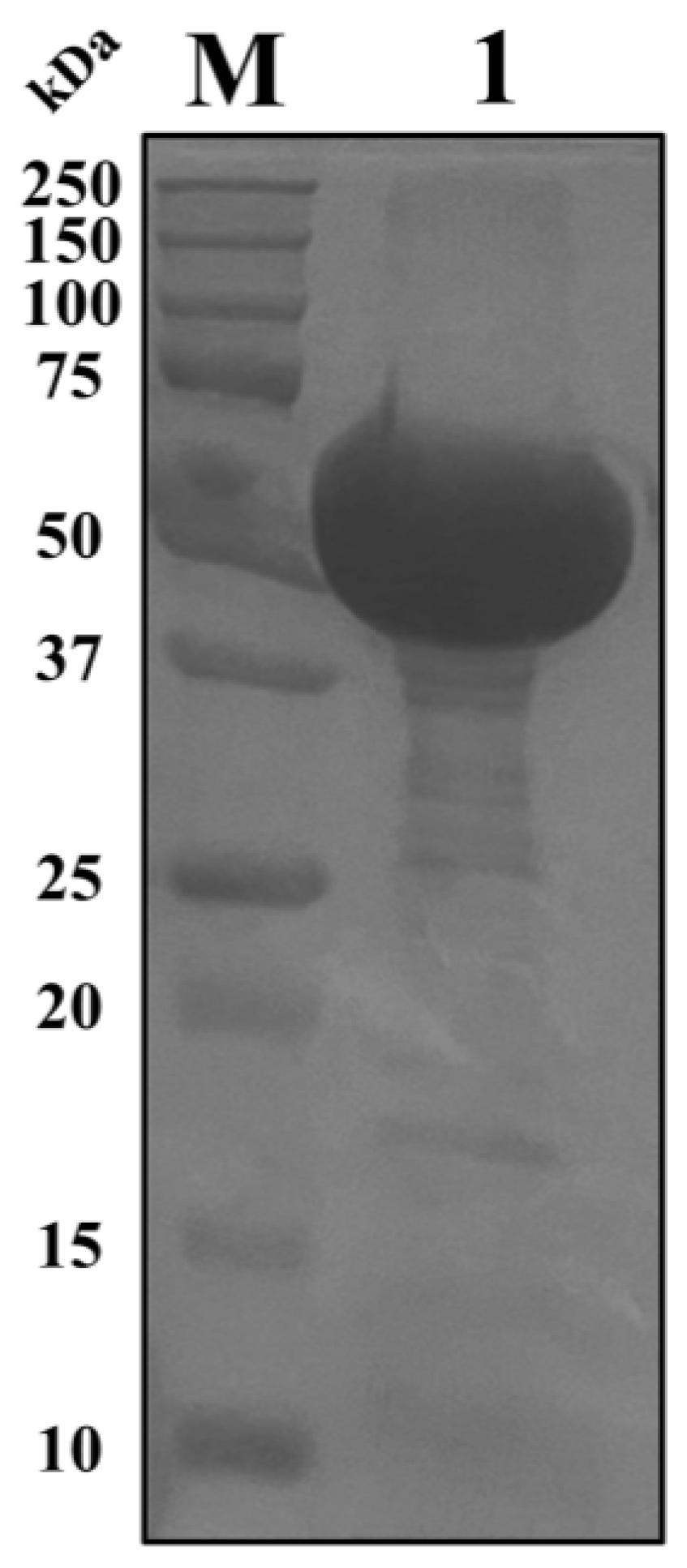
4.2. Preparation of the Cry1Ab1 Protein
4.3. Bioassay of the Cry1Ab1 Protein Against M. persicae
4.4. Pull-Down Experiment and LC-MS/MS Analysis
4.5. Functional Analysis of Cry1Ab1-Binding Proteins
4.6. RNA Sequencing
4.7. Transcriptome Assembly and Functional Annotation
4.8. DEGs Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.; Li, H.; Tang, Q.; Zhang, H.; Liu, T.; Wang, Y. Trends in the global commercialization of genetically modified crops in 2023. J. Integr. Agric. 2024, 23, 3943–3952. [Google Scholar] [CrossRef]
- Park, Y.; González-Martínez, R.M.; Navarro-Cerrillo, G.; Chakroun, M.; Kim, Y.; Ziarsolo, P.; Blanca, J.; Cañizares, J.; Ferré, J.; Herrero, S. ABCC transporters mediate insect resistance to multiple Bt toxins revealed by bulk segregant analysis. BMC Biol. 2014, 12, 46. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberón, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef]
- Pigott, C.R.; Ellar, D.J. Role of Receptors in Bacillus thuringiensis Crystal Toxin Activity. Microbiol. Mol. Biol. Rev. 2007, 71, 255–281. [Google Scholar] [CrossRef]
- Yao, X.; Duan, Y.; Deng, Z.; Zhao, W.; Wei, J.; Li, X.; An, S. ATP Synthase Subunit α from Helicoverpa armigera Acts as a Receptor of Bacillus thuringiensis Cry1Ac and Synergizes Cry1Ac Toxicity. J. Agric. Food Chem. 2023, 71, 6155–6163. [Google Scholar] [CrossRef]
- Yao, X.; Liu, C.; Duan, Y.; An, S.; Wei, J.; Liang, G. ABCC2 is a functional receptor of Bacillus thuringiensis Cry1Ca in Spodoptera litura. Int. J. Biol. Macromol. 2022, 194, 9–16. [Google Scholar] [CrossRef]
- Meng, M.; Shen, C.; Lin, M.; Jin, J.; Chen, W.; Zhang, X.; Xu, C.; Hu, X.; Zhu, Q.; Chen, C.; et al. Characterization of the individual domains of the Bacillus thuringiensis Cry2Aa implicates Domain I as a possible binding site to Helicoverpa armigera. J. Invertebr. Pathol. 2024, 205, 108129. [Google Scholar] [CrossRef]
- Zhang, F.; Peng, D.; Cheng, C.; Zhou, W.; Ju, S.; Wan, D.; Yu, Z.; Shi, J.; Deng, Y.; Wang, F.; et al. Bacillus thuringiensis Crystal Protein Cry6Aa Triggers Caenorhabditis elegans Necrosis Pathway Mediated by Aspartic Protease (ASP-1). PLOS Pathog. 2016, 12, e1005389. [Google Scholar] [CrossRef]
