Cathepsin K Inhibitors as Potential Drugs for the Treatment of Osteoarthritis
Abstract
1. Introduction
2. Current Treatments of Osteoarthritis and Promising Approaches
| Current Treatments | Types | Effects | Ref. |
|---|---|---|---|
| Physical exercise | Running | Relief of OA symptoms | [24] |
| Walking | Relief of OA symptoms | [25] | |
| Weight management | Relief of OA symptoms | [26] | |
| Acetominophen | Acetominophem, similar to NSAIDs | Analgesic and antipyretic | [31] |
| Duloxetine | Instead of NSAIDs and acetominophen | Release pain | [27,31] |
| NSAIDs | Celecoxib | Anti-inflammatory | [27] |
| Diacerin | Anti-inflammatory | [27] | |
| Diclefenac | Antipyretic, anti-inflammatory | [28,29,30] | |
| Etoricoxib | Anti-inflammatory | [29] | |
| Ibuprofen | Anti-inflammatory | [32] | |
| Naproxen | Anti-inflammatory | [32] | |
| Tramadol | Tramadol not used for first-time treatment | Opioid, relief of pain | [31] |
| Intra-articular injection | Hyaluronic acid intra-articular injection | Improves integrity of joints | [33,34,35] |
| Corticosteroid intra-articular injection | Anti-inflammatory | [26] | |
| Surgery | Joint replacement | Relieves pain and OA symptoms | [36,37,38,39,40,41] |
| Promising Treatments | Types | Effects | Ref. |
|---|---|---|---|
| Acupotomy | Acupotomy | Improves subchondral bone in rabbit | [69,70] |
| Chondroitin sulfate | Chondroitin sulfate | Protective effects on joints | [44,45] |
| Chondrocyte transplantation | Chondrocyte transplantation | Cells did not reach cartilage | [88,89] |
| Cordycepin | Cordycepin | Chondroprotective effects on explants | [46] |
| Melatonin | Melatonin | Protects chondrocytes and cartilage | [53] |
| Inhibitors of chondrocyte receptors | Inhibition of Asporin | May prevent cartilage degradation | [51] |
| Inhibition of CXCR4 | Activates cartilage progenitor cells | [51] | |
| Inhibition of DDR2 | Prevents mice cartilage degeneration | [52] | |
| Inhibitors of metalloproteases | Inhibitors of metalloproteases | Proposed as a treatment of OA | [42,43] |
| Intra-articular injection of EVs | Intra-articular injections of EVs | Reduces cartilage loss in animals | [92,108,109,110,111,112] |
| Intra-articular injection of MSCs | Intra-articular injections of MSCs | Clinical trials hold promise | [71,72,73,74,75,76,77,78,79,80,81,82,83,84,85] |
| Nutraceuticals | Curcumin | Reduces pain but less than NSAIDs | [57,58] |
| Flavonoids | More clinical trials are needed | [59,60,61] | |
| Ginseng | Anti-oxidant and anti-inflammatory | [62] | |
| Grapefruit | Chondroprotective effects on rat OA | [63] | |
| Omega-3 fatty acids | No changes in OA parameters | [57,64] | |
| Polyphenols | More clinical trials are needed | [65] | |
| Vitamin D | Inconclusive | [66,67,68] | |
| Strontium ranelate | Strontium ranelate | Reduces the progression of dog OA | [47] |
3. Development of Epiphyseal Cartilage
4. Articular Cartilage
5. Degradation of Cartilage
5.1. Pathological Mechanisms of OA
5.2. Enzymatic Degradation of Cartilage
6. Functions of Cathepsin K
6.1. Involvement of Cathepsin K in Osteoarthritis Physiopathology
6.2. Function and Level of Expression of Cathepsin K
7. Design and In Vitro Effects of Cathepsin K Inhibitors
8. Pre-Clinical and Clinical Trials on Cathepsin K Inhibitors
| Inhibitors | INN | Target Diseases | Clinical Trial | Benefits | Side Effects | Ref. |
|---|---|---|---|---|---|---|
| Odanacatib | MK-0822 | Osteoporosis | NCT00620113 NCT00729183 NCT00529373 NCT01120600 | Reduction in bone fracture rates | Increased risk of cardiovascular events | [293,294,295] |
| Balicatib | AAE581 | Knee OA or osteoporosis | NCT00371670 NCT00170911 NCT00100607 | Improvement of bone and cartilage structure | Skin rashes and dermal fibrosis | [296,297] |
| MIV-711 | Knee OA | NCT02705625 | Reduction in medial femoral cartilage thickness | No change in NRS pain scores compared with placebo | [303,304] | |
| MIV-711 | Knee OA | NCT03037489 | Reduced change in bone area Greater reduction in unilateral knee joint pain | No difference in cartilage thickness | [305] |
| Inhibitors/ INN | Target Diseases | Admnist. | In Vivo Models | Benefits | Side Effects | Ref. |
|---|---|---|---|---|---|---|
| Balicatib/AAE581 | Osteoporosis | Gavage | Ovariectomized monkeys | Stimulation of periosteal bone formation (spine and femur) | Not described | [298] |
| SB-553484 | Knee OA | Oral | Female beagle dogs with partial medial meniscectomy | Reduction of cartilage degradation; reduced urine levels of CTX-I and CTX-II | Not described | [299] |
| AZ12606133 | OA | Oral | Guinea pig (spontaneous model) | Decreased joint pain; reduced urine levels of CTX-II | Not described | [235] |
| L-006235 | Knee OA | Encapsulated in hydrogel and knee insertion | DMM mice | Reduced cartilage degeneration | Not described | [300] |
| L-006235 | Knee OA | Oral | ACLT mice and rabbits | Protection of cartilage damage | Not described | [301] |
| MIV-711 | Knee OA | Oral | ACLT rabbits and dogs | Attenuation of joint degradation | Well tolerated | [302] |
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salman, L.A.; Ahmed, G.; Dakin, S.G.; Kendrick, B.; Price, A. Osteoarthritis: A Narrative Review of Molecular Approaches to Disease Management. Arthritis Res. Ther. 2023, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Belluzzi, E.; Todros, S.; Pozzuoli, A.; Ruggieri, P.; Carniel, E.L.; Berardo, A. Human Cartilage Biomechanics: Experimental and Theoretical Approaches towards the Identification of Mechanical Properties in Healthy and Osteoarthritic Conditions. Processes 2023, 11, 1014. [Google Scholar] [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, M.; Namane, M.; Cicuttini, F. Osteoarthritis. Lancet 2025, 405, 71–85. [Google Scholar] [CrossRef]
- Moseng, T.; Vliet Vlieland, T.P.M.; Battista, S.; Beckwée, D.; Boyadzhieva, V.; Conaghan, P.G.; Costa, D.; Doherty, M.; Finney, A.G.; Georgiev, T.; et al. EULAR Recommendations for the Non-Pharmacological Core Management of Hip and Knee Osteoarthritis: 2023 Update. Ann. Rheum. Dis. 2024, 83, 730–740. [Google Scholar] [CrossRef]
- Arruda, A.; Katsoula, G.; Chen, S.; Reimann, E.; Kreitmaier, P.; Zeggini, E. The Genetics and Functional Genomics of Osteoarthritis. Annu. Rev. Genom. Hum. Genet. 2024, 25, 239–257. [Google Scholar] [CrossRef]
- Roemer, F.W.; Kwoh, C.K.; Hayashi, D.; Felson, D.T.; Guermazi, A. The Role of Radiography and MRI for Eligibility Assessment in DMOAD Trials of Knee OA. Nat. Rev. Rheumatol. 2018, 14, 372–380. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Zaucke, F. Osteoarthritis Year in Review 2024: Biology. Osteoarthr. Cartil. 2025, 33, 58–66. [Google Scholar] [CrossRef]
- van den Bosch, M.H.J.; Blom, A.B.; van der Kraan, P.M. Inflammation in Osteoarthritis: Our View on Its Presence and Involvement in Disease Development over the Years. Osteoarthr. Cartil. 2024, 32, 355–364. [Google Scholar] [CrossRef]
- Peshkova, M.; Lychagin, A.; Lipina, M.; Di Matteo, B.; Anzillotti, G.; Ronzoni, F.; Kosheleva, N.; Shpichka, A.; Royuk, V.; Fomin, V.; et al. Gender-Related Aspects in Osteoarthritis Development and Progression: A Review. Int. J. Mol. Sci. 2022, 23, 2767. [Google Scholar] [CrossRef] [PubMed]
- Binvignat, M.; Sellam, J.; Berenbaum, F.; Felson, D.T. The Role of Obesity and Adipose Tissue Dysfunction in Osteoarthritis Pain. Nat. Rev. Rheumatol. 2024, 20, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Robbins, S.R.; McDougall, J.J. Osteoarthritis: The Genesis of Pain. Rheumatology 2018, 57, iv43–iv50. [Google Scholar] [CrossRef] [PubMed]
- Spector, T.D.; Cooper, C. Radiographic Assessment of Osteoarthritis in Population Studies: Whither Kellgren and Lawrence? Osteoarthr. Cartil. 1993, 1, 203–206. [Google Scholar] [CrossRef]
- Pelletier, J.-P.; Cooper, C.; Peterfy, C.; Reginster, J.-Y.; Brandi, M.-L.; Bruyère, O.; Chapurlat, R.; Cicuttini, F.; Conaghan, P.G.; Doherty, M.; et al. What Is the Predictive Value of MRI for the Occurrence of Knee Replacement Surgery in Knee Osteoarthritis? Ann. Rheum. Dis. 2013, 72, 1594–1604. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic Signaling Pathways and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Cook, A.D.; Hamilton, J.A.; Tak, P.P. Therapeutic Options for Targeting Inflammatory Osteoarthritis Pain. Nat. Rev. Rheumatol. 2019, 15, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Knights, A.J.; Redding, S.J.; Maerz, T. Inflammation in Osteoarthritis: The Latest Progress and Ongoing Challenges. Curr. Opin. Rheumatol. 2023, 35, 128–134. [Google Scholar] [CrossRef]
- Wang, M.G.; Seale, P.; Furman, D. The Infrapatellar Fat Pad in Inflammaging, Knee Joint Health, and Osteoarthritis. NPJ Aging 2024, 10, 34. [Google Scholar] [CrossRef]
- Wood, M.J.; Miller, R.E.; Malfait, A.-M. The Genesis of Pain in Osteoarthritis: Inflammation as a Mediator of Osteoarthritis Pain. Clin. Geriatr. Med. 2022, 38, 221–238. [Google Scholar] [CrossRef]
- Yu, H.; Huang, T.; Lu, W.W.; Tong, L.; Chen, D. Osteoarthritis Pain. Int. J. Mol. Sci. 2022, 23, 4642. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. 2020, 104, 293–311. [Google Scholar] [CrossRef]
- Dodds, R.A.; Connor, J.R.; Drake, F.H.; Gowen, M. Expression of Cathepsin K Messenger RNA in Giant Cells and Their Precursors in Human Osteoarthritic Synovial Tissues. Arthritis Rheum. 1999, 42, 1588–1593. [Google Scholar] [CrossRef]
- Morko, J.P.; Söderström, M.; Säämänen, A.-M.K.; Salminen, H.J.; Vuorio, E.I. Up Regulation of Cathepsin K Expression in Articular Chondrocytes in a Transgenic Mouse Model for Osteoarthritis. Ann. Rheum. Dis. 2004, 63, 649–655. [Google Scholar] [CrossRef]
- Goldring, M.B.; Berenbaum, F. Emerging Targets in Osteoarthritis Therapy. Curr. Opin. Pharmacol. 2015, 22, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Wilken, F.; Buschner, P.; Benignus, C.; Behr, A.-M.; Rieger, J.; Beckmann, J. Pharmatherapeutic Treatment of Osteoarthrosis-Does the Pill against Already Exist? A Narrative Review. J. Pers. Med. 2023, 13, 1087. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Inoue, H.; Arai, Y.; Shimomura, S.; Nakagawa, S.; Kishida, T.; Tsuchida, S.; Kamada, Y.; Kaihara, K.; Shirai, T.; et al. Treadmill Running in Established Phase Arthritis Inhibits Joint Destruction in Rat Rheumatoid Arthritis Models. Int. J. Mol. Sci. 2019, 20, 5100. [Google Scholar] [CrossRef]
- Drummen, S.J.J.; Balogun, S.; Lahham, A.; Bennell, K.; Hinman, R.S.; Callisaya, M.; Cai, G.; Otahal, P.; Winzenberg, T.; Wang, Z.; et al. A Pilot Randomized Controlled Trial Evaluating Outdoor Community Walking for Knee Osteoarthritis: Walk. Clin. Rheumatol. 2023, 42, 1409–1421. [Google Scholar] [CrossRef]
- Gibbs, A.J.; Gray, B.; Wallis, J.A.; Taylor, N.F.; Kemp, J.L.; Hunter, D.J.; Barton, C.J. Recommendations for the Management of Hip and Knee Osteoarthritis: A Systematic Review of Clinical Practice Guidelines. Osteoarthr. Cartil. 2023, 31, 1280–1292. [Google Scholar] [CrossRef]
- Pelletier, J.-P.; Raynauld, J.-P.; Dorais, M.; Bessette, L.; Dokoupilova, E.; Morin, F.; Pavelka, K.; Paiement, P.; Martel-Pelletier, J. DISSCO Trial Investigator Group An International, Multicentre, Double-Blind, Randomized Study (DISSCO): Effect of Diacerein vs Celecoxib on Symptoms in Knee Osteoarthritis. Rheumatology 2020, 59, 3858–3868. [Google Scholar] [CrossRef]
