Recent Advances in the Application of Hydrogels as Drug Carriers in Inflammatory Bowel Disease: A Review
Abstract
1. Introduction
2. Response Mechanism of Hydrogels
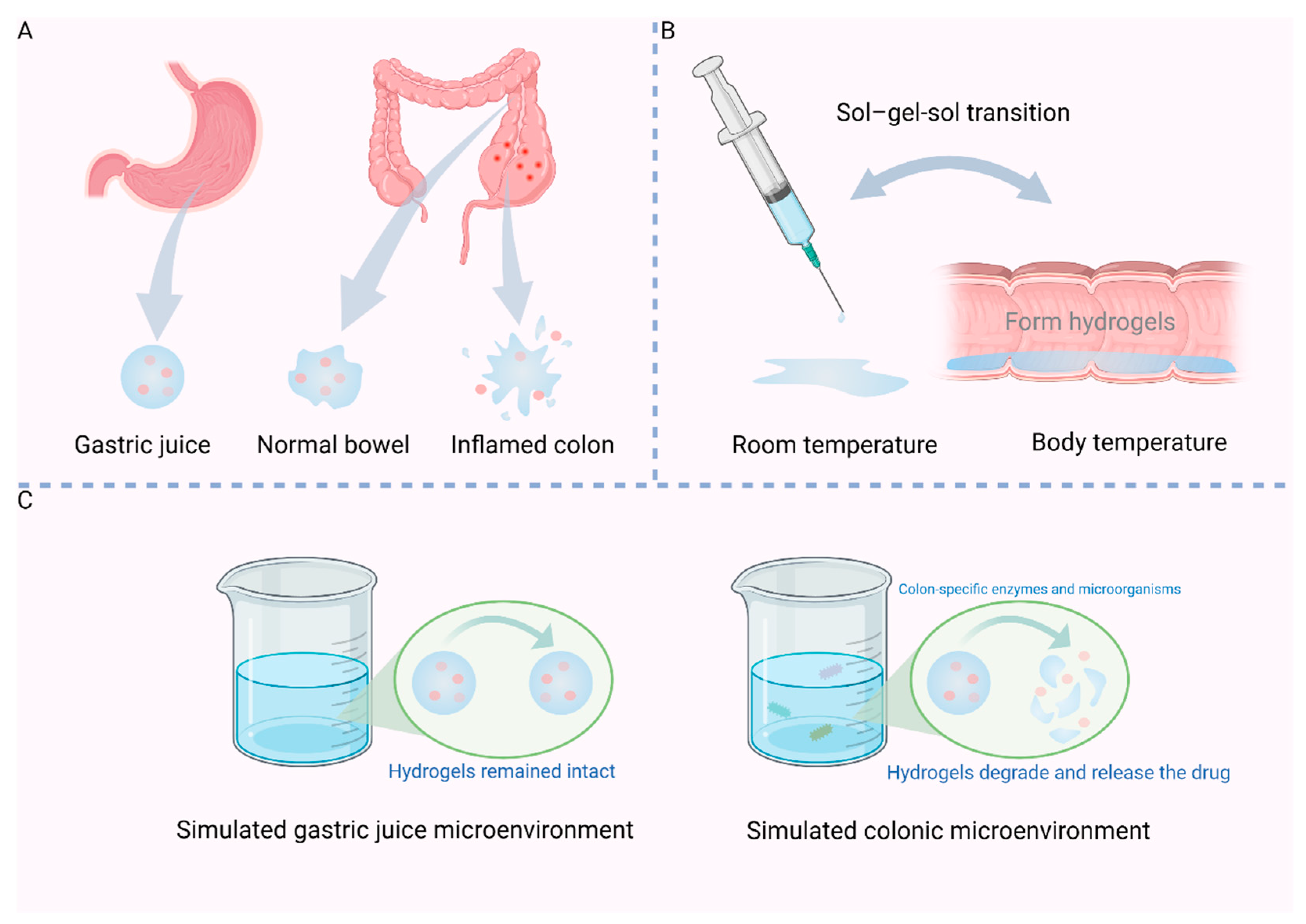
2.1. pH-Sensitive
2.2. Temperature-Sensitive
2.3. Enzyme Sensitivity
2.4. ROS Sensitive
3. Application of Hydrogels as Drug Carriers in the Treatment of IBD
3.1. Maintained the Intestinal Barrier
3.2. Regulate the Immune System
3.3. Regulate Gut Microbiota
3.4. Clear Intracellular ROS
3.5. Adhesion
3.6. Anti-Fibrosis
3.7. Relieve Anxiety and Depression
4. Mode of Administration
4.1. Oral Administration
4.2. Rectal Administration
4.3. Endoscopic Administration
4.4. Subcutaneous Administration
5. Conclusions and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Z.; Wan, X. Global, regional, and national burden of inflammatory bowel disease and its associated anemia, 1990 to 2019 and predictions to 2050: An analysis of the global burden of disease study 2019. Autoimmun. Rev. 2024, 23, 103498. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, B.; Sun, Y.; Li, J.; Shen, P.; Hu, L.; Liu, G.; Wang, J.; Duan, L.; Zhan, S.; et al. Incidence of Inflammatory Bowel Disease in Urban China: A Nationwide Population-based Study. Clin. Gastroenterol. Hepatol. 2023, 21, 3379–3386.e29. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.S.; Ferreira, D.; Paige, E.; Gedye, C.; Boyle, M. Infectious Complications of Biological and Small Molecule Targeted Immunomodulatory Therapies. Clin. Microbiol. Rev. 2020, 33, 1110–1128. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Peyrin-Biroulet, L.; Panaccione, R.; Rutgeerts, P.; Hanauer, S.B.; Ghosh, S.; Van Assche, G.; Robinson, A.M.; et al. Long-term safety of adalimumab in clinical trials in adult patients with Crohn’s disease or ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 219–228. [Google Scholar] [CrossRef]
- Murray, A.; Nguyen, T.M.; Parker, C.E.; Feagan, B.G.; MacDonald, J.K. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2020, 8, Cd000543. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Pagnini, C.; Pizarro, T.T.; Cominelli, F. Novel Pharmacological Therapy in Inflammatory Bowel Diseases: Beyond Anti-Tumor Necrosis Factor. Front. Pharmacol. 2019, 10, 671. [Google Scholar] [CrossRef]
- Gutierrez, A.M.; Frazar, E.M.; MV, X.K.; Paul, P.; Hilt, J.Z. Hydrogels and Hydrogel Nanocomposites: Enhancing Healthcare through Human and Environmental Treatment. Adv. Healthc. Mater. 2022, 11, e2101820. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, e2306152. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, X.; Liu, R.; Zhao, Y.; Sun, L. ECM-Inspired Hydrogels with ADSCs Encapsulation for Rheumatoid Arthritis Treatment. Adv. Sci. 2023, 10, e2206253. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, R.; Wu, H.; Yao, H.; Yan, Y.; Liu, J.; Ran, L.; Sun, Z.; Yi, L.; Dang, L.; et al. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat. Commun. 2019, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Kuang, S.; Zhang, Y.; Yang, M.; Qin, W.; Shi, X.; Lin, Z. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact. Mater. 2020, 5, 1113–1126. [Google Scholar] [CrossRef]
