Synthesis, Characterization, and Preliminary Analysis of Squid Pen Trypsin Hydrolysates and Chitosan Microcapsules
Abstract
1. Introduction
2. Results and Discussion
2.1. SP Composition
2.2. Preparation Conditions and Encapsulation Efficiency of Microcapsules
2.3. Bulk Density of Microcapsules
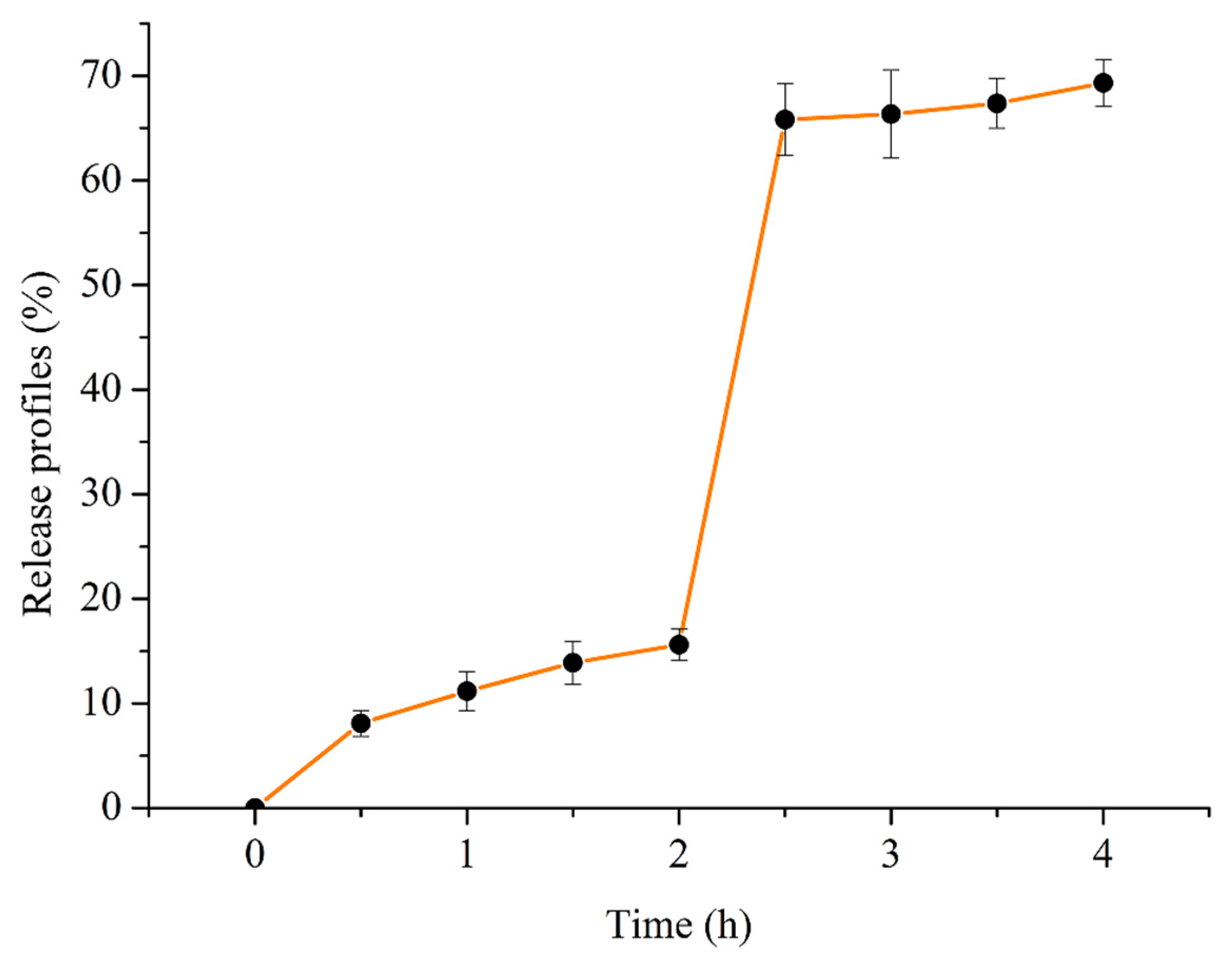
2.4. In Vitro Release Profiles During Simulated Gastrointestinal Digestion
2.5. Characterization of SPTH-SPC Microcapsules
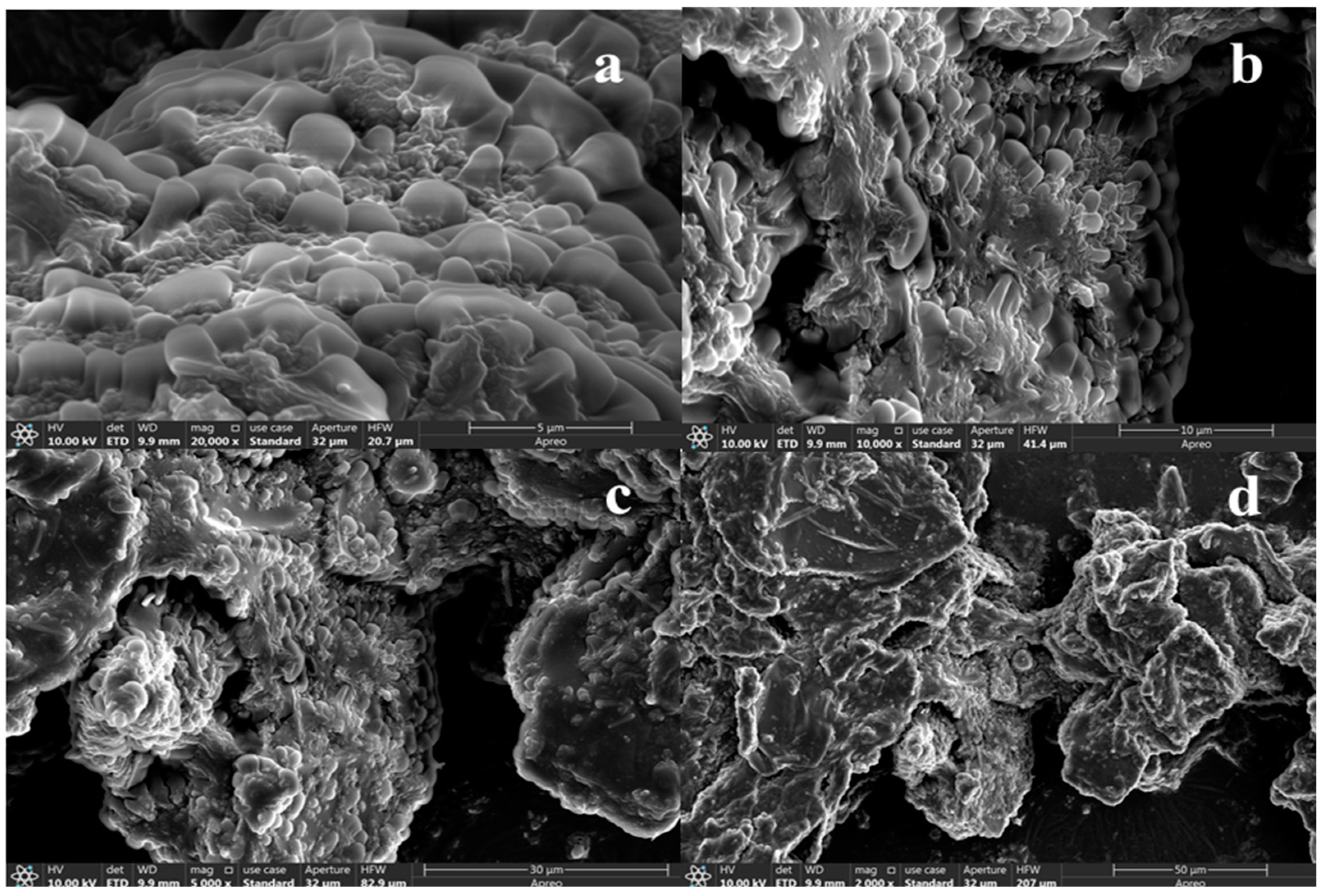
2.5.1. SEM of SPTH-SPC Microcapsules
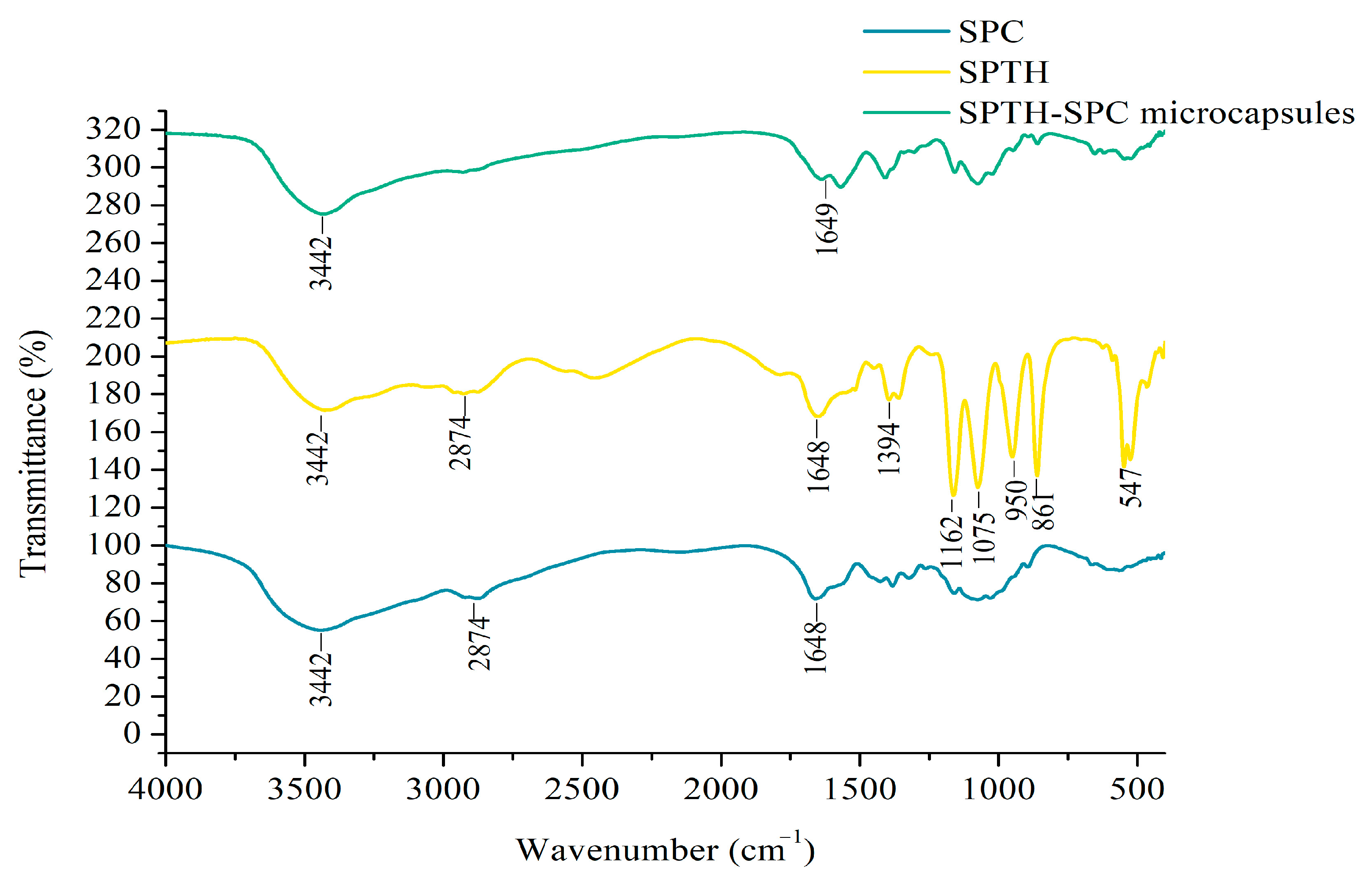
2.5.2. FTIR of SPTH-SPC Microcapsules
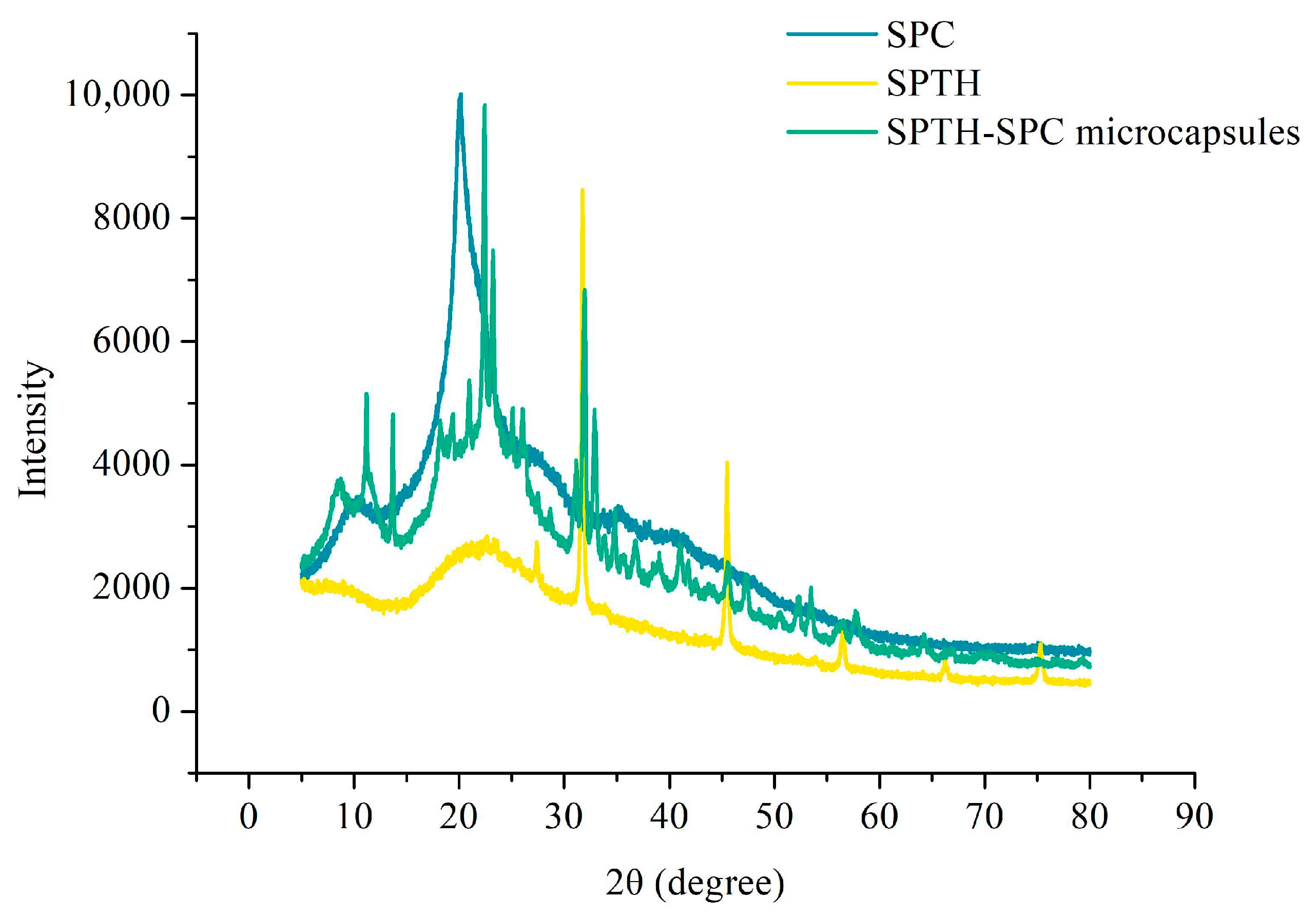
2.5.3. XRD of SPTH-SPC Microcapsules
2.5.4. DSC of SPTH-SPC Microcapsules
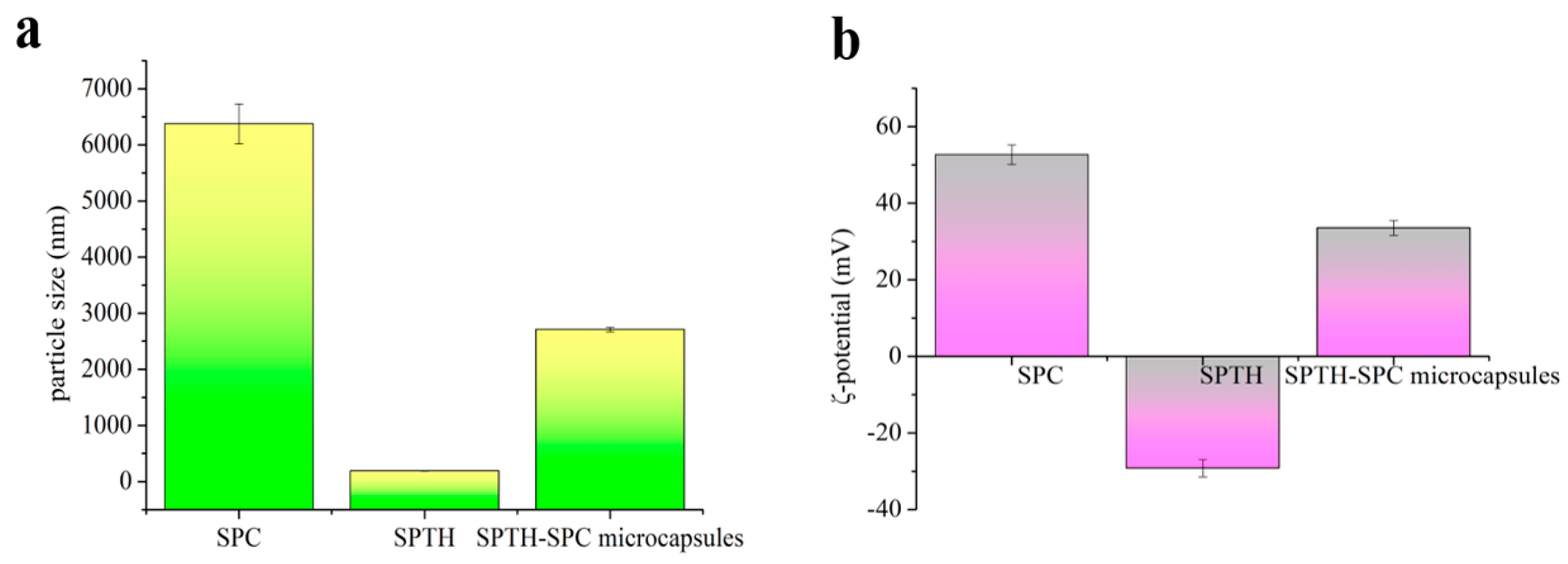
2.5.5. Particle Size and ζ-Potential of SPTH-SPC Microcapsules
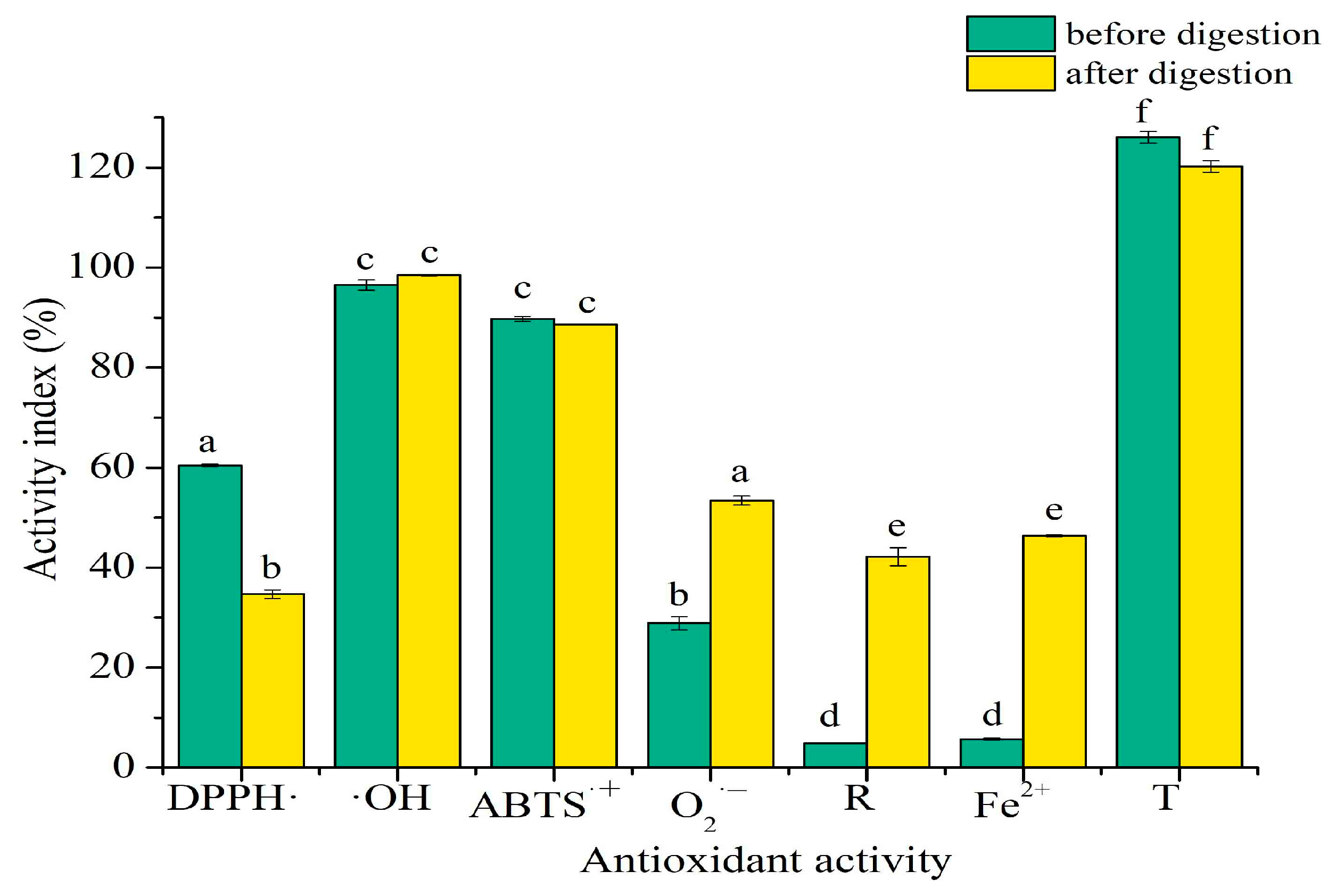
2.6. Antioxidant Activity In Vitro of SPTH-SPC Microcapsules
3. Materials and Methods
3.1. Materials and Reagents
3.2. Determination of the Composition of SP
3.3. Preparation of SPTH and SPC
3.4. Preparation of Microcapsules
3.5. Determination of Encapsulation Efficacy
3.6. Determination of Dispersibility
3.7. Determination of Stacking Density
3.8. In Vitro Release Patterns During Simulated Gastrointestinal Digestion
3.9. Characterization of SPTH-SPC Microcapsules
3.9.1. Fourier Transform Infrared Spectroscopy (FTIR) and the Degree of Deacetylation (DD)
3.9.2. Scanning Electron Microscopy (SEM)
3.9.3. X-Ray Diffraction (XRD)
3.9.4. Differential Scanning Calorimeter (DSC)
3.9.5. Particle Size and Zeta Potential of the SPTH-SPC Microcapsules
3.10. Antioxidant Activity Assay In Vitro
3.10.1. DPPH Radical Scavenging Activity Assay
3.10.2. Hydroxyl Radical Scavenging Activity Assay
3.10.3. ABTS·+ Scavenging Activity Assay
3.10.4. O2− Scavenging Activity Assay
3.10.5. Reducing Power Assay
3.10.6. Ferrous Ions Chelating Activity Assay
3.10.7. Total Antioxidant Activity Assay
