Impact of SGLT2 Inhibitors on Magnesium in Kidney Transplant Patients with and Without Diabetes
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Mg2+ | Magnesium |
| CKD | Chronic kidney disease |
| CNi | Calcineurin inhibitors |
| SGLT2i | Sodium-glucose cotransporter 2 inhibitors |
| KTR | Kidney transplant recipient |
| eGFR | Estimated glomerular filtration rate |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| IFG | Impaired fasting glucose |
References
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S.; Van Biesen, W. Hypomagnesaemia in Kidney Transplantation. Transplant. Rev. 2015, 29, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Ehrenpreis, E.D.; Jarrouj, G.; Meader, R.; Wagner, C.; Ellis, M. A Comprehensive Review of Hypomagnesemia. Dis. Mon. 2022, 68, 101285. [Google Scholar] [CrossRef]
- Ahmed, F.; Mohammed, A. Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia. Med. Sci. 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Bulló, M.; Estruch, R.; Corella, D.; Martínez-González, M.A.; Ros, E.; Covas, M.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary Magnesium Intake Is Inversely Associated with Mortality in Adults at High Cardiovascular Disease Risk. J. Nutr. 2014, 144, 55–60. [Google Scholar] [CrossRef]
- Akarolo-Anthony, S.N.; Jiménez, M.C.; Chiuve, S.E.; Spiegelman, D.; Willett, W.C.; Rexrode, K.M. Plasma Magnesium and Risk of Ischemic Stroke among Women. Stroke 2014, 45, 2881–2886. [Google Scholar] [CrossRef]
- Peacock, J.M.; Ohira, T.; Post, W.; Sotoodehnia, N.; Rosamond, W.; Folsom, A.R. Serum Magnesium and Risk of Sudden Cardiac Death in the Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2010, 160, 464–470. [Google Scholar] [CrossRef]
- Van Laecke, S.; Nagler, E.V.; Verbeke, F.; Van Biesen, W.; Vanholder, R. Hypomagnesemia and the Risk of Death and GFR Decline in Chronic Kidney Disease. Am. J. Med. 2013, 126, 825–831. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Isaka, Y. Hypomagnesemia Is a Significant Predictor of Cardiovascular and Non-Cardiovascular Mortality in Patients Undergoing Hemodialysis. Kidney Int. 2014, 85, 174–181. [Google Scholar] [CrossRef]
- Barton, C.H.; Vaziri, N.D.; Martin, D.C.; Choi, S.; Alikhani, S. Hypomagnesemia and Renal Magnesium Wasting in Renal Transplant Recipients Receiving Cyclosporine. Am. J. Med. 1987, 83, 693–699. [Google Scholar] [CrossRef]
- Higgins, R.M.; Hart, P.; Lam, F.T.; Kashi, H. Conversion from Tacrolimus to Cyclosporine in Stable Renal Transplant Patients: Safety, Metabolic Changes, and Pharmacokinetic Comparison. Transplantation 2000, 69, 1736–1739. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Sankarasubbaiyan, S.; Gross, M.D.; Jeevanantham, V.; Monk, R.D. Tacrolimus-Associated Hypomagnesemia in Renal Transplant Recipients. Transplant. Proc. 2006, 38, 1320–1322. [Google Scholar] [CrossRef]
- Mazzola, B.L.; Vannini, S.D.P.; Truttmann, A.C.; von Vigier, R.O.; Wermuth, B.; Ferrari, P.; Bianchetti, M.G. Long-Term Calcineurin Inhibition and Magnesium Balance after Renal Transplantation. Transplant. Int. 2003, 16, 76–81. [Google Scholar] [CrossRef]