- Yan, N.; Xu, J.; Liu, G.; Ma, C.; Bao, L.; Cong, Y.; Wang, Z.; Zhao, Y.; Xu, W.; Chen, C. Penetrating Macrophage-Based Nanoformulation for Periodontitis Treatment. ACS Nano 2022, 16, 18253–18265. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Bai, J.; Li, X.; Chen, X.; Li, Z.; Pan, H.; Li, S.; Gao, Q.; Zhao, N.; et al. Multifunctional Hydrogel Eye Drops for Synergistic Treatment of Ocular Inflammatory Disease. ACS Nano 2023, 17, 25377–25390. [Google Scholar] [CrossRef]
- Fang, G.; Wang, Q.; Yang, X.; Qian, Y.; Zhang, G.; Tang, B. γ-Cyclodextrin-based polypseudorotaxane hydrogels for ophthalmic delivery of flurbiprofen to treat anterior uveitis. Carbohydr. Polym. 2022, 277, 118889. [Google Scholar] [CrossRef]
- Lin, D.; Xu, X.; Chen, L.; Chen, L.; Deng, M.; Chen, J.; Ren, Z.; Lei, L.; Wang, J.; Deng, J.; et al. Supramolecular nanofiber of indomethacin derivative confers highly cyclooxygenase-2 (COX-2) selectivity and boosts anti-inflammatory efficacy. J. Control. Release 2023, 364, 272–282. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, L.; Hu, S.; Hu, J.; Fu, Y.; Hu, Y.; Yang, X. Carboxymethyl chitosan microspheres loaded hyaluronic acid/gelatin hydrogels for controlled drug delivery and the treatment of inflammatory bowel disease. Int. J. Biol. Macromol. 2021, 167, 1598–1612. [Google Scholar] [CrossRef]
- Huang, H.B.; Gong, W.; Hou, Y.Y.; He, W.Y.; Wang, R.; Wang, X.C.; Hu, J.N. Mucoadhesive Hydrogel with Anti-gastric Acid and Sustained-Release Functions for Amelioration of DSS-Induced Ulcerative Colitis. J. Agric. Food Chem. 2023, 71, 4016–4028. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Bai, Y.; Zhang, Z.; Mei, H.; Li, J.; Pu, Y.; Zhao, N.; Gao, W.; Wu, F.; He, B.; et al. Thermosensitive polymer hydrogel as a physical shield on colonic mucosa for colitis treatment. J. Mater. Chem. B 2021, 9, 3874–3884. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ning, X.; Wu, J.; Wang, Q.; Wang, Z.; Chen, Z.; Tang, X.; Bai, P.; Pu, K.; Li, L.; et al. Metabolic Nanoregulator Remodels Gut Microenvironment for Treatment of Inflammatory Bowel Disease. ACS Nano 2024, 18, 7123–7135. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Chen, H.; Liu, E.; Zhang, M.; Zeng, F.; Shen, H.; Fang, Y.; Muhitdinov, B.; Huang, Y. Oral pectin/oligochitosan microspheres for colon-specific controlled release of quercetin to treat inflammatory bowel disease. Carbohydr. Polym. 2023, 316, 121025. [Google Scholar] [CrossRef]
- Huai, M.; Pei, M.; Pan, J.; Zhu, Y.; Chen, Y.; Du, P.; Duan, Y.; Xu, H.; Ge, W. Oral colon-targeted responsive alginate/hyaluronic acid-based hydrogel propels the application of infliximab in colitis. Int. J. Biol. Macromol. 2023, 249, 125952. [Google Scholar] [CrossRef]
- Zhang, G.; Song, D.; Ma, R.; Li, M.; Liu, B.; He, Z.; Fu, Q. Artificial mucus layer formed in response to ROS for the oral treatment of inflammatory bowel disease. Sci. Adv. 2024, 10, eado8222. [Google Scholar] [CrossRef]
- Liu, L.; Yao, W.; Rao, Y.; Lu, X.; Gao, J. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef]
- Hendi, A.; Umair Hassan, M.; Elsherif, M.; Alqattan, B.; Park, S.; Yetisen, A.K.; Butt, H. Healthcare Applications of pH-Sensitive Hydrogel-Based Devices: A Review. Int. J. Nanomed. 2020, 15, 3887–3901. [Google Scholar] [CrossRef]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin maintenance therapy for ulcerative colitis: Randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef]