3.11. Statistic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osada, M.; Nishiwaki, M.; Watanabe, T. Environment-friendly utilization of squid pen with water: Production of beta-chitin nanofibers and peptides for lowering blood pressure. Int. J. Biol. Macromol. 2021, 189, 921–929. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Wang, Z. Synthesis, characterization, and application of chitosan nanomaterials loaded with other met-als/elements in plant. J. Adv. Nanomater. Agric. 2024, 35, 456–470. [Google Scholar]
- Assadpour, E.; Mahdi Jafari, S. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. 2018, 59, 3129–3151. [Google Scholar] [CrossRef]
- Li, R.M.; Wang, Q.T.; Shen, Y.Y.; Li, M.B.; Sun, L.L. Integrated extraction, structural characterization, and activity assessment of squid pen protein hydrolysates and β-chitin with different protease hydrolysis. Int. J. Biol. Macromol. 2024, 262, 130069. [Google Scholar] [CrossRef]
- Meng, Q.; Zhong, S.; Wang, J.; Gao, Y.; Cui, X. Advances in chitosan-based microcapsules and their applications. Carbohyd Polym. 2023, 300, 110766. [Google Scholar] [CrossRef]
- Anderson, L.A.; Islam, M.A.; Prather, K.L.J. Synthetic biology strategies for improving microbial synthesis of “green” biopolymers. J. Biol. Chem. 2018, 293, 5053–5061. [Google Scholar] [CrossRef]
- Lang, X.; Wang, T.; Sun, M.; Chen, X.; Liu, Y. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. J. Biol. Macromol. 2020, 154, 433–445. [Google Scholar] [CrossRef]
- Hamdine, M.; Heuzey, M.C.; Bégin, A. Effect of organic and inorganic acids on concentrated chitosan solutions and gels. Int. J. Biol. Macromol. 2005, 37, 134–142. [Google Scholar] [CrossRef]
- Lv, J.; Fang, Y.; Wang, D.; Wu, M.; Zhang, W.; Ou, X.; Li, H.; Shang, L.; Li, Z.; Zhao, Y. Green preparation of β-chitins from squid pens by using alkaline deep eutectic solvents. Int. J. Biol. Macromol. 2023, 253, 126767. [Google Scholar] [CrossRef]
- Facchinatto, W.M.; dos Santos, D.M.; de Lacerda Bukzem, A.; Moraes, T.B.; Habitzreuter, F.; de Azevedo, E.R.; Colnago, L.A.; Campana-Filho, S.P. Insight into morphological, physicochemical and spectroscopic properties of β-chitin nanocrystalline structures. Carbohyd Polym. 2021, 273, 118563. [Google Scholar] [CrossRef]