- van Walsem, A.; Pandhi, S.; Nixon, R.M.; Guyot, P.; Karabis, A.; Moore, R.A. Relative Benefit-Risk Comparing Diclofenac to Other Traditional Non-Steroidal Anti-Inflammatory Drugs and Cyclooxygenase-2 Inhibitors in Patients with Osteoarthritis or Rheumatoid Arthritis: A Network Meta-Analysis. Arthritis Res. Ther. 2015, 17, 66. [Google Scholar] [CrossRef]
- da Costa, B.R.; Pereira, T.V.; Saadat, P.; Rudnicki, M.; Iskander, S.M.; Bodmer, N.S.; Bobos, P.; Gao, L.; Kiyomoto, H.D.; Montezuma, T.; et al. Effectiveness and Safety of Non-Steroidal Anti-Inflammatory Drugs and Opioid Treatment for Knee and Hip Osteoarthritis: Network Meta-Analysis. BMJ 2021, 375, n2321. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.G.; Christophersen, C.; Brown, S.M.; Mulcahey, M.K. Topical Nonsteroidal Anti-Inflammatory Drugs in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Phys. Sport. 2021, 49, 381–391. [Google Scholar] [CrossRef]
- Richard, M.J.; Driban, J.B.; McAlindon, T.E. Pharmaceutical Treatment of Osteoarthritis. Osteoarthr. Cartil. 2023, 31, 458–466. [Google Scholar] [CrossRef]
- Nissen, S.E.; Yeomans, N.D.; Solomon, D.H.; Lüscher, T.F.; Libby, P.; Husni, M.E.; Graham, D.Y.; Borer, J.S.; Wisniewski, L.M.; Wolski, K.E.; et al. Cardiovascular Safety of Celecoxib, Naproxen, or Ibuprofen for Arthritis. N. Engl. J. Med. 2016, 375, 2519–2529. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Wildi, L.M.; Pelletier, J.-P. Future Therapeutics for Osteoarthritis. Bone 2012, 51, 297–311. [Google Scholar] [CrossRef]
- Chavda, S.; Rabbani, S.A.; Wadhwa, T. Role and Effectiveness of Intra-Articular Injection of Hyaluronic Acid in the Treatment of Knee Osteoarthritis: A Systematic Review. Cureus 2022, 14, e24503. [Google Scholar] [CrossRef]
- Webner, D.; Huang, Y.; Hummer, C.D. Intraarticular Hyaluronic Acid Preparations for Knee Osteoarthritis: Are Some Better Than Others? Cartilage 2021, 13, 1619S–1636S. [Google Scholar] [CrossRef] [PubMed]
- Gelber, A.C. Knee Osteoarthritis. Ann. Intern. Med. 2024, 177, ITC129–ITC144. [Google Scholar] [CrossRef]
- Madry, H. Surgical Therapy in Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 1019–1034. [Google Scholar] [CrossRef]
- Zaballa, E.; Dennison, E.; Walker-Bone, K. Function and Employment after Total Hip Replacement in Older Adults: A Narrative Review. Maturitas 2023, 167, 8–16. [Google Scholar] [CrossRef]
- Martina, K.; Hunter, D.J.; Salmon, L.J.; Roe, J.P.; Dowsey, M.M. Surgery for Osteoarthritis: Total Joint Arthroplasty, Realistic Expectations of Rehabilitation and Surgical Outcomes: A Narrative Review. Clin. Geriatr. Med. 2022, 38, 385–396. [Google Scholar] [CrossRef]
- Konnyu, K.J.; Pinto, D.; Cao, W.; Aaron, R.K.; Panagiotou, O.A.; Bhuma, M.R.; Adam, G.P.; Balk, E.M.; Thoma, L.M. Rehabilitation for Total Hip Arthroplasty: A Systematic Review. Am. J. Phys. Med. Rehabil. 2023, 102, 11–18. [Google Scholar] [CrossRef] [PubMed]
- De Nanteuil, G.; Portevin, B.; Benoist, A. Disease-Modifying Anti-Osteoarthritic Drugs: Current Therapies and New Prospects around Protease Inhibition. Farmaco 2001, 56, 107–112. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Cooper, C.; Oreffo, R.O.C.; Price, A.J.; Kaux, J.F.; Maheu, E.; Cutolo, M.; Honvo, G.; Conaghan, P.G.; Berenbaum, F.; et al. Alternative and Complementary Therapies in Osteoarthritis and Cartilage Repair. Aging Clin. Exp. Res. 2020, 32, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Das, B. The Role of Inflammatory Mediators and Matrix Metalloproteinases (MMPs) in the Progression of Osteoarthritis. Biomater. Biosyst. 2024, 13, 100090. [Google Scholar] [CrossRef]
- Minoretti, P.; Santiago Sáez, A.; Liaño Riera, M.; Gómez Serrano, M.; García Martín, Á. Efficacy and Safety of Two Chondroprotective Supplements in Patients With Knee Osteoarthritis: A Randomized, Single-Blind, Pilot Study. Cureus 2024, 16, e57579. [Google Scholar] [CrossRef]
- Uebelhart, D. Clinical Review of Chondroitin Sulfate in Osteoarthritis. Osteoarthr. Cartil. 2008, 16 (Suppl. S3), S19–S21. [Google Scholar] [CrossRef]
- Hu, P.; Chen, W.; Bao, J.; Jiang, L.; Wu, L. Cordycepin Modulates Inflammatory and Catabolic Gene Expression in Interleukin-1beta-Induced Human Chondrocytes from Advanced-Stage Osteoarthritis: An in Vitro Study. Int. J. Clin. Exp. Pathol. 2014, 7, 6575–6584. [Google Scholar]
- Pelletier, J.-P.; Kapoor, M.; Fahmi, H.; Lajeunesse, D.; Blesius, A.; Maillet, J.; Martel-Pelletier, J. Strontium Ranelate Reduces the Progression of Experimental Dog Osteoarthritis by Inhibiting the Expression of Key Proteases in Cartilage and of IL-1β in the Synovium. Ann. Rheum. Dis. 2013, 72, 250–257. [Google Scholar] [CrossRef]
- Beyer, C.; Schett, G. Pharmacotherapy: Concepts of Pathogenesis and Emerging Treatments. Novel Targets in Bone and Cartilage. Best. Pract. Res. Clin. Rheumatol. 2010, 24, 489–496. [Google Scholar] [CrossRef]
- Zhai, G. Clinical Relevance of Biochemical and Metabolic Changes in Osteoarthritis. Adv. Clin. Chem. 2021, 101, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Kihara, S.; Hayashi, S.; Hashimoto, S.; Kanzaki, N.; Takayama, K.; Matsumoto, T.; Chinzei, N.; Iwasa, K.; Haneda, M.; Takeuchi, K.; et al. Cyclin-Dependent Kinase Inhibitor-1-Deficient Mice Are Susceptible to Osteoarthritis Associated with Enhanced Inflammation. J. Bone Miner. Res. 2017, 32, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Palit, P.; Thomas, S.; Gupta, G.; Ghosh, P.; Goswami, R.P.; Kumar Maity, T.; Dutta Choudhury, M. Osteoarthritis: Prognosis and Emerging Therapeutic Approach for Disease Management. Drug Dev. Res. 2021, 82, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Liao, Z.; Li, J.; Wang, Y.; Zhang, Y.; Cai, L.; Lu, W.W.; Yang, F.; Pan, H.; Chen, D. MSAB Limits Osteoarthritis Development and Progression through Inhibition of β-Catenin-DDR2 Signaling. Bioact. Mater. 2025, 46, 259–272. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Yang, H.; He, F.; Zhu, X. Melatonin: A Novel Candidate for the Treatment of Osteoarthritis. Ageing Res. Rev. 2022, 78, 101635. [Google Scholar] [CrossRef]
- Li, Z.; Dai, A.; Yang, M.; Chen, S.; Deng, Z.; Li, L. p38MAPK Signaling Pathway in Osteoarthritis: Pathological and Therapeutic Aspects. J. Inflamm. Res. 2022, 15, 723–734. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Afzal, O.; Altamimi, A.S.A.; Almalki, W.H.; Kazmi, I.; Al-Abbasi, F.A.; Alzarea, S.I.; Makeen, H.A.; Albratty, M. Potential Role of Nutraceuticals via Targeting a Wnt/β-Catenin and NF-κB Pathway in Treatment of Osteoarthritis. J. Food Biochem. 2022, 46, e14427. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, Z.; Liu, L.; Xiao, Y. Natural Compounds Protect against the Pathogenesis of Osteoarthritis by Mediating the NRF2/ARE Signaling. Front. Pharmacol. 2023, 14, 1188215. [Google Scholar] [CrossRef]
- Mathieu, S.; Soubrier, M.; Peirs, C.; Monfoulet, L.-E.; Boirie, Y.; Tournadre, A. A Meta-Analysis of the Impact of Nutritional Supplementation on Osteoarthritis Symptoms. Nutrients 2022, 14, 1607. [Google Scholar] [CrossRef]
- Swallow, J.; Seidler, K.; Barrow, M. The Mechanistic Role of Curcumin on Matrix Metalloproteinases in Osteoarthritis. Fitoterapia 2024, 174, 105870. [Google Scholar] [CrossRef]
- Ye, Y.; Zhou, J. The Protective Activity of Natural Flavonoids against Osteoarthritis by Targeting NF-κB Signaling Pathway. Front. Endocrinol. 2023, 14, 1117489. [Google Scholar] [CrossRef]
- Naselli, F.; Bellavia, D.; Costa, V.; De Luca, A.; Raimondi, L.; Giavaresi, G.; Caradonna, F. Osteoarthritis in the Elderly Population: Preclinical Evidence of Nutrigenomic Activities of Flavonoids. Nutrients 2023, 16, 112. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, F.; Liu, A.; Zhang, C.; Li, Q.; Zhang, C.; He, F.; Shang, M. Icariin: A Potential Molecule for Treatment of Knee Osteoarthritis. Front. Pharmacol. 2022, 13, 811808. [Google Scholar] [CrossRef]
- Chen, J.; Huang, L.; Liao, X. Protective Effects of Ginseng and Ginsenosides in the Development of Osteoarthritis (Review). Exp. Ther. Med. 2023, 26, 465. [Google Scholar] [CrossRef] [PubMed]
- Alazragi, R.S.; Baeissa, H.M. Chondroprotective Effects of Grapefruit (Citrus Paradisi Macfad.) Juice in a Complete Freund’s Adjuvant Rat Model of Knee Osteoarthritis. Nutrients 2023, 15, 798. [Google Scholar] [CrossRef]
- Cordingley, D.M.; Cornish, S.M. Omega-3 Fatty Acids for the Management of Osteoarthritis: A Narrative Review. Nutrients 2022, 14, 3362. [Google Scholar] [CrossRef]
- Valsamidou, E.; Gioxari, A.; Amerikanou, C.; Zoumpoulakis, P.; Skarpas, G.; Kaliora, A.C. Dietary Interventions with Polyphenols in Osteoarthritis: A Systematic Review Directed from the Preclinical Data to Randomized Clinical Studies. Nutrients 2021, 13, 1420. [Google Scholar] [CrossRef]
- Zhao, Z.-X.; He, Y.; Peng, L.-H.; Luo, X.; Liu, M.; He, C.-S.; Chen, J. Does Vitamin D Improve Symptomatic and Structural Outcomes in Knee Osteoarthritis? A Systematic Review and Meta-Analysis. Aging Clin. Exp. Res. 2021, 33, 2393–2403. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, D.; Feng, D.; Zhao, J. Association between Vitamin D and Knee Osteoarthritis: A PRISMA-Compliant Meta-Analysis. Z. Orthop. Unfall. 2021, 159, 281–287. [Google Scholar] [CrossRef]
- Chevalley, T.; Brandi, M.L.; Cashman, K.D.; Cavalier, E.; Harvey, N.C.; Maggi, S.; Cooper, C.; Al-Daghri, N.; Bock, O.; Bruyère, O.; et al. Role of Vitamin D Supplementation in the Management of Musculoskeletal Diseases: Update from an European Society of Clinical and Economical Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) Working Group. Aging Clin. Exp. Res. 2022, 34, 2603–2623. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Y.; Shi, X.-W.; Gao, Y.; Zhang, J.-Y.; Wang, C.-J.; Yang, X.; Shu, Q.; Chen, X.-L.; Fu, X.-Y.; et al. Acupotomy Contributes to Suppressing Subchondral Bone Resorption in KOA Rabbits by Regulating the OPG/RANKL Signaling Pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 8168657. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, Y.; Lu, J.; Qin, L.; Hu, T.; Zeng, X.; Wang, X.; Zhang, A.; Zhuang, Y.; Zhong, H.; et al. Acupotomy Ameliorates Subchondral Bone Absorption and Mechanical Properties in Rabbits with Knee Osteoarthritis by Regulating Bone Morphogenetic Protein 2-Smad1 Pathway. J. Tradit. Chin. Med. 2023, 43, 734–743. [Google Scholar] [CrossRef]
- Di Matteo, B.; Vandenbulcke, F.; Vitale, N.D.; Iacono, F.; Ashmore, K.; Marcacci, M.; Kon, E. Minimally Manipulated Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Systematic Review of Clinical Evidence. Stem Cells Int. 2019, 2019, 1735242. [Google Scholar] [CrossRef]
- Najar, M.; Melki, R.; Khalife, F.; Lagneaux, L.; Bouhtit, F.; Moussa Agha, D.; Fahmi, H.; Lewalle, P.; Fayyad-Kazan, M.; Merimi, M. Therapeutic Mesenchymal Stem/Stromal Cells: Value, Challenges and Optimization. Front. Cell Dev. Biol. 2021, 9, 716853. [Google Scholar] [CrossRef]
- Wei, P.; Bao, R. Intra-Articular Mesenchymal Stem Cell Injection for Knee Osteoarthritis: Mechanisms and Clinical Evidence. Int. J. Mol. Sci. 2022, 24, 59. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, Z.; Lu, Z. Clinical Efficacy and Safety of the Combination of Mesenchymal Stem Cells and Scaffolds in the Treatment of Knee Osteoarthritis: Protocol for Systematic Review and Meta-Analysis. Medicine 2022, 101, e31638. [Google Scholar] [CrossRef] [PubMed]