- Aditya, N.P.; Hamilton, I.E.; Norton, I.T. Amorphous nano-curcumin stabilized oil in water emulsion: Physico chemical characterization. Food Chem. 2017, 224, 191–200. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, P.; Shen, H. Corrigendum to “Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis” [Int. Immunopharmacol. 67 (2019) 129-137]. Int. Immunopharmacol. 2021, 99, 107815. [Google Scholar] [CrossRef] [PubMed]
- Shahrzad, S.; Aoyagi, K.; Winter, A.; Koyama, A.; Bitsch, I. Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J. Nutr. 2001, 131, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wei, C.; Liu, T.; Lv, F.; Qian, Z. Real-Time Fluorescence Tracking of Protoporphyrin Incorporated Thermosensitive Hydrogel and Its Drug Release in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 5104–5113. [Google Scholar] [CrossRef]
- Park, M.H.; Joo, M.K.; Choi, B.G.; Jeong, B. Biodegradable thermogels. Acc. Chem. Res. 2012, 45, 424–433. [Google Scholar] [CrossRef]
- Zhang, W.; Mahuta, K.M.; Mikulski, B.A.; Harvestine, J.N.; Crouse, J.Z.; Lee, J.C.; Kaltchev, M.G.; Tritt, C.S. Novel pectin-based carriers for colonic drug delivery. Pharm. Dev. Technol. 2016, 21, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Emami, J.; Tavakoli, N.; Minaiyan, M.; Rahmani, N.; Dorkoosh, F.; Mahzouni, P. Pectin Film Coated Pellets for Colon-targeted Delivery of Budesonide: In-vitro/In-vivo Evaluation in Induced Ulcerative Colitis in Rat. Iran. J. Pharm. Res. 2012, 11, 733–745. [Google Scholar]
- Pandey, M.; Choudhury, H.; SK, D.O.S.S.; Chetty Annan, N.; Bhattamisra, S.K.; Gorain, B.; Mohd Amin, M.C.I. Budesonide-Loaded Pectin/Polyacrylamide Hydrogel for Sustained Delivery: Fabrication, Characterization and In Vitro Release Kinetics. Molecules 2021, 26, 2704. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Fang, Z.; Ng, K. Dietary fiber-based colon-targeted delivery systems for polyphenols. Trends Food Sci. Technol. 2020, 100, 333–348. [Google Scholar] [CrossRef]
- Lee, Y.; Sugihara, K.; Gillilland, M.G., 3rd; Jon, S.; Kamada, N.; Moon, J.J. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 2020, 19, 118–126. [Google Scholar] [CrossRef]
- Leichner, C.; Jelkmann, M.; Bernkop-Schnürch, A. Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature. Adv. Drug Deliv. Rev. 2019, 151–152, 191–221. [Google Scholar] [CrossRef]
- Roldo, M.; Hornof, M.; Caliceti, P.; Bernkop-Schnürch, A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: Synthesis and in vitro evaluation. Eur. J. Pharm. Biopharm. 2004, 57, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pan, Y.; Guo, Z.; Fan, X.; Pan, Q.; Gao, W.; Luo, K.; Pu, Y.; He, B. An olsalazine nanoneedle-embedded inulin hydrogel reshapes intestinal homeostasis in inflammatory bowel disease. Bioact. Mater. 2024, 33, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, X.; Wang, L.; Guo, H.; Han, M.; Guo, H.; Chen, Y.; Wu, A.; Li, H.; Chen, S.; et al. Mn3O4 Nanozyme Loaded Thermosensitive PDLLA-PEG-PDLLA Hydrogels for the Treatment of Inflammatory Bowel Disease. ACS Appl. Mater. Interfaces 2023, 15, 33273–33287. [Google Scholar] [CrossRef]
