NCKAP1 Inhibits the Progression of Renal Carcinoma via Modulating Immune Responses and the PI3K/AKT/mTOR Signaling Pathway
Abstract
:1. Introduction
2. Results
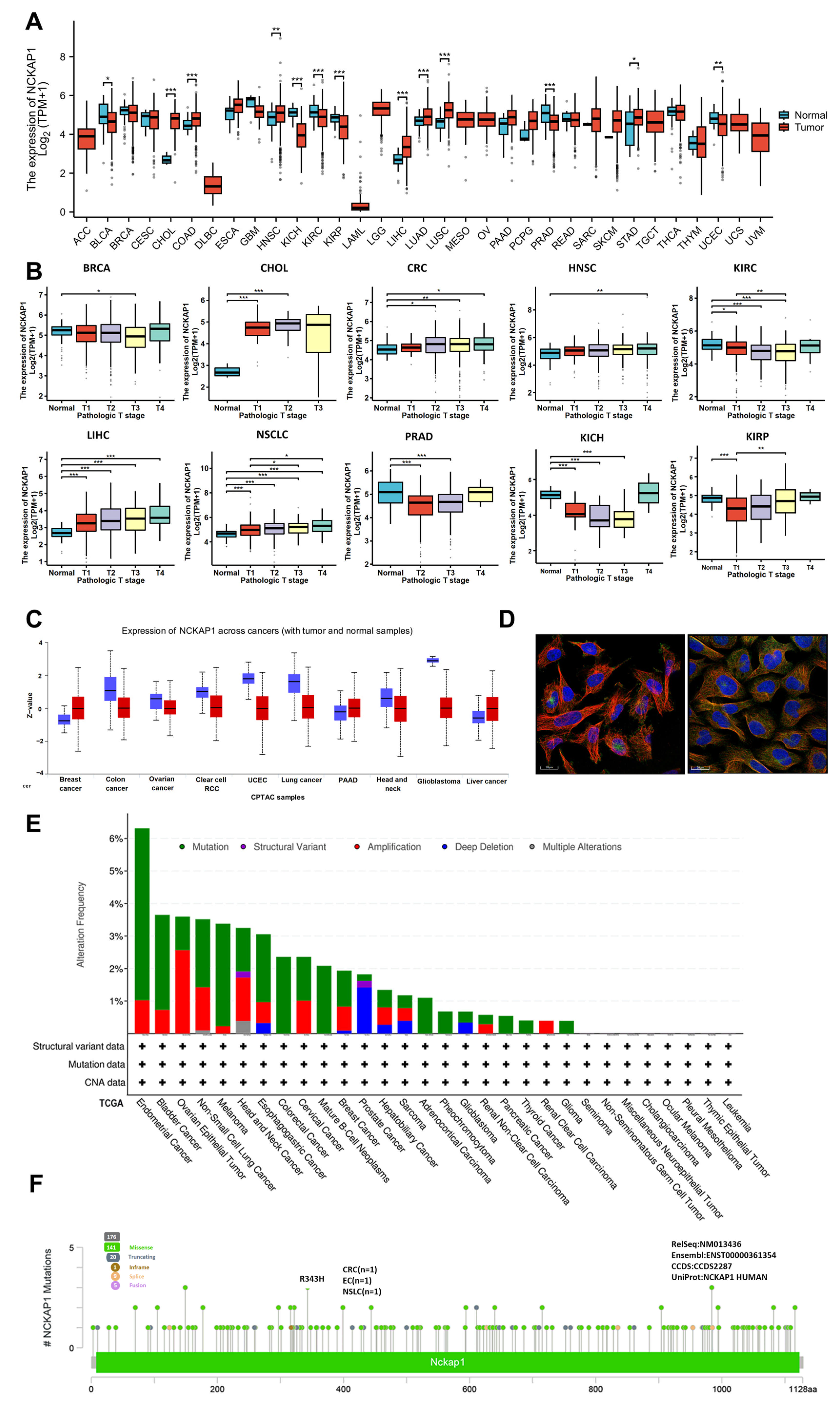
2.1. Analyses of NCKAP1 Expression and Genetic Alterations Across Tumors
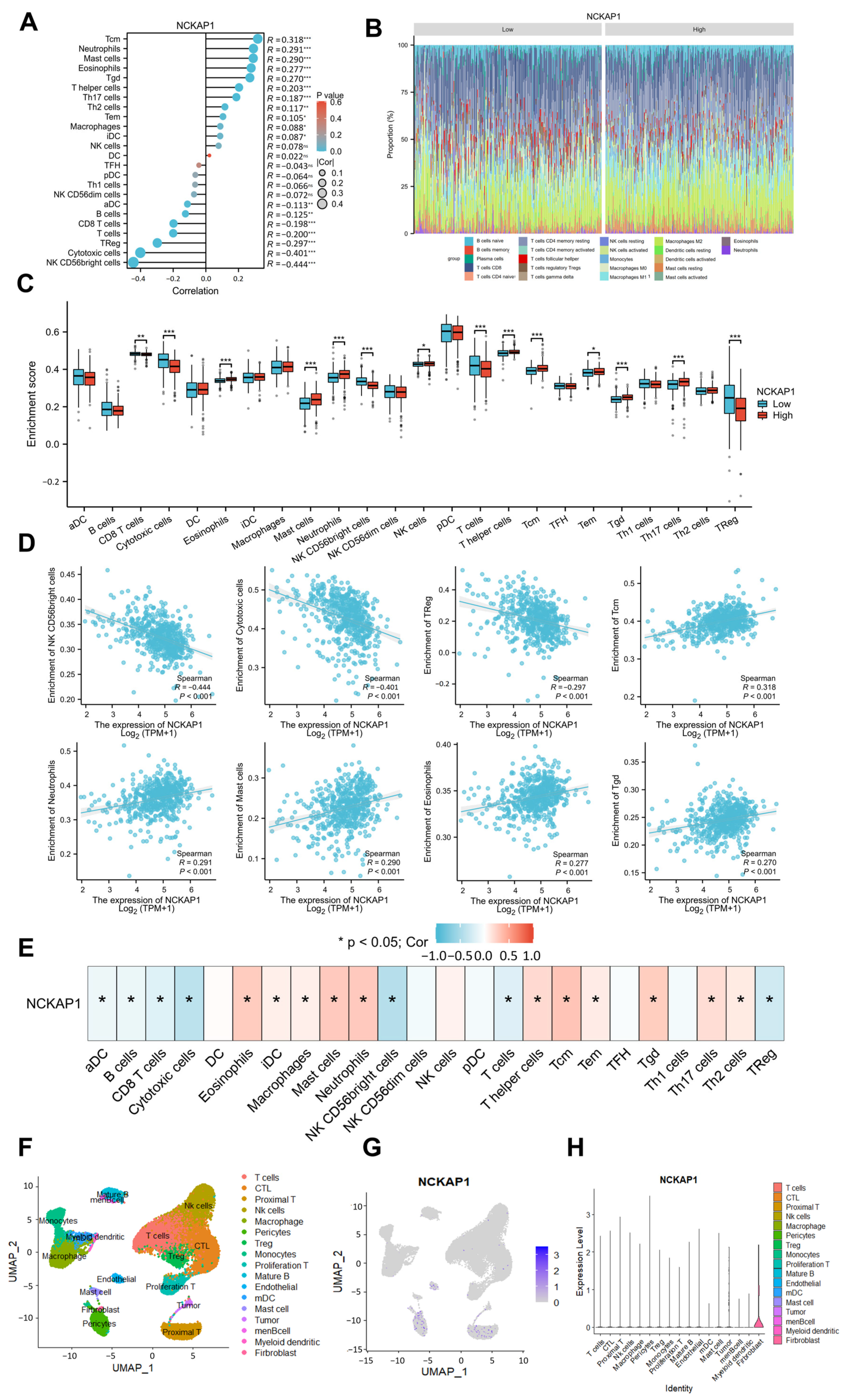
2.2. CIBERSORT and Single-Cell Transcriptomics Revealed a Significant Correlation Between NCKAP1 and Immune Cell Infiltration in the Kidney Tumor Microenvironment
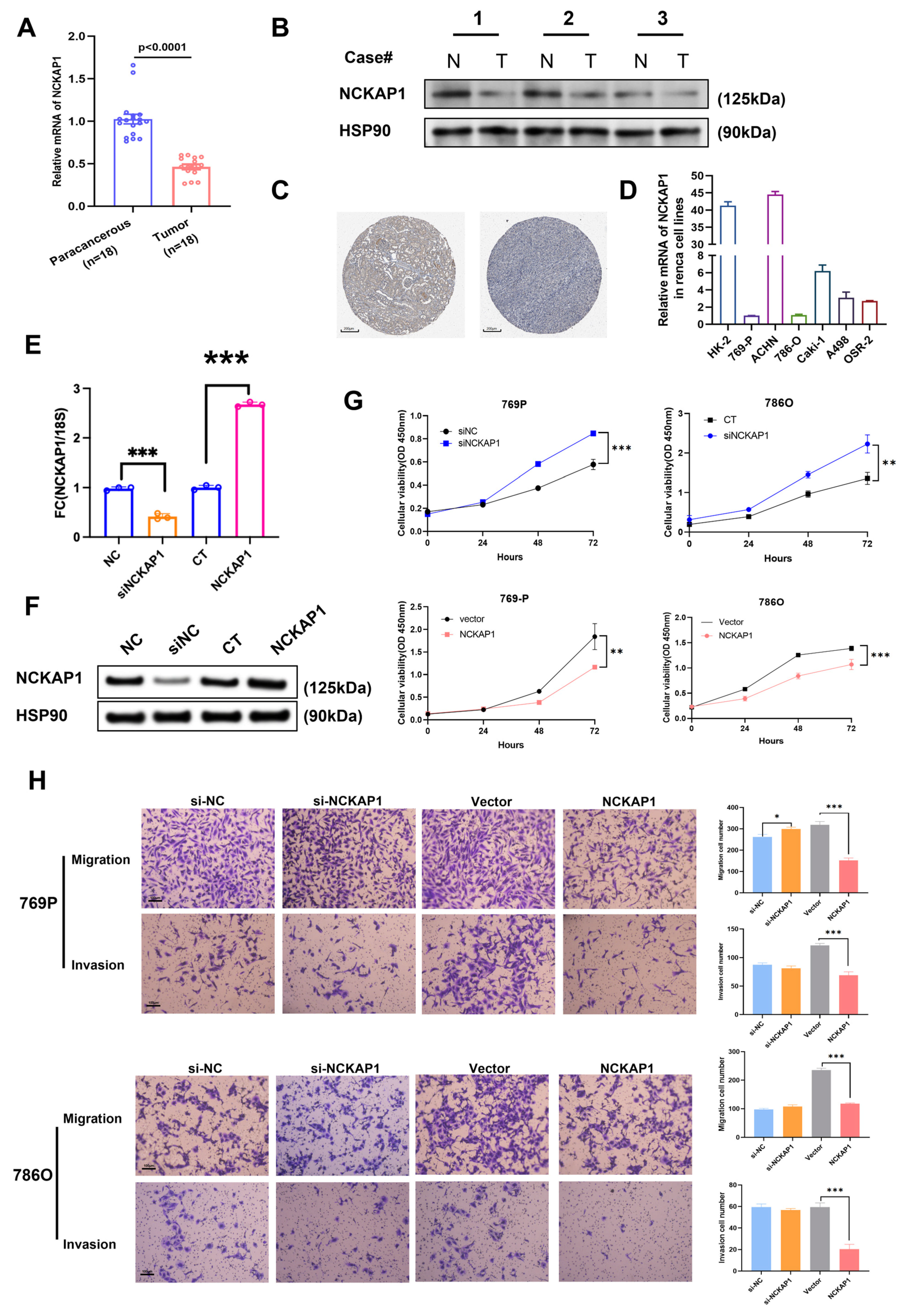
2.3. Reduced NCKAP1 Expression Was Correlated with More Advanced Stages of Pathological T Stage, Pathological Stage, and Higher Histological Grade and Significantly Affected the Overall Survival of Patients with Renal Cancer
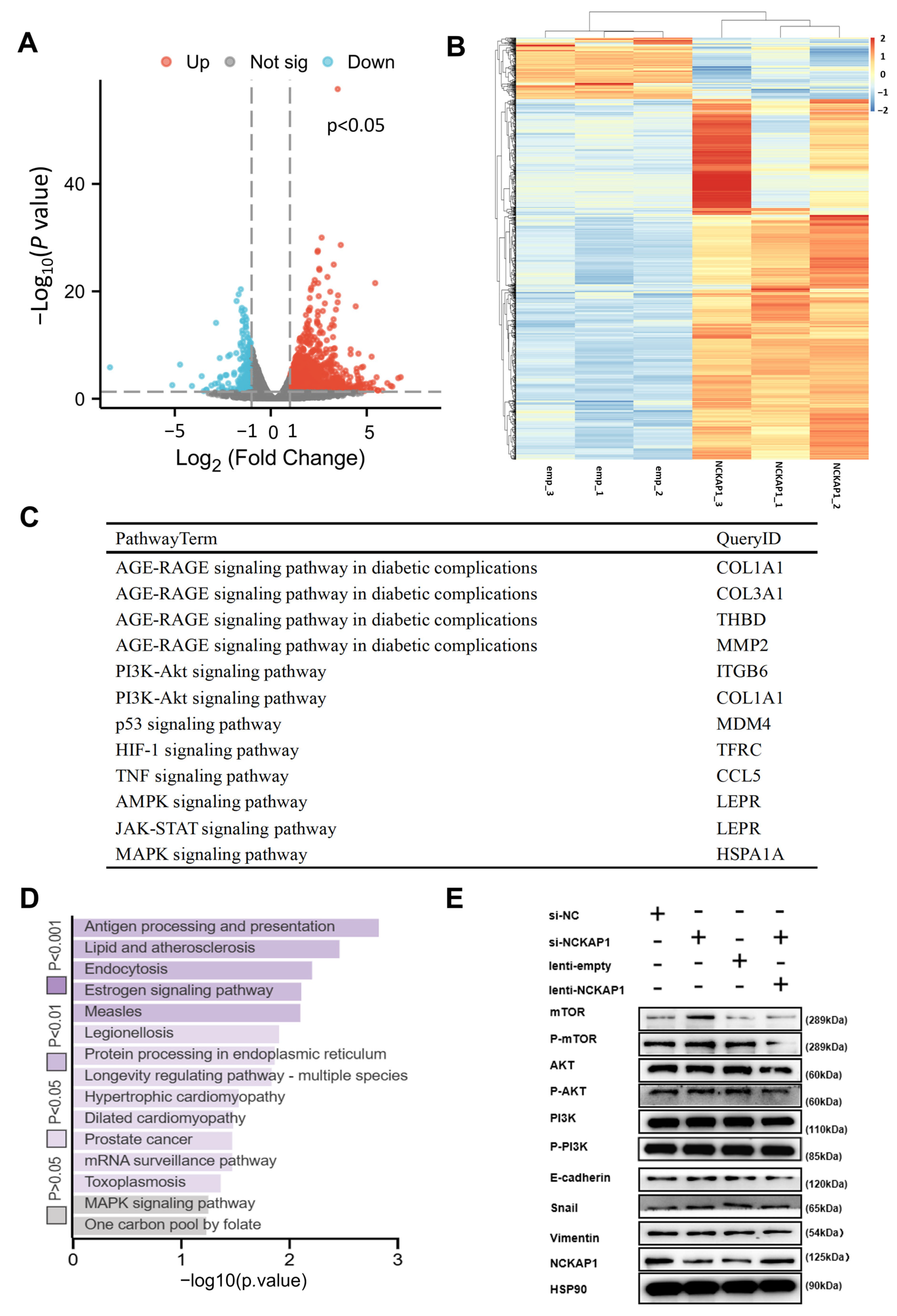
2.4. NCKAP1 Inhibits the Proliferation and Invasion of Renal Cancer Cells by the PI3K/AKT/mTOR Signaling Pathway
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Clinical Characteristics and Genetic Alteration Analyses
5.2. Survival and Similar Gene Analysis
5.3. Gene Enrichment Analysis
5.4. Human Proteome Atlas and Protein–Protein Interaction Network Analysis
5.5. Single-Cell Transcriptomic Sequencing Data Analysis
5.6. Immune Cell Infiltration and Correlation Analysis Between Immune Cells and NCKAP1
5.7. Cell Culture and Samples
5.8. CCK8 Assay
5.9. Transwell Assay
5.10. Quantitative Real-Time PCR
5.11. Immunoblots