- Shegos, C.J.; Chaudhry, A.F. A Narrative Review of Mesenchymal Stem Cells Effect on Osteoarthritis. Ann. Jt. 2022, 7, 26. [Google Scholar] [CrossRef]
- Wong, K.L.; Lee, K.B.L.; Tai, B.C.; Law, P.; Lee, E.H.; Hui, J.H.P. Injectable Cultured Bone Marrow-Derived Mesenchymal Stem Cells in Varus Knees with Cartilage Defects Undergoing High Tibial Osteotomy: A Prospective, Randomized Controlled Clinical Trial with 2 Years’ Follow-Up. Arthroscopy 2013, 29, 2020–2028. [Google Scholar] [CrossRef]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef]
- Entessari, M.; Oliveira, L.P. Current Evidence on Mesenchymal Stem Cells for Hip Osteoarthritis: A Narrative Review. Regen. Med. 2023, 18, 749–758. [Google Scholar] [CrossRef]
- Lv, Z.; Cai, X.; Bian, Y.; Wei, Z.; Zhu, W.; Zhao, X.; Weng, X. Advances in Mesenchymal Stem Cell Therapy for Osteoarthritis: From Preclinical and Clinical Perspectives. Bioengineering 2023, 10, 195. [Google Scholar] [CrossRef]
- Carneiro, D.d.C.; Araújo, L.T.d.; Santos, G.C.; Damasceno, P.K.F.; Vieira, J.L.; Santos, R.R.D.; Barbosa, J.D.V.; Soares, M.B.P. Clinical Trials with Mesenchymal Stem Cell Therapies for Osteoarthritis: Challenges in the Regeneration of Articular Cartilage. Int. J. Mol. Sci. 2023, 24, 9939. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, T.; Pitsilos, C.; Iosifidou, M.; Tzaveas, A.; Gigis, I.; Ditsios, K.; Iosifidis, M. Stem Cells for the Treatment of Early to Moderate Osteoarthritis of the Knee: A Systematic Review. J. Exp. Orthop. 2023, 10, 102. [Google Scholar] [CrossRef]
- Razak, H.R.B.A.; Corona, K.; Totlis, T.; Chan, L.Y.T.; Salreta, J.F.; Sleiman, O.; Vasso, M.; Baums, M.H. Mesenchymal Stem Cell Implantation Provides Short-Term Clinical Improvement and Satisfactory Cartilage Restoration in Patients with Knee Osteoarthritis but the Evidence Is Limited: A Systematic Review Performed by the Early-Osteoarthritis Group of ESSKA-European Knee Associates Section. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5306–5318. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Su, S.; Yu, Y.; Liang, S.; Ma, C.; Jiao, Y.; Xing, W.; Tian, Z.; Jiang, T.; Wang, J. Revolutionizing Osteoarthritis Treatment: How Mesenchymal Stem Cells Hold the Key. Biomed. Pharmacother. 2024, 173, 116458. [Google Scholar] [CrossRef]
- Ehioghae, M.; Vippa, T.K.; Askins, D.; Slusarczyk, S.; Bobo, E.; Montoya, A.; Anderson, D.; Robinson, C.L.; Kaye, A.D.; Urits, I. Exploring Orthopedic Stem-Cell Approaches for Osteoarthritis Management: Current Trends and Future Horizons. Curr. Pain Headache Rep. 2024, 28, 27–35. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, S.; Cao, M.; Lin, Z.; Kong, L.; Wu, X.; Guo, Q.; Ouyang, Y.; Song, Y. What Is the Optimal Dose of Adipose-Derived Mesenchymal Stem Cells Treatment for Knee Osteoarthritis? A Conventional and Network Meta-Analysis of Randomized Controlled Trials. Stem Cell Res. Ther. 2023, 14, 245. [Google Scholar] [CrossRef]
- Kim, K.-I.; Kim, M.-S.; Kim, J.-H. Intra-Articular Injection of Autologous Adipose-Derived Stem Cells or Stromal Vascular Fractions: Are They Effective for Patients With Knee Osteoarthritis? A Systematic Review With Meta-Analysis of Randomized Controlled Trials. Am. J. Sports Med. 2023, 51, 837–848. [Google Scholar] [CrossRef]
- Nogueira, L.F.B.; Maniglia, B.C.; Buchet, R.; Millán, J.L.; Ciancaglini, P.; Bottini, M.; Ramos, A.P. Three-Dimensional Cell-Laden Collagen Scaffolds: From Biochemistry to Bone Bioengineering. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 967–983. [Google Scholar] [CrossRef]
- Albrecht, C.; Tichy, B.; Nürnberger, S.; Hosiner, S.; Zak, L.; Aldrian, S.; Marlovits, S. Gene Expression and Cell Differentiation in Matrix-Associated Chondrocyte Transplantation Grafts: A Comparative Study. Osteoarthr. Cartil. 2011, 19, 1219–1227. [Google Scholar] [CrossRef]
- Dai, W.; Kawazoe, N.; Lin, X.; Dong, J.; Chen, G. The Influence of Structural Design of PLGA/Collagen Hybrid Scaffolds in Cartilage Tissue Engineering. Biomaterials 2010, 31, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.B.; Shon, O.-J.; Seo, M.-S.; Choi, Y.; Park, W.T.; Lee, G.W. Mesenchymal Stem Cell-Derived Exosomes and Their Therapeutic Potential for Osteoarthritis. Biology 2021, 10, 285. [Google Scholar] [CrossRef]
- Chen, A.; Chen, Y.; Rong, X.; You, X.; Wu, D.; Zhou, X.; Zeng, W.; Zhou, Z. The Application of Exosomes in the Early Diagnosis and Treatment of Osteoarthritis. Front. Pharmacol. 2023, 14, 1154135. [Google Scholar] [CrossRef]
- Zhou, Q.; Cai, Y.; Jiang, Y.; Lin, X. Exosomes in Osteoarthritis and Cartilage Injury: Advanced Development and Potential Therapeutic Strategies. Int. J. Biol. Sci. 2020, 16, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, L.; Lin, J.; Liu, Q. The Role of Extracellular Vesicles in the Pathogenesis, Diagnosis, and Treatment of Osteoarthritis. Molecules 2021, 26, 4987. [Google Scholar] [CrossRef] [PubMed]
- Maehara, M.; Toyoda, E.; Takahashi, T.; Watanabe, M.; Sato, M. Potential of Exosomes for Diagnosis and Treatment of Joint Disease: Towards a Point-of-Care Therapy for Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2666. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuang, Y.; Fang, L.; Yuan, C.; Wang, X.; Lin, K. Breakthrough of Extracellular Vesicles in Pathogenesis, Diagnosis and Treatment of Osteoarthritis. Bioact. Mater. 2023, 22, 423–452. [Google Scholar] [CrossRef]
- Zhuang, Y.; Jiang, S.; Yuan, C.; Lin, K. The Potential Therapeutic Role of Extracellular Vesicles in Osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10, 1022368. [Google Scholar] [CrossRef]
- Bryk, M.; Karnas, E.; Mlost, J.; Zuba-Surma, E.; Starowicz, K. Mesenchymal Stem Cells and Extracellular Vesicles for the Treatment of Pain: Current Status and Perspectives. Br. J. Pharmacol. 2022, 179, 4281–4299. [Google Scholar] [CrossRef]
- Ruiz, M.; Cosenza, S.; Maumus, M.; Jorgensen, C.; Noël, D. Therapeutic Application of Mesenchymal Stem Cells in Osteoarthritis. Expert Opin. Biol. Ther. 2016, 16, 33–42. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Hui, J.H.P.; Lim, S.K. MSC Exosome as a Cell-Free MSC Therapy for Cartilage Regeneration: Implications for Osteoarthritis Treatment. Semin. Cell Dev. Biol. 2017, 67, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Zhu, J. Stem-Cell Derived Exosomes for the Treatment of Osteoarthritis. Curr. Stem Cell Res. Ther. 2020, 15, 597–601. [Google Scholar] [CrossRef]
- Ng, C.Y.; Chai, J.Y.; Foo, J.B.; Mohamad Yahaya, N.H.; Yang, Y.; Ng, M.H.; Law, J.X. Potential of Exosomes as Cell-Free Therapy in Articular Cartilage Regeneration: A Review. Int. J. Nanomed. 2021, 16, 6749–6781. [Google Scholar] [CrossRef]
- Jousheghan, S.S.; Sajjadi, M.M.; Jousheghan, S.S.; Hosseininejad, S.-M.; Maleki, A. Extracellular Vesicles as Novel Approaches for the Treatment of Osteoarthritis: A Narrative Review on Potential Mechanisms. J. Mol. Histol. 2021, 52, 879–891. [Google Scholar] [CrossRef]
- Boulestreau, J.; Maumus, M.; Jorgensen, C.; Noël, D. Extracellular Vesicles from Mesenchymal Stromal Cells: Therapeutic Perspectives for Targeting Senescence in Osteoarthritis. Adv. Drug Deliv. Rev. 2021, 175, 113836. [Google Scholar] [CrossRef]
- Hormozi, A.; Hasanzadeh, S.; Ebrahimi, F.; Daei, N.; Hajimortezayi, Z.; Mehdizadeh, A.; Zamani, M. Treatment with Exosomes Derived from Mesenchymal Stem Cells: A New Window of Healing Science in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2024, 19, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Pourakbari, R.; Khodadadi, M.; Aghebati-Maleki, A.; Aghebati-Maleki, L.; Yousefi, M. The Potential of Exosomes in the Therapy of the Cartilage and Bone Complications; Emphasis on Osteoarthritis. Life Sci. 2019, 236, 116861. [Google Scholar] [CrossRef]
- Torrecillas-Baena, B.; Pulido-Escribano, V.; Dorado, G.; Gálvez-Moreno, M.Á.; Camacho-Cardenosa, M.; Casado-Díaz, A. Clinical Potential of Mesenchymal Stem Cell-Derived Exosomes in Bone Regeneration. J. Clin. Med. 2023, 12, 4385. [Google Scholar] [CrossRef]
- Miyaki, S.; Lotz, M.K. Extracellular Vesicles in Cartilage Homeostasis and Osteoarthritis. Curr. Opin. Rheumatol. 2018, 30, 129–135. [Google Scholar] [CrossRef]
- Li, J.J.; Hosseini-Beheshti, E.; Grau, G.E.; Zreiqat, H.; Little, C.B. Stem Cell-Derived Extracellular Vesicles for Treating Joint Injury and Osteoarthritis. Nanomaterials 2019, 9, 261. [Google Scholar] [CrossRef]
- Mustonen, A.-M.; Nieminen, P. Extracellular Vesicles and Their Potential Significance in the Pathogenesis and Treatment of Osteoarthritis. Pharmaceuticals 2021, 14, 315. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Duong, C.M.; Nguyen, X.-H.; Than, U.T.T. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Osteoarthritis Treatment: Extracellular Matrix Protection, Chondrocyte and Osteocyte Physiology, Pain and Inflammation Management. Cells 2021, 10, 2887. [Google Scholar] [CrossRef] [PubMed]
- To, K.; Romain, K.; Mak, C.; Kamaraj, A.; Henson, F.; Khan, W. The Treatment of Cartilage Damage Using Human Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Systematic Review of in Vivo Studies. Front. Bioeng. Biotechnol. 2020, 8, 580. [Google Scholar] [CrossRef]
- Jiang, Y.; Lv, H.; Shen, F.; Fan, L.; Zhang, H.; Huang, Y.; Liu, J.; Wang, D.; Pan, H.; Yang, J. Strategies in Product Engineering of Mesenchymal Stem Cell-Derived Exosomes: Unveiling the Mechanisms Underpinning the Promotive Effects of Mesenchymal Stem Cell-Derived Exosomes. Front. Bioeng. Biotechnol. 2024, 12, 1363780. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, S.; Sun, Z.; Zhai, C.; Xia, J.; Wen, C.; Zhang, Y.; Zhang, Y. Enhancement of the Therapeutic Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Osteoarthritis. Cell Mol. Biol. Lett. 2023, 28, 75. [Google Scholar] [CrossRef]
- van de Looij, S.M.; de Jong, O.G.; Vermonden, T.; Lorenowicz, M.J. Injectable Hydrogels for Sustained Delivery of Extracellular Vesicles in Cartilage Regeneration. J. Control Release 2023, 355, 685–708. [Google Scholar] [CrossRef]
- Amizuka, N.; Hasegawa, T.; Oda, K.; Luiz de Freitas, P.H.; Hoshi, K.; Li, M.; Ozawa, H. Histology of Epiphyseal Cartilage Calcification and Endochondral Ossification. Front. Biosci. Elite Ed. 2012, 4, 2085–2100. [Google Scholar] [CrossRef]
- Poole, A.R. The Growth Plate: Cellular Physiology, Cartilage Assembly and Mineralization. In Cartilage: Molecular Aspects; Hall, B., Newman, S., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 179–211. Available online: https://www.google.com/search?client=firefox-b-e&sca_esv=776364c56d1effde&sxsrf=ADLYWIK0FRK44fN_x5DQeziYou79nTurfw:1735066871249&q=Poole,+A.R.+(1991)+The+Growth+Plate:+Cellular+Physiology,+Cartilage+Assembly+and+Mineralization.+In:+Hall,+B.+and+Newman,+S.,+Eds.,+Cartilage:+Molecular+Aspects,+CRC+Press,+Boca+Raton,+FL,+179-211&source=lnms&fbs=AEQNm0CbCVgAZ5mWEJDg6aoPVcBgTlosgQSuzBMlnAdio07UCCJug3WzoI_0_7bcYmDUufwL4h0h9JsKaCFDU5b_RnWAz4amEGUtY-9QgtLUxVwALu4YzL6k1GV8L0y209eXy6wQPk0bFkwMnygY6tpmF9UkDDoT7Ro4RiUTMjqWyIaEvMfCrPbhkhjpTW5YBEuVTmi8BtT1&sa=X&ved=2ahUKEwixxJbki8GKAxXJdqQEHcz1HfgQ0pQJegQIDRAB&biw=1271&bih=550&dpr=1.5 (accessed on 24 December 2024).