- Adomako, E.A.; Yu, A.S.L. Magnesium Disorders: Core Curriculum 2024. Am. J. Kidney Dis. 2024, 83, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Szumilas, K.; Wilk, A.; Wiśniewski, P.; Gimpel, A.; Dziedziejko, V.; Kipp, M.; Pawlik, A. Current Status Regarding Immunosuppressive Treatment in Patients after Renal Transplantation. Int. J. Mol. Sci. 2023, 24, 10301. [Google Scholar] [CrossRef] [PubMed]
- Ranade, V.V.; Somberg, J.C. Bioavailability and Pharmacokinetics of Magnesium After Administration of Magnesium Salts to Humans. Am. J. Ther. 2001, 8, 345. [Google Scholar]
- Toto, R.D.; Goldenberg, R.; Chertow, G.M.; Cain, V.; Stefánsson, B.V.; Sjöström, C.D.; Sartipy, P. Correction of Hypomagnesemia by Dapagliflozin in Patients with Type 2 Diabetes: A Post Hoc Analysis of 10 Randomized, Placebo-Controlled Trials. J. Diabetes Its Complicat. 2019, 33, 107402. [Google Scholar] [CrossRef]
- Cianciolo, G.; De Pascalis, A.; Capelli, I.; Gasperoni, L.; Di Lullo, L.; Bellasi, A.; La Manna, G. Mineral and Electrolyte Disorders with SGLT2i Therapy. JBMR Plus 2019, 3, e10242. [Google Scholar] [CrossRef]
- Sánchez Fructuoso, A.I.; Bedia Raba, A.; Banegas Deras, E.; Vigara Sánchez, L.A.; Valero San Cecilio, R.; Franco Esteve, A.; Cruzado Vega, L.; Gavela Martínez, E.; González Garcia, M.E.; Saurdy Coronado, P.; et al. Sodium-Glucose Cotransporter-2 Inhibitor Therapy in Kidney Transplant Patients with Type 2 or Post-Transplant Diabetes: An Observational Multicentre Study. Clin. Kidney J. 2023, 16, 1022–1034. [Google Scholar] [CrossRef]
- Song, C.C.; Brown, A.; Winstead, R.; Yakubu, I.; Demehin, M.; Kumar, D.; Gupta, G. Early Initiation of Sodium-Glucose Linked Transporter Inhibitors (SGLT-2i) and Associated Metabolic and Electrolyte Outcomes in Diabetic Kidney Transplant Recipients. Endocrinol. Diabetes Metab. 2021, 4, e00185. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, X.; Zhang, J.; Li, Y.; Del Gobbo, L.C.; Zhai, S.; Song, Y. Elevated Serum Magnesium Associated with SGLT2 Inhibitor Use in Type 2 Diabetes Patients: A Meta-Analysis of Randomised Controlled Trials. Diabetologia 2016, 59, 2546–2551. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Alon, I.; Almoznino-Sarafian, D.; Zaidenstein, R.; Weissgarten, J.; Gorelik, O.; Berman, S.; Modai, D.; Golik, A. Metabolic and Clinical Effects of Oral Magnesium Supplementation in Furosemide-Treated Patients with Severe Congestive Heart Failure. Clin. Cardiol. 2000, 23, 433–436. [Google Scholar] [CrossRef]
- Mondellini, G.M.; Verbrugge, F.H. Evaluation and Management of Hyponatremia in Heart Failure. Curr. Heart Fail. Rep. 2024, 21, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Leary, W.P.; Reyes, A.J. Diuretic-Induced Magnesium Losses. Drugs 1984, 28 (Suppl. S1), 182–187. [Google Scholar] [CrossRef] [PubMed]
- Bilancio, G.; Celano, M.; Cozza, V.; Zingone, F.; Palladino, G.; Cirillo, M. Early Prediction of Cardiovascular Disease in Kidney Transplant Recipients. Transplant. Proc. 2017, 49, 2092–2098. [Google Scholar] [CrossRef]
- Agenzia Italiana del Farmaco—Nota 100. Available online: https://www.aifa.gov.it/nota-100 (accessed on 25 February 2025).
