A TMT-Based Proteomic Analysis of Osmoregulation in the Gills of Oreochromis mossambicus Exposed to Three Osmotic Stresses
Abstract
1. Introduction
2. Results
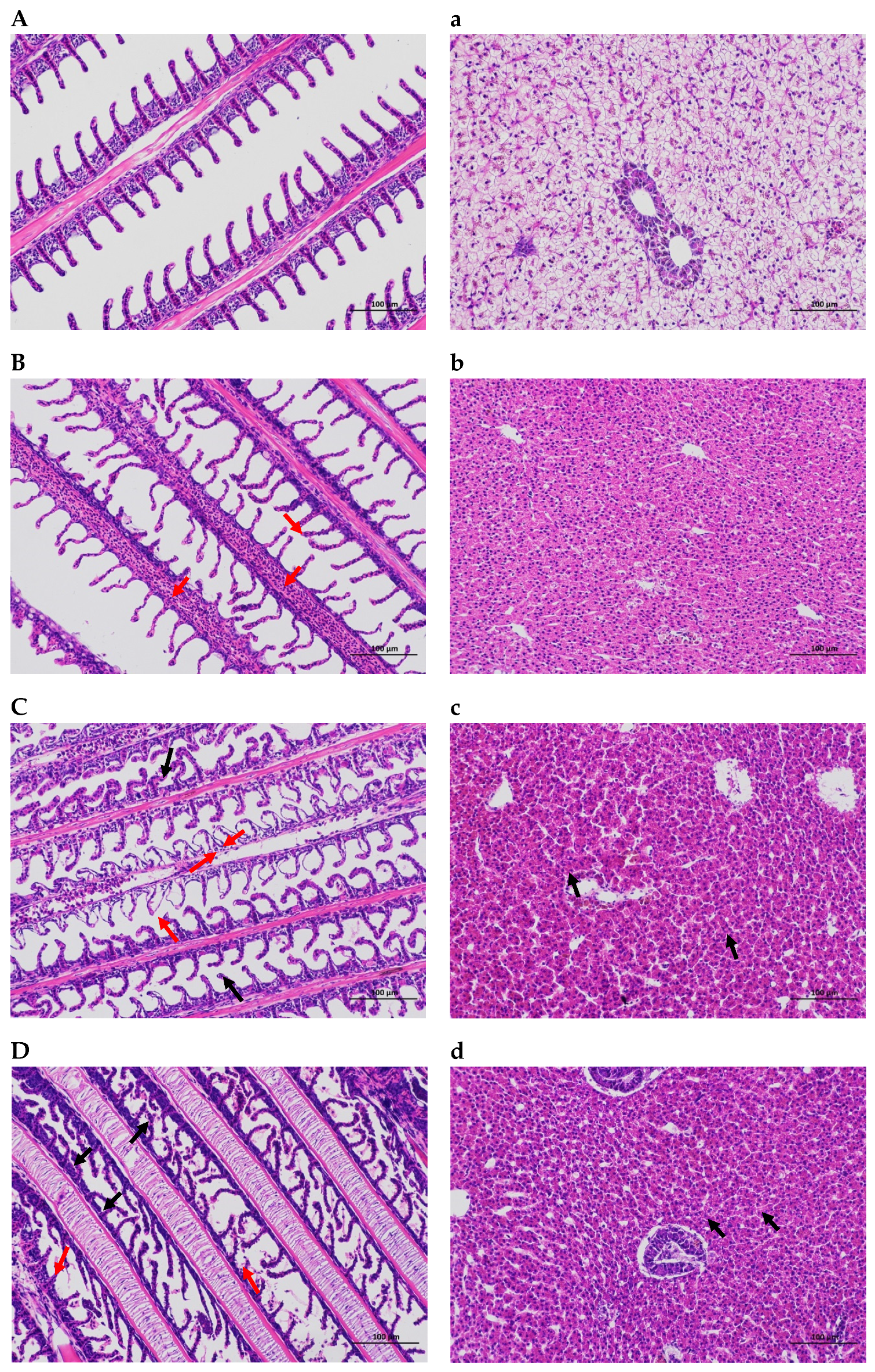
2.1. Gill and Hepatic Histological
2.2. Biochemical Activity Analysis
2.3. Proteomic Profiles in the Gills Under Osmotic Stress
2.3.1. Proteomic Profiles in the Gills Under Salinity Stress
2.3.2. Proteomic Profiles in the Gills Under Alkalinity Stress
2.3.3. Proteomic Profiles in the Gills Under Saline–Alkalinity Stress
2.4. Compared Proteomic Differences Between Osmotic Stresses
2.5. PRM Validation
2.6. Osmoregulation Genes Expression
2.7. Observation on the Apoptosis of Branchial Tissue Cells
3. Discussions
3.1. Histologic Changes
3.2. Oxidative Stress Changes
3.3. Gill Proteomics in Response to Osmotic Stresses
3.3.1. DEPs Connected to Osmoregulation
3.3.2. Osmoregulation-Related Pathways
4. Methods and Materials
4.1. Animals, Stress Exposure, and Sample Collection
4.2. Histomorphological Observation and Antioxidant Enzyme Analysis
4.3. Proteomic Analysis
4.3.1. Proteins Extraction and Digestion
4.3.2. TMT Labeling and Separation of Fractions
4.3.3. High-Performance Liquid Chromatography Analysis
4.3.4. Identification and Quantification of Proteins
4.3.5. Functional Analysis of Proteins and DEPs
4.4. Verification of Protein Identification by Parallel Reaction Monitoring (PRM)
4.5. qPCR Analysis of Key Gene Expressions
4.6. Tunel Staining
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, T.G.; Kültz, D. The cellular stress response in fish exposed to salinity fluctuations. J. Exp. Zool. Part A: Ecol. Integr. Physiol. 2020, 333, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Surendran, S.N. Global climate change and its potential impact on disease transmission by salinity-tolerant mosquito vectors in coastal zones. Front. Physiol. 2012, 3, 198. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Jiao, J.J. Seawater intrusion and coastal aquifer management in China: A review. Environ. Earth Sci. 2014, 72, 2811–2819. [Google Scholar] [CrossRef]
- Huang, M.; Dong, Y.; Zhang, Y.; Chen, Q.; Xie, J.; Xu, C.; Zhao, Q.; Li, E. Growth and lipidomic responses of juvenile Pacific white shrimp Litopenaeus vannamei to low salinity. Front. Physiol. 2019, 10, 1087. [Google Scholar] [CrossRef]
- Lu, Z.; Huang, W.; Wang, S.; Shan, X.; Ji, C.; Wu, H. Liver transcriptome analysis reveals the molecular responses to low-salinity in large yellow croaker Larimichthys crocea. Aquaculture 2020, 517, 734827. [Google Scholar] [CrossRef]
