Abstract
Sleep is a fundamental process essential for all organisms. Sleep deprivation can lead to significant detrimental effects, contributing to various physiological disorders and elevating the risk of several diseases. Investigating the relationship between sleep and human diseases offers valuable insights into the molecular mechanisms governing sleep regulation, potentially guiding the development of more effective treatments for sleep disorders and associated diseases. This study explored the roles of sleep-related genes in biological processes and their associations with chronic diseases, mainly including neurological, metabolic, cardiovascular diseases, and cancer. Additionally, an analysis on the sleep-related genes was also performed to understand the potential role in tumorigenesis. This review aims to enhance the understanding of the link between sleep-related genes and chronic diseases, contributing to the development of novel therapeutic approaches targeting sleep and circadian rhythm-related chronic diseases.
1. Introduction
Sleep is a fundamental and vital behavior in most organisms, influencing various biological aspects, including cognitive function, emotion, and memory [1]. Humans exhibit a unique sleep pattern, consisting of two distinct phases: rapid eye movement (REM) sleep and non-rapid eye movement (NREM) sleep. According to established standards, NREM sleep is subdivided into 4 Stages, while REM sleep is characterized by rapid eye movements and heightened brain wave activity [2]. As individuals transition into Stage 1, consciousness gradually fades, with a decrease in α waves and the emergence of θ waves. Stage 2 sleep is marked by the appearance of K complexes, which consist of negative sharp waves followed by high-amplitude positive slow waves, and sleep spindles, characterized by 12–16 Hz oscillations. Stages 3 and 4 represent deep sleep, dominated by δ waves. In Stage 3, δ waves account for 20–50% of the time, whereas in Stage 4, they exceed 50%. At these stages, muscle activity decreases, body temperature and metabolic rate drop, and individuals become harder to awaken. REM sleep, distinguished by rapid eye movements, low muscle tone, and active brain waves, is typically associated with dreaming [2]. REM sleep is a critical phase of the sleep cycle, closely linked to memory consolidation and emotional regulation. NREM sleep is involved in energy conservation and neural recovery. Both stages are essential for maintaining physiological balance: NREM sleep facilitates recovery and REM sleep supports periodic brain activation, local restoration, and emotional regulation [1,3,4]. Chronic sleep and wake disturbances can lead to long-term health consequences. Various sleep disorders, such as delayed sleep onset, frequent awakenings, and daytime sleepiness, may signal suboptimal health conditions and even contribute to the onset of certain diseases. Sleep regulation mechanisms are complex, and there is growing interest in understanding the molecular pathways governing sleep and wakefulness. Recent advances in technology have identified numerous genes associated with sleep duration and sleep homeostasis maintenance, including those related to neural circuits such as ion channels (e.g., Kcnn2, Kcnn3), neuropeptides (e.g., orexin), and neurotransmitters (e.g., dopamine, histamine) [5,6,7,8]. Increasing evidence links sleep dysfunction with human chronic diseases, including neurodegenerative disorders [9,10,11,12,13,14,15] and disease pathogenesis [16,17,18]. However, the underlying etiology remains poorly understood. This review aims to further elucidate the potential connections between sleep-related genes and diverse human chronic diseases, offering insights into the molecular alterations of these genes and their potential roles in disease diagnosis and treatment.
2. Sleep-Related Genes Contribute to Multiple Biological Processes
Based on the definitions, characteristics, and functions of sleep-related genes, these genes can be categorized into circadian clock genes, sleep structure regulation genes, sleep drive regulation genes, and sleep duration regulation genes (Figure 1A). For instance, the PER2 gene influences the biological clock’s oscillation cycle by regulating the transcriptional activation of BMAL1 [19]. Mutations in PER2 can lead to advanced sleep phase syndrome, resulting in significantly early sleep and wake times. Numerous non-coding RNAs (ncRNAs), including the widely studied microRNAs (miRNAs), regulate sleep by targeting sleep-related mRNAs within miRNA-mediated regulatory networks (Figure 1B). The complex miRNA–mRNA interactions play a pivotal role in regulating sleep-related processes via dynamically modulating gene expression, and dysregulation of regulatory networks may be linked to sleep disorders, highlighting their importance in maintaining sleep quality and neural health.
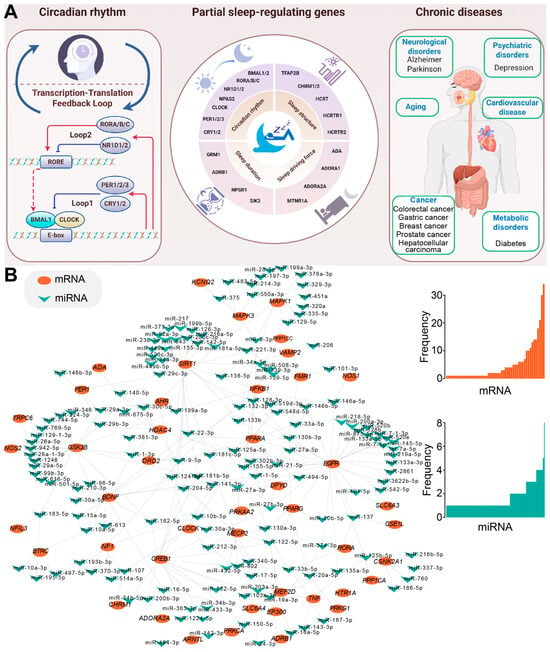
Figure 1.
The schema of sleep-related genes and human diseases. (A) A schematic overview of sleep-related genes and their association with human diseases created using Figdraw. The left panel depicts the basic circadian rhythm, the middle panel highlights key genes involved in sleep regulation, and the right panel illustrates how dysregulated sleep-related genes and sleep disorders may contribute to a range of chronic human diseases. (B) An example illustrating a sleep-related miRNA-mRNA regulatory network. Experimentally validated miRNA–mRNA interactions are sourced from the starBase database [20], and the network is constructed by Cytoscape (3.6.1) [21]. The detailed distributions of interaction numbers are also presented on the right, indicating the complex interactions in miRNA-mediated regulatory networks.
The evolutionarily conserved miRNA, miR-137, targets the neuropeptide hypocretin/orexin (HCRT), thereby modulating the wake–sleep ratio [22]. Dysregulated miRNAs are implicated in the pathophysiology of central hypersomnias [23]. Specifically, a study found significant changes in the plasma levels of four miRNAs in patients with central hypersomnias: miR-30c, let-7f, and miR-26a were upregulated, while miR-130a was downregulated. The dysregulation of miRNAs may influence the disease’s progression by regulating neuronal differentiation and REM sleep. Another highly conserved miRNA, let-7, targets Cry2 mRNA, influencing the stability and translation of Cry2 [24]. miR-132 plays a critical role in the regulation of the PER gene, a key component of the circadian rhythm [25]. Light stimulation in the suprachiasmatic nucleus (SCN) induces the transcription of miR-132 via activation of the MAPK/ERK and CREB signaling pathways. miR-132, acting as a negative regulator, inhibits light-induced PER gene expression, thereby attenuating the light’s reset effect on circadian rhythms. The potential links between these sleep-related genes and factors like sleep duration and periodicity are increasingly studied based on their distinct functions. Critical sleep-related genes, particularly those involved in circadian rhythms and core regulatory pathways, have been summarized according to their effects on sleep.
2.1. Circadian Rhythm-Related Genes Are Associated with Sleep
A number of key genes involved in regulating the circadian rhythm have garnered considerable attention due to their critical roles in the sleep–wake cycle, with their regulatory mechanisms exhibiting hierarchical network characteristics. Studies have revealed significant abnormalities in the circadian rhythm of peripheral clock genes (such as Per2, Bmal1, and Nr1d1) in patients with idiopathic REM sleep behavior disorder (RBD) [26]. The melatonin curve in patients with RBD was delayed by 2 h compared to healthy controls, and their sleep stages were delayed by 1 h. These findings highlight the close association between circadian rhythm-related genes and sleep. For instance, the BMAL1 gene forms a heterodimer with the CLOCK gene, which binds to the E-box sequence, thereby activating the transcription of downstream circadian genes such as Per1, Per2, Cry1, and Cry2 [27]. Furthermore, RORE—the binding site for ROR and REV-ERB proteins—plays a pivotal role in the regulation of BMAL1 transcription. ROR activates BMAL1 transcription via RORE, while REV-ERB inhibits this process, thereby establishing a stable feedback loop that maintains the molecular clock cycle [28]. In BMAL1-deficient mice, both sleep and activity cycles were disordered, with no distinct circadian rhythm under constant darkness. These mice exhibited an approximately 6.3% increase in total sleep time, sleep fragmentation, and other disruptions [27]. BMAL1 expression in skeletal muscle is essential for regulating total sleep, influencing not only sleep volume but potentially affecting the brain’s sleep regulation mechanisms by releasing certain factors [29]. Moreover, in a mouse model with a C-terminal truncated BMAL1 gene, homozygous mutant mice displayed irregular mRNA and protein expression in the suprachiasmatic nucleus and liver, indicating disruption of the molecular clock mechanism [30]. The C-terminal region of BMAL1 is pivotal for maintaining biological rhythms and regulating physiological functions. Additionally, sleep deprivation significantly reduced the expression of CLOCK and BMAL1 genes while increasing the expression of the PER1 gene [31].