- Zhao, X.-D.; Zhang, B.-W.; Fu, L.-J.; Li, Q.-L.; Lin, Y.; Yu, X.-Q. Possible Insecticidal Mechanism of Cry41-Related Toxin against Myzus persicae by Enhancing Cathepsin B Activity. J. Agric. Food Chem. 2020, 68, 4607–4615. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Hu, X.-H.; Wu, S.-Q.; Batool, K.; Chowdhury, M.; Lin, Y.; Zhang, J.; Gill, S.S.; Guan, X.; Yu, X.-Q. Aedes aegypti Galectin Competes with Cry11Aa for Binding to ALP1 To Modulate Cry Toxicity. J. Agric. Food Chem. 2018, 66, 13435–13443. [Google Scholar] [CrossRef] [PubMed]
- Favret, C.; Miller, G.L.; Nafría, J.M.N.; Gabaudan, F.C. Catalog of the Aphid Genera Described from the New World. Trans. Am. Èntomol. Soc. 2007, 133, 363–412. [Google Scholar] [CrossRef]
- Yusuf, S.W.; Collins, G.G. Effect of Soil Sulphur Levels on Feeding Preference of Brevicoryne brassicae on Brussels Sprouts. J. Chem. Ecol. 1998, 24, 417–424. [Google Scholar] [CrossRef]
- Farooq, A.V.; Valyi-Nagy, T.; Shukla, D. Mediators and Mechanisms of Herpes Simplex Virus Entry into Ocular Cells. Curr. Eye Res. 2010, 35, 445–450. [Google Scholar] [CrossRef]
- Scholthof, K.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Chougule, N.P.; Bonning, B.C. Toxins for Transgenic Resistance to Hemipteran Pests. Toxins 2012, 4, 405–429. [Google Scholar] [CrossRef]
- Noack, F.; Engist, D.; Gantois, J.; Gaur, V.; Hyjazie, B.F.; Larsen, A.; M’gonigle, L.K.; Missirian, A.; Qaim, M.; Sargent, R.D.; et al. Environmental impacts of genetically modified crops. Science 2024, 385, eado9340. [Google Scholar] [CrossRef]
- Ramirez-Romero, R.; Desneux, N.; Chaufaux, J.; Kaiser, L. Bt-maize effects on biological parameters of the non-target aphid Sitobion avenae (Homoptera: Aphididae) and Cry1Ab toxin detection. Pestic. Biochem. Physiol. 2008, 91, 110–115. [Google Scholar] [CrossRef]
- Romeis, J.; McLean, M.A.; Shelton, A.M. When bad science makes good headlines: Bt maize and regulatory bans. Nat. Biotechnol. 2013, 31, 386–387. [Google Scholar] [CrossRef]
- Zhang, J.-H. Long-term effects of transgenic Bacillus thuringiensis cotton on the non-target Aphis gossypii (Homoptera: Aphididae) maintained for multiple generations. Afr. J. Biotechnol. 2012, 11, 9873–9880. [Google Scholar] [CrossRef][Green Version]