- Araki, T.; Mitsuyama, K.; Yamasaki, H.; Morita, M.; Tsuruta, K.; Mori, A.; Yoshimura, T.; Fukunaga, S.; Kuwaki, K.; Yoshioka, S.; et al. Therapeutic Potential of a Self-Assembling Peptide Hydrogel to Treat Colonic Injuries Associated with Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cai, Z.; Wang, F.; Hong, L.; Deng, L.; Zhong, J.; Wang, Z.; Cui, W. Colon-Targeted Adhesive Hydrogel Microsphere for Regulation of Gut Immunity and Flora. Adv. Sci. 2021, 8, e2101619. [Google Scholar] [CrossRef]
- Ouyang, J.; Deng, B.; Zou, B.; Li, Y.; Bu, Q.; Tian, Y.; Chen, M.; Chen, W.; Kong, N.; Chen, T.; et al. Oral Hydrogel Microbeads-Mediated In Situ Synthesis of Selenoproteins for Regulating Intestinal Immunity and Microbiota. J. Am. Chem. Soc. 2023, 145, 12193–12205. [Google Scholar] [CrossRef]
- Lei, F.; Zeng, F.; Yu, X.; Deng, Y.; Zhang, Z.; Xu, M.; Ding, N.; Tian, J.; Li, C. Oral hydrogel nanoemulsion co-delivery system treats inflammatory bowel disease via anti-inflammatory and promoting intestinal mucosa repair. J. Nanobiotechnol. 2023, 21, 275. [Google Scholar] [CrossRef]
- Kim, D.W.; Jeong, H.S.; Kim, E.; Lee, H.; Choi, C.H.; Lee, S.J. Oral delivery of stem-cell-loaded hydrogel microcapsules restores gut inflammation and microbiota. J. Control. Release 2022, 347, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Xia, G.; Zhang, X.; Deng, S.; Zhao, X.; Xu, Y.; Chang, G.; Tao, Y.; Li, M.; et al. Oral Delivery of Bioactive Glass-Loaded Core-Shell Hydrogel Microspheres for Effective Treatment of Inflammatory Bowel Disease. Adv. Sci. 2023, 10, e2207418. [Google Scholar] [CrossRef]
- Cao, X.; Duan, L.; Hou, H.; Liu, Y.; Chen, S.; Zhang, S.; Liu, Y.; Wang, C.; Qi, X.; Liu, N.; et al. IGF-1C hydrogel improves the therapeutic effects of MSCs on colitis in mice through PGE(2)-mediated M2 macrophage polarization. Theranostics 2020, 10, 7697–7709. [Google Scholar] [CrossRef]
- Poláková, L.; Raus, V.; Cuchalová, L.; Poręba, R.; Hrubý, M.; Kučka, J.; Větvička, D.; Trhlíková, O.; Sedláková, Z. SHARP hydrogel for the treatment of inflammatory bowel disease. Int. J. Pharm. 2022, 613, 121392. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.T.; Kuo, S.N.; Hung, S.W.; Yang, C.Y. Combined Treatment with Hyaluronic Acid and Mesalamine Protects Rats from Inflammatory Bowel Disease Induced by Intracolonic Administration of Trinitrobenzenesulfonic Acid. Molecules 2017, 22, 904. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Jin, K.; Wang, R.; Chen, B.; Zhang, J.; Ren, C.; Chen, X.; Lu, J.; Zhou, M. Microalgae-Based Hydrogel for Inflammatory Bowel Disease and Its Associated Anxiety and Depression. Adv. Mater. 2024, 36, e2312275. [Google Scholar] [CrossRef]
- Buckley, A.; Turner, J.R. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Jahanbazi Jahan-Abad, A.; Karima, S.; Sahab Negah, S.; Noorbakhsh, F.; Borhani-Haghighi, M.; Gorji, A. Therapeutic potential of conditioned medium derived from oligodendrocytes cultured in a self-assembling peptide nanoscaffold in experimental autoimmune encephalomyelitis. Brain Res. 2019, 1711, 226–235. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Ma, H.; Yang, Q.; Shang, Q.; Song, L.; Zheng, Z.; Zhang, S.; Pan, Y.; Huang, P.; et al. Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superoxide-dependent AMPK activation, Hif-1α upregulation and autophagy. Free Radic. Biol. Med. 2020, 161, 339–350. [Google Scholar] [CrossRef]