The Transcription–Translation Feedback Loop (TTFL) is a fundamental component of the cellular circadian clockwork. Positive regulatory molecules, such as BMAL1, CLOCK, and NPAS2, form heterodimers and bind to the E-box elements of target genes, thereby activating the transcription of PER and CRY. In contrast, negative regulatory molecules PER and CRY inhibit the transcriptional activity of BMAL1, CLOCK, and NPAS2, creating a negative feedback loop [32,33] (Figure 1A). A positive correlation can be detected between the expression of PER1 and total sleep time, as well as NREM sleep duration, while a negative correlation was detected with sleep latency and the α, β, and δ waves in EEG [31]. The PER2 gene mutation, specifically phosphorylation at the S662 site, is associated with familial advanced sleep phase syndrome (FASPS). The hPER2 S662G mutation linked to FASPS alters the phosphorylation level of PER2 in vitro through tyrosine kinase I (CKI), thereby impacting its circadian clock regulatory function [19]. Additionally, the PER3 VNTR polymorphism (4 or 5 repeats) is associated with variations in sleep duration and responses to sleep deprivation. In Per3 (5/5) mice with 5 repeats, EEG power loss after sleep deprivation could be fully compensated, whereas Per3 (4/4) mice with 4 repeats failed to compensate fully [34]. The CRY1 gene primarily functions as an inhibitory component in the CLOCK-BMAL1-mediated transcription–translation negative feedback loop. Mutant CRY1 mice exhibit a significantly prolonged free-running cycle of 28 h, indicating disruption to the stability of their biological clock [35]. CRY1-deficient mice display an accelerated free activity cycle, while CRY2-deficient mice exhibit a delayed activity cycle [36]. When both CRY1 and CRY2 are absent, mice completely lose their free activity rhythm, leading to a disordered biological clock. FASPS is linked to mutations in the PER2 gene, while the CRY1 gene is associated with familial delayed sleep phase syndrome (FDSPS) [37].
2.2. Other Sleep-Related Regulatory Genes
Due to the unique structure of human sleep, several genes play specific roles in determining the proportions and characteristics of different sleep stages, including non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. For instance, GAD67-positive GABAergic neurons in the ventral tegmental area (VTA) are considered crucial for regulating NREM sleep. These neurons release GABA to inhibit arousal-promoting neurons (e.g., neurons containing appetite neuropeptides), thereby facilitating NREM sleep [38]. Chemical genetic activation of GAD67+ neurons in the VTA significantly increases NREM sleep duration and slow-wave activity, while optogenetic inhibition of these neurons causes rapid awakening from NREM sleep without affecting REM sleep [38]. The Tfap2b gene, an upstream regulator, influences the function of GABAergic neurons by affecting the expression of genes related to glutamate decarboxylase (GAD1 and GAD2) and the GABA transporter (Vgat) [39]. The NALCN gene significantly impacts REM sleep duration; its missense mutation (Nalcn Drl) results in a substantial reduction in REM sleep time in mice [40]. Two important muscarinic acetylcholine receptors, Chrm1 and Chrm3, play pivotal roles in the structure of human REM and NREM sleep. Double knockout of Chrm1 and Chrm3 leads to a dramatic reduction in REM sleep, nearly eliminating it [41]. Orexin-A, another critical sleep regulator, inhibits both REM and NREM duration. Produced in the hypothalamus, orexin-A promotes wakefulness while inhibiting NREM and REM sleep through two receptors, OX1R and OX2R. Notably, OX2R plays a primary role in mediating the promotion of wakefulness and inhibition of NREM sleep [42]. In OX2R knockout mice, the effects of orexin-A on wakefulness and NREM sleep were significantly attenuated. OX1R also contributed to maintaining wakefulness, though to a lesser extent than OX2R [42]. The orexin system also sustains wakefulness by regulating factors such as histaminergic neuron activity in the tuberomammillary nucleus (TM) and subsequently regulating the transition from wakefulness to NREM sleep through OX2R [43].
Several genes have been implicated in the regulation of sleep-driving forces, the maintenance of sleep or wakefulness homeostasis, and other related functions as demonstrated through various experimental models—including sleep deprivation and physical stimulation. NPAS2 plays a pivotal role in sleep homeostasis regulation. Under baseline conditions, NPAS2-deficient mice exhibited a 41 min reduction in NREM sleep (NREMS) compared to wild-type mice [44]. Furthermore, NPAS2 deficiency led to more pronounced reductions in NREMS and increased arousal following acute and chronic fentanyl administration [45]. These findings underscore the potential role of NPAS2 in regulating drug-induced alterations in sleep and wakefulness. Adenosine, a key sleep-promoting substance, acts as an endogenous sleep inducer. Within the A1 receptor/BF pathway, adenosine functions as an inhibitory neuromodulator, suppressing cholinergic neurons in the basal forebrain (BF) via activation of the A1 receptor [46]. Additionally, adenosine inhibits histaminergic neuron activity by activating ADORA1 receptors [47]. Another adenosine receptor, ADORA2A, also influences sleep regulation. Two distinct types of sleep neurons—Type-1 and Type-2—reside in the ventrolateral preoptic nucleus (VLPO). Type-1 neurons exhibit inhibitory responses to adenosine, whereas Type-2 neurons are excited by 5-hydroxytryptamine and adenosine. Notably, Type-2 neurons are activated by the A2A adenosine receptors, thereby promoting sleep [48]. Melatonin, another key sleep regulator, acts as a sleep driver. Upon binding to the MT1 receptor, melatonin activates the associated G protein, resulting in the dissociation of the Gα subunit and the Gβγ subunit. The released Gβγ subunit subsequently activates the BK potassium channel which inhibits neurotransmitter release, thus promoting sleep [49].
Mutations in certain sleep-related genes can significantly impact the overall sleep duration of individuals. These mutations not only affect total sleep time but can also alter sleep and wake cycles. For instance, the DEC2 gene mutation (hDEC2-P385R) is associated with a short sleep phenotype, causing affected individuals to sleep considerably less than the average population [50]. Similarly, mutations in the glutamate receptor GRM1 gene, specifically GRM1b-R889W and GRM1-S458A, have been linked to natural short sleep [51]. Individuals with the rare A187V mutation in the ADRB1 gene typically sleep only 4–6 h per night yet report feeling well-rested [52]. Using optogenetics and calcium imaging, researchers confirmed that ADRB1-positive neurons are active during REM sleep and wakefulness, with mutations enhancing neuronal activity, thereby promoting wakefulness [52]. The Y206H mutation in the NPSR1 gene is also associated with a natural short sleep phenotype. When NPS binds to NPSR1 it activates downstream signaling pathways, leading to increased phosphorylation of cAMP response element-binding protein (CREB) [53]. This effect was particularly pronounced in NPSR1-Y206H mutant mice, where the mutation heightened receptor sensitivity to NPS, thereby promoting wakefulness and reducing sleep duration [53]. Additionally, SIK3, which plays a role in intracellular signaling, regulates sleep needs and self-adjusts daily sleep volume, further influencing overall sleep duration [40].
2.3. Sleep-Related Genes Are Associated with Multiple Biological Processes
Individuals with poor sleep quality often experience reduced immunity, making them more susceptible to illness. A number of sleep-related genes play a significant role in modulating immune function. For example, activation of ADORA2A can increase intracellular cAMP levels, which inhibits the effector function and anti-tumor activity of T cells [54]. Deletion of ADORA2A using CRISPR/Cas9 has been shown to enhance the function of CAR T cells, with ADORA2A-deficient CAR T cells demonstrating improved tolerance in mice. ADORA2A also plays a critical role in tumor immune escape; elevated levels of adenosine in the tumor microenvironment activate A2A receptors via the CD39/CD73 axis, promoting immune evasion [55]. In tumor models, combining ADORA2A receptor inhibition with anti-PD-1 therapy significantly improves therapeutic outcomes [55]. Additionally, ADRB1 influences T cell exhaustion by inhibiting cytokine production and proliferation through the activation of adenylate cyclase and an increase in intracellular cAMP levels [56]. The inhibitory neurotransmitter GABA, involved in sleep regulation, also impacts the immune system, particularly the maturation and inflammatory response of macrophages. GABA enhances the succinate-flavin adenine dinucleotide (FAD)-lysine-specific demethylase 1 (LSD1) signaling pathway [57]. Additionally, 5-HT modulates sleep by influencing the polarization of human macrophages through various receptors, including 5HT2B and 5HT7, which play a critical role in regulating inflammation [58]. Specifically, 5-HT alters the gene expression profile of macrophages via activation of 5HT2B and 5HT7 receptors, promoting the expression of anti-inflammatory genes while inhibiting the expression of pro-inflammatory genes.