- Xiong, Y.; Dong, S.; Huang, M.; Li, Y.; Wang, X.; Wang, F.; Ma, S.; Zhou, Y. Growth, osmoregulatory response, adenine nucleotide contents, and liver transcriptome analysis of steelhead trout (Oncorhynchus mykiss) under different salinity acclimation methods. Aquaculture 2020, 520, 734937. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Y.; Liu, Y.; Qiao, F.; Chen, L.; Liu, W.T.; Du, Z.; Li, E. Response of gut microbiota to salinity change in two euryhaline aquatic animals with reverse salinity preference. Aquaculture 2016, 454, 72–80. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Qin, J.G.; Wang, X.; Chen, L.; Xu, C.; Li, E. Dietary prebiotic inulin benefits on growth performance, antioxidant capacity, immune response and intestinal microbiota in Pacific white shrimp (Litopenaeus vannamei) at low salinity. Aquaculture 2020, 518, 734847. [Google Scholar] [CrossRef]
- Nakamura, K.; Iwaizumi, K.; Yamada, S. Hemolymph patterns of free amino acids in the brine shrimp Artemia franciscana after three days starvation at different salinities. Comp. Biochem. Physiol. A 2007, 147, 254–259. [Google Scholar] [CrossRef]
- McFarland, K.; Ludovic, D.; Aswani, K.V. Effect of acute salinity changes on hemolymph osmolality and clearance rate of the nonnative mussel, Perna viridis, and the native oyster, Crassostrea virginica, in Southwest Florida. Aquat. Invasions 2013, 8, 299–310. [Google Scholar] [CrossRef]
- Chong-Robles, J.; Charmantier, G.; Boulo, V.; Lizárraga-Valdéz, J.; Enríquez-Paredes, L.; Giffard-Mena, I. Osmoregulation pattern and salinity tolerance of the white shrimp Litopenaeus vannamei (Boone, 1931) during post-embryonic development. Aquaculture 2014, 422, 261–267. [Google Scholar] [CrossRef]
- Yang, W.K.; Chung, C.H.; Hui, C.C.; Tang, C.H.; Lee, T.H. Different expression patterns of renal Na(+)/K(+)-ATPaseα-isoform-like proteins between tilapia and milkfish following salinity challenges. Comp. Biochem. Physiol. B 2016, 202, 23–30. [Google Scholar] [CrossRef]
- Yao, Z.; Lai, Q.; Zhou, K.; Rizalita, R.E.; Wang, H. Developmental biology of medaka fish (Oryzias latipes) exposed to alkalinity stress. J. Appl. Ichthyol. 2010, 26, 397–402. [Google Scholar] [CrossRef]
- Saha, N.; Kharbuli, Z.Y.; Bhattacharjee, A.; Goswami, C.; Häussingerb, D. Effect of alkalinity (pH 10) on ureogenesis in the air-breathing walking catfish, Clarias batrachus. Comp. Biochem. Physiol. Part A 2002, 132, 353–364. [Google Scholar] [CrossRef]