- Pan, X.; Zhu, Q.; Pan, L.L.; Sun, J. Macrophage immunometabolism in inflammatory bowel diseases: From pathogenesis to therapy. Pharmacol. Ther. 2022, 238, 108176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Luo, Z.; Ma, L.; Zhu, S.; Wang, Z.; Wen, J.; Cheng, S.; Gu, W.; Lian, Q.; et al. ECM1 is an essential factor for the determination of M1 macrophage polarization in IBD in response to LPS stimulation. Proc. Natl. Acad. Sci. USA 2020, 117, 3083–3092. [Google Scholar] [CrossRef]
- Rao, S.; Lin, Y.; Lin, R.; Liu, J.; Wang, H.; Hu, W.; Chen, B.; Chen, T. Traditional Chinese medicine active ingredients-based selenium nanoparticles regulate antioxidant selenoproteins for spinal cord injury treatment. J. Nanobiotechnol. 2022, 20, 278. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.; Wu, Y.; Yuan, M.; Huang, J.; Xia, Y.; Ling, Q.; Hoffmann, P.R.; Huang, Z.; Chen, T. Atherosclerotic plaque-targeted nanotherapeutics ameliorates atherogenesis by blocking macrophage-driven inflammation. Nano Today 2022, 42, 101351. [Google Scholar] [CrossRef]
- Dionne, S.; Duchatelier, C.F.; Seidman, E.G. The influence of vitamin D on M1 and M2 macrophages in patients with Crohn’s disease. Innate Immun. 2017, 23, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Xu, L.; Sheng, Y.; Wang, J.; Zhou, X.; Zhang, C.; Guo, L.; Li, W.; Han, C. Th1 promotes M1 polarization of intestinal macrophages to regulate colitis-related mucosal barrier damage. Aging 2023, 15, 6721–6735. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Somineni, H.K.; Kugathasan, S. The Microbiome in Patients With Inflammatory Diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 243–255. [Google Scholar] [CrossRef]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Krishnan, S.; Ramakrishna, B.S.; Mathan, M.; Pulimood, A.B.; Murthy, S.N. Butyrate and glucose metabolism by colonocytes in experimental colitis in mice. Gut 2000, 46, 493–499. [Google Scholar] [CrossRef]
- De, A.; Chen, W.; Li, H.; Wright, J.R.; Lamendella, R.; Lukin, D.J.; Szymczak, W.A.; Sun, K.; Kelly, L.; Ghosh, S.; et al. Bacterial Swarmers Enriched During Intestinal Stress Ameliorate Damage. Gastroenterology 2021, 161, 211–224. [Google Scholar] [CrossRef]
- Priya, S.; Burns, M.B.; Ward, T.; Mars, R.A.T.; Adamowicz, B.; Lock, E.F.; Kashyap, P.C.; Knights, D.; Blekhman, R. Identification of shared and disease-specific host gene-microbiome associations across human diseases using multi-omic integration. Nat. Microbiol. 2022, 7, 780–795. [Google Scholar] [CrossRef]
- Alipour, M.; Zaidi, D.; Valcheva, R.; Jovel, J.; Martínez, I.; Sergi, C.; Walter, J.; Mason, A.L.; Wong, G.K.; Dieleman, L.A.; et al. Mucosal Barrier Depletion and Loss of Bacterial Diversity are Primary Abnormalities in Paediatric Ulcerative Colitis. J. Crohns Colitis 2016, 10, 462–471. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. 2016, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; DeGraff, W.; Hankovszky, O.H.; Sár, C.P.; Kálai, T.; Jeko, J.; Russo, A.; Mitchell, J.B.; Hideg, K. Studies of structure-activity relationship of nitroxide free radicals and their precursors as modifiers against oxidative damage. J. Med. Chem. 1998, 41, 3477–3492. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Strandman, S.; Zhu, J.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Stidham, R.W.; Xu, J.; Johnson, L.A.; Kim, K.; Moons, D.S.; McKenna, B.J.; Rubin, J.M.; Higgins, P.D. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology 2011, 141, 819–826.e811. [Google Scholar] [CrossRef]