3. Dysregulated Sleep-Related Genes Have a Critical Role in Human Diseases
3.1. Sleep-Related Genes May Be Associated with Metabolism-Related Diseases
The fast-paced nature of modern life significantly impacts sleep, with sleep deprivation being closely linked to increased stress. Insufficient sleep disrupts eating patterns, leading individuals to reduce main meal intake while increasing snacking and high-calorie food consumption, particularly during the evening [59,60]. Interestingly, although extended wakefulness due to sleep deprivation theoretically increases energy expenditure, it is insufficient to offset the additional calories consumed, potentially due to a reduction in leptin levels which normally suppress appetite [59,61]. Circadian clock genes play a key role in regulating metabolism. For instance, PER2 influences lipid metabolism by inhibiting PPARγ, and the loss of PER2 leads to increased PPARγ activity, resulting in metabolic disruptions [62]. Another key circadian gene, CRY1, also impacts metabolic processes. CRY1 mutant mice exhibit polyuria and excessive thirst at 20 weeks of age, suggesting a potential connection to diabetes [35].
Orexin regulates both sleep architecture and wakefulness, while also serving as a critical metabolic modulator. In high-fat diet-induced obese mice, selective inactivation of Ox1R reduced glucose utilization in brown adipose tissue and skeletal muscle, ultimately impairing insulin sensitivity [63]. This highlights the dual role of the orexin system in glucose homeostasis: Ox1R activation typically enhances insulin sensitivity and promotes BAT thermogenesis, whereas Ox2R activation is linked to diminished glucose tolerance and insulin resistance [63]. Furthermore, circadian clock gene disruptions not only precipitate insulin resistance, diabetes, and obesity, but also exacerbate the risk of kidney stone disease through oxidative stress and inflammation [64]. For instance, insulin resistance can lead to increased urine acidification and calcium excretion, while diabetes induces oxidative damage to renal tubular cells, thereby promoting stone formation [64]. Additionally, insufficient sleep or circadian rhythm disruptions often manifest as hormonal imbalances, resulting in symptoms such as skin discoloration and emotional instability. Specific circadian genes regulate hormone secretion, as evidenced by PER2-deficient mice, which lack normal cortisol rhythms during the light–dark cycle [65]. In these mice, cortisol levels significantly increased after mild restraint stress and remained elevated long-term, indicating a dysfunction in the hypothalamus–pituitary–adrenal (HPA) axis [65]. The liver’s circadian clock can regulate choline kinase (CHK) gene expression via the BMAL1-REV-ERBα axis, influencing lipid metabolism [66]. REV-ERBα exhibits a clear circadian rhythm by inhibiting CHK expression, thus reducing phosphatidylcholine synthesis. Disruption of this rhythm in the absence of BMAL1 leads to elevated phosphatidylcholine levels, resulting in altered lipoprotein profiles, an increase in non-HDL cholesterol, and the development of conditions such as fatty liver, dyslipidemia, and metabolic syndrome. Additionally, REV-ERBα regulates transcription by directly binding to the RORE sequence in the CHK gene promoter [66].
3.2. Sleep-Related Genes May Be Associated with Cardiovascular Diseases
The aging population, dietary changes, and other societal factors have increasingly highlighted cardiovascular health concerns. Sleep and circadian rhythms are critical in regulating cardiovascular functions. Healthy sleep patterns significantly reduce the risk of cardiovascular disease (CVD), coronary heart disease (CHD), and stroke [67]. PER2-deficient mice exhibit larger myocardial infarct sizes and reduced lactate production during cardiac ischemia, potentially impairing the heart’s energy supply. As a circadian gene, PER2 may offer a protective role in the cardiovascular system. Treatment with TNF-α or H2O2 disrupts mitochondrial membrane potential, and PER2 knockdown exacerbates this damage [68]. The BK channel, another factor promoting sleep, also plays a protective role in cardiovascular health by enhancing Ang II’s inhibitory effect on BK channels [69]. Pharmacological activation of BK channels or their associated genes can safeguard cardiac function in diabetic mice and mitigate myocardial ischemia/reperfusion injury [69]. Moreover, circadian rhythms regulate the sympathetic–parasympathetic balance of the heart, with PER3 gene polymorphisms influencing autonomic nervous system (ANS) activity [70]. Sleep deprivation leads to reduced heart rate variability and heightened sympathetic nerve activity. Other circadian genes, such as CRY1, have been linked to hypertension risk, with serum levels serving as diagnostic markers for individuals with abnormal anthropometric indicators [71].
As a key regulator of sleep, 5-HT receptors also play a role in maintaining normal cardiac function. For instance, the absence of 5-HT(2B) receptors results in cardiomyocyte dysfunction [72]. 5-HT(2B) receptor-deficient mice exhibit left ventricular dilation and impaired systolic function indicative of myocardial tissue loss and disrupted muscle fiber alignment [72]. The 5-HT(2A) receptor mediates cardiac cell hypertrophic responses to 5-HT and is associated with HDAC4 phosphorylation [73]. Selective 5-HT2A receptor antagonists significantly reduce the phosphorylation of CaMKII and HDAC4 in mice with aortic coarctation, thereby inhibiting cardiac hypertrophy [73].
3.3. Sleep-Related Genes Are Associated with Cancer
Insomniacs face a 24% higher cancer risk compared to the general population, likely due to factors such as chronic inflammation, impaired immune function, and hormonal alterations caused by sleep deprivation [74]. One sleep-related gene, the circadian clock gene BMAL1, is involved in cell proliferation and apoptosis [75]. Circadian clock genes are crucial for regulating the cell cycle, linking them closely to cancer development. Loss of Per1 significantly impacts the proliferation and apoptosis of oral squamous cell carcinoma (OSCC) cells. Cells overexpressing Per1 show a marked reduction in proliferation and a substantial increase in apoptosis [76]. The loss of PER1 may influence OSCC progression via the AKT/mTOR signaling pathway, while PER1 modulates cell proliferation through glycolysis regulation and the PI3K/AKT pathway, positioning it as a tumor suppressor [77]. In trastuzumab-resistant gastric cancer cells, PER1 silencing disrupts the circadian rhythm of the PER1-HK2 axis, potentially reversing drug resistance [78]. Specifically, PER1 interacts with PPARγ to enhance its transcriptional activity, promoting HK2 expression, altering glucose metabolism, and impacting the proliferation and survival of cancer cells. This suggests that targeting PER1 could be a promising strategy for overcoming trastuzumab resistance in gastric cancer [78]. Moreover, PER1 may modulate immune pathways through NK cell clocks [79], and its role in regulating the cell cycle and DNA damage repair underscores its potential as a tumor suppressor [80,81], making it a valuable target for cancer treatment [82,83,84,85]. Similarly, the circadian clock gene PER2 is closely linked to tumor suppression. Mice deficient in PER2 exhibit heightened cancer susceptibility, particularly following radiation exposure [86]. Lymphocytes from mPer2 mutant mice display resistance to apoptosis after radiation, leading to the accumulation of damaged cells. As a tumor suppressor, PER2 exerts significant inhibitory effects on breast cancer cells. Overexpression of PER2 in the MCF-7 breast cancer cell line leads to a notable increase in the proportion of cells in the G1 phase and a decrease in the S phase. This effect is more pronounced when PER2 and Cry2 are co-expressed [87]. Additionally, PER2 expression induces apoptosis in MCF-7 cells. As a key regulator of the circadian cycle and transcriptional control of other clock genes, PER2 modulates various physiological systems, including metabolism, sleep, immune response, cardiovascular health, and renal function, highlighting its complex role in sleep disorders and their assessment and treatment [88]. BMAL1 also contributes to colorectal cancer metastasis by stimulating exosome secretion, providing insight into the role of circadian rhythms in cancer progression [89]. BMAL1 depletion enhances the sensitivity of adrenocortical carcinoma cells to DNA damage-based therapies both in vitro and in vivo, implicating BMAL1 as a potential target in cancer therapeutics [90]. Its influence on cancer cell behavior suggests that BMAL1 may be a promising therapeutic target in colorectal cancer [91]. Moreover, BMAL1 regulates the stemness and tumorigenesis of gliomas via the Wnt/β-catenin signaling pathway, with its interaction with glioma stem cells significantly impacting glioma initiation and progression [92].