- Anderson, H.C. Molecular Biology of Matrix Vesicles. Clin. Orthop. Relat. Res. 1995, 314, 266–280. [Google Scholar] [CrossRef]
- Żylińska, B.; Sobczyńska-Rak, A.; Lisiecka, U.; Stodolak-Zych, E.; Jarosz, Ł.; Szponder, T. Structure and Pathologies of Articular Cartilage. In Vivo 2021, 35, 1355–1363. [Google Scholar] [CrossRef]
- Bhosale, A.M.; Richardson, J.B. Articular Cartilage: Structure, Injuries and Review of Management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef]
- Ge, Z.; Li, C.; Heng, B.C.; Cao, G.; Yang, Z. Functional Biomaterials for Cartilage Regeneration. J. Biomed. Mater. Res. A 2012, 100, 2526–2536. [Google Scholar] [CrossRef]
- Mollenhauer, J.A. Perspectives on Articular Cartilage Biology and Osteoarthritis. Injury 2008, 39 (Suppl. S1), S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Buchet, R.; Mebarek, S.; Pikula, S.; Strzelecka-Kiliszek, A.; Magne, D.; Duffles, L.F.; Taira, T.M.; Bottini, M.; Ciancaglini, P.; Millán, J.L.; et al. Chapter 2—Physiological Biomineralization. The Properties and Role of Matrix Vesicles in Skeletal and Dental Calcifications. In Mineralizing Vesicles; Bottini, M., Ramos, A.P., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 29–59. ISBN 978-0-323-99158-2. [Google Scholar]
- Hunziker, E.B. Mechanism of Longitudinal Bone Growth and Its Regulation by Growth Plate Chondrocytes. Microsc. Res. Tech. 1994, 28, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.Y. Analysis of Matrix Vesicles and Their Role in the Calcification of Epiphyseal Cartilage. Fed. Proc. 1976, 35, 135–142. [Google Scholar]
- Anderson, H.C. Vesicles Associated with Calcification in the Matrix of Epiphyseal Cartilage. J. Cell Biol. 1969, 41, 59–72. [Google Scholar] [CrossRef]
- Bonucci, E. Fine Structure of Early Cartilage Calcification. J. Ultrastruct. Res. 1967, 20, 33–50. [Google Scholar] [CrossRef]
- Anderson, H.C. Electron Microscopic Studies of Induced Cartilage Development and Calcification. J. Cell Biol. 1967, 35, 81–101. [Google Scholar] [CrossRef]
- Boskey, A.L. Mineral-Matrix Interactions in Bone and Cartilage. Clin. Orthop. Relat. Res. 1992, 281, 244–274. [Google Scholar]
- Anderson, H.C. Matrix Vesicles and Calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef]
- Golub, E.E. Role of Matrix Vesicles in Biomineralization. Biochim. Biophys. Acta 2009, 1790, 1592–1598. [Google Scholar] [CrossRef]
- Wuthier, R.E.; Lipscomb, G.F. Matrix Vesicles: Structure, Composition, Formation and Function in Calcification. Front. Biosci. 2011, 16, 2812–2902. [Google Scholar] [CrossRef]
- Bottini, M.; Mebarek, S.; Anderson, K.L.; Strzelecka-Kiliszek, A.; Bozycki, L.; Simão, A.M.S.; Bolean, M.; Ciancaglini, P.; Pikula, J.B.; Pikula, S.; et al. Matrix Vesicles from Chondrocytes and Osteoblasts: Their Biogenesis, Properties, Functions and Biomimetic Models. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Mebarek, S.; Buchet, R.; Pikula, S.; Strzelecka-Kiliszek, A.; Brizuela, L.; Corti, G.; Collacchi, F.; Anghieri, G.; Magrini, A.; Ciancaglini, P.; et al. Do Media Extracellular Vesicles and Extracellular Vesicles Bound to the Extracellular Matrix Represent Distinct Types of Vesicles? Biomolecules 2023, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, T.; von der Mark, K. Remodelling of Collagen Types I, II and X and Calcification of Human Fetal Cartilage. Bone Miner. 1992, 18, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Descalzi Cancedda, F.; Gentili, C.; Manduca, P.; Cancedda, R. Hypertrophic Chondrocytes Undergo Further Differentiation in Culture. J. Cell Biol. 1992, 117, 427–435. [Google Scholar] [CrossRef]
- Gentili, C.; Bianco, P.; Neri, M.; Malpeli, M.; Campanile, G.; Castagnola, P.; Cancedda, R.; Cancedda, F.D. Cell Proliferation, Extracellular Matrix Mineralization, and Ovotransferrin Transient Expression during in Vitro Differentiation of Chick Hypertrophic Chondrocytes into Osteoblast-like Cells. J. Cell Biol. 1993, 122, 703–712. [Google Scholar] [CrossRef]
- Aghajanian, P.; Xing, W.; Cheng, S.; Mohan, S. Epiphyseal Bone Formation Occurs via Thyroid Hormone Regulation of Chondrocyte to Osteoblast Transdifferentiation. Sci. Rep. 2017, 7, 10432. [Google Scholar] [CrossRef]
- Roach, H.I.; Erenpreisa, J.; Aigner, T. Osteogenic Differentiation of Hypertrophic Chondrocytes Involves Asymmetric Cell Divisions and Apoptosis. J. Cell Biol. 1995, 131, 483–494. [Google Scholar] [CrossRef]
- Gerber, H.P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF Couples Hypertrophic Cartilage Remodeling, Ossification and Angiogenesis during Endochondral Bone Formation. Nat. Med. 1999, 5, 623–628. [Google Scholar] [CrossRef]
- Zeyland, J.; Lipiński, D.; Juzwa, W.; Pławski, A.; Słomski, R. Structure and Application of Select Glycosaminoglycans. Med. Weter. 2006, 62, 139–144. [Google Scholar]
- Wilusz, R.E.; Sanchez-Adams, J.; Guilak, F. The Structure and Function of the Pericellular Matrix of Articular Cartilage. Matrix Biol. 2014, 39, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Kelly, D.C.; Curtin, C.M.; O’Brien, F.J. Mechanosignalling in Cartilage: An Emerging Target for the Treatment of Osteoarthritis. Nat. Rev. Rheumatol. 2022, 18, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Andriacchi, T.P.; Favre, J. The Nature of in Vivo Mechanical Signals That Influence Cartilage Health and Progression to Knee Osteoarthritis. Curr. Rheumatol. Rep. 2014, 16, 463. [Google Scholar] [CrossRef]
- Guo, H.; Maher, S.A.; Torzilli, P.A. A Biphasic Finite Element Study on the Role of the Articular Cartilage Superficial Zone in Confined Compression. J. Biomech. 2015, 48, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Segarra-Queralt, M.; Crump, K.; Pascuet-Fontanet, A.; Gantenbein, B.; Noailly, J. The Interplay between Biochemical Mediators and Mechanotransduction in Chondrocytes: Unravelling the Differential Responses in Primary Knee Osteoarthritis. Phys. Life Rev. 2024, 48, 205–221. [Google Scholar] [CrossRef]
- Mow, V.C.; Ratcliffe, A.; Poole, A.R. Cartilage and Diarthrodial Joints as Paradigms for Hierarchical Materials and Structures. Biomaterials 1992, 13, 67–97. [Google Scholar] [CrossRef] [PubMed]
- Arokoski, J.P.; Jurvelin, J.S.; Väätäinen, U.; Helminen, H.J. Normal and Pathological Adaptations of Articular Cartilage to Joint Loading. Scand. J. Med. Sci. Sports 2000, 10, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Chwastek, J.; Kędziora, M.; Borczyk, M.; Korostyński, M.; Starowicz, K. Inflammation-Driven Secretion Potential Is Upregulated in Osteoarthritic Fibroblast-Like Synoviocytes. Int. J. Mol. Sci. 2022, 23, 11817. [Google Scholar] [CrossRef]
- Arora, D.; Taneja, Y.; Sharma, A.; Dhingra, A.; Guarve, K. Role of Apoptosis in the Pathogenesis of Osteoarthritis: An Explicative Review. Curr. Rheumatol. Rev. 2024, 20, 2–13. [Google Scholar] [CrossRef]
- O’Neill, T.W.; Felson, D.T. Mechanisms of Osteoarthritis (OA) Pain. Curr. Osteoporos. Rep. 2018, 16, 611–616. [Google Scholar] [CrossRef]
- Zhou, S.; Thornhill, T.S.; Meng, F.; Xie, L.; Wright, J.; Glowacki, J. Influence of Osteoarthritis Grade on Molecular Signature of Human Cartilage. J. Orthop. Res. 2016, 34, 454–462. [Google Scholar] [CrossRef]
- Lotz, M. Cytokines in Cartilage Injury and Repair. Clin. Orthop. Relat. Res. 2001, 391, S108–S115. [Google Scholar] [CrossRef]
- Goldring, M.B. The Role of the Chondrocyte in Osteoarthritis. Arthritis Rheum. 2000, 43, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Hayami, T.; Pickarski, M.; Zhuo, Y.; Wesolowski, G.A.; Rodan, G.A.; Duong, L.T. Characterization of Articular Cartilage and Subchondral Bone Changes in the Rat Anterior Cruciate Ligament Transection and Meniscectomized Models of Osteoarthritis. Bone 2006, 38, 234–243. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef]
- Ko, F.C.; Dragomir, C.L.; Plumb, D.A.; Hsia, A.W.; Adebayo, O.O.; Goldring, S.R.; Wright, T.M.; Goldring, M.B.; van der Meulen, M.C.H. Progressive Cell-Mediated Changes in Articular Cartilage and Bone in Mice Are Initiated by a Single Session of Controlled Cyclic Compressive Loading. J. Orthop. Res. 2016, 34, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Manon-Jensen, T.; Arendt-Nielsen, L.; Petersen, K.K.; Christiansen, T.; Samuels, J.; Abramson, S.; Karsdal, M.A.; Attur, M.; Bay-Jensen, A.C. Potential Diagnostic Value of a Type X Collagen Neo-Epitope Biomarker for Knee Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 611–620. [Google Scholar] [CrossRef]
- Bouaziz, W.; Funck-Brentano, T.; Lin, H.; Marty, C.; Ea, H.-K.; Hay, E.; Cohen-Solal, M. Loss of Sclerostin Promotes Osteoarthritis in Mice via β-Catenin-Dependent and -Independent Wnt Pathways. Arthritis Res. Ther. 2015, 17, 24. [Google Scholar] [CrossRef]
- Wu, J.; Ma, L.; Wu, L.; Jin, Q. Wnt-β-Catenin Signaling Pathway Inhibition by Sclerostin May Protect against Degradation in Healthy but Not Osteoarthritic Cartilage. Mol. Med. Rep. 2017, 15, 2423–2432. [Google Scholar] [CrossRef]
- Niu, Q.; Li, F.; Zhang, L.; Xu, X.; Liu, Y.; Gao, J.; Feng, X. Role of the Wnt/β-Catenin Signaling Pathway in the Response of Chondrocytes to Mechanical Loading. Int. J. Mol. Med. 2016, 37, 755–762. [Google Scholar] [CrossRef]