5.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Aljahdali, I.A.M.; Zhang, R.; Nastiuk, K.L.; Krolewski, J.J.; Ling, X. Kidney cancer biomarkers and targets for therapeutics: Survivin (BIRC5), XIAP, MCL-1, HIF1α, HIF2α, NRF2, MDM2, MDM4, p53, KRAS and AKT in renal cell carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 254. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Lei, Y.; Li, J.K.; Du, W.X.; Li, R.G.; Yang, J.; Li, J.; Li, F.; Tan, H.B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Ye, J.; Xu, J.; Liu, S.; Wang, L.; Li, Z.; Li, Q.; Liu, F.; He, X.; Zou, H.; et al. Comprehensive RNA-seq reveals molecular changes in kidney malignancy among people living with HIV. Mol. Ther. Nucleic Acids 2022, 29, 91–101. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Liu, X.; Nie, L.; Zhang, Y.; Yan, Y.; Wang, C.; Colic, M.; Olszewski, K.; Horbath, A.; Chen, X.; Lei, G.; et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat. Cell Biol. 2023, 25, 404–414. [Google Scholar] [CrossRef]
- Xu, K.; Li, D.; Qian, J.; Zhang, Y.; Zhang, M.; Zhou, H.; Hou, X.; Jiang, J.; Zhang, Z.; Sun, H.; et al. Single-cell disulfidptosis regulator patterns guide intercellular communication of tumor microenvironment that contribute to kidney renal clear cell carcinoma progression and immunotherapy. Front. Immunol. 2024, 15, 1288240. [Google Scholar] [CrossRef]
- Whitelaw, J.A.; Swaminathan, K.; Kage, F.; Machesky, L.M. The WAVE Regulatory Complex Is Required to Balance Protrusion and Adhesion in Migration. Cells 2020, 9, 7. [Google Scholar] [CrossRef]