- Cui, J.J.; Xia, J.Y. Effects of Bt (Bacillus thuringiensis) transgenic cotton on the dynamics of pest population and their enemies. Acta Phytophylacica Sin. 2000, 27, 141–145. [Google Scholar]
- Paula, D.P.; Andow, D.A. Differential Cry toxin detection and effect on Brevicoryne brassicae and Myzus persicae feeding on artificial diet. Èntomol. Exp. Appl. 2016, 159, 54–60. [Google Scholar] [CrossRef]
- Porcar, M.; Grenier, A.-M.; Federici, B.; Rahbé, Y. Effects of Bacillus thuringiensis δ-Endotoxins on the Pea Aphid (Acyrthosiphon pisum). Appl. Environ. Microbiol. 2009, 75, 4897–4900. [Google Scholar] [CrossRef]
- Hurej, M.; Twardowski, J.P.; Beres, P.; Klukowski, Z. Population Development of Bird Cherry-Oat Aphid Rhopalosiphum padi (L.) (Hemiptera, Aphididae) on Conventional and Bt-Maize Expressing the Insecticidal Protein Cry1Ab. Bulg. J. Agric. Sci. 2014, 20, 658–665. [Google Scholar]
- Shu, Y.; Du, Y.; Wang, J. Presence of Cry1Ab in the Bt maize—aphid (Rhopalosiphum maidis)—ladybeetle (Propylea japonica) system has no adverse effects on insect biological parameters. Èntomol. Exp. Appl. 2019, 167, 553–560. [Google Scholar] [CrossRef]
- Koyama, N.; Sano, Y.; Tanaka, M.; Haginoya, K.; Promodonkoy, B.; Angsuthanasombat, C.; Mitsui, T.; Khoya, T.; Tanigushi, M.; Okazaki, K.; et al. Bacillus thuringiensis cytotoxin, Cyt2Aa killed Myzus persicae (Sulzer) (Hemiptera: Aphididae). Bull. Fac. Agric. Niigata Univ. 2011, 63, 77–81. [Google Scholar]
- Tian, J.-C.; Yao, J.; Long, L.-P.; Romeis, J.; Shelton, A.M. Bt crops benefit natural enemies to control non-target pests. Sci. Rep. 2015, 5, 16636. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, G.; Hu, X.; Liu, J.; Li, M.; Batool, K.; Chen, M.; Wang, J.; Xu, J.; Huang, T.; et al. Cry11Aa Interacts with the ATP-Binding Protein from Culex quinquefasciatus To Improve the Toxicity. J. Agric. Food Chem. 2017, 65, 10884–10890. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Z.; Liu, Y.; Liang, G.; Shu, C.; Song, F.; Zhou, X.; Bravo, A.; Soberón, M.; Zhang, J. Identification of ABCC2 as a binding protein of Cry1Ac on brush border membrane vesicles from Helicoverpa armigera by an improved pull-down assay. Microbiologyopen 2016, 5, 659–669. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Jurat-Fuentes, J.; McNall, R.; Andacht, T.; Adang, M.J. Identification of novel Cry1Ac binding proteins in midgut membranes from Heliothis virescens using proteomic analyses. Insect Biochem. Mol. Biol. 2007, 37, 189–201. [Google Scholar] [CrossRef]