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Mason, J.B. Vitamins, Trace Minerals, and Other Micronutrients. In Goldman’s Cecil Medicine; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1397–1406. ISBN 978-1-4377-1604-7. [Google Scholar]
| Number of patients | 63 |
| Sex, male, n (%) | 52 (82.5%) |
| Age, years (median, IQR) | 55 (41.8–55) |
| Diabetes, n (%) | 20 (31.7%) |
| IFG | 8 (12.7%) |
| Weight, kg | 81.8 ± 15.7 |
| SBP, mmHg | 139 ± 15 |
| DBP, mmHg | 83 ± 10 |
| Hypertension | 63 (100%) |
| Diuretic therapy | 11 (17.5%) |
| Mg2+ supplements | 21 (33.3%) |
| Dyslipidemia | 56 (89.9%) |
| Tacrolimus, n (%) | 40 (63.5%) |
| Ciclosporine, n (%) | 20 (31.7%) |
| Everolimus, n (%) | 15 (23.8%) |
| Mycophenolate, n (%) | 44 (69.8%) |
| Steroids, n (%) | 60 (95.2%) |
| Creatinine, mg/dL | 1.45 ± 0.45 |
| eGFR mL/min/1.73 m2 | 56 ± 19 |
| Glucose, mg/dL | 95 (83–119) |
| Urate, mg/dL | 6.2 ± 1.3 |
| Sodium, mEq/L | 139 ± 2.6 |
| Potassium, mEq/L | 4.3 (3.9–4.6) |
| Calcium, mg/dL | 9.5 ± 0.5 |
| Phosphorus, mg/dL | 3.7 ± 0.8 |
| Magnesium, mg/dL | 1.7 ± 0.2 |
| Hemoglobin, g/dL | 13.2 ± 1.53 |
| T0 | T1 | T2 | |
|---|---|---|---|
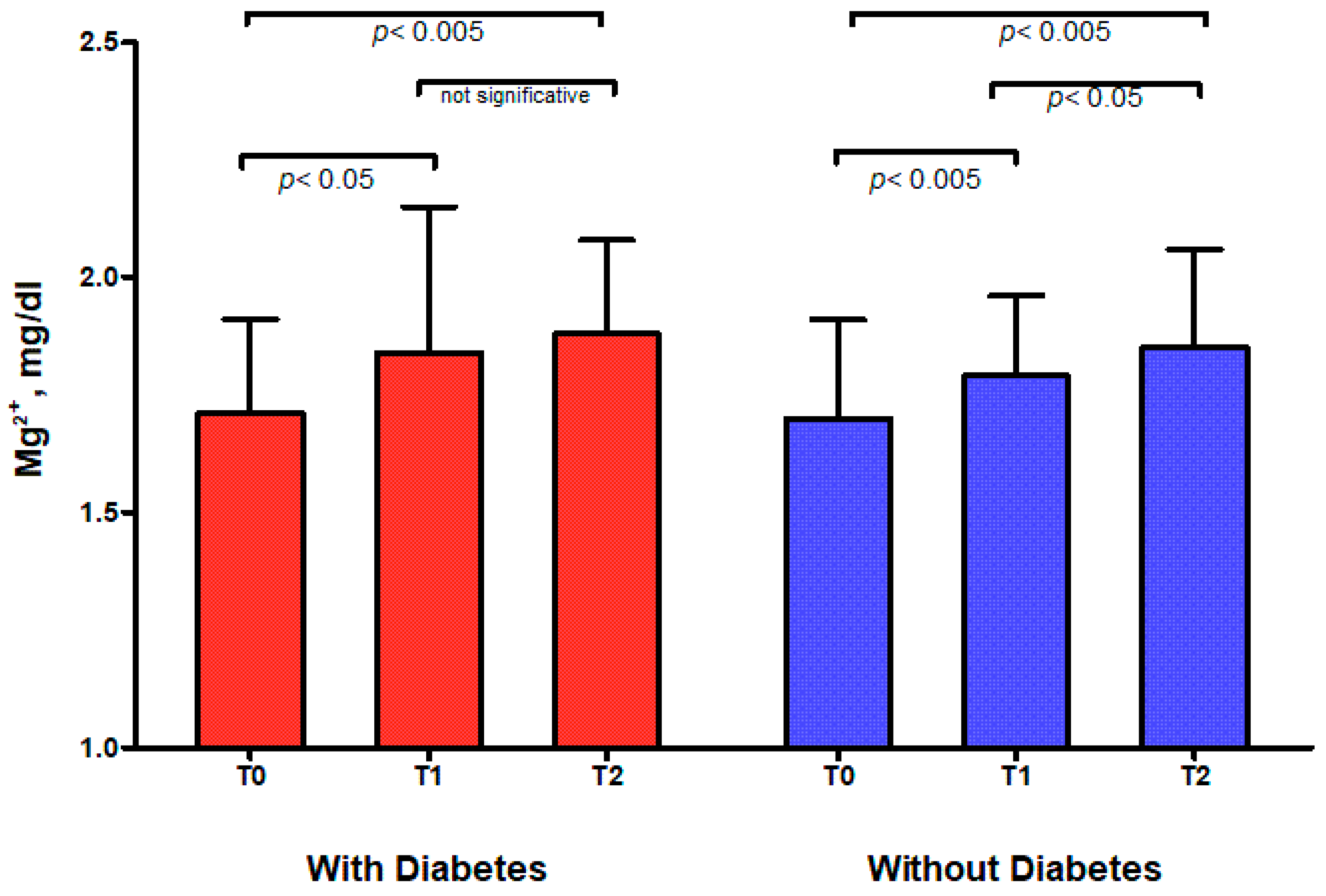
| Whole cohort | 1.70 ± 0.21 | 1.81 ± 0.22 ** | 1.86 ± 0.21 ‡ |
| With Diabetes | 1.71 ± 0.20 | 1.84 ± 0.31 * | 1.88 ± 0.20 ns |