- Teng, Y.; Qin, H.; Bahassan, A.; Bendzunas, N.G.; Kennedy, E.J.; Cowell, J.K. The WASF3-NCKAP1-CYFIP1 Complex Is Essential for Breast Cancer Metastasis. Cancer Res. 2016, 76, 5133–5142. [Google Scholar] [CrossRef]
- Xiong, Y.; He, L.; Shay, C.; Lang, L.; Loveless, J.; Yu, J.; Chemmalakuzhy, R.; Jiang, H.; Liu, M.; Teng, Y. Nck-associated protein 1 associates with HSP90 to drive metastasis in human non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2019, 38, 122. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Greening, D.W.; Xu, R.; Suwakulsiri, W.; Simpson, R.J. Exosomes Derived from the Human Primary Colorectal Cancer Cell Line SW480 Orchestrate Fibroblast-Led Cancer Invasion. Proteomics 2020, 20, e2000016. [Google Scholar] [PubMed]
- Zhang, F.; Xie, S.; Zhang, Z.; Zhao, H.; Zhao, Z.; Sun, H.; Zheng, J. A Novel Risk Model Based on Autophagy Pathway Related Genes for Survival Prediction in Lung Adenocarcinoma. Med. Sci. Monit. 2020, 26, e924710. [Google Scholar] [PubMed]
- Chen, J.; Ge, J.; Zhang, W.; Xie, X.; Zhong, X.; Tang, S. NCKAP1 is a Prognostic Biomarker for Inhibition of Cell Growth in Clear Cell Renal Cell Carcinoma. Front. Genet. 2022, 13, 764957. [Google Scholar] [CrossRef]
- Cowell, J.K.; Teng, Y.; Bendzunas, N.G.; Ara, R.; Arbab, A.S.; Kennedy, E.J. Suppression of Breast Cancer Metastasis Using Stapled Peptides Targeting the WASF Regulatory Complex. Cancer Growth Metast. 2017, 10, 1179064417713197. [Google Scholar]