NPAS2, a gene that promotes sleep recovery, also acts as a circadian regulator. In prostate cancer, it enhances cell survival by promoting glycolysis and inhibiting oxidative phosphorylation. Overexpression of NPAS2 increases glucose uptake and lactate production, thereby improving cellular glycolytic capacity [93]. In hepatocellular carcinoma, NPAS2 promotes cell survival and proliferation by upregulating phosphatase CDC25A expression through direct binding to the E-box element in the CDC25A promoter [94]. This leads to the dephosphorylation of CDK2, CDK4, and CDK6, activating these kinases and promoting cell cycle progression [94]. Another sleep regulator, SIK3, promotes cancer cell proliferation through the mTOR signaling pathway [95]. Mutations in the GRM1 gene, which is involved in regulating sleep duration, are associated with decreased sleep duration, as seen with specific mutations such as GRM1b-R889W and GRM1-S458A linked to natural short sleep. In mouse models, inducible RNA interference-mediated inhibition of GRM1 expression results in reduced cell proliferation, increased apoptosis, and suppression of downstream MAPK and PI3K/AKT pathways [96]. Inhibition of GRM1 is associated with increased apoptosis markers and reduced proliferation markers such as Ki-67. Additionally, GAD1, which regulates human sleep architecture by synthesizing the GABA-inhibitory neurotransmitters, facilitates tumor cell proliferation through GABA-mediated β-catenin activation. This occurs via the inhibition of glycogen synthase kinase 3β through GABA_B receptor activation, enhancing β-catenin signaling [97]. In a mouse model, knockout of GAD1 significantly slowed tumor growth. GABA induced a non-T cell inflammatory tumor microenvironment by inhibiting the activity and infiltration of CD8+ T cells, and it also suppressed the production of chemotactic factors, such as CCL4 and CCL5, which typically recruit T cells and dendritic cells to the tumor site [97]. This highlights GABA’s role in immune evasion. As the connection between sleep genes and cancer becomes clearer, researchers have begun exploring chronotherapy as a potential treatment approach. Circadian rhythm genes offer an optimized framework for improving therapeutic outcomes. Since different cell types exhibit varying drug sensitivities, administering certain chemotherapy drugs at specific times of day can reduce toxicity and enhance efficacy [98,99].
These sleep-related genes have garnered significant attention in cancer research due to the intricate relationship between sleep and tumorigenesis. They are distributed on most human chromosomes, especially on Chromosomes 1 and 17 (Figure 2A). Positive or negative expression relationships can be detected between some sleep-related genes (Figure 2B), and some genes play a role in cancer-related pathways, such as GPCR-related signaling pathways, apoptosis, the cell cycle, DNA damage, and RTK pathways, indicating the potential contributions to tumorigenesis (Figure 2). Dysregulation of gene expression in specific cancers indicates the potential involvement in both physiological and pathological processes, indicating that gaining insights into the mechanisms of sleep regulation could facilitate the development of cancer therapies.
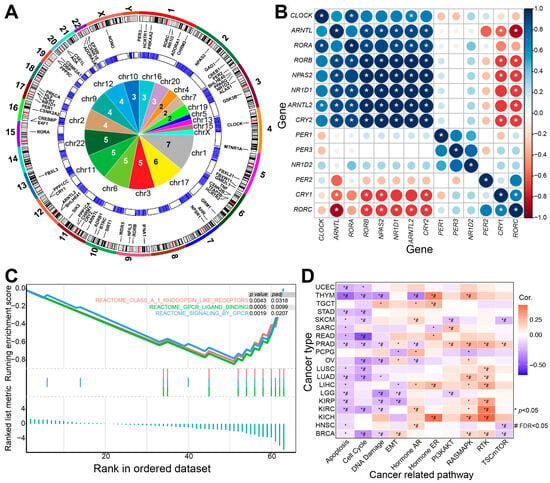
Figure 2.
The potential role of sleep-related genes in tumorigenesis. (A) Gene distributions of sleep-related genes on human chromosomes. The outer circle shows the distributions of core circadian rhythm-related genes on human chromosomes. The middle circle displays the expression distributions in normal brain tissues (data obtained from The Cancer Genome Atlas (TCGA) using “TCGAbiolinks” [100]) and the inner panel provides detailed pie distributions of genes on human chromosomes. Chromosomes 1 and 17 contain more genes than other chromosomes. (B) Expression correlations of core sleep-related genes based on the glioblastoma multiforme (GBM) dataset from TCGA (TCGA-GBM). * indicates a significant Spearman correlation (p < 0.05). (C) Gene set enrichment analysis (GSEA) reveals that these sleep-related genes are significantly enriched in specific biological pathways. (D) These genes may contribute to tumorigenesis by interacting with specific pathways (analyzed using the GSCA platform [101]).
3.4. Sleep and Mental Health
As the central regulator of sleep, the nervous system is profoundly impacted by sleep loss or other sleep disorders. Common neurological diseases, such as Alzheimer’s disease, Parkinson’s disease, and schizophrenia are often closely linked to sleep disturbances. Many patients with neurological conditions experience varying degrees of sleep difficulties, including sleep fragmentation. Variations or functional alterations in genes that regulate sleep increase the risk of these neurological disorders. Patients with schizophrenia, for example, typically exhibit poor sleep quality, with disrupted sleep structure characterized by reduced deep sleep and shortened REM latency [102]. Dysregulation of the circadian rhythm genes CRY1 and PER2 is commonly observed in these patients. In individuals with first-episode schizophrenia, the mRNA expression of CLOCK, PER2, and CRY1 genes significantly decreases in the early stages, suggesting that the circadian rhythm disturbance may be part of the pathological process of schizophrenia rather than a result of long-term drug treatment [103]. In contrast to the worsening effects of sleep gene loss on neurodegenerative diseases, experimental mice lacking BMAL1 demonstrated improved performance. BMAL1 deficiency inhibited the aggregation of tau protein and α-synuclein (aSyn), as well as related pathological changes [104]. Astrocytes lacking BMAL1 showed enhanced autophagy and phagocytosis, likely due to increased BAG3 expression. Overexpression of BAG3 significantly reduced the spread of α-synuclein. BAG3 was found to be highly expressed in the astrocytes of patients with Alzheimer’s disease, suggesting a potential therapeutic target for astrocyte-specific interventions in neurodegenerative diseases [104]. Additionally, the complex between the 5-HT2A receptor and mGluR2 receptor has been extensively studied in relation to schizophrenia. These receptors can form functional complexes, integrating serotonin and glutamate signaling to regulate G-protein coupling patterns [105]. Activation of mGluR2 can inhibit behavioral responses, such as head torsion induced by 5-HT2A receptor agonists. This receptor complex plays a key role in regulating hallucination-related behaviors and may represent a promising therapeutic target for schizophrenia [105].
Depression remains a major global health burden, with many antidepressants facing challenges related to poor efficacy or side effects. Sleep-related genes may offer promising new therapeutic targets. Up-regulation of ADORA2A (A2AR), a receptor for adenosine, has been linked to the onset of depressive symptoms, and ADORA2A antagonists are considered potential antidepressants [106]. A2AR activation increases the firing rate of A2AR-positive neurons in the lateral ventricle, inhibiting the activity of neighboring neurons. This activation can induce depression-like behavior while inhibiting A2AR-positive neurons reduces these behaviors [106]. Stress-induced up-regulation of ADORA2A can lead to alterations in neuronal function, promoting depression-like symptoms. Therefore, ADORA2A up-regulation may serve as both a biomarker and a therapeutic target for depression. In addition, GABA, an inhibitory neurotransmitter, plays a pivotal role in sleep regulation and significantly affects appetite and mood. A long-term high-fat diet can reduce the responsiveness of AgRP neurons to hunger, anxiety, and depression stimuli [107]. This decreased sensitivity weakens the GABAergic output of AgRP neurons to downstream MC4R dBNST neurons, contributing to severe psychological disorders. Enhancing GABAA R-α5 signaling or inhibiting 5-HT3 receptor signaling can significantly alleviate anxiety and depression induced by a high-fat diet, while also inhibiting excessive food intake and weight gain [107]. Furthermore, the ERK MAPK signaling pathway is vital in regulating sleep and plays a key role in learning and memory. Activation of this pathway promotes the expression of genes involved in neuroplasticity, which are essential for stress-induced learning and memory processes [108]. Specifically, ERK MAPK activation leads to the phosphorylation (Ser10) and acetylation (Lys14) of histone H3 through downstream molecules like MSK1 and Elk-1, forming the dual modification H3S10p-K14ac. This modification enhances the transcriptional activation of immediate early genes such as c-fos, which is considered a key marker for memory consolidation.