- Dell’accio, F.; De Bari, C.; Eltawil, N.M.; Vanhummelen, P.; Pitzalis, C. Identification of the Molecular Response of Articular Cartilage to Injury, by Microarray Screening: Wnt-16 Expression and Signaling after Injury and in Osteoarthritis. Arthritis Rheum. 2008, 58, 1410–1421. [Google Scholar] [CrossRef]
- Nalesso, G.; Thomas, B.L.; Sherwood, J.C.; Yu, J.; Addimanda, O.; Eldridge, S.E.; Thorup, A.-S.; Dale, L.; Schett, G.; Zwerina, J.; et al. WNT16 Antagonises Excessive Canonical WNT Activation and Protects Cartilage in Osteoarthritis. Ann. Rheum. Dis. 2017, 76, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.-H.; Wang, C.-J.; Ko, J.-Y.; Sun, Y.-C.; Su, Y.-S.; Wang, F.-S. Inflammation Induction of Dickkopf-1 Mediates Chondrocyte Apoptosis in Osteoarthritic Joint. Osteoarthr. Cartil. 2009, 17, 933–943. [Google Scholar] [CrossRef]
- Voorzanger-Rousselot, N.; Ben-Tabassi, N.C.; Garnero, P. Opposite Relationships between Circulating Dkk-1 and Cartilage Breakdown in Patients with Rheumatoid Arthritis and Knee Osteoarthritis. Ann. Rheum. Dis. 2009, 68, 1513–1514. [Google Scholar] [CrossRef] [PubMed]
- Salter, D.M.; Millward-Sadler, S.J.; Nuki, G.; Wright, M.O. Integrin-Interleukin-4 Mechanotransduction Pathways in Human Chondrocytes. Clin. Orthop. Relat. Res. 2001, 391, S49–S60. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Bao, L.; Li, J.; Shi, Z.; Wang, J. Cyclic Hydrostatic Compress Force Regulates Apoptosis of Meniscus Fibrochondrocytes via Integrin Alpha5beta1. Physiol. Res. 2019, 68, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Han, S. Osteoarthritis Year in Review 2022: Biology. Osteoarthr. Cartil. 2022, 30, 1575–1582. [Google Scholar] [CrossRef]
- Del Carlo, M.; Schwartz, D.; Erickson, E.A.; Loeser, R.F. Endogenous Production of Reactive Oxygen Species Is Required for Stimulation of Human Articular Chondrocyte Matrix Metalloproteinase Production by Fibronectin Fragments. Free Radic. Biol. Med. 2007, 42, 1350–1358. [Google Scholar] [CrossRef]
- Fang, T.; Zhou, X.; Jin, M.; Nie, J.; Li, X. Molecular Mechanisms of Mechanical Load-Induced Osteoarthritis. Int. Orthop. 2021, 45, 1125–1136. [Google Scholar] [CrossRef]
- Burr, D.B. Anatomy and Physiology of the Mineralized Tissues: Role in the Pathogenesis of Osteoarthrosis. Osteoarthr. Cartil. 2004, 12 (Suppl. A), S20–S30. [Google Scholar] [CrossRef]
- Burr, D.B.; Schaffler, M.B. The Involvement of Subchondral Mineralized Tissues in Osteoarthrosis: Quantitative Microscopic Evidence. Microsc. Res. Tech. 1997, 37, 343–357. [Google Scholar] [CrossRef]
- Imhof, H.; Sulzbacher, I.; Grampp, S.; Czerny, C.; Youssefzadeh, S.; Kainberger, F. Subchondral Bone and Cartilage Disease: A Rediscovered Functional Unit. Investig. Radiol. 2000, 35, 581–588. [Google Scholar] [CrossRef]
- Mebarek, S.; Hamade, E.; Thouverey, C.; Bandorowicz-Pikula, J.; Pikula, S.; Magne, D.; Buchet, R. Ankylosing Spondylitis, Late Osteoarthritis, Vascular Calcification, Chondrocalcinosis and Pseudo Gout: Toward a Possible Drug Therapy. Curr. Med. Chem. 2011, 18, 2196–2203. [Google Scholar] [CrossRef]
- Zhang, W.; Doherty, M.; Bardin, T.; Barskova, V.; Guerne, P.-A.; Jansen, T.L.; Leeb, B.F.; Perez-Ruiz, F.; Pimentao, J.; Punzi, L.; et al. European League Against Rheumatism Recommendations for Calcium Pyrophosphate Deposition. Part I: Terminology and Diagnosis. Ann. Rheum. Dis. 2011, 70, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.K.; Ryan, L.M. Calcium Pyrophosphate Deposition Disease. N. Engl. J. Med. 2016, 374, 2575–2584. [Google Scholar] [CrossRef]
- Pritzker, K.P.H. Counterpoint: Hydroxyapatite Crystal Deposition Is Not Intimately Involved in the Pathogenesis and Progression of Human Osteoarthritis. Curr. Rheumatol. Rep. 2009, 11, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, R.; Houben, A.; Tohidnezhad, M.; Kweider, N.; Fragoulis, A.; Wruck, C.J.; Brandenburg, L.O.; Hermanns-Sachweh, B.; Goldring, M.B.; Pufe, T.; et al. Mechanical Forces Induce Changes in VEGF and VEGFR-1/sFlt-1 Expression in Human Chondrocytes. Int. J. Mol. Sci. 2014, 15, 15456–15474. [Google Scholar] [CrossRef]
- Ashraf, S.; Wibberley, H.; Mapp, P.I.; Hill, R.; Wilson, D.; Walsh, D.A. Increased Vascular Penetration and Nerve Growth in the Meniscus: A Potential Source of Pain in Osteoarthritis. Ann. Rheum. Dis. 2011, 70, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, T.; Guan, M.; Zhao, W.; Leung, F.-K.-L.; Pan, H.; Cao, X.; Guo, X.E.; Lu, W.W. Bone Turnover and Articular Cartilage Differences Localized to Subchondral Cysts in Knees with Advanced Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2174–2183. [Google Scholar] [CrossRef]
- Tonna, S.; Poulton, I.J.; Taykar, F.; Ho, P.W.M.; Tonkin, B.; Crimeen-Irwin, B.; Tatarczuch, L.; McGregor, N.E.; Mackie, E.J.; Martin, T.J.; et al. Chondrocytic Ephrin B2 Promotes Cartilage Destruction by Osteoclasts in Endochondral Ossification. Development 2016, 143, 648–657. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Z.; Bai, L. Cell Interplay in Osteoarthritis. Front. Cell Dev. Biol. 2021, 9, 720477. [Google Scholar] [CrossRef]
- Walsh, D.A.; McWilliams, D.F.; Turley, M.J.; Dixon, M.R.; Fransès, R.E.; Mapp, P.I.; Wilson, D. Angiogenesis and Nerve Growth Factor at the Osteochondral Junction in Rheumatoid Arthritis and Osteoarthritis. Rheumatology 2010, 49, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Fransès, R.E.; McWilliams, D.F.; Mapp, P.I.; Walsh, D.A. Osteochondral Angiogenesis and Increased Protease Inhibitor Expression in OA. Osteoarthr. Cartil. 2010, 18, 563–571. [Google Scholar] [CrossRef]
- Walsh, D.A.; Bonnet, C.S.; Turner, E.L.; Wilson, D.; Situ, M.; McWilliams, D.F. Angiogenesis in the Synovium and at the Osteochondral Junction in Osteoarthritis. Osteoarthr. Cartil. 2007, 15, 743–751. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.-J. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J. Bone Miner. Res. 2016, 31, 911–924. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020, 11, 111. [Google Scholar] [CrossRef]
- Nagase, H.; Kashiwagi, M. Aggrecanases and Cartilage Matrix Degradation. Arthritis Res. Ther. 2003, 5, 94–103. [Google Scholar] [CrossRef]
- Bondeson, J.; Wainwright, S.; Hughes, C.; Caterson, B. The Regulation of the ADAMTS4 and ADAMTS5 Aggrecanases in Osteoarthritis: A Review. Clin. Exp. Rheumatol. 2008, 26, 139–145. [Google Scholar]
- Verma, P.; Dalal, K. ADAMTS-4 and ADAMTS-5: Key Enzymes in Osteoarthritis. J. Cell Biochem. 2011, 112, 3507–3514. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM Metalloproteinases in Osteoarthritis—Looking beyond the “Usual Suspects”. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef]
- Dean, D.D.; Martel-Pelletier, J.; Pelletier, J.P.; Howell, D.S.; Woessner, J.F. Evidence for Metalloproteinase and Metalloproteinase Inhibitor Imbalance in Human Osteoarthritic Cartilage. J. Clin. Investig. 1989, 84, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Karila, T.; Tervahartiala, T.; Cohen, B.; Sorsa, T. The Collagenases: Are They Tractable Targets for Preventing Cartilage Destruction in Osteoarthritis? Expert Opin. Ther. Targets 2022, 26, 93–105. [Google Scholar] [CrossRef]
- Salminen-Mankonen, H.J.; Morko, J.; Vuorio, E. Role of Cathepsin K in Normal Joints and in the Development of Arthritis. Curr. Drug Targets 2007, 8, 315–323. [Google Scholar] [CrossRef]
- Lis, K.; Odrowaz-Sypniewska, G. Role of Cathepsin K in the Pathogenesis of Degenerative Changes in Joints. Ortop. Traumatol. Rehabil. 2005, 7, 361–364. [Google Scholar]
- Miller, R.E.; Lu, Y.; Tortorella, M.D.; Malfait, A.-M. Genetically Engineered Mouse Models Reveal the Importance of Proteases as Osteoarthritis Drug Targets. Curr. Rheumatol. Rep. 2013, 15, 350. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.A.; Ranganath, L.R.; Boyde, A. Lessons from Rare Diseases of Cartilage and Bone. Curr. Opin. Pharmacol. 2015, 22, 107–114. [Google Scholar] [CrossRef]
- Hou, W.S.; Li, Z.; Gordon, R.E.; Chan, K.; Klein, M.J.; Levy, R.; Keysser, M.; Keyszer, G.; Brömme, D. Cathepsin k Is a Critical Protease in Synovial Fibroblast-Mediated Collagen Degradation. Am. J. Pathol. 2001, 159, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Kiviranta, R.; Morko, J.; Uusitalo, H.; Aro, H.T.; Vuorio, E.; Rantakokko, J. Accelerated Turnover of Metaphyseal Trabecular Bone in Mice Overexpressing Cathepsin K. J. Bone Miner. Res. 2001, 16, 1444–1452. [Google Scholar] [CrossRef]
- Morko, J.; Kiviranta, R.; Joronen, K.; Säämänen, A.-M.; Vuorio, E.; Salminen-Mankonen, H. Spontaneous Development of Synovitis and Cartilage Degeneration in Transgenic Mice Overexpressing Cathepsin K. Arthritis Rheum. 2005, 52, 3713–3717. [Google Scholar] [CrossRef]
- Morko, J.; Kiviranta, R.; Hurme, S.; Rantakokko, J.; Vuorio, E. Differential Turnover of Cortical and Trabecular Bone in Transgenic Mice Overexpressing Cathepsin K. Bone 2005, 36, 854–865. [Google Scholar] [CrossRef]