| Without Diabetes | 1.70 ± 0.21 | 1.79 ± 0.17 ** | 1.85 ± 0.21 ‡ |
| Time Points | Whole Cohort | With Diabetes | Without Diabetes |
|---|---|---|---|
| T0, n (%) | 24 (38.1%) | 7 (11.1%) | 17 (27%) |
| T1 *, n (%) | 13 (20.6%) * | 5 (7.9%) | 8 (12.7%) |
| T2 **, n (%) | 10 (15.9%) ** | 2 (3.2%) | 8 (12.7%) |
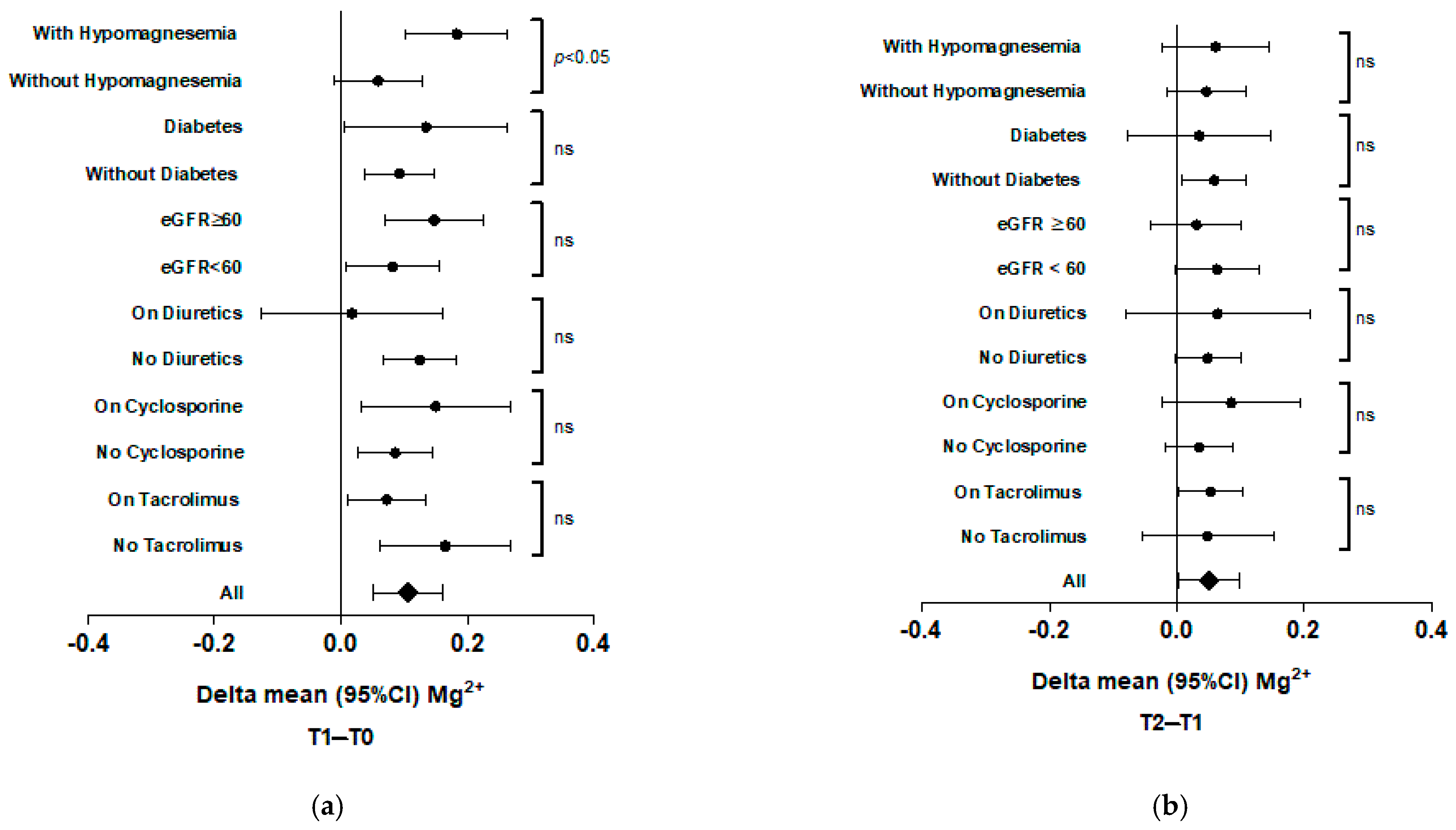
| Delta | |||||
|---|---|---|---|---|---|
| T1 | T2 | T1-T0 | T2-T0 | T2-T1 | |
| Body weight, kg | 80.6 ± 15.8 | 78.5 ± 18.3 | −0.93 ** | −2.6 ns | −1.62 ns |
| Systolic blood pressure, mmHg | 133 ± 13 | 135 ± 15 | −5.87 ** | −4.8 * | 0.29 ns |
| Diastolic blood pressure, mmHg | 81 ± 9 | 79 ± 8 | −2.87 * | −4.7 ** | −2.06 ns |
| Creatinine, mg/dL | 1.55 ± 0.5 | 1.57 ± 0.6 | 0.1 * | 0.11 ns | 0.003 ns |
| eGFR, mL/min/1.73 m2 | 53 ± 19 | 53 ± 20 | −3.49 * | −3.42 ns | 0.27 ns |
| Urate, mg/dL | 5.7 ± 1.3 | 5.6 ± 1.6 | −0.53 * | −0.60 ** | −0.09 ns |
| Sodium, mEq/L | 140 ± 2 | 139 ± 3 | 0.31 ns | −0.08 ns | −0.33 ns |
| Calcium, mg/dL | 9.6 ± 0.6 | 9.6 ± 0.6 | 0.05 ns | 0.14 ns | 0.01 ns |
| Phosphorus, mg/dL | 3.7 ± 1.1 | 3.5 ± 0.8 | 0.07 ns | −0.05 ns | −0.2 ns |
| Hemoglobin, g/dL | 13.4 ± 1.7 | 13.3 ± 1.8 | 0.13 ns | 0.11 ns | −0.02 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secondulfo, C.; Vecchione, N.; Russo, D.; Hamzeh, S.; Iacuzzo, C.; Apicella, L.; Di