- Speca, S.; Giusti, I.; Rieder, F.; Latella, G. Cellular and molecular mechanisms of intestinal fibrosis. World J. Gastroenterol. 2012, 18, 3635–3661. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, T.H.; Allin, K.H.; Keefer, L.; Ananthakrishnan, A.N.; Jess, T. Depression and anxiety in inflammatory bowel disease: Epidemiology, mechanisms and treatment. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 717–726. [Google Scholar] [CrossRef]
- Obrenovich, M.E.M. Leaky Gut, Leaky Brain? Microorganisms 2018, 6, 107. [Google Scholar] [CrossRef]
- Ohara, T.E.; Hsiao, E.Y. Microbiota–neuroepithelial signalling across the gut–brain axis.
- Pei, R.; Jiang, Y.; Lei, G.; Chen, J.; Liu, M.; Liu, S. Rhein Derivatives, A Promising Pivot? Mini Rev. Med. Chem. 2021, 21, 554–575. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Qu, Y.; Yang, Y.; Guo, Z.N. Brain injury biomarkers and applications in neurological diseases. Chin. Med. J. 2025, 138, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Shen, R.; Wei, L.; Xu, S.; Xia, W.; Hou, Y.; Cui, J.; Qu, R.; Luo, J.; Cao, J.; et al. Designing a microbial fermentation-functionalized alginate microsphere for targeted release of 5-ASA using nano dietary fiber carrier for inflammatory bowel disease treatment. J. Nanobiotechnol. 2023, 21, 344. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Zhang, M.; Yu, Z.; Zhang, M. Oral Administration of Platinum Nanoparticles with SOD/CAT Cascade Catalytic Activity to Alleviate Ulcerative Colitis. J. Funct. Biomater. 2023, 14, 548. [Google Scholar] [CrossRef]
- Zhang, S.; Ermann, J.; Succi, M.D.; Zhou, A.; Hamilton, M.J.; Cao, B.; Korzenik, J.R.; Glickman, J.N.; Vemula, P.K.; Glimcher, L.H.; et al. An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci. Transl. Med. 2015, 7, 300ra128. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Lee, S.; Kim, T.Y.; Yu, S.E.; Kim, H.S.; Chung, Y.S.; Chung, S.; Park, S.; Shin, Y.C.; Wang, E.K.; et al. Sprayable nanomicelle hydrogels and inflammatory bowel disease patient cell chips for development of intestinal lesion-specific therapy. Bioact. Mater. 2022, 18, 433–445. [Google Scholar] [CrossRef]
- DeFrates, K.G.; Tong, E.; Cheng, J.; Heber-Katz, E.; Messersmith, P.B. A Pro-Regenerative Supramolecular Prodrug Protects Against and Repairs Colon Damage in Experimental Colitis. Adv. Sci. 2024, 11, e2304716. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Knapczyk, J.; Krowczynski, L.; Krzck, J.; Brzeski, M.; Nirnberg, E.; Schenk, D.; Struszcyk, H. Requirements of chitosan for pharmaceutical and biomedical applications. In Chitin and Chitosan: Sources, Chemistry, Biochemistry, Physical Properties and Applications; Elsevier: London, UK, 1989. [Google Scholar]
- Hirano, S.; Seino, H.; Akiyama, Y.; Nonaka, I. Chitosan: A Biocompatible Material for Oral and Intravenous Administrations. In Progress in Biomedical Polymers; Springer: Boston, MA, USA, 1990. [Google Scholar]
- Adamiak, K.; Sionkowska, A. State of Innovation in Alginate-Based Materials. Mar. Drugs 2023, 21, 353. [Google Scholar] [CrossRef]