- Kozawa, E.; Nishida, Y.; Cheng, X.W.; Urakawa, H.; Arai, E.; Futamura, N.; Shi, G.-P.; Kuzuya, M.; Hu, L.; Sasaki, T.; et al. Osteoarthritic Change Is Delayed in a Ctsk-Knockout Mouse Model of Osteoarthritis. Arthritis Rheum. 2012, 64, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Hayami, T.; Zhuo, Y.; Wesolowski, G.A.; Pickarski, M.; Duong, L.T. Inhibition of Cathepsin K Reduces Cartilage Degeneration in the Anterior Cruciate Ligament Transection Rabbit and Murine Models of Osteoarthritis. Bone 2012, 50, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Soki, F.N.; Yoshida, R.; Paglia, D.N.; Duong, L.T.; Hansen, M.F.; Drissi, H. Articular Cartilage Protection in Ctsk-/- Mice Is Associated with Cellular and Molecular Changes in Subchondral Bone and Cartilage Matrix. J. Cell Physiol. 2018, 233, 8666–8676. [Google Scholar] [CrossRef]
- Vinardell, T.; Dejica, V.; Poole, A.R.; Mort, J.S.; Richard, H.; Laverty, S. Evidence to Suggest That Cathepsin K Degrades Articular Cartilage in Naturally Occurring Equine Osteoarthritis. Osteoarthr. Cartil. 2009, 17, 375–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mort, J.S.; Beaudry, F.; Théroux, K.; Emmott, A.A.; Richard, H.; Fisher, W.D.; Lee, E.R.; Poole, A.R.; Laverty, S. Early Cathepsin K Degradation of Type II Collagen in Vitro and in Vivo in Articular Cartilage. Osteoarthr. Cartil. 2016, 24, 1461–1469. [Google Scholar] [CrossRef]
- Rantakokko, J.; Aro, H.T.; Savontaus, M.; Vuorio, E. Mouse Cathepsin K: cDNA Cloning and Predominant Expression of the Gene in Osteoclasts, and in Some Hypertrophying Chondrocytes during Mouse Development. FEBS Lett. 1996, 393, 307–313. [Google Scholar] [CrossRef]
- Dodds, R.A.; James, I.E.; Rieman, D.; Ahern, R.; Hwang, S.M.; Connor, J.R.; Thompson, S.D.; Veber, D.F.; Drake, F.H.; Holmes, S.; et al. Human Osteoclast Cathepsin K Is Processed Intracellularly Prior to Attachment and Bone Resorption. J. Bone Miner. Res. 2001, 16, 478–486. [Google Scholar] [CrossRef]
- Söderström, M.; Salminen, H.; Glumoff, V.; Kirschke, H.; Aro, H.; Vuorio, E. Cathepsin Expression during Skeletal Development. Biochim. Biophys. Acta 1999, 1446, 35–46. [Google Scholar] [CrossRef]
- Uusitalo, H.; Hiltunen, A.; Söderström, M.; Aro, H.T.; Vuorio, E. Expression of Cathepsins B, H, K, L, and S and Matrix Metalloproteinases 9 and 13 during Chondrocyte Hypertrophy and Endochondral Ossification in Mouse Fracture Callus. Calcif. Tissue Int. 2000, 67, 382–390. [Google Scholar] [CrossRef]
- Nakase, T.; Kaneko, M.; Tomita, T.; Myoui, A.; Ariga, K.; Sugamoto, K.; Uchiyama, Y.; Ochi, T.; Yoshikawa, H. Immunohistochemical Detection of Cathepsin D, K, and L in the Process of Endochondral Ossification in the Human. Histochem. Cell Biol. 2000, 114, 21–27. [Google Scholar] [CrossRef]
- Kozawa, E.; Cheng, X.W.; Urakawa, H.; Arai, E.; Yamada, Y.; Kitamura, S.; Sato, K.; Kuzuya, M.; Ishiguro, N.; Nishida, Y. Increased Expression and Activation of Cathepsin K in Human Osteoarthritic Cartilage and Synovial Tissues. J. Orthop. Res. 2016, 34, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, Y.T.; Mandelin, J.; Li, T.-F.; Salo, J.; Lassus, J.; Liljeström, M.; Hukkanen, M.; Takagi, M.; Virtanen, I.; Santavirta, S. Acidic Cysteine Endoproteinase Cathepsin K in the Degeneration of the Superficial Articular Hyaline Cartilage in Osteoarthritis. Arthritis Rheum. 2002, 46, 953–960. [Google Scholar] [CrossRef]
- Khoshdel, A.; Forootan, M.; Afsharinasab, M.; Rezaian, M.; Abbasifard, M. Assessment of the Circulatory Concentrations of Cathepsin D, Cathepsin K, and Alpha-1 Antitrypsin in Patients with Knee Osteoarthritis. Ir. J. Med. Sci. 2023, 192, 1191–1196. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Zhu, J.; Zhou, G.; Zhang, W.J.; Cao, Y.; Liu, W. Enhanced Cartilage Formation by Inhibiting Cathepsin K Expression in Chondrocytes Expanded in Vitro. Biomaterials 2012, 33, 7394–7404. [Google Scholar] [CrossRef]
- Dai, R.; Wu, Z.; Chu, H.Y.; Lu, J.; Lyu, A.; Liu, J.; Zhang, G. Cathepsin K: The Action in and Beyond Bone. Front. Cell Dev. Biol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, Molecular Target and Current Status on Drug Development. Curr. Med. Chem. 2021, 28, 1489–1507. [Google Scholar] [CrossRef] [PubMed]
- Mijanović, O.; Jakovleva, A.; Branković, A.; Zdravkova, K.; Pualic, M.; Belozerskaya, T.A.; Nikitkina, A.I.; Parodi, A.; Zamyatnin, A.A. Cathepsin K in Pathological Conditions and New Therapeutic and Diagnostic Perspectives. Int. J. Mol. Sci. 2022, 23, 13762. [Google Scholar] [CrossRef] [PubMed]
- Anish, R.J.; Nair, A. Osteoporosis Management-Current and Future Perspectives—A Systemic Review. J. Orthop. 2024, 53, 101–113. [Google Scholar] [CrossRef]
- Turk, B.; Turk, V.; Turk, D. Structural and Functional Aspects of Papain-like Cysteine Proteinases and Their Protein Inhibitors. Biol. Chem. 1997, 378, 141–150. [Google Scholar]
- Lecaille, F.; Brömme, D.; Lalmanach, G. Biochemical Properties and Regulation of Cathepsin K Activity. Biochimie 2008, 90, 208–226. [Google Scholar] [CrossRef]
- Brömme, D.; Lecaille, F. Cathepsin K Inhibitors for Osteoporosis and Potential Off-Target Effects. Expert Opin. Investig. Drugs 2009, 18, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Novinec, M.; Lenarčič, B. Cathepsin K: A Unique Collagenolytic Cysteine Peptidase. Biol. Chem. 2013, 394, 1163–1179. [Google Scholar] [CrossRef]
- Kafienah, W.; Brömme, D.; Buttle, D.J.; Croucher, L.J.; Hollander, A.P. Human Cathepsin K Cleaves Native Type I and II Collagens at the N-Terminal End of the Triple Helix. Biochem. J. 1998, 331 Pt 3, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Delaissé, J.-M.; Andersen, T.L.; Engsig, M.T.; Henriksen, K.; Troen, T.; Blavier, L. Matrix Metalloproteinases (MMP) and Cathepsin K Contribute Differently to Osteoclastic Activities. Microsc. Res. Tech. 2003, 61, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Amar, S.; Smith, L.; Fields, G.B. Matrix Metalloproteinase Collagenolysis in Health and Disease. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Delaissé, J.M.; Engsig, M.T.; Everts, V.; del Carmen Ovejero, M.; Ferreras, M.; Lund, L.; Vu, T.H.; Werb, Z.; Winding, B.; Lochter, A.; et al. Proteinases in Bone Resorption: Obvious and Less Obvious Roles. Clin. Chim. Acta 2000, 291, 223–234. [Google Scholar] [CrossRef]
- Demeuse, J.; Massonnet, P.; Schoumacher, M.; Grifnée, E.; Huyghebaert, L.; Dubrowski, T.; Peeters, S.; Le Goff, C.; Cavalier, E. Innovative Workflow for the Identification of Cathepsin K Cleavage Sites in Type I Collagen. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2023, 1228, 123864. [Google Scholar] [CrossRef]
- Helali, A.M.; Iti, F.M.; Mohamed, I.N. Cathepsin K Inhibitors: A Novel Target but Promising Approach in the Treatment of Osteoporosis. Curr. Drug Targets 2013, 14, 1591–1600. [Google Scholar] [CrossRef]
- Ruettger, A.; Schueler, S.; Mollenhauer, J.A.; Wiederanders, B. Cathepsins B, K, and L Are Regulated by a Defined Collagen Type II Peptide via Activation of Classical Protein Kinase C and P38 MAP Kinase in Articular Chondrocytes. J. Biol. Chem. 2008, 283, 1043–1051. [Google Scholar] [CrossRef]
- Dejica, V.M.; Mort, J.S.; Laverty, S.; Percival, M.D.; Antoniou, J.; Zukor, D.J.; Poole, A.R. Cleavage of Type II Collagen by Cathepsin K in Human Osteoarthritic Cartilage. Am. J. Pathol. 2008, 173, 161–169. [Google Scholar] [CrossRef]
- Dejica, V.M.; Mort, J.S.; Laverty, S.; Antoniou, J.; Zukor, D.J.; Tanzer, M.; Poole, A.R. Increased Type II Collagen Cleavage by Cathepsin K and Collagenase Activities with Aging and Osteoarthritis in Human Articular Cartilage. Arthritis Res. Ther. 2012, 14, R113. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Du, X.; Sharma, V.; Lamour, G.; Castro, M.; Li, H.; Brömme, D. Effects of Cysteine Proteases on the Structural and Mechanical Properties of Collagen Fibers. J. Biol. Chem. 2013, 288, 5940–5950. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.J.; Schuelert, N.; Bowyer, J. Cathepsin K Inhibition Reduces CTXII Levels and Joint Pain in the Guinea Pig Model of Spontaneous Osteoarthritis. Osteoarthr. Cartil. 2010, 18, 1355–1357. [Google Scholar] [CrossRef]
- Li, Z.; Hou, W.S.; Brömme, D. Collagenolytic Activity of Cathepsin K Is Specifically Modulated by Cartilage-Resident Chondroitin Sulfates. Biochemistry 2000, 39, 529–536. [Google Scholar] [CrossRef]
- Li, Z.; Yasuda, Y.; Li, W.; Bogyo, M.; Katz, N.; Gordon, R.E.; Fields, G.B.; Brömme, D. Regulation of Collagenase Activities of Human Cathepsins by Glycosaminoglycans. J. Biol. Chem. 2004, 279, 5470–5479. [Google Scholar] [CrossRef]
- Tatara, Y.; Suto, S.; Itoh, K. Novel Roles of Glycosaminoglycans in the Degradation of Type I Collagen by Cathepsin K. Glycobiology 2017, 27, 1089–1098. [Google Scholar] [CrossRef]
- Brito, R.; Costa, D.; Dias, C.; Cruz, P.; Barros, P. Chondroitin Sulfate Supplements for Osteoarthritis: A Critical Review. Cureus 2023, 15, e40192. [Google Scholar] [CrossRef]
- Henrotin, Y.; Marty, M.; Mobasheri, A. What Is the Current Status of Chondroitin Sulfate and Glucosamine for the Treatment of Knee Osteoarthritis? Maturitas 2014, 78, 184–187. [Google Scholar] [CrossRef]
- Fernández-Martín, S.; González-Cantalapiedra, A.; Muñoz, F.; García-González, M.; Permuy, M.; López-Peña, M. Glucosamine and Chondroitin Sulfate: Is There Any Scientific Evidence for Their Effectiveness as Disease-Modifying Drugs in Knee Osteoarthritis Preclinical Studies?-A Systematic Review from 2000 to 2021. Animals 2021, 11, 1608. [Google Scholar] [CrossRef]