Brain functions, such as attention and memory, are closely linked to sleep quality and status. Genes involved in regulating sleep or circadian rhythms may also influence other functions in the same or different brain regions, including memory, learning, and social interaction. For instance, deletion of the circadian rhythm gene BMAL1 results in significant social impairments and repetitive stereotyped behaviors in mice, resembling autism spectrum disorders [109]. BMAL1 deficiency may also induce dry mouth and eyes through down-regulation of ITPR2/3, suggesting a potential therapeutic avenue for these symptoms [110].
4. Conclusions
To explore the molecular alterations of sleep-related genes and their potential associations with human diseases, this study discussed the complex mechanisms of sleep regulation, particularly their roles in chronic human diseases. The relationships between sleep-related genes and various conditions, including neurological, metabolic, cardiovascular diseases, and cancer were further examined, along with the central genes involved. Given the profound connection between sleep and disease, research efforts are increasingly focusing on utilizing genetic and circadian rhythm information to regulate sleep quality. This approach is paving the way for the diagnosis and treatment of related diseases based on insights from human circadian and sleep gene data. The sleep regulation mechanisms will support the development of new therapeutic strategies and pharmacological treatments for diseases associated with sleep and circadian rhythms.
Funding
This work was supported by National Natural Science Foundation of China (No. 62171236), the key project of social development in Jiangsu Province (No. BE2022799), the key projects of Natural Science Research in Universities of Jiangsu Province (No. 22KJA180006), funding from Shandong Provincial Key Laboratory of Biophysics, Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX24_1220), and the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, J.M. Clues to the functions of mammalian sleep. Nature 2005, 437, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Sugita, Y.; Koga, E.; Shirakawa, S.; Inoue, K.; Uchida, S.; Kuwahara, H.; Kousaka, M.; Kobayashi, T.; Tsuji, Y.; et al. Proposed supplements and amendments to ’A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects’, the Rechtschaffen & Kales (1968) standard. Psychiatry Clin. Neurosci. 2001, 55, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, L.; Yang, G.; Gan, W.B. REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci. 2017, 20, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Filho, D.G.; Queiroz, C.M.; Ribeiro, S. Memory corticalization triggered by REM sleep: Mechanisms of cellular and systems consolidation. Cell Mol. Life Sci. 2018, 75, 3715–3740. [Google Scholar] [CrossRef]
- Cade, B.E.; Gottlieb, D.J.; Lauderdale, D.S.; Bennett, D.A.; Buchman, A.S.; Buxbaum, S.G.; De Jager, P.L.; Evans, D.S.; Fülöp, T.; Gharib, S.A.; et al. Common variants in DRD2 are associated with sleep duration: The CARe consortium. Hum. Mol. Genet. 2016, 25, 167–179. [Google Scholar] [CrossRef]
- Chemelli, R.M.; Willie, J.T.; Sinton, C.M.; Elmquist, J.K.; Scammell, T.; Lee, C.; Richardson, J.A.; Williams, S.C.; Xiong, Y.; Kisanuki, Y.; et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 1999, 98, 437–451. [Google Scholar] [CrossRef]
- Naganuma, F.; Nakamura, T.; Yoshikawa, T.; Iida, T.; Miura, Y.; Kárpáti, A.; Matsuzawa, T.; Yanai, A.; Mogi, A.; Mochizuki, T.; et al. Histamine N-methyltransferase regulates aggression and the sleep-wake cycle. Sci. Rep. 2017, 7, 15899. [Google Scholar] [CrossRef]
- Tatsuki, F.; Sunagawa, G.A.; Shi, S.; Susaki, E.A.; Yukinaga, H.; Perrin, D.; Sumiyama, K.; Ukai-Tadenuma, M.; Fujishima, H.; Ohno, R.; et al. Involvement of Ca2+-Dependent Hyperpolarization in Sleep Duration in Mammals. Neuron 2016, 90, 70–85. [Google Scholar] [CrossRef]
- Schenck, C.H.; Boeve, B.F.; Mahowald, M.W. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: A 16-year update on a previously reported series. Sleep Med. 2013, 14, 744–748. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Lam, S.P.; Chan, J.W.; Mok, V.; Chan, A.; Li, S.X.; Liu, Y.; Tang, X.; Yung, W.H.; et al. Excessive Daytime Sleepiness Predicts Neurodegeneration in Idiopathic REM Sleep Behavior Disorder. Sleep 2017, 40, zsx041. [Google Scholar] [CrossRef]
- Ju, Y.E.; McLeland, J.S.; Toedebusch, C.D.; Xiong, C.; Fagan, A.M.; Duntley, S.P.; Morris, J.C.; Holtzman, D.M. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013, 70, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.S.; Kowgier, M.; Yu, L.; Buchman, A.S.; Bennett, D.A. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep 2013, 36, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Hahn, E.A.; Wang, H.X.; Andel, R.; Fratiglioni, L. A change in sleep pattern may predict Alzheimer disease. Am. J. Geriatr. Psychiatry 2014, 22, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, P.N.G.; Villaraza, S.G.; Rosa, J.C.D. The association between sleep and Alzheimer’s disease: A systematic review. Dement. Neuropsychol. 2024, 18, e20230049. [Google Scholar] [CrossRef]
- Lacerda, R.A.V.; Desio, J.A.F.; Kammers, C.M.; Henkes, S.; Freitas de Sa, M.; de Souza, E.F.; da Silva, D.M.; Teixeira Pinheiro Gusmao, C.; Santos, J. Sleep disorders and risk of alzheimer’s disease: A two-way road. Ageing Res. Rev. 2024, 101, 102514. [Google Scholar] [CrossRef]
- Kang, J.E.; Lim, M.M.; Bateman, R.J.; Lee, J.J.; Smyth, L.P.; Cirrito, J.R.; Fujiki, N.; Nishino, S.; Holtzman, D.M. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009, 326, 1005–1007. [Google Scholar] [CrossRef]
- Di Meco, A.; Joshi, Y.B.; Pratico, D. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol. Aging 2014, 35, 1813–1820. [Google Scholar] [CrossRef]
- Scott-Massey, A.; Boag, M.K.; Magnier, A.; Bispo, D.; Khoo, T.K.; Pountney, D.L. Glymphatic System Dysfunction and Sleep Disturbance May Contribute to the Pathogenesis and Progression of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 12928. [Google Scholar] [CrossRef]
- Xu, Y.; Toh, K.L.; Jones, C.R.; Shin, J.Y.; Fu, Y.H.; Ptácek, L.J. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell 2007, 128, 59–70. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, S.; Zhou, H.; Qu, L.H.; Yang, J.H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.; Possovre, M.L.; Bandarabadi, M.; Moseholm, K.F.; Justinussen, J.L.; Bozic, I.; Lemcke, R.; Arribat, Y.; Amati, F.; Silahtaroglu, A.; et al. The evolutionarily conserved miRNA-137 targets the neuropeptide hypocretin/orexin and modulates the wake to sleep ratio. Proc. Natl. Acad. Sci. USA 2022, 119, e2112225119. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.; Bang-Berthelsen, C.H.; Knudsen, S.; Kornum, B.R.; Modvig, S.; Jennum, P.; Gammeltoft, S. miRNA profiles in plasma from patients with sleep disorders reveal dysregulation of miRNAs in narcolepsy and other central hypersomnias. Sleep 2014, 37, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Sinigaglia, K.; Wiatrek, D.; Khan, A.; Michalik, D.; Sambrani, N.; Sedmík, J.; Vukić, D.; O’Connell, M.A.; Keegan, L.P. ADAR RNA editing in innate immune response phasing, in circadian clocks and in sleep. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Saavedra, M.; Antoun, G.; Yanagiya, A.; Oliva-Hernandez, R.; Cornejo-Palma, D.; Perez-Iratxeta, C.; Sonenberg, N.; Cheng, H.Y. miRNA-132 orchestrates chromatin remodeling and translational control of the circadian clock. Hum. Mol. Genet. 2011, 20, 731–751. [Google Scholar] [CrossRef]