- Dong, S.; Hu, S.M.; Yu, S.J.; Zhou, S.; Zhou, T. Soybean protein isolate/chitosan complex-rutin microcapsules. Int. J. Biol. Macromol. 2023, 243, 125323. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Córdoba, L.J.; Norton, I.T.; Batchelor, H.K.; Gkatzionis, K.; Spyropoulos, F.; Sobral, P.J.A. Physico-chemical, antimicrobial and antioxidant properties of gelatin-chitosan based films loaded with nanoemulsions encapsulating active compounds. Food Hydrocoll. 2018, 79, 544–559. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Yuen, M.C.W.; Lam, P.L.; Gambari, R.; Wong, R.S.M.; Cheng, G.Y.M.; Lai, P.B.S.; Tong, S.W.; Chan, K.W.; Lau, F.Y.; et al. Synthesis, characterization and preliminary analysis of in vivo biological activity of chitosan/celecoxib microcapsules. Bioorg Med. Chem. Lett. 2010, 20, 4147–4151. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhao, S.; Yang, M.; Song, M.; Li, J.; Zheng, J. Structurally stable sustained-release microcapsules stabilized by self-assembly of pectin-chitosan-collagen in aqueous two-phase system. Food Hydrocoll. 2022, 125, 107413. [Google Scholar] [CrossRef]
- Susana Cortizo, M.; Berghoff, C.F.; Alessandrini, J.L. Characterization of chitin from Illex argentinus squid pen. Carbohydrate Polymers 2008, 74, 10–15. [Google Scholar] [CrossRef]
- Hoffer, L. Parenteral Nutrition: Amino Acids. Nutrients 2017, 9, 257. [Google Scholar] [CrossRef]
- Imprimir, P. Protein and energy requirements: A joint FAO/WHO memorandum. B World Health Organ 1979, 57, 65–79. [Google Scholar]
- Luo, Q.; Wang, Y.; Han, Q.; Ji, L.; Zhang, H.; Fei, Z.; Wang, Y. Comparison of the physicochemical, rheological, and morphologic properties of chitosan from four insects. Carbohydr. Polym. 2019, 209, 266–275. [Google Scholar] [CrossRef]