- Golovach, I.; Rekalov, D.; Akimov, O.Y.; Kostenko, H.; Kostenko, V.; Mishchenko, A.; Solovyova, N.; Kostenko, V. Molecular Mechanisms and Potential Applications of Chondroitin Sulphate in Managing Post-Traumatic Osteoarthritis. Reumatologia 2023, 61, 395–407. [Google Scholar] [CrossRef]
- Yang, W.; Sun, C.; He, S.Q.; Chen, J.Y.; Wang, Y.; Zhuo, Q. The Efficacy and Safety of Disease-Modifying Osteoarthritis Drugs for Knee and Hip Osteoarthritis-a Systematic Review and Network Meta-Analysis. J. Gen. Intern. Med. 2021, 36, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Mankin, H.J.; Lippiello, L. The Glycosaminoglycans of Normal and Arthritic Cartilage. J. Clin. Investig. 1971, 50, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Lipson, S.J.; Muir, H. Vertebral Osteophyte Formation in Experimental Disc Degeneration. Morphologic and Proteoglycan Changes over Time. Arthritis Rheum. 1980, 23, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.-S.; Li, Z.; Büttner, F.H.; Bartnik, E.; Brömme, D. Cleavage Site Specificity of Cathepsin K toward Cartilage Proteoglycans and Protease Complex Formation. Biol. Chem. 2003, 384, 891–897. [Google Scholar] [CrossRef]
- Yamashita, D.S.; Dodds, R.A. Cathepsin K and the Design of Inhibitors of Cathepsin K. Curr. Pharm. Des. 2000, 6, 1–24. [Google Scholar] [CrossRef]
- Gowen, M. Inhibition of Cathepsin K—A Novel Approach to Antiresorptive Therapy. Expert Opin. Investig. Drugs 1997, 6, 1199–1202. [Google Scholar] [CrossRef]
- Yasuda, Y.; Kaleta, J.; Brömme, D. The Role of Cathepsins in Osteoporosis and Arthritis: Rationale for the Design of New Therapeutics. Adv. Drug Deliv. Rev. 2005, 57, 973–993. [Google Scholar] [CrossRef]
- Costa, A.G.; Cusano, N.E.; Silva, B.C.; Cremers, S.; Bilezikian, J.P. Cathepsin K: Its Skeletal Actions and Role as a Therapeutic Target in Osteoporosis. Nat. Rev. Rheumatol. 2011, 7, 447–456. [Google Scholar] [CrossRef]
- Stone, J.A.; McCrea, J.B.; Witter, R.; Zajic, S.; Stoch, S.A. Clinical and Translational Pharmacology of the Cathepsin K Inhibitor Odanacatib Studied for Osteoporosis. Br. J. Clin. Pharmacol. 2019, 85, 1072–1083. [Google Scholar] [CrossRef]
- Elahmer, N.R.; Wong, S.K.; Mohamed, N.; Alias, E.; Chin, K.-Y.; Muhammad, N. Mechanistic Insights and Therapeutic Strategies in Osteoporosis: A Comprehensive Review. Biomedicines 2024, 12, 1635. [Google Scholar] [CrossRef]
- Vasiljeva, O.; Reinheckel, T.; Peters, C.; Turk, D.; Turk, V.; Turk, B. Emerging Roles of Cysteine Cathepsins in Disease and Their Potential as Drug Targets. Curr. Pharm. Des. 2007, 13, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.; Hörler, D.; Baici, A. The Relative Importance of Cysteine Peptidases in Osteoarthritis. J. Rheumatol. 2000, 27, 1970–1979. [Google Scholar] [PubMed]
- Alcaraz, M.J.; Guillén, M.I.; Ferrándiz, M.L. Emerging Therapeutic Agents in Osteoarthritis. Biochem. Pharmacol. 2019, 165, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Guay, J.; Falgueyret, J.P.; Ducret, A.; Percival, M.D.; Mancini, J.A. Potency and Selectivity of Inhibition of Cathepsin K, L and S by Their Respective Propeptides. Eur. J. Biochem. 2000, 267, 6311–6318. [Google Scholar] [CrossRef]
- Goričan, T.; Ciber, L.; Petek, N.; Svete, J.; Novinec, M. Synthesis and Kinetic Characterization of Hyperbolic Inhibitors of Human Cathepsins K and S Based on a Succinimide Scaffold. Bioorg. Chem. 2021, 115, 105213. [Google Scholar] [CrossRef]
- Rüttger, A.; Mollenhauer, J.; Löser, R.; Gütschow, M.; Wiederanders, B. Microplate Assay for Quantitative Determination of Cathepsin Activities in Viable Cells Using Derivatives of 4-Methoxy-Beta-Naphthylamide. Biotechniques 2006, 41, 469–473. [Google Scholar] [CrossRef]
- Dodds, R.A. A Cytochemical Assay for Osteoclast Cathepsin K Activity. Cell Biochem. Funct. 2003, 21, 231–234. [Google Scholar] [CrossRef]
- Noé, B.; Poole, A.R.; Mort, J.S.; Richard, H.; Beauchamp, G.; Laverty, S. C2K77 ELISA Detects Cleavage of Type II Collagen by Cathepsin K in Equine Articular Cartilage. Osteoarthr. Cartil. 2017, 25, 2119–2126. [Google Scholar] [CrossRef]
- Satkunananthan, P.B.; Anderson, M.J.; De Jesus, N.M.; Haudenschild, D.R.; Ripplinger, C.M.; Christiansen, B.A. In Vivo Fluorescence Reflectance Imaging of Protease Activity in a Mouse Model of Post-Traumatic Osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1461–1469. [Google Scholar] [CrossRef]
- Villalvilla, A.; da Silva, J.A.; Largo, R.; Gualillo, O.; Vieira, P.C.; Herrero-Beaumont, G.; Gómez, R. 6-Shogaol Inhibits Chondrocytes’ Innate Immune Responses and Cathepsin-K Activity. Mol. Nutr. Food Res. 2014, 58, 256–266. [Google Scholar] [CrossRef]
- Yuan, X.-Y.; Ren, Z.; Wu, Y.; Bougault, C.; Brizuela, L.; Magne, D.; Buchet, R.; Mebarek, S. Design, Synthesis and Biological Evaluation of Inhibitors of Cathepsin K on Dedifferentiated Chondrocytes. Bioorg Med. Chem. 2019, 27, 1034–1042. [Google Scholar] [CrossRef]
- Boyd, M.J.; Crane, S.N.; Robichaud, J.; Scheigetz, J.; Black, W.C.; Chauret, N.; Wang, Q.; Massé, F.; Oballa, R.M. Investigation of Ketone Warheads as Alternatives to the Nitrile for Preparation of Potent and Selective Cathepsin K Inhibitors. Bioorg Med. Chem. Lett. 2009, 19, 675–679. [Google Scholar] [CrossRef]
- Gontijo, T.B.; Lima, P.S.; Icimoto, M.Y.; Neves, R.L.; de Alvarenga, É.C.; Carmona, A.K.; de Castro, A.A.; Ramalho, T.C.; da Silva Júnior, E.N.; de Freitas, R.P. Cathepsin K Inhibitors Based on 2-Amino-1,3,4-Oxadiazole Derivatives. Bioorg Chem. 2021, 109, 104662. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.-Y.; Machuca-Gayet, I.; Domenget, C.; Buchet, R.; Wu, Y.; Jurdic, P.; Mebarek, S. Azanitrile Cathepsin K Inhibitors: Effects on Cell Toxicity, Osteoblast-Induced Mineralization and Osteoclast-Mediated Bone Resorption. PLoS ONE 2015, 10, e0132513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mons, E.; Jansen, I.D.C.; Loboda, J.; van Doodewaerd, B.R.; Hermans, J.; Verdoes, M.; van Boeckel, C.A.A.; van Veelen, P.A.; Turk, B.; Turk, D.; et al. The Alkyne Moiety as a Latent Electrophile in Irreversible Covalent Small Molecule Inhibitors of Cathepsin K. J. Am. Chem. Soc. 2019, 141, 3507–3514. [Google Scholar] [CrossRef]
- Bansal, S.; Bala, M.; Suthar, S.K.; Choudhary, S.; Bhattacharya, S.; Bhardwaj, V.; Singla, S.; Joseph, A. Design and Synthesis of Novel 2-Phenyl-5-(1,3-Diphenyl-1H-Pyrazol-4-Yl)-1,3,4-Oxadiazoles as Selective COX-2 Inhibitors with Potent Anti-Inflammatory Activity. Eur. J. Med. Chem. 2014, 80, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Abd-Ellah, H.S.; Abdel-Aziz, M.; Shoman, M.E.; Beshr, E.A.M.; Kaoud, T.S.; Ahmed, A.-S.F.F. Novel 1,3,4-Oxadiazole/Oxime Hybrids: Synthesis, Docking Studies and Investigation of Anti-Inflammatory, Ulcerogenic Liability and Analgesic Activities. Bioorg Chem. 2016, 69, 48–63. [Google Scholar] [CrossRef]
- Hannoun, M.H.; Hagras, M.; Kotb, A.; El-Attar, A.-A.M.M.; Abulkhair, H.S. Synthesis and Antibacterial Evaluation of a Novel Library of 2-(Thiazol-5-Yl)-1,3,4-Oxadiazole Derivatives against Methicillin-Resistant Staphylococcus Aureus (MRSA). Bioorg Chem. 2020, 94, 103364. [Google Scholar] [CrossRef]
- SciELO. Brazil-1,2,4- and 1,3,4-Oxadiazoles as Scaffolds in the Development of Antiparasitic Agents 1,2,4- and 1,3,4-Oxadiazoles as Scaffolds in the Development of Antiparasitic Agents. Available online: https://www.scielo.br/j/jbchs/a/C3vbJtND6RKQB7KCDFRVFRr/?lang=en (accessed on 25 December 2024).
- Altıntop, M.D.; Sever, B.; Akalın Çiftçi, G.; Turan-Zitouni, G.; Kaplancıklı, Z.A.; Özdemir, A. Design, Synthesis, in Vitro and in Silico Evaluation of a New Series of Oxadiazole-Based Anticancer Agents as Potential Akt and FAK Inhibitors. Eur. J. Med. Chem. 2018, 155, 905–924. [Google Scholar] [CrossRef]
- Ahsan, M.J. 1,3,4-Oxadiazole Containing Compounds As Therapeutic Targets For Cancer Therapy. Mini Rev. Med. Chem. 2022, 22, 164–197. [Google Scholar] [CrossRef]
- de Miguel, R.; Montejano, R.; Stella-Ascariz, N.; Arribas, J.R. A Safety Evaluation of Raltegravir for the Treatment of HIV. Expert Opin. Drug Saf. 2018, 17, 217–223. [Google Scholar] [CrossRef]
- Hassan, A.; Khan, A.H.; Saleem, F.; Ahmad, H.; Khan, K.M. A Patent Review of Pharmaceutical and Therapeutic Applications of Oxadiazole Derivatives for the Treatment of Chronic Diseases (2013–2021). Expert Opin. Ther. Pat. 2022, 32, 969–1001. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.T.; Hirschbein, B.L.; Cheung, H.; McCarter, J.; Janc, J.W.; Yu, Z.W.; Wesolowski, G. Keto-1,3,4-Oxadiazoles as Cathepsin K Inhibitors. Bioorg Med. Chem. Lett. 2006, 16, 2909–2914. [Google Scholar] [CrossRef] [PubMed]
- Tavares, F.X.; Deaton, D.N.; Miller, A.B.; Miller, L.R.; Wright, L.L. Ketoheterocycle-Based Inhibitors of Cathepsin K: A Novel Entry into the Synthesis of Peptidic Ketoheterocycles. Bioorg Med. Chem. Lett. 2005, 15, 3891–3895. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.L.; Bartolomeu, A.d.A.; Jesus, H.C.R.d.; Oliveira, K.T.d.; Fernandes, J.B.; Brömme, D.; Vieira, P.C. New Synthetic Quinolines as Cathepsin K Inhibitors. J. Braz. Chem. Soc. 2020, 31, 1605–1613. [Google Scholar] [CrossRef]