- Weissová, K.; Škrabalová, J.; Skálová, K.; Červená, K.; Bendová, Z.; Miletínová, E.; Kopřivová, J.; Šonka, K.; Dudysová, D.; Bartoš, A.; et al. Circadian rhythms of melatonin and peripheral clock gene expression in idiopathic REM sleep behavior disorder. Sleep Med. 2018, 52, 1–6. [Google Scholar] [CrossRef]
- Laposky, A.; Easton, A.; Dugovic, C.; Walisser, J.; Bradfield, C.; Turek, F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep 2005, 28, 395–409. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Chung, S.; Park, N.; Son, G.H.; An, H.; Jang, J.; Chang, D.J.; Suh, Y.G.; Kim, K. Identification of a novel circadian clock modulator controlling BMAL1 expression through a ROR/REV-ERB-response element-dependent mechanism. Biochem. Biophys. Res. Commun. 2016, 469, 580–586. [Google Scholar] [CrossRef]
- Ehlen, J.C.; Brager, A.J.; Baggs, J.; Pinckney, L.; Gray, C.L.; DeBruyne, J.P.; Esser, K.A.; Takahashi, J.S.; Paul, K.N. Bmal1 function in skeletal muscle regulates sleep. Elife 2017, 6, e26557. [Google Scholar] [CrossRef]
- Park, N.; Kim, H.D.; Cheon, S.; Row, H.; Lee, J.; Han, D.H.; Cho, S.; Kim, K. A Novel Bmal1 Mutant Mouse Reveals Essential Roles of the C-Terminal Domain on Circadian Rhythms. PLoS ONE 2015, 10, e0138661. [Google Scholar] [CrossRef]
- Sochal, M.; Ditmer, M.; Tarasiuk-Zawadzka, A.; Binienda, A.; Turkiewicz, S.; Wysokiński, A.; Karuga, F.F.; Białasiewicz, P.; Fichna, J.; Gabryelska, A. Circadian Rhythm Genes and Their Association with Sleep and Sleep Restriction. Int. J. Mol. Sci. 2024, 25, 10445. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, D.; Shostak, A.; Oster, H. Clock genes and sleep. Pflug. Arch. 2012, 463, 3–14. [Google Scholar] [CrossRef]
- Piggins, H.D. Human clock genes. Ann. Med. 2002, 34, 394–400. [Google Scholar] [CrossRef]
- Hasan, S.; van der Veen, D.R.; Winsky-Sommerer, R.; Hogben, A.; Laing, E.E.; Koentgen, F.; Dijk, D.J.; Archer, S.N. A human sleep homeostasis phenotype in mice expressing a primate-specific PER3 variable-number tandem-repeat coding-region polymorphism. FASEB J. 2014, 28, 2441–2454. [Google Scholar] [CrossRef] [PubMed]
- Okano, S.; Akashi, M.; Hayasaka, K.; Nakajima, O. Unusual circadian locomotor activity and pathophysiology in mutant CRY1 transgenic mice. Neurosci. Lett. 2009, 451, 246–251. [Google Scholar] [CrossRef]
- van der Horst, G.T.; Muijtjens, M.; Kobayashi, K.; Takano, R.; Kanno, S.; Takao, M.; de Wit, J.; Verkerk, A.; Eker, A.P.; van Leenen, D.; et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 1999, 398, 627–630. [Google Scholar] [CrossRef]
- Jagannath, A.; Taylor, L.; Wakaf, Z.; Vasudevan, S.R.; Foster, R.G. The genetics of circadian rhythms, sleep and health. Hum. Mol. Genet. 2017, 26, R128–R138. [Google Scholar] [CrossRef]
- Chowdhury, S.; Matsubara, T.; Miyazaki, T.; Ono, D.; Fukatsu, N.; Abe, M.; Sakimura, K.; Sudo, Y.; Yamanaka, A. GABA neurons in the ventral tegmental area regulate non-rapid eye movement sleep in mice. Elife 2019, 8, e44928. [Google Scholar] [CrossRef]
- Hu, Y.; Bringmann, H. Tfap2b acts in GABAergic neurons to control sleep in mice. Sci. Rep. 2023, 13, 8026. [Google Scholar] [CrossRef]
- Funato, H.; Miyoshi, C.; Fujiyama, T.; Kanda, T.; Sato, M.; Wang, Z.; Ma, J.; Nakane, S.; Tomita, J.; Ikkyu, A.; et al. Forward-genetics analysis of sleep in randomly mutagenized mice. Nature 2016, 539, 378–383. [Google Scholar] [CrossRef]
- Niwa, Y.; Kanda, G.N.; Yamada, R.G.; Shi, S.; Sunagawa, G.A.; Ukai-Tadenuma, M.; Fujishima, H.; Matsumoto, N.; Masumoto, K.H.; Nagano, M.; et al. Muscarinic Acetylcholine Receptors Chrm1 and Chrm3 Are Essential for REM Sleep. Cell Rep. 2018, 24, 2231–2247.e2237. [Google Scholar] [CrossRef] [PubMed]
- Mieda, M.; Hasegawa, E.; Kisanuki, Y.Y.; Sinton, C.M.; Yanagisawa, M.; Sakurai, T. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J. Neurosci. 2011, 31, 6518–6526. [Google Scholar] [CrossRef] [PubMed]
- Willie, J.T.; Chemelli, R.M.; Sinton, C.M.; Tokita, S.; Williams, S.C.; Kisanuki, Y.Y.; Marcus, J.N.; Lee, C.; Elmquist, J.K.; Kohlmeier, K.A.; et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: Molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron 2003, 38, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Franken, P.; Dudley, C.A.; Estill, S.J.; Barakat, M.; Thomason, R.; O’Hara, B.F.; McKnight, S.L. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: Genotype and sex interactions. Proc. Natl. Acad. Sci. USA 2006, 103, 7118–7123. [Google Scholar] [CrossRef]
- Gamble, M.C.; Chuan, B.; Gallego-Martin, T.; Shelton, M.A.; Puig, S.; O’Donnell, C.P.; Logan, R.W. A role for the circadian transcription factor NPAS2 in the progressive loss of non-rapid eye movement sleep and increased arousal during fentanyl withdrawal in male mice. Psychopharmacology 2022, 239, 3185–3200. [Google Scholar] [CrossRef]
- Alam, M.N.; Szymusiak, R.; Gong, H.; King, J.; McGinty, D. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: Neuronal recording with microdialysis. J. Physiol. 1999, 521 Pt 3, 679–690. [Google Scholar] [CrossRef]
- Oishi, Y.; Huang, Z.L.; Fredholm, B.B.; Urade, Y.; Hayaishi, O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc. Natl. Acad. Sci. USA 2008, 105, 19992–19997. [Google Scholar] [CrossRef]
- Gallopin, T.; Luppi, P.H.; Cauli, B.; Urade, Y.; Rossier, J.; Hayaishi, O.; Lambolez, B.; Fort, P. The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus. Neuroscience 2005, 134, 1377–1390. [Google Scholar] [CrossRef]
- Niu, L.; Li, Y.; Zong, P.; Liu, P.; Shui, Y.; Chen, B.; Wang, Z.W. Melatonin promotes sleep by activating the BK channel in C. elegans. Proc. Natl. Acad. Sci. USA 2020, 117, 25128–25137. [Google Scholar] [CrossRef]
- He, Y.; Jones, C.R.; Fujiki, N.; Xu, Y.; Guo, B.; Holder, J.L., Jr.; Rossner, M.J.; Nishino, S.; Fu, Y.H. The transcriptional repressor DEC2 regulates sleep length in mammals. Science 2009, 325, 866–870. [Google Scholar] [CrossRef]
- Shi, G.; Yin, C.; Fan, Z.; Xing, L.; Mostovoy, Y.; Kwok, P.Y.; Ashbrook, L.H.; Krystal, A.D.; Ptáček, L.J.; Fu, Y.H. Mutations in Metabotropic Glutamate Receptor 1 Contribute to Natural Short Sleep Trait. Curr. Biol. 2021, 31, 13–24.e14. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Xing, L.; Wu, D.; Bhattacharyya, B.J.; Jones, C.R.; McMahon, T.; Chong, S.Y.C.; Chen, J.A.; Coppola, G.; Geschwind, D.; et al. A Rare Mutation of β(1)-Adrenergic Receptor Affects Sleep/Wake Behaviors. Neuron 2019, 103, 1044–1055.e1047. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Shi, G.; Mostovoy, Y.; Gentry, N.W.; Fan, Z.; McMahon, T.B.; Kwok, P.Y.; Jones, C.R.; Ptáček, L.J.; Fu, Y.H. Mutant neuropeptide S receptor reduces sleep duration with preserved memory consolidation. Sci. Transl. Med. 2019, 11, eaax2014. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, L.; Sek, K.; Henderson, M.A.; Lai, J.; Chen, A.X.Y.; Meyran, D.; Todd, K.L.; Petley, E.V.; Mardiana, S.; Mølck, C.; et al. CRISPR/Cas9 mediated deletion of the adenosine A2A receptor enhances CAR T cell efficacy. Nat. Commun. 2021, 12, 3236. [Google Scholar] [CrossRef]