- Zhao, M.; He, H.; Guo, D.; Zhang, X.; Jia, L.; Hou, T.; Ma, A. Chitosan oligosaccharides-tripolyphosphate microcapsules as efficient vehicles for desalted duck egg white peptides-calcium: Fabrication, entrapment mechanism and in vivo calcium absorption studies. LWT-Food Sci. Technol. 2022, 154, 112869. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Błaszczak, A.; Musiał, M.; Wawrzyniak-Wróbel, M.; Witrowa-Rajchert, D. Microencapsulation of Bioactive Compounds: A Review on Wall Materials, Technologies, and Characterization Methods. Appl. Sci. 2020, 10, 6049. [Google Scholar] [CrossRef]
- Cian, R.E.; Proaño, J.L.; Salgado, P.R.; Mauri, A.N.; Drago, S.R. High iron bioaccessibility from co-microencapsulated iron/ascorbic acid using chelating polypeptides from brewers’ spent grain protein as wall material. LWT-Food Sci. Technol. 2021, 139, 110579. [Google Scholar] [CrossRef]
- Saji, R.; Ramani, A.; Gandhi, K.; Seth, R.; Sharma, R. Application of FTIR spectroscopy in dairy products: A systematic review. Food Humanit. 2024, 2, 100239. [Google Scholar] [CrossRef]
- Remanan, M.K.; Zhu, F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chem. 2021, 353, 128534. [Google Scholar] [CrossRef]
- Su, Y.R.; Yu, S.H.; Chao, A.C.; Wu, J.Y.; Lin, Y.F.; Lu, K.Y.; Mi, F.L. Preparation and properties of pH-responsive, self-assembled colloidal nanoparticles from guanidine-containing polypeptide and chitosan for antibiotic delivery. Colloid. Surface A 2016, 494, 9–20. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.; Wang, Q. Nanoparticles Synthesized from Soy Protein: Preparation, Characterization, and Application for Nutraceutical Encapsulation. J. Agr. Food Chem. 2012, 60, 2712–2720. [Google Scholar] [CrossRef]
- Jia, J.; Song, N.; Gai, Y.; Zhang, L.; Zhao, Y. Release-controlled curcumin proliposome produced by ultrasound-assisted supercritical antisolvent method. J. Supercrit. Fluid. 2016, 113, 150–157. [Google Scholar] [CrossRef]
- Tang, Q.; Li, Z.; Xu, X.; Wang, P.; Chen, B.; Yang, Y.; Jin, G.; Sheng, L. Enhancing stability of 1,3-dioleic acid-2-palmitate (OPO) through microencapsulation: A comparative study of single- and double-layer microcapsules. Food Hydrocoll. 2023, 145, 109151. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, L.; McClements, D.J.; Wang, X.; Ye, J.; Liu, C. Spray drying and rehydration of macadamia oil-in-water emulsions: Impact of macadamia protein isolate to chitosan hydrochloride ratio. Food Chem. 2021, 342, 128380. [Google Scholar] [CrossRef]