- Chen, L.; Liang, G.; Zhang, J.; Wu, K.; Guo, Y.; Rector, B.G. Proteomic analysis of novel Cry1Ac binding proteins in Helicoverpa armigera (Hübner). Arch. Insect Biochem. Physiol. 2010, 73, 61–73. [Google Scholar] [CrossRef]
- Bourne, H.R.; Sanders, D.A.; McCormick, F. The GTPase superfamily: A conserved switch for diverse cell functions. Nature 1990, 348, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Mandava, C.S.; Guo, Q.; Wang, J.; Cao, W.; Li, N.; Zhang, Y.; Zhang, Y.; Wang, Z.; Wu, J.; et al. Structural and Functional Insights into the Mode of Action of a Universally Conserved Obg GTPase. PLOS Biol. 2014, 12, e1001866. [Google Scholar] [CrossRef]
- Sprang, S.R. G PROTEIN MECHANISMS: Insights from Structural Analysis. Annu. Rev. Biochem. 1997, 66, 639–678. [Google Scholar] [CrossRef]
- Li, L.-L.; Wang, Z.-Y.; He, K.-L.; Bai, S.-X.; Hua, L. Effects of transgenic corn expressing Bacillus thuringiensis cry1Ab toxin on population increase of Rhopalosiphum maidis Fitch. J. Appl. Ecol. 2007, 18, 1077–1080. [Google Scholar]
- Christen, V.; Mittner, F.; Fent, K. Molecular Effects of Neonicotinoids in Honey Bees (Apis mellifera). Environ. Sci. Technol. 2016, 50, 4071–4081. [Google Scholar] [CrossRef]
- Ichimiya, T.; Yamakawa, T.; Hirano, T.; Yokoyama, Y.; Hayashi, Y.; Hirayama, D.; Wagatsuma, K.; Itoi, T.; Nakase, H. Autophagy and Autophagy-Related Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 8974. [Google Scholar] [CrossRef]
- Ktistakis, N.T.; Tooze, S.A. Digesting the Expanding Mechanisms of Autophagy. Trends Cell Biol. 2016, 26, 624–635. [Google Scholar] [CrossRef]
- Jiang, K.; Hou, X.-Y.; Tan, T.-T.; Cao, Z.-L.; Mei, S.-Q.; Yan, B.; Chang, J.; Han, L.; Zhao, D.; Cai, J. Scavenger receptor-C acts as a receptor for Bacillus thuringiensis vegetative insecticidal protein Vip3Aa and mediates the internalization of Vip3Aa via endocytosis. PLOS Pathog. 2018, 14, e1007347. [Google Scholar] [CrossRef]
- Jin, M.; Shan, Y.; Peng, Y.; Wang, P.; Li, Q.; Yu, S.; Zhang, L.; Xiao, Y. An Integrative Analysis of Transcriptomics and Proteomics Reveals Novel Insights into the Response in the Midgut of Spodoptera frugiperda Larvae to Vip3Aa. Toxins 2022, 14, 55. [Google Scholar] [CrossRef]