- Leone, R.D.; Sun, I.M.; Oh, M.H.; Sun, I.H.; Wen, J.; Englert, J.; Powell, J.D. Inhibition of the adenosine A2a receptor modulates expression of T cell coinhibitory receptors and improves effector function for enhanced checkpoint blockade and ACT in murine cancer models. Cancer Immunol. Immunother. 2018, 67, 1271–1284. [Google Scholar] [CrossRef]
- Globig, A.M.; Zhao, S.; Roginsky, J.; Maltez, V.I.; Guiza, J.; Avina-Ochoa, N.; Heeg, M.; Araujo Hoffmann, F.; Chaudhary, O.; Wang, J.; et al. The β(1)-adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature 2023, 622, 383–392. [Google Scholar] [CrossRef]
- Lewis, D.A. GABAergic local circuit neurons and prefrontal cortical dysfunction in schizophrenia. Brain Res. Brain Res. Rev. 2000, 31, 270–276. [Google Scholar] [CrossRef]
- de las Casas-Engel, M.; Domínguez-Soto, A.; Sierra-Filardi, E.; Bragado, R.; Nieto, C.; Puig-Kroger, A.; Samaniego, R.; Loza, M.; Corcuera, M.T.; Gómez-Aguado, F.; et al. Serotonin skews human macrophage polarization through HTR2B and HTR7. J. Immunol. 2013, 190, 2301–2310. [Google Scholar] [CrossRef]
- Pack, A.I.; Pien, G.W. Update on sleep and its disorders. Annu. Rev. Med. 2011, 62, 447–460. [Google Scholar] [CrossRef]
- Konttinen, H. Emotional eating and obesity in adults: The role of depression, sleep and genes. Proc. Nutr. Soc. 2020, 79, 283–289. [Google Scholar] [CrossRef]
- Reutrakul, S.; Van Cauter, E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism 2018, 84, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, B.; Bellet, M.M.; Katada, S.; Astarita, G.; Hirayama, J.; Amin, R.H.; Granneman, J.G.; Piomelli, D.; Leff, T.; Sassone-Corsi, P. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010, 12, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yeghiazaryan, G.; Hess, S.; Klemm, P.; Sieben, A.; Kleinridders, A.; Morgan, D.A.; Wunderlich, F.T.; Rahmouni, K.; Kong, D.; et al. Orexin receptors 1 and 2 in serotonergic neurons differentially regulate peripheral glucose metabolism in obesity. Nat. Commun. 2021, 12, 5249. [Google Scholar] [CrossRef] [PubMed]
- He, S.K.; Wang, J.H.; Li, T.; Yin, S.; Cui, J.W.; Xiao, Y.F.; Tang, Y.; Wang, J.; Bai, Y.J. Sleep and circadian rhythm disturbance in kidney stone disease: A narrative review. Front. Endocrinol. 2023, 14, 1293685. [Google Scholar] [CrossRef]
- Russell, A.L.; Miller, L.; Yi, H.; Keil, R.; Handa, R.J.; Wu, T.J. Knockout of the circadian gene, Per2, disrupts corticosterone secretion and results in depressive-like behaviors and deficits in startle responses. BMC Neurosci. 2021, 22, 5. [Google Scholar] [CrossRef]
- Gréchez-Cassiau, A.; Feillet, C.; Guérin, S.; Delaunay, F. The hepatic circadian clock regulates the choline kinase α gene through the BMAL1-REV-ERBα axis. Chronobiol. Int. 2015, 32, 774–784. [Google Scholar] [CrossRef]
- Bonney, S.; Kominsky, D.; Brodsky, K.; Eltzschig, H.; Walker, L.; Eckle, T. Cardiac Per2 functions as novel link between fatty acid metabolism and myocardial inflammation during ischemia and reperfusion injury of the heart. PLoS ONE 2013, 8, e71493. [Google Scholar] [CrossRef]
- Bhaskara, M.; Anjorin, O.; Yoniles, A.; Liu, J.; Wang, M. Importance of Per2 in cardiac mitochondrial protection during stress. Sci. Rep. 2024, 14, 1290. [Google Scholar] [CrossRef]
- Lu, T.; Jiang, B.; Wang, X.L.; Lee, H.C. Coronary arterial BK channel dysfunction exacerbates ischemia/reperfusion-induced myocardial injury in diabetic mice. Appl. Physiol. Nutr. Metab. 2016, 41, 992–1001. [Google Scholar] [CrossRef]
- Viola, A.U.; James, L.M.; Archer, S.N.; Dijk, D.J. PER3 polymorphism and cardiac autonomic control: Effects of sleep debt and circadian phase. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2156–H2163. [Google Scholar] [CrossRef]
- Mikulska, A.A.; Grzelak, T.; Pelczyńska, M.; Bogdański, P.; Czyżewska, K. Assessment of Selected Clock Proteins (CLOCK and CRY1) and Their Relationship with Biochemical, Anthropometric, and Lifestyle Parameters in Hypertensive Patients. Biomolecules 2021, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Nebigil, C.G.; Hickel, P.; Messaddeq, N.; Vonesch, J.L.; Douchet, M.P.; Monassier, L.; György, K.; Matz, R.; Andriantsitohaina, R.; Manivet, P.; et al. Ablation of serotonin 5-HT(2B) receptors in mice leads to abnormal cardiac structure and function. Circulation 2001, 103, 2973–2979. [Google Scholar] [CrossRef] [PubMed]
- Lairez, O.; Cognet, T.; Schaak, S.; Calise, D.; Guilbeau-Frugier, C.; Parini, A.; Mialet-Perez, J. Role of serotonin 5-HT2A receptors in the development of cardiac hypertrophy in response to aortic constriction in mice. J. Neural Transm. 2013, 120, 927–935. [Google Scholar] [CrossRef]
- Mogavero, M.P.; DelRosso, L.M.; Fanfulla, F.; Bruni, O.; Ferri, R. Sleep disorders and cancer: State of the art and future perspectives. Sleep Med. Rev. 2021, 56, 101409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Meng, X.; Li, Y.; Liu, L.; He, Q.; Jiang, J.; Chen, Y.; Li, X.; Li, Y.; Tang, Y.; et al. Circadian clock gene BMAL1 inhibits the proliferation and tumor-formation ability of nasopharyngeal carcinoma cells and increases the sensitivity of radiotherapy. Chronobiol. Int. 2022, 39, 1340–1351. [Google Scholar] [CrossRef]
- Yang, G.; Yang, Y.; Tang, H.; Yang, K. Loss of the clock gene Per1 promotes oral squamous cell carcinoma progression via the AKT/mTOR pathway. Cancer Sci. 2020, 111, 1542–1554. [Google Scholar] [CrossRef]
- Gong, X.; Tang, H.; Yang, K. PER1 suppresses glycolysis and cell proliferation in oral squamous cell carcinoma via the PER1/RACK1/PI3K signaling complex. Cell Death Dis. 2021, 12, 276. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Q.; Hu, X.; Zhang, S.; Jiang, Y.; Yao, G.; Hu, K.; Xu, X.; Liang, B.; Wu, Q.; et al. Disrupting Circadian Rhythm via the PER1-HK2 Axis Reverses Trastuzumab Resistance in Gastric Cancer. Cancer Res. 2022, 82, 1503–1517. [Google Scholar] [CrossRef]
- Logan, R.W.; Wynne, O.; Levitt, D.; Price, D.; Sarkar, D.K. Altered circadian expression of cytokines and cytolytic factors in splenic natural killer cells of Per1(-/-) mutant mice. J. Interferon Cytokine Res. 2013, 33, 108–114. [Google Scholar] [CrossRef]
- Hernandez-Rosas, F.; Hernandez-Oliveras, A.; Flores-Peredo, L.; Rodriguez, G.; Zarain-Herzberg, A.; Caba, M.; Santiago-Garcia, J. Histone deacetylase inhibitors induce the expression of tumor suppressor genes Per1 and Per2 in human gastric cancer cells. Oncol. Lett. 2018, 16, 1981–1990. [Google Scholar] [CrossRef]