- Petek, N.; Štefane, B.; Novinec, M.; Svete, J. Synthesis and Biological Evaluation of 7-(Aminoalkyl)Pyrazolo [1,5-a]Pyrimidine Derivatives as Cathepsin K Inhibitors. Bioorg Chem. 2019, 84, 226–238. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, T.; Xiong, H.; Hu, W.; Zhu, X.; Ma, Y.; Zhang, Z. Synthesis and Biological Evaluation of Novel Piperidine-3-Carboxamide Derivatives as Anti-Osteoporosis Agents Targeting Cathepsin K. Molecules 2024, 29, 4011. [Google Scholar] [CrossRef]
- Cardoso Prado Martins, F.; Dos Reis Rocho, F.; Bonatto, V.; Jatai Batista, P.H.; Lameira, J.; Leitão, A.; Montanari, C.A. Novel Selective Proline-Based Peptidomimetics for Human Cathepsin K Inhibition. Bioorg Med. Chem. Lett. 2024, 110, 129887. [Google Scholar] [CrossRef]
- Falgueyret, J.-P.; Desmarais, S.; Oballa, R.; Black, W.C.; Cromlish, W.; Khougaz, K.; Lamontagne, S.; Massé, F.; Riendeau, D.; Toulmond, S.; et al. Lysosomotropism of Basic Cathepsin K Inhibitors Contributes to Increased Cellular Potencies against Off-Target Cathepsins and Reduced Functional Selectivity. J. Med. Chem. 2005, 48, 7535–7543. [Google Scholar] [CrossRef]
- Wang, D.; Pechar, M.; Li, W.; Kopecková, P.; Brömme, D.; Kopecek, J. Inhibition of Cathepsin K with Lysosomotropic Macromolecular Inhibitors. Biochemistry 2002, 41, 8849–8859. [Google Scholar] [CrossRef]
- Gauthier, J.Y.; Chauret, N.; Cromlish, W.; Desmarais, S.; Duong, L.T.; Falgueyret, J.-P.; Kimmel, D.B.; Lamontagne, S.; Léger, S.; LeRiche, T.; et al. The Discovery of Odanacatib (MK-0822), a Selective Inhibitor of Cathepsin K. Bioorg Med. Chem. Lett. 2008, 18, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Stoch, S.A.; Zajic, S.; Stone, J.A.; Miller, D.L.; van Bortel, L.; Lasseter, K.C.; Pramanik, B.; Cilissen, C.; Liu, Q.; Liu, L.; et al. Odanacatib, a Selective Cathepsin K Inhibitor to Treat Osteoporosis: Safety, Tolerability, Pharmacokinetics and Pharmacodynamics--Results from Single Oral Dose Studies in Healthy Volunteers. Br. J. Clin. Pharmacol. 2013, 75, 1240–1254. [Google Scholar] [CrossRef] [PubMed]
- Lark, M.W.; Stroup, G.B.; James, I.E.; Dodds, R.A.; Hwang, S.M.; Blake, S.M.; Lechowska, B.A.; Hoffman, S.J.; Smith, B.R.; Kapadia, R.; et al. A Potent Small Molecule, Nonpeptide Inhibitor of Cathepsin K (SB 331750) Prevents Bone Matrix Resorption in the Ovariectomized Rat. Bone 2002, 30, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Ochi, Y.; Yamada, H.; Mori, H.; Kawada, N.; Kayasuga, R.; Nakanishi, Y.; Tanaka, M.; Imagawa, A.; Ohmoto, K.; Kawabata, K. ONO-5334, a Cathepsin K Inhibitor, Improves Bone Strength by Preferentially Increasing Cortical Bone Mass in Ovariectomized Rats. J. Bone Miner. Metab. 2014, 32, 645–652. [Google Scholar] [CrossRef]
- Yamashita, T.; Hagino, H.; Hayashi, I.; Hayashibara, M.; Tanida, A.; Nagira, K.; Fukui, R.; Nagashima, H. Effect of a Cathepsin K Inhibitor on Arthritis and Bone Mineral Density in Ovariectomized Rats with Collagen-Induced Arthritis. Bone Rep. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Vashum, Y.; Premsingh, R.; Kottaiswamy, A.; Soma, M.; Padmanaban, A.; Kalaiselvan, P.; Samuel, S. Inhibitory Effect of Cathepsin K Inhibitor (ODN-MK-0822) on Invasion, Migration and Adhesion of Human Breast Cancer Cells in Vitro. Mol. Biol. Rep. 2021, 48, 105–116. [Google Scholar] [CrossRef]
- Duong, L.T.; Leung, A.T.; Langdahl, B. Cathepsin K Inhibition: A New Mechanism for the Treatment of Osteoporosis. Calcif. Tissue Int. 2016, 98, 381–397. [Google Scholar] [CrossRef]
- Rocho, F.R.; Bonatto, V.; Lameiro, R.F.; Lameira, J.; Leitão, A.; Montanari, C.A. A Patent Review on Cathepsin K Inhibitors to Treat Osteoporosis (2011–2021). Expert Opin. Ther. Pat. 2022, 32, 561–573. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Zhang, L.; Hu, B.; Hu, S.; Zhang, X.; Hu, J. Small Molecule Inhibitors of Osteoarthritis: Current Development and Future Perspective. Front. Physiol. 2023, 14, 1156913. [Google Scholar] [CrossRef]
- Brömme, D.; Panwar, P.; Turan, S. Cathepsin K Osteoporosis Trials, Pycnodysostosis and Mouse Deficiency Models: Commonalities and Differences. Expert Opin. Drug Discov. 2016, 11, 457–472. [Google Scholar] [CrossRef]
- McClung, M.R.; O’Donoghue, M.L.; Papapoulos, S.E.; Bone, H.; Langdahl, B.; Saag, K.G.; Reid, I.R.; Kiel, D.P.; Cavallari, I.; Bonaca, M.P.; et al. Odanacatib for the Treatment of Postmenopausal Osteoporosis: Results of the LOFT Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial and LOFT Extension Study. Lancet Diabetes Endocrinol. 2019, 7, 899–911. [Google Scholar] [CrossRef]
- Binkley, N.; Orwoll, E.; Chapurlat, R.; Langdahl, B.L.; Scott, B.B.; Giezek, H.; Santora, A.C. Randomized, Controlled Trial to Assess the Safety and Efficacy of Odanacatib in the Treatment of Men with Osteoporosis. Osteoporos. Int. 2021, 32, 173–184. [Google Scholar] [CrossRef]
- Berenbaum, F. Targeted Therapies in Osteoarthritis: A Systematic Review of the Trials on www.Clinicaltrials.gov. Best Pract. Res. Clin. Rheumatol. 2010, 24, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Peroni, A.; Zini, A.; Braga, V.; Colato, C.; Adami, S.; Girolomoni, G. Drug-Induced Morphea: Report of a Case Induced by Balicatib and Review of the Literature. J. Am. Acad. Dermatol. 2008, 59, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Jerome, C.; Missbach, M.; Gamse, R. Balicatib, a Cathepsin K Inhibitor, Stimulates Periosteal Bone Formation in Monkeys. Osteoporos. Int. 2012, 23, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; LePage, C.; Swift, B.A.; Yamashita, D.; Bendele, A.M.; Maul, D.; Kumar, S. Protective Effects of a Cathepsin K Inhibitor, SB-553484, in the Canine Partial Medial Meniscectomy Model of Osteoarthritis. Osteoarthr. Cartil. 2009, 17, 1236–1243. [Google Scholar] [CrossRef]
- Svelander, L.; Erlandsson-Harris, H.; Astner, L.; Grabowska, U.; Klareskog, L.; Lindstrom, E.; Hewitt, E. Inhibition of Cathepsin K Reduces Bone Erosion, Cartilage Degradation and Inflammation Evoked by Collagen-Induced Arthritis in Mice. Eur. J. Pharmacol. 2009, 613, 155–162. [Google Scholar] [CrossRef]
- Joshi, N.; Yan, J.; Dang, M.; Slaughter, K.; Wang, Y.; Wu, D.; Ung, T.; Pandya, V.; Chen, M.X.; Kaur, S.; et al. A Mechanically Resilient Soft Hydrogel Improves Drug Delivery for Treating Post-Traumatic Osteoarthritis in Physically Active Joints. bioRxiv 2024. 2024.05.16.594611. [Google Scholar] [CrossRef]
- Lindström, E.; Rizoska, B.; Tunblad, K.; Edenius, C.; Bendele, A.M.; Maul, D.; Larson, M.; Shah, N.; Yoder Otto, V.; Jerome, C.; et al. The Selective Cathepsin K Inhibitor MIV-711 Attenuates Joint Pathology in Experimental Animal Models of Osteoarthritis. J. Transl. Med. 2018, 16, 56. [Google Scholar] [CrossRef]
- Lindström, E.; Rizoska, B.; Henderson, I.; Terelius, Y.; Jerling, M.; Edenius, C.; Grabowska, U. Nonclinical and Clinical Pharmacological Characterization of the Potent and Selective Cathepsin K Inhibitor MIV-711. J. Transl. Med. 2018, 16, 125. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Bowes, M.A.; Kingsbury, S.R.; Brett, A.; Guillard, G.; Rizoska, B.; Sjögren, N.; Graham, P.; Jansson, Å.; Wadell, C.; et al. Disease-Modifying Effects of a Novel Cathepsin K Inhibitor in Osteoarthritis: A Randomized Controlled Trial. Ann. Intern. Med. 2020, 172, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Bihlet, A.R.; Byrjalsen, I.; Andersen, J.R.; Öberg, F.; Herder, C.; Bowes, M.A.; Conaghan, P.G. Symptomatic and Structural Benefit of Cathepsin K Inhibition by MIV-711 in a Subgroup with Unilateral Pain: Post-Hoc Analysis of a Randomised Phase 2a Clinical Trial. Clin. Exp. Rheumatol. 2022, 40, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Hedström, H. Press Release. Available online: https://www.medivir.com/investors/press-releases/press-release (accessed on 6 March 2025).
- Nwosu, L.N.; Gowler, P.R.W.; Burston, J.J.; Rizoska, B.; Tunblad, K.; Lindström, E.; Grabowska, U.; Li, L.; McWilliams, D.F.; Walsh, D.A.; et al. Analgesic Effects of the Cathepsin K Inhibitor L-006235 in the Monosodium Iodoacetate Model of Osteoarthritis Pain. Pain Rep. 2018, 3, e685. [Google Scholar] [CrossRef] [PubMed]

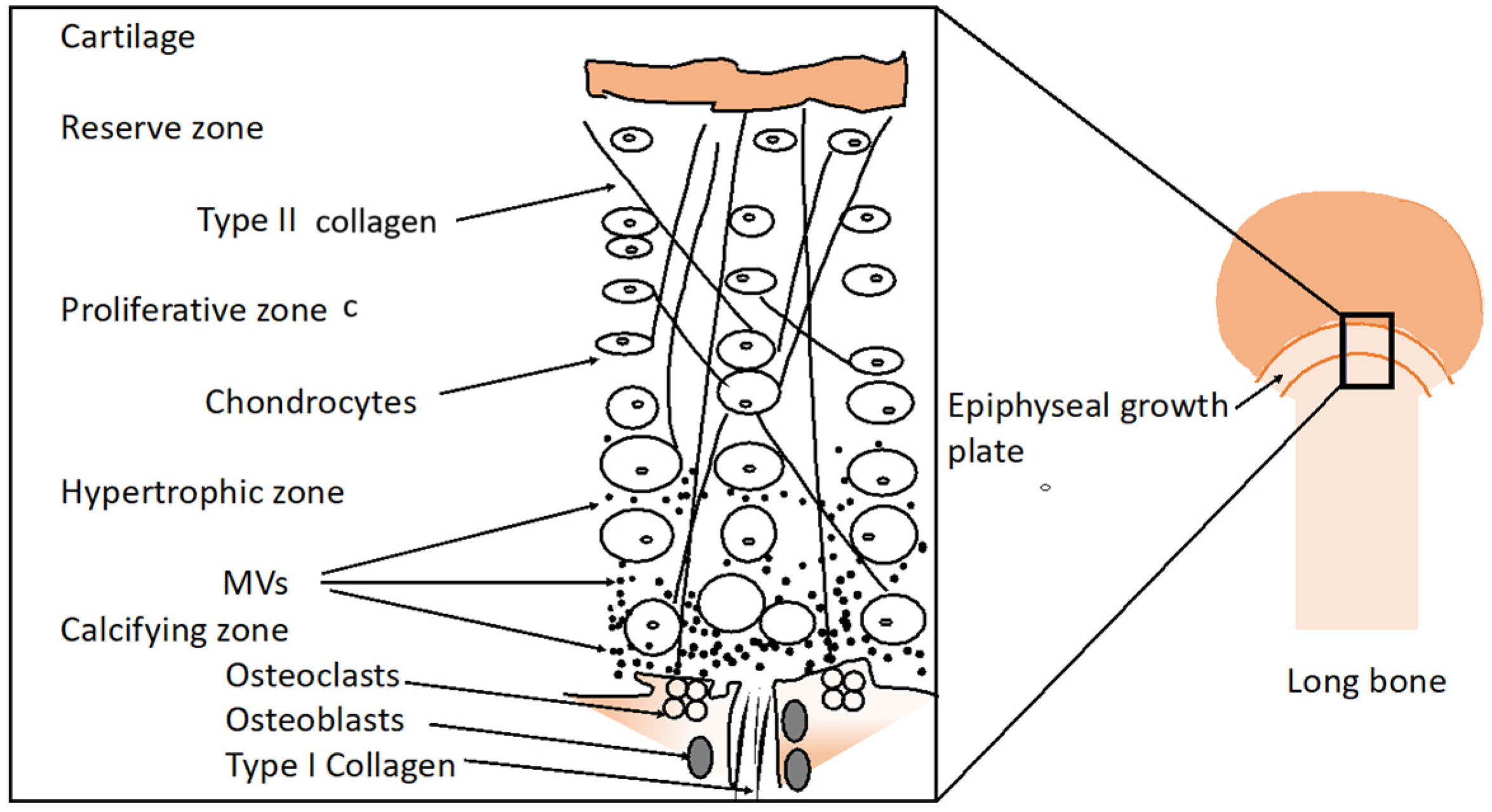
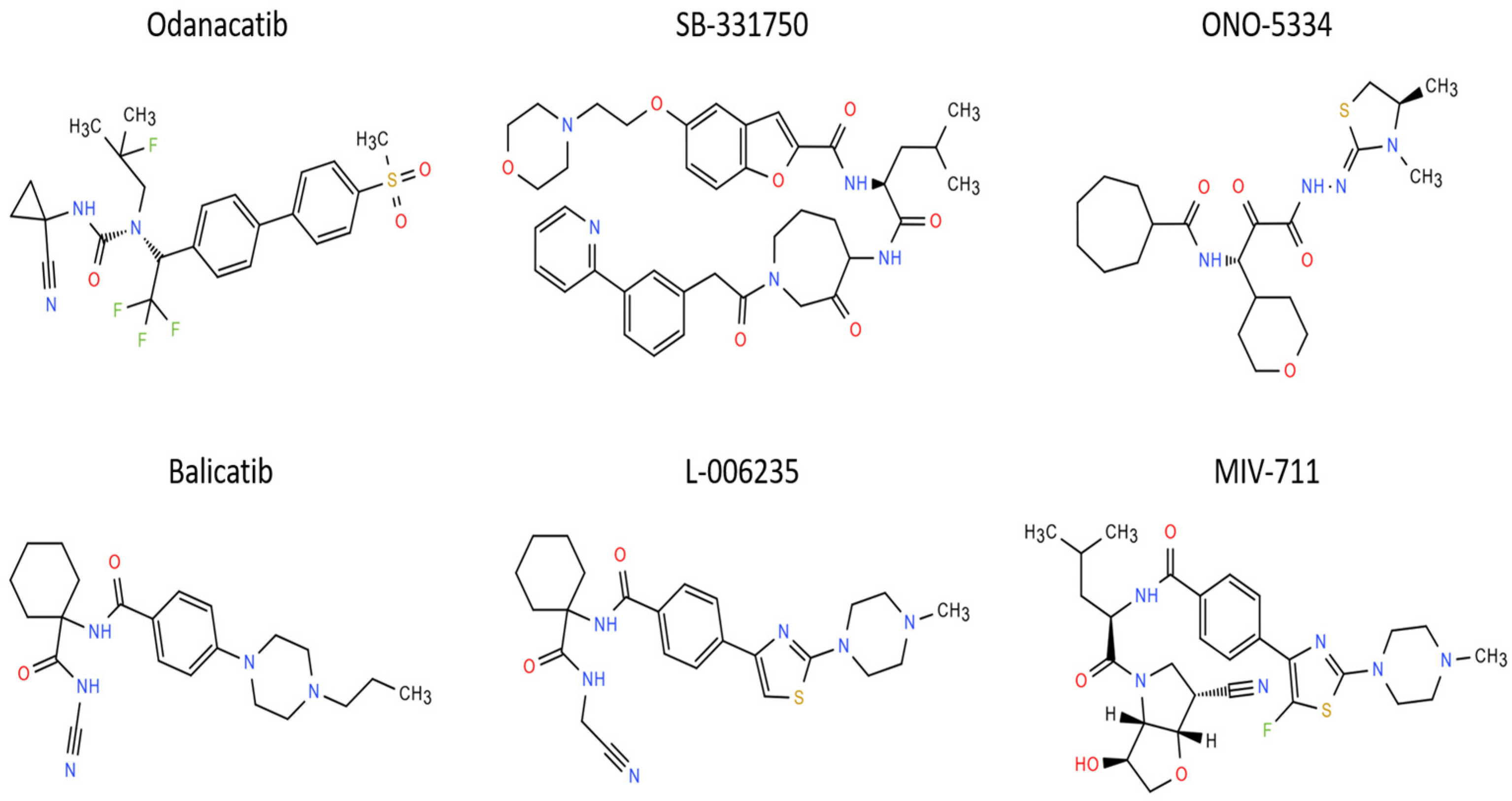
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brizuela, L.; Buchet, R.; Bougault, C.; Mebarek, S. Cathepsin K Inhibitors as Potential Drugs for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2025, 26, 2896. https://doi.org/10.3390/ijms26072896
Brizuela L, Buchet R, Bougault C, Mebarek S. Cathepsin K Inhibitors as Potential Drugs for the Treatment of Osteoarthritis. International Journal of Molecular Sciences. 2025; 26(7):2896. https://doi.org/10.3390/ijms26072896
Chicago/Turabian StyleBrizuela, Leyre, Rene Buchet, Carole Bougault, and Saida Mebarek. 2025. "Cathepsin K Inhibitors as Potential Drugs for the Treatment of Osteoarthritis" International Journal of Molecular Sciences 26, no. 7: 2896. https://doi.org/10.3390/ijms26072896
APA StyleBrizuela, L., Buchet, R., Bougault, C., & Mebarek, S. (2025). Cathepsin K Inhibitors as Potential Drugs for the Treatment of Osteoarthritis. International Journal of Molecular Sciences, 26(7), 2896. https://doi.org/10.3390/ijms26072896