- Zhong, X.P.; Kan, A.; Ling, Y.H.; Lu, L.H.; Mei, J.; Wei, W.; Li, S.H.; Guo, R.P. NCKAP1 improves patient outcome and inhibits cell growth by enhancing Rb1/p53 activation in hepatocellular carcinoma. Cell Death Dis. 2019, 10, 369. [Google Scholar] [CrossRef]
- Zhu, A.; Zong, Y.; Wei, S.; Li, Y.; Fan, Y.; Liu, S.; Gao, X. Pancancer Analysis of the Disulfidptosis-related Gene NCKAP1 and Its Prognostic Value for Lung Adenocarcinoma. J. Cancer 2023, 14, 3351–3367. [Google Scholar]
- Ni, L.; Yang, H.; Wu, X.; Zhou, K.; Wang, S. The expression and prognostic value of disulfidptosis progress in lung adenocarcinoma. Aging 2023, 15, 7741–7759. [Google Scholar]
- Kwon, M.R.; Lee, J.H.; Park, J.; Park, S.S.; Ju, E.J.; Ko, E.J.; Shin, S.H.; Son, G.W.; Lee, H.W.; Kim, Y.J.; et al. NCK-associated protein 1 regulates metastasis and is a novel prognostic marker for colorectal cancer. Cell Death Discov. 2023, 9, 7. [Google Scholar] [CrossRef]
- Yan, J.; Fang, Z.; Shi, M.; Tu, C.; Zhang, S.; Jiang, C.; Li, Q.; Shao, Y. Clinical Significance of Disulfidptosis-related Genes and Functional Analysis in Gastric Cancer. J. Cancer 2024, 15, 1053–1066. [Google Scholar]
- Liang, X.; Hong, A.; Shen, R.; Zhu, M.; Tian, W. NCKAP1 as a prognostic and immunological biomarker: Pancancer analysis and validation in renal clear cell carcinoma. Am. J. Transl. Res. 2024, 16, 4083–4100. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Wiestner, A. Targeting B-cell receptor signaling in cancer: Preclinical and clinical advances. Nat. Rev. Cancer 2018, 18, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Tolosano, E.; Fagoonee, S.; Morello, N.; Vinchi, F.; Fiorito, V. Heme scavenging and the other facets of hemopexin. Antioxid. Redox Signal. 2010, 12, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Alborzian Deh Sheikh, A.; Akatsu, C.; Abdu-Allah, H.H.M.; Suganuma, Y.; Imamura, A.; Ando, H.; Takematsu, H.; Ishida, H.; Tsubata, T. The Protein Tyrosine Phosphatase SHP-1 (PTPN6) but Not CD45 (PTPRC) Is Essential for the Ligand-Mediated Regulation of CD22 in BCR-Ligated B Cells. J. Immunol. 2021, 206, 2544–2551. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Huang, J.T.; Wang, J.; Srivastava, V.; Sen, S.; Liu, S.M. MicroRNA Machinery Genes as Novel Biomarkers for Cancer. Front. Oncol. 2014, 4, 113. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]

| Characteristics | KIRC | p Value | KIRP | p Value | KICH | p Value | |||
|---|---|---|---|---|---|---|---|---|---|
| NCKAP1Low | NCKAP1High | NCKAP1Low | NCKAP1High | NCKAP1Low | NCKAP1High | ||||
| n | 270 | 271 | 145 | 146 | 32 | 33 | |||
| Gender, n (%) | 0.016 | <0.001 | 0.362 | ||||||
| Female | 80 (14.8%) | 107 (19.8%) | 19 (6.5%) | 58 (19.9%) | 11 (16.9%) | 15 (23.1%) | |||
| Male | 190 (35.1%) | 164 (30.3%) | 126 (43.3%) | 88 (30.2%) | 21 (32.3%) | 18 (27.7%) | |||
| Pathologic T stage, n (%) | 0.036 | 0.013 | 0.535 | ||||||