- Minh, N.C.; Nguyen, V.H.; Schwarz, S.; Stevens, W.F.; Trung, T.S. Preparation of water soluble hydrochloric chitosan from low molecular weight chitosan in the solid state. Int. J. Biol. Macromol. 2019, 121, 718–726. [Google Scholar] [CrossRef]
- Šurlan, J.; Šereš, Z.; Dokić, L.; Krstonošić, V.; Maravić, N. Evaluation of sugar beet pectin viscosity, surface activity, conductivity and zeta potential in sodium chloride aqueous solutions. Food Hydrocoll. 2023, 139, 108490. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, J.; Chu, Y.; Zhu, P.; Zhang, T.; Mao, L.; Gao, Y.; Chen, L.; Yuan, F. Sonochemical synthesis of γ-CD-MOFs microcapsule for myricetin delivery: Study of adsorption mechanism, molecular simulation, solubility, antioxidation, biocompatibility, and in vitro digestion. Food Hydrocoll. 2024, 147, 109318. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Anal. Method. 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Bonifácio-Lopes, T.M.G.; Castro, L.; Vilas-Boas, A.; Campos, D.; Teixeira, J.A.; Pintado, M. Impact of gastrointestinal digestion simulation on brewer’s spent grain green extracts and their prebiotic activity. Food Res. Int. 2023, 165, 112515. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, Z. Double-layer mucin microencapsulation enhances the stress tolerance and oral delivery of probiotics. Food Res Int 2024, 176, 113101. [Google Scholar]
- Li, X.; Zhang, Y.; Wang, Z. Impact of chitosa-based microcapsules on the stability and bioactivity of polyphenols during in vitro digestion. Food Chem. 2023, 415, 135678. [Google Scholar] [CrossRef]
- Smith, A.; Johnson, B. Lipid-based delivery systems for vitamin E: Enhanced stability and bioavailability through nanoencapsulation. J. Nanobiotechnol. 2023, 21, 1–15. [Google Scholar]