- Nizet, V. Bacteria and Phagocytes: Mortal Enemies. J. Innate Immun. 2010, 2, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Stuart, L.M.; Ezekowitz, R.A. Phagocytosis and comparative innate immunity: Learning on the fly. Nat. Rev. Immunol. 2008, 8, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, C.J.; Han, Q.; Mehere, P.; Ding, H.; Christensen, B.M.; Li, J. Tyrosine metabolic enzymes from insects and mammals: A comparative perspective. Insect Sci. 2014, 21, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Islinger, M.; Voelkl, A.; Fahimi, H.D.; Schrader, M. The peroxisome: An update on mysteries 2.0. Histochem. Cell Biol. 2018, 150, 443–471. [Google Scholar] [CrossRef]
- Edgar, B.A. How flies get their size: Genetics meets physiology. Nat. Rev. Genet. 2006, 7, 907–916. [Google Scholar] [CrossRef]
- Wilson, D.F. Oxidative phosphorylation: Regulation and role in cellular and tissue metabolism. J. Physiol. 2017, 595, 7023–7038. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, X.-D.; Liao, J.-A.; Fu, L.-J.; Lin, Y. Cry41-Related Mutants against Myzus persicae Based on Its Interaction with Cathepsin B. ACS Agric. Sci. Technol. 2023, 3, 350–358. [Google Scholar] [CrossRef]
- Aggarwal, N.; Sloane, B.F. Cathepsin B: Multiple roles in cancer. Proteom.-Clin. Appl. 2014, 8, 427–437. [Google Scholar] [CrossRef]
- Droga-Mazovec, G.; Bojič, L.; Petelin, A.; Ivanova, S.; Romih, R.; Repnik, U.; Salvesen, G.S.; Stoka, V.; Turk, V.; Turk, B. Cysteine Cathepsins Trigger Caspase-dependent Cell Death through Cleavage of Bid and Antiapoptotic Bcl-2 Homologues. J. Biol. Chem. 2008, 283, 19140–19150. [Google Scholar] [CrossRef]
- Harrison, R.L.; Bonning, B.C. Proteases as Insecticidal Agents. Toxins 2010, 2, 935–953. [Google Scholar] [CrossRef]
- Lumbierres, B.; Albajes, R.; Pons, X. Transgenic Bt maize and Rhopalosiphum padi (Hom., Aphididae) performance. Ecol. Èntomol. 2004, 29, 309–317. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, X.-D.; Lu, J.-W.; Zhang, B.-W.; Lei, Y.-Q.; Lin, Y. Protection of HSP60 in Myzus persicae Treated with Cry7Ab4 Toxin. ACS Agric. Sci. Technol. 2023, 3, 211–221. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Zhang, Y.; Chen, F.; Sun, M.; Li, S.; Zhang, J.; Zhang, F. Resistance to both aphids and nematodes in tobacco plants expressing a Bacillus thuringiensis crystal protein. Pest Manag. Sci. 2024, 80, 3098–3106. [Google Scholar] [CrossRef]