- Liang, S.; Xu, J.; Weng, L.; Dai, H.; Zhang, X.; Zhang, L. Protein diffusion in agarose hydrogel in situ measured by improved refractive index method. J. Control. Release 2006, 115, 189–196. [Google Scholar] [CrossRef]
- van Hoogdalem, E.J.; de Boer, A.G.; Breimer, D.D. Pharmacokinetics of rectal drug administration, Part II. Clinical applications of peripherally acting drugs, and conclusions. Clin. Pharmacokinet. 1991, 21, 110–128. [Google Scholar] [CrossRef]
- Jiao, Y.; Xie, S.; Baseer, A.; Ud-Din, F. Rectal Administration of Celecoxib Liquid Suppositories with Enhanced Bioavailability and Safety in Rats. Curr. Drug Deliv. 2023, 20, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Prantera, C.; Rizzi, M. 5-ASA in ulcerative colitis: Improving treatment compliance. World J. Gastroenterol. 2009, 15, 4353–4355. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Del Rio, L.; Diaz-Rodriguez, P.; Landin, M. New tools to design smart thermosensitive hydrogels for protein rectal delivery in IBD. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110252. [Google Scholar] [CrossRef]
- Zebrowitz, E.; Aslanukov, A.; Kajikawa, T.; Bedelbaeva, K.; Bollinger, S.; Zhang, Y.; Sarfatti, D.; Cheng, J.; Messersmith, P.B.; Hajishengallis, G.; et al. Prolyl-hydroxylase inhibitor-induced regeneration of alveolar bone and soft tissue in a mouse model of periodontitis through metabolic reprogramming. Front. Dent. Med. 2022, 3, 992722. [Google Scholar] [CrossRef]
- Zhang, Y.; Strehin, I.; Bedelbaeva, K.; Gourevitch, D.; Clark, L.; Leferovich, J.; Messersmith, P.B.; Heber-Katz, E. Drug-induced regeneration in adult mice. Sci. Transl. Med. 2015, 7, 290ra292. [Google Scholar] [CrossRef]

| Response Mechanism | Advantages | Hydrogels | Mechanism | References |
|---|---|---|---|---|
| pH-sensitive | pH-sensitive hydrogels can release drugs at a specific PH. | HA-GE hydrogel | When pH = 6.8/7.4, the polyanionic nature facilitates drug release from the hydrogel via swelling and degradation of the hydrogel network. | [20] |
| GA/SA hybrid hydrogel (GAS) | At lower pH, ionic interactions between hydrogen ions and deprotonated sodium alginate help maintain hydrogel stability. | [21] | ||
| Temperature-Sensitive | Thermosensitive hydrogels exhibiting excellent injectability and rapid, uncomplicated gelation properties, facilitating enema administration. | PDLLA-PEG-PDLLA | With the temperature change, sol-gel-sol conversion can be achieved. | [22] |
| Thermosensitive injectable metabolic nanoregulator (TMNR) | [23] | |||
| Enzyme Sensitivity | Enzyme-sensitive hydrogels can achieve drug release in the colon microenvironment and improve targeting. | COS-CaP-QT | COS-CaP-QT microspheres are capable of colon-specific microenvironmental responses. | [24] |
| AL/HA hydrogel | HA is susceptible to degradation by ROS and hyaluronidase in the intestines. | [25] | ||
| ROS Sensitive | ROS Sensitive hydrogels are selectively formed at inflammatory sites within the colon. | HASA | Sulfhydryl-substituted polymers can be oxidized to disulfide bonds by ROS to specifically form hydrogels. | [26] |
| Effects | Hydrogels | Drugs | Subjects | References |
|---|---|---|---|---|