- Cuong, H.N.; Minh, N.C.; Van Hoa, N.; Trung, T.S. Preparation and characterization of high purity β-chitin from squid pens (Loligo chenisis). Int. J. Biol. Macromol. 2016, 93, 442–447. [Google Scholar] [CrossRef]
- Pęksa, A.; Miedzianka, J.; Nemś, A. Amino acid composition of flesh-coloured potatoes as affected by storage conditions. Food Chem. 2018, 266, 335–342. [Google Scholar] [CrossRef]
- Lv, S.H.; Zhang, S.S.; Zuo, J.J.; Liang, S.; Yang, J.H.; Wang, J.L.; Wei, D.Q. Progress in preparation and properties of chitosan-based hydrogels. Int. J. Biol. Macromol. 2023, 242, 124915. [Google Scholar] [CrossRef]
- Soon, C.Y.; Tee, Y.B.; Tan, C.H.; Rosnita, A.T.; Khalina, A. Extraction and physicochemical characterization of chitin and chitosan from Zophobas morio larvae in varying sodium hydroxide concentration. Int. J. Biol. Macromol. 2018, 108, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Rosebrough, N.; Farr, A.L. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Gao, J.; Ye, H.; Ren, G.; Ma, X.; Xie, H.; Fang, S.; Lei, Q.; Fang, W. Development of ovalbumin-pectin nanocomplexes for vitamin D3 encapsulation: Enhanced storage stability and sustained release in simulated gastrointestinal digestion. Food Hydrocoll. 2020, 106, 105926. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Chen, K.; Chen, Y.L.; Zhang, C.; Xie, Y.Y.; Hider, R.C.; Zhou, T. Design and synthesis of novel stilbene-hydroxypyridinone hybrids as tyrosinase inhibitors and their application in the anti-browning of freshly-cut apples. Food Chem. 2022, 385, 132730. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Zhang, Q.; Chen, F.; Yue, Z.; Zhang, T.; Deng, H.; Huselstein, C.; Anderson, D.P.; Chang, P.R.; et al. Accelerated skin wound healing by soy protein isolate–modified hydroxypropyl chitosan composite films. Int. J. Biol. Macromol. 2018, 118, 1293–1302. [Google Scholar] [CrossRef]
- Yu, F.; Wei, Z.; Chen, J.; Long, Y.; Qing, Q.; Li, B.; Zhang, X.; Chen, H.; Lan, T.; Zhu, P.; et al. Preparation of curcumin-loaded MPEG-PTMC nanoparticles: Physicochemical properties, antioxidant activity, and in vivo pharmacokinetic behavior. Powder Technol. 2023, 423, 118492. [Google Scholar] [CrossRef]