- Pardo-López, L.; Soberón, M.; Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Vílchez, S. Making 3D-Cry Toxin Mutants: Much More Than a Tool of Understanding Toxins Mechanism of Action. Toxins 2020, 12, 600. [Google Scholar] [CrossRef]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef]
- Smedley, D.P.; Ellar, D.J. Mutagenesis of three surface-exposed loops of a Bacillus thuringiensis insecticidal toxin reveals residues important for toxicity, receptor recognition and possibly membrane insertion. Microbiology 1996, 142, 1617–1624. [Google Scholar] [CrossRef][Green Version]
- Wang, F.; Liu, Y.; Zhang, F.; Chai, L.; Ruan, L.; Peng, D.; Sun, M. Improvement of Crystal Solubility and Increasing Toxicity against Caenorhabditis elegans by Asparagine Substitution in Block 3 of Bacillus thuringiensis Crystal Protein Cry5Ba. Appl. Environ. Microbiol. 2012, 78, 7197–7204. [Google Scholar] [CrossRef]
- Wu, S.-J.; Dean, D.H. Functional Significance of Loops in The Receptor Binding Domain of Bacillus thuringiensis CryIIIA δ-Endotoxin. J. Mol. Biol. 1996, 255, 628–640. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, B.; Lu, J.; Liao, J.; Zhu, Q.; Lin, Y.; Yu, X. The mechanism of Cry41-related toxin against Myzus persicae based on its interaction with Buchnera-derived ATP-dependent 6-phosphofructokinase. Pest Manag. Sci. 2023, 79, 1684–1691. [Google Scholar] [CrossRef]
- Lu, J.-W.; Jin, L.; Li, M.-G.; Yu, B.Q.; Wen, Y.-F.; Gu, Y.-Q.; Lin, Y.; Yu, X.-Q. A possible mechanism of Cry7Ab4 protein in delaying pupation of Plutella xylostella larvae. Front. Immunol. 2022, 13, 849620. [Google Scholar] [CrossRef]
- Lu, K.; Gu, Y.; Liu, X.; Lin, Y.; Yu, X.-Q. Possible Insecticidal Mechanisms Mediated by Immune-Response-Related Cry-Binding Proteins in the Midgut Juice of Plutella xylostella and Spodoptera exigua. J. Agric. Food Chem. 2017, 65, 2048–2055. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, L.; Zhang, Y.; Zhang, T.; Feng, Y.; Lu, X.; Lan, W.; Wang, J.; Wu, H.; Cao, C.; et al. Highly Efficient Production of Soluble Proteins from Insoluble Inclusion Bodies by a Two-Step-Denaturing and Refolding Method. PLoS ONE 2011, 6, e22981. [Google Scholar] [CrossRef]