| Maintained the intestine barrier | PDLLA-PEG-PDLLA | Mesalazine | DSS-induced acute UC mouse models | [22] |
| Inulin gel | Olsalazine | DSS-induced acute UC mouse models | [42] | |
| DSS-induced UC rats with delayed treatment | ||||
| TNBS-induced CD mouse and rat models | ||||
| PDLLA-PEG-PDLLA | Mn3O4 nanozyme | DSS-induced acute UC mouse models | [43] | |
| SAPH | - | TNBS-induced acute UC rat models | [44] | |
| Regulate the immune system | HAMs | - | iBMDM cells DSS-induced acute UC mouse models | [45] |
| SA, HA | Selenoprotein | iBMDM cells DSS-induced acute UC mouse models | [46] | |
| CS, SA | Emodin, curcumin | DSS-induced acute UC mouse models | [47] | |
| Regulate gut microbiota | PEG hydrogel | UCB-MSCs | DSS-induced UC mouse models | [48] |
| SA | Bioactive glass | DSS-induced acute UC mouse models | [49] | |
| DSS-induced chronic UC mouse models | ||||
| Clear intracellular ROS | CS-IGF-1C | hP-MSCs | TNBS-induced UC rat models | [50] |
| SHARP | - | in vitro ROO·, ·OH, and O2·− models | [51] | |
| Adhesion | HA | Mesalazine | TNBS-induced UC rat models | [52] |
| Anti-fibrosis | SA | Bioactive glass | DSS-induced chronic UC mouse models | [49] |
| Relieve anxiety and depression | SP | Rh | DSS-induced chronic UC mouse models | [53] |
| Mode of Administration | Hydrogels | Advantages | References |
|---|---|---|---|
| Oral administration | SA | Hydrogels serve as carriers for oral medications, shielding them from breakdown caused by stomach acid and digestive enzymes, which enhances the drugs’ stability and efficiency. | [83] |
| CS, SA | [84] | ||
| Rectal administration | AP | Incorporating drugs into hydrogels may extend their duration of action within the intestinal tract and improve their therapeutic effectiveness. | [85] |
| Endoscopic administration | Peptide-hydrogel | The gel’s characteristics can significantly enhance the duration of contact between the drug and the intestinal wall, thereby extending the therapeutic effect. | [86] |
| Subcutaneous administration | PEG-DPCA | Subcutaneous administration has a long time of action, slow release, and simple administration process, which can enhance patient adherence. | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Lv, B.; Li, M.; Zhang, T.; Li, H.; Tian, H.; Yu, Y. Recent Advances in the Application of Hydrogels as Drug Carriers in Inflammatory Bowel Disease: A Review. Int. J. Mol. Sci. 2025, 26, 2894. https://doi.org/10.3390/ijms26072894
Zhang Q, Lv B, Li M, Zhang T, Li H, Tian H, Yu Y. Recent Advances in the Application of Hydrogels as Drug Carriers in Inflammatory Bowel Disease: A Review. International Journal of Molecular Sciences. 2025; 26(7):2894. https://doi.org/10.3390/ijms26072894
Chicago/Turabian StyleZhang, Qingrui, Bingxuan Lv, Manyu Li, Tiancai Zhang, Haoyu Li, Huimin Tian, and Yanbo Yu. 2025. "Recent Advances in the Application of Hydrogels as Drug Carriers in Inflammatory Bowel Disease: A Review" International Journal of Molecular Sciences 26, no. 7: 2894. https://doi.org/10.3390/ijms26072894
APA StyleZhang, Q., Lv, B., Li, M., Zhang, T., Li, H., Tian, H., & Yu, Y. (2025). Recent Advances in the Application of Hydrogels as Drug Carriers in Inflammatory Bowel Disease: A Review. International Journal of Molecular Sciences, 26(7), 2894. https://doi.org/10.3390/ijms26072894