- Gery, S.; Komatsu, N.; Baldjyan, L.; Yu, A.; Koo, D.; Koeffler, H.P. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol. Cell 2006, 22, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, L.; Liu, X.; Ma, Z.; Lv, L. PER1 Is a Prognostic Biomarker and Correlated With Immune Infiltrates in Ovarian Cancer. Front. Genet. 2021, 12, 697471. [Google Scholar] [CrossRef] [PubMed]
- Bellet, M.M.; Stincardini, C.; Costantini, C.; Gargaro, M.; Pieroni, S.; Castelli, M.; Piobbico, D.; Sassone-Corsi, P.; Della-Fazia, M.A.; Romani, L.; et al. The Circadian Protein PER1 Modulates the Cellular Response to Anticancer Treatments. Int. J. Mol. Sci. 2021, 22, 2974. [Google Scholar] [CrossRef]
- Li, H.X.; Fu, X.J.; Yang, K.; Chen, D.; Tang, H.; Zhao, Q. The clock gene PER1 suppresses expression of tumor-related genes in human oral squamous cell carcinoma. Oncotarget 2016, 7, 20574–20583. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Q.; Hu, Y.; Wang, F. The Circadian Gene Per1 Plays an Important Role in Radiation-Induced Apoptosis and DNA Damage in Glioma. Asian Pac. J. Cancer Prev. 2019, 20, 2195–2201. [Google Scholar] [CrossRef]
- Fu, L.; Pelicano, H.; Liu, J.; Huang, P.; Lee, C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 2002, 111, 41–50. [Google Scholar] [CrossRef]
- Xiang, S.; Coffelt, S.B.; Mao, L.; Yuan, L.; Cheng, Q.; Hill, S.M. Period-2: A tumor suppressor gene in breast cancer. J. Circadian Rhythm. 2008, 6, 4. [Google Scholar] [CrossRef]
- Pavithra, S.; Aich, A.; Chanda, A.; Zohra, I.F.; Gawade, P.; Das, R.K. PER2 gene and its association with sleep-related disorders: A review. Physiol. Behav. 2024, 273, 114411. [Google Scholar] [CrossRef]
- Dong, P.; Wang, Y.; Liu, Y.; Zhu, C.; Lin, J.; Qian, R.; Hua, L.; Lu, C. BMAL1 induces colorectal cancer metastasis by stimulating exosome secretion. Mol. Biol. Rep. 2022, 49, 373–384. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Sun, L.; Jin, H.; Ren, K.; Liu, S.; Qian, Y.; Li, S.; Li, F.; Zhu, C.; et al. BMAL1 collaborates with CLOCK to directly promote DNA double-strand break repair and tumor chemoresistance. Oncogene 2023, 42, 967–979. [Google Scholar] [CrossRef]
- Shan, L.; Zheng, W.; Bai, B.; Hu, J.; Lv, Y.; Chen, K.; Wang, X.; Pan, Y.; Huang, X.; Zhu, H.; et al. BMAL1 promotes colorectal cancer cell migration and invasion through ERK- and JNK-dependent c-Myc expression. Cancer Med. 2023, 12, 4472–4485. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Li, M.; Su, D.; Fan, H.; Xia, H. Bmal1 regulates the stemness and tumorigenesis of gliomas with the Wnt/beta-catenin signaling pathway. Gene 2025, 933, 148940. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Chen, Y.; Quan, P.; Zhang, J.; Han, S.; Wang, G.; Qi, R.; Zhang, X.; Wang, F.; Yuan, J.; et al. NPAS2 promotes aerobic glycolysis and tumor growth in prostate cancer through HIF-1A signaling. BMC Cancer 2023, 23, 280. [Google Scholar] [CrossRef]
- Yuan, P.; Li, J.; Zhou, F.; Huang, Q.; Zhang, J.; Guo, X.; Lyu, Z.; Zhang, H.; Xing, J. NPAS2 promotes cell survival of hepatocellular carcinoma by transactivating CDC25A. Cell Death Dis. 2017, 8, e2704. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, L.; Kothandan, G.; Manoharan, R. Berberine and Emodin abrogates breast cancer growth and facilitates apoptosis through inactivation of SIK3-induced mTOR and Akt signaling pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165897. [Google Scholar] [CrossRef]
- Wangari-Talbot, J.; Wall, B.A.; Goydos, J.S.; Chen, S. Functional effects of GRM1 suppression in human melanoma cells. Mol. Cancer Res. 2012, 10, 1440–1450. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y.; Thompson, J.W.; Yin, T.; Alexander, P.B.; Qin, D.; Mudgal, P.; Wu, H.; Liang, Y.; Tan, L.; et al. Cancer-cell-derived GABA promotes β-catenin-mediated tumour growth and immunosuppression. Nat. Cell Biol. 2022, 24, 230–241. [Google Scholar] [CrossRef]
- Miro, C.; Docimo, A.; Barrea, L.; Verde, L.; Cernea, S.; Sojat, A.S.; Marina, L.V.; Docimo, G.; Colao, A.; Dentice, M.; et al. “Time” for obesity-related cancer: The role of the circadian rhythm in cancer pathogenesis and treatment. Semin. Cancer Biol. 2023, 91, 99–109. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Nice, E.; Huang, C.; Zhang, W.; Tang, Y. Circadian rhythms and cancers: The intrinsic links and therapeutic potentials. J. Hematol. Oncol. 2022, 15, 21. [Google Scholar] [CrossRef]
- Colaprico, A.; Silva, T.C.; Olsen, C.; Garofano, L.; Cava, C.; Garolini, D.; Sabedot, T.S.; Malta, T.M.; Pagnotta, S.M.; Castiglioni, I.; et al. TCGAbiolinks: An R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016, 44, e71. [Google Scholar] [CrossRef]
- Liu, C.J.; Hu, F.F.; Xie, G.Y.; Miao, Y.R.; Li, X.W.; Zeng, Y.; Guo, A.Y. GSCA: An integrated platform for gene set cancer analysis at genomic, pharmacogenomic and immunogenomic levels. Brief. Bioinform. 2023, 24, bbac558. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M.; Monti, D. Sleep disturbance in schizophrenia. Int. Rev. Psychiatry 2005, 17, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.S.; Owe-Larsson, B.; Hetta, J.; Lundkvist, G.B. Altered circadian clock gene expression in patients with schizophrenia. Schizophr. Res. 2016, 174, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, P.W.; Nadarajah, C.J.; Kanan, M.F.; Patterson, J.N.; Novotny, B.; Lawrence, J.H.; King, M.W.; Brase, L.; Inman, C.E.; Yuede, C.M.; et al. An astrocyte BMAL1-BAG3 axis protects against alpha-synuclein and tau pathology. Neuron 2023, 111, 2383–2398.e2387. [Google Scholar] [CrossRef]
- González-Maeso, J.; Ang, R.L.; Yuen, T.; Chan, P.; Weisstaub, N.V.; López-Giménez, J.F.; Zhou, M.; Okawa, Y.; Callado, L.F.; Milligan, G.; et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 2008, 452, 93–97. [Google Scholar] [CrossRef]
- Wang, M.; Li, P.; Li, Z.; da Silva, B.S.; Zheng, W.; Xiang, Z.; He, Y.; Xu, T.; Cordeiro, C.; Deng, L.; et al. Lateral septum adenosine A(2A) receptors control stress-induced depressive-like behaviors via signaling to the hypothalamus and habenula. Nat. Commun. 2023, 14, 1880. [Google Scholar] [CrossRef]
- Xia, G.; Han, Y.; Meng, F.; He, Y.; Srisai, D.; Farias, M.; Dang, M.; Palmiter, R.D.; Xu, Y.; Wu, Q. Reciprocal control of obesity and anxiety-depressive disorder via a GABA and serotonin neural circuit. Mol. Psychiatry 2021, 26, 2837–2853. [Google Scholar] [CrossRef]
- Trollope, A.F.; Gutièrrez-Mecinas, M.; Mifsud, K.R.; Collins, A.; Saunderson, E.A.; Reul, J.M. Stress, epigenetic control of gene expression and memory formation. Exp. Neurol. 2012, 233, 3–11. [Google Scholar] [CrossRef]
- Liu, D.; Nanclares, C.; Simbriger, K.; Fang, K.; Lorsung, E.; Le, N.; Amorim, I.S.; Chalkiadaki, K.; Pathak, S.S.; Li, J.; et al. Autistic-like behavior and cerebellar dysfunction in Bmal1 mutant mice ameliorated by mTORC1 inhibition. Mol. Psychiatry 2023, 28, 3727–3738. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, G.; He, Y.; Tang, Q.; Yin, Y.; Jie, Y. BMAL1 deficiency provokes dry mouth and eyes by down-regulating ITPR2/3. Ocul. Surf. 2024, 34, 430–440. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).