| T1 and T2 | 163 (30.1%) | 187 (34.6%) | 121 (41.9%) | 106 (36.7%) | 21 (32.3%) | 24 (36.9%) | |||
| T3 and T4 | 107 (19.8%) | 84 (15.5%) | 22 (7.6%) | 40 (13.8%) | 11 (16.9%) | 9 (13.8%) | |||
| Pathologic N stage, n (%) | 0.540 | 0.020 | 0.198 | ||||||
| N0 | 117 (45.3%) | 125 (48.4%) | 28 (35.9%) | 22 (28.2%) | 24 (54.5%) | 15 (34.1%) | |||
| N1 | 9 (3.5%) | 7 (2.7%) | 8 (10.3%) | 20 (25.6%) | 1 (2.3%) | 4 (9.1%) | |||
| Pathologic M stage, n (%) | 0.051 | 0.605 | 1.000 | ||||||
| M0 | 204 (40.2%) | 225 (44.3%) | 46 (44.2%) | 49 (47.1%) | 21 (58.3%) | 13 (36.1%) | |||
| M1 | 47 (9.3%) | 32 (6.3%) | 3 (2.9%) | 6 (5.8%) | 1 (2.8%) | 1 (2.8%) | |||
| Pathologic stage, n (%) | 0.006 | 0.022 | 0.535 | ||||||
| Stage I and Stage II | 150 (27.9%) | 182 (33.8%) | 101 (38.7%) | 93 (35.6%) | 21 (32.3%) | 24 (36.9%) | |||
| Stage III and Stage IV | 118 (21.9%) | 88 (16.4%) | 24 (9.2%) | 43 (16.5%) | 11 (16.9%) | 9 (13.8%) | |||
| Histologic grade, n (%) | <0.001 | ||||||||
| G1 and G2 | 105 (19.7%) | 145 (27.2%) | |||||||
| G3 and G4 | 162 (30.4%) | 121 (22.7%) | |||||||
| OS event, n (%) | <0.001 | 0.529 | 0.096 | ||||||
| Alive | 161 (29.8%) | 205 (37.9%) | 125 (43%) | 122 (41.9%) | 30 (46.2%) | 25 (38.5%) | |||
| Dead | 109 (20.1%) | 66 (12.2%) | 20 (6.9%) | 24 (8.2%) | 2 (3.1%) | 8 (12.3%) | |||
| DSS event, n (%) | <0.001 | 0.125 | 0.066 | ||||||
| Yes | 75 (14.2%) | 34 (6.4%) | 10 (3.5%) | 18 (6.3%) | 1 (1.5%) | 7 (10.8%) | |||