Pietro, R.A.; Pisani, A.; Amicone, M.; Cirillo, M.; et al. Impact of SGLT2 Inhibitors on Magnesium in Kidney Transplant Patients with and Without Diabetes. Int. J. Mol. Sci. 2025, 26, 2904. https://doi.org/10.3390/ijms26072904
Secondulfo C, Vecchione N, Russo D, Hamzeh S, Iacuzzo C, Apicella L, Di Pietro RA, Pisani A, Amicone M, Cirillo M, et al. Impact of SGLT2 Inhibitors on Magnesium in Kidney Transplant Patients with and Without Diabetes. International Journal of Molecular Sciences. 2025; 26(7):2904. https://doi.org/10.3390/ijms26072904
Chicago/Turabian StyleSecondulfo, Carmine, Nicoletta Vecchione, Dora Russo, Sarah Hamzeh, Candida Iacuzzo, Luca Apicella, Renata Angela Di Pietro, Antonio Pisani, Maria Amicone, Massimo Cirillo, and et al. 2025. "Impact of SGLT2 Inhibitors on Magnesium in Kidney Transplant Patients with and Without Diabetes" International Journal of Molecular Sciences 26, no. 7: 2904. https://doi.org/10.3390/ijms26072904
APA StyleSecondulfo, C., Vecchione, N., Russo, D., Hamzeh, S., Iacuzzo, C., Apicella, L., Di Pietro, R. A., Pisani, A., Amicone, M., Cirillo, M., & Bilancio, G. (2025). Impact of SGLT2 Inhibitors on Magnesium in Kidney Transplant Patients with and Without Diabetes. International Journal of Molecular Sciences, 26(7), 2904. https://doi.org/10.3390/ijms26072904