- Hu, B.; Wang, Y.; Xie, M.; Hu, G.; Ma, F.; Zeng, X. Polymer nanoparticles composed with gallic acid grafted chitosan and bioactive peptides combined antioxidant, anticancer activities and improved delivery property for labile polyphenols. J. Funct. Foods 2015, 15, 593–603. [Google Scholar] [CrossRef]
- Ge, S.; Tong, X.; Gao, C.; Xu, H.; He, R.; Wu, Q.; Wang, J. Kinetics of silkworm pupae protein extraction at different ultrasonic frequency and temperature: Effects on physicochemical properties, functional properties and oxidation resistance. Process Biochem. 2022, 122, 36–52. [Google Scholar] [CrossRef]
- Qing, M.; Zang, J.; Ma, Y.; Chi, Y.; Chi, Y. Effects of rice vinegar treatment on the antioxidant activities and protein structures of whole egg liquid before and after gastrointestinal digestion. Food Chem. 2023, 404, 134574. [Google Scholar] [CrossRef]
- Wang, W.H.; Li, W.L.; Chen, C.Y.; Chang, M.Y.; Huang, S.L.; Shih, C.H.; Lin, Y.S. Antioxidant ability of Chenopodium formosanum extracted using an ethanol-ammonium sulfate two-phase system. Chem. Biol. Technol. Ag. 2022, 9, 1–8. [Google Scholar] [CrossRef]
- Zhou, C.; Mi, S.; Li, J.; Gao, J.; Wang, X.; Sang, Y. Purification, characterisation and antioxidant activities of chondroitin sulphate extracted from Raja porosa cartilage. Carbohydr. Polym. 2020, 241, 116306. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Lin, W.S.; Tung, J.W.; Cheng, Y.C.; Chang, M.Y.; Chen, C.Y.; Huang, S.L. Antioxidant Capacities of Jujube Fruit Seeds and Peel Pulp. Appl. Sci. 2020, 10, 6007. [Google Scholar] [CrossRef]
- Chen, C.Y.; Shih, C.H.; Lin, T.C.; Zheng, J.H.; Hsu, C.C.; Chen, K.M.; Lin, Y.S.; Wu, C.T.; Cilindre, C. Antioxidation and Tyrosinase Inhibitory Ability of Coffee Pulp Extract by Ethanol. J. Chem. 2021, 10, 8649618. [Google Scholar] [CrossRef]
- Canabady-Rochelle, L.L.S.; Harscoat-Schiavo, C.; Kessler, V.; Aymes, A.; Fournier, F.; Girardet, J.M. Determination of reducing power and metal chelating ability of antioxidant peptides: Revisited methods. Food Chem. 2015, 183, 129–135. [Google Scholar] [CrossRef]