| No | 189 (35.7%) | 232 (43.8%) | 132 (46%) | 127 (44.3%) | 31 (47.7%) | 26 (40%) | |||
| PFI event, n (%) | 0.002 | 0.115 | 0.063 | ||||||
| Yes | 97 (17.9%) | 65 (12%) | 24 (8.2%) | 35 (12%) | 3 (4.6%) | 9 (13.8%) | |||
| No | 173 (32%) | 206 (38.1%) | 121 (41.6%) | 111 (38.1%) | 29 (44.6%) | 24 (36.9%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ye, J.; Sun, L.; Xu, W.; He, X.; Bao, J.; Wang, J. NCKAP1 Inhibits the Progression of Renal Carcinoma via Modulating Immune Responses and the PI3K/AKT/mTOR Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 2813. https://doi.org/10.3390/ijms26062813
Zhang X, Ye J, Sun L, Xu W, He X, Bao J, Wang J. NCKAP1 Inhibits the Progression of Renal Carcinoma via Modulating Immune Responses and the PI3K/AKT/mTOR Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(6):2813. https://doi.org/10.3390/ijms26062813
Chicago/Turabian StyleZhang, Xin, Jianqing Ye, Lixiang Sun, Wanli Xu, Xiaomeng He, Juan Bao, and Jin Wang. 2025. "NCKAP1 Inhibits the Progression of Renal Carcinoma via Modulating Immune Responses and the PI3K/AKT/mTOR Signaling Pathway" International Journal of Molecular Sciences 26, no. 6: 2813. https://doi.org/10.3390/ijms26062813
APA StyleZhang, X., Ye, J., Sun, L., Xu, W., He, X., Bao, J., & Wang, J. (2025). NCKAP1 Inhibits the Progression of Renal Carcinoma via Modulating Immune Responses and the PI3K/AKT/mTOR Signaling Pathway. International Journal of Molecular Sciences, 26(6), 2813. https://doi.org/10.3390/ijms26062813