- Shang, J.; Yao, Y.-S.; Chen, L.-L.; Zhu, X.-Z.; Niu, L.; Gao, X.-K.; Luo, J.-Y.; Ji, J.-C.; Cui, J.-J. Sublethal Exposure to Deltamethrin Stimulates Reproduction and Alters Symbiotic Bacteria in Aphis gossypii. J. Agric. Food Chem. 2021, 69, 15097–15107. [Google Scholar] [CrossRef] [PubMed]
- Cun, Y.; Fröhlich, H. Network and Data Integration for Biomarker Signature Discovery via Network Smoothed T-Statistics. PLoS ONE 2013, 8, e73074. [Google Scholar] [CrossRef]
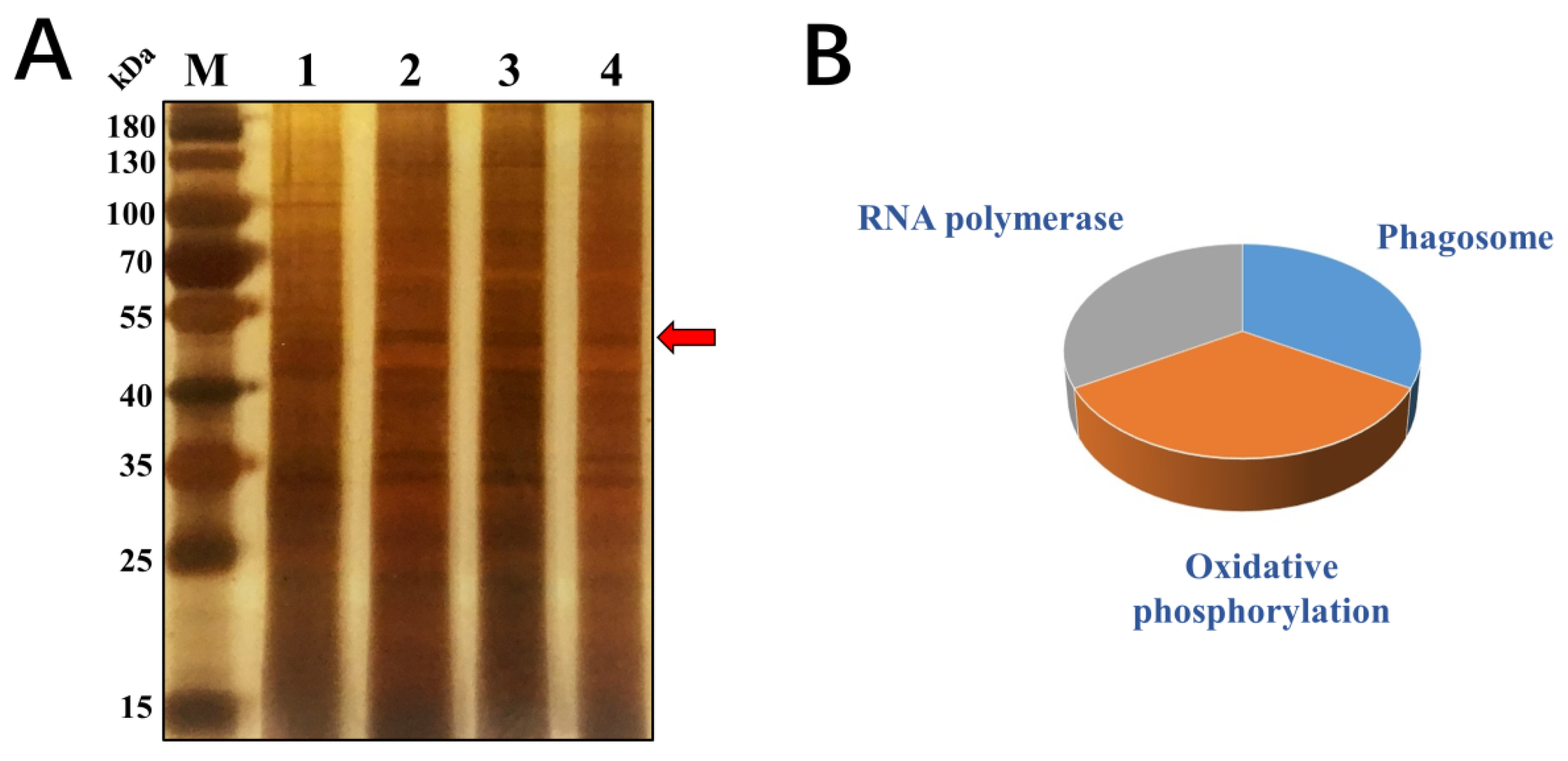

| Sample | Accession Number | Score | Protein Description |
|---|---|---|---|
| 1 | tr|T1UMS9|T1UMS9_APHGO | 82 | Beta-actin OS = Aphis gossypii |
| 2 | tr|J9K1R5|J9K1R5_ACYPI | 31 | GPN-loop GTPase 2 OS = Acyrthosiphon pisum |
| 3 | tr|J9M0Y0|J9M0Y0_ACYPI | 29 | Uncharacterized protein OS = Acyrthosiphon pisum |
| 4 | tr|Q5XUA1|Q5XUA1_TOXCI | 28 | ATP synthase subunit alpha OS = Toxoptera citricida |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Zhang, B.; Aguila, L.C.R.; Lu, J.; Gao, X.; Luo, J.; Cui, J.; Lin, Y. Potential Mechanisms Underlying the Minimal Impact of Cry1Ab1 Protein on Myzus persicae. Int. J. Mol. Sci. 2025, 26, 2924. https://doi.org/10.3390/ijms26072924
Jin L, Zhang B, Aguila LCR, Lu J, Gao X, Luo J, Cui J, Lin Y. Potential Mechanisms Underlying the Minimal Impact of Cry1Ab1 Protein on Myzus persicae. International Journal of Molecular Sciences. 2025; 26(7):2924. https://doi.org/10.3390/ijms26072924
Chicago/Turabian StyleJin, Liang, Binwu Zhang, Luis Carlos Ramos Aguila, Jingwen Lu, Xueke Gao, Junyu Luo, Jinjie Cui, and Yi Lin. 2025. "Potential Mechanisms Underlying the Minimal Impact of Cry1Ab1 Protein on Myzus persicae" International Journal of Molecular Sciences 26, no. 7: 2924. https://doi.org/10.3390/ijms26072924
APA StyleJin, L., Zhang, B., Aguila, L. C. R., Lu, J., Gao, X., Luo, J., Cui, J., & Lin, Y. (2025). Potential Mechanisms Underlying the Minimal Impact of Cry1Ab1 Protein on Myzus persicae. International Journal of Molecular Sciences, 26(7), 2924. https://doi.org/10.3390/ijms26072924