| Component | Content (%) |
|---|---|
| Water | 6.02 ± 0.76 |
| Ash | 3.26 ± 0.22 |
| Protein | 64.41 ± 1.24 |
| Chitin | 26.03 ± 0.73 |
| Amino Acid Separation | Amino Acid Name | Content (g/100 g) |
|---|---|---|
| Essential amino acid (EAA) | Val | 3.48 |
| Ile | 1.77 | |
| Leu | 4.2 | |
| Phe | 1.06 | |
| Met | 13.67 | |
| Thr | 2.18 | |
| Lys | 2.18 | |
| Non-essential amino acid (NEAA) | Glu | 2.67 |
| Asp | 4.14 | |
| Ala | 6.41 | |
| Gly | 5.82 | |
| Tyr | 9.81 | |
| His | 7.12 | |
| Ser | 1.89 | |
| Arg | 1.18 | |
| Cys | 1.74 | |
| Pro | 6.68 | |
| EAA | 28.53 | |
| NEAA | 47.46 | |
| Total amino acids (TAA) | 75.99 | |
| EAA/TAA (%) | 37.55 | |
| EAA/NEAA (%) | 60.13 |
| SPC Concentration | SPTH Concentration | Core-to-Wall Ratio | Sonication Time | Encapsulation Efficiency |
|---|---|---|---|---|
| 2% | 4 mg/mL | 1:3 | 20 min | 58.95 ± 2.53% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Song, W.; Huang, S.; Liu, C.; Li, M.; Sun, L. Synthesis, Characterization, and Preliminary Analysis of Squid Pen Trypsin Hydrolysates and Chitosan Microcapsules. Int. J. Mol. Sci. 2025, 26, 2885. https://doi.org/10.3390/ijms26072885
Li R, Song W, Huang S, Liu C, Li M, Sun L. Synthesis, Characterization, and Preliminary Analysis of Squid Pen Trypsin Hydrolysates and Chitosan Microcapsules. International Journal of Molecular Sciences. 2025; 26(7):2885. https://doi.org/10.3390/ijms26072885
Chicago/Turabian StyleLi, Ruimin, Wenkui Song, Shijia Huang, Chuyi Liu, Mingbo Li, and Leilei Sun. 2025. "Synthesis, Characterization, and Preliminary Analysis of Squid Pen Trypsin Hydrolysates and Chitosan Microcapsules" International Journal of Molecular Sciences 26, no. 7: 2885. https://doi.org/10.3390/ijms26072885
APA StyleLi, R., Song, W., Huang, S., Liu, C., Li, M., & Sun, L. (2025). Synthesis, Characterization, and Preliminary Analysis of Squid Pen Trypsin Hydrolysates and Chitosan Microcapsules. International Journal of Molecular Sciences, 26(7), 2885. https://doi.org/10.3390/ijms26072885