- Velasco-Santamaría, Y.M.; Cruz-Casallas, P.E. Behavioural and gill histopathological effects of acute exposure to sodium chloride in moneda (Metynnis orinocensis). Environ. Toxicol. Pharmacol. 2008, 25, 365–372. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Zhang, P.; Liu, J.; Wang, B.; Bu, X.; Wei, Q.Y.; Liu, S.J.; Li, Y.H. Effects of dietary supplementation with Bacillus subtilis on immune, antioxidant, and histopathological parameters of Carassius auratus gibelio juveniles exposed to acute saline-alkaline conditions. Aquacult. Int. 2022, 30, 2295–2310. [Google Scholar] [CrossRef]
- Su, H.H.; Ma, D.M.; Zhu, H.P.; Liu, Z.G.; Gao, F.Y. Transcriptomic response to three osmotic stresses in gills of hybrid tilapia (Oreochromis mossambicus female × O. urolepis hornorum male). BMC Genom. 2020, 2020, 110. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G.B.N. Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to changing salinity. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Long, X.W.; Wu, X.G.; Zhao, L.; Ye, H.; Zeng, C. Physiological responses and ovarian development of female Chinese mitten crab Eriocheir sinensis subjected to different salinity conditions. Front. Physiol. 2018, 8, 1072. [Google Scholar] [CrossRef]
- Chang, C.H.; Mayer, M.; Rivera-Ingraham, G.; Blondeau-Bidet, E.; Wu, W.Y.; Lorin-Nebel, C.; Lee, T.H. Effects of temperature and salinity on antioxidant responses in livers of temperate (Dicentrarchus labrax) and tropical (Chanos Chanos) marine euryhaline fish. J. Therm. Biol. 2021, 99, 103016. [Google Scholar] [CrossRef]
- Mozanzadeh, O.S.; Rahim, O.; Shapour, M.; Mojtaba, Z.N.; Seyed, J.H.; Hamid, S.; Javad, M. The effect of salinity on growth performance, digestive and antioxidant enzymes, humoral immunity and stress indices in two euryhaline fish species: Yellowfin seabream (Acanthopagrus latus) and Asian seabass (Lates calcarifer). Aquaculture 2021, 2021, 736329. [Google Scholar] [CrossRef]
- Tian, L.; Tan, P.; Yang, L.; Zhu, W.L.; Xu, D.D. Effects of salinity on the growth, plasma ion concentrations, osmoregulation, non-specific immunity, and intestinal microbiota of the yellow drum (Nibea albiflora). Aquaculture 2020, 528, 735470. [Google Scholar] [CrossRef]
- Martínez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Shin, H.S.; Yoo, J.H.; Min, T.S.; Lee, K.Y.; Choi, C.Y. The effects of quercetin on physiological characteristics and oxidative stress resistance in olive flounder, Paralichthys olivaceus. Asian-Australas. Assoc. Anim. Prod. Soc. 2010, 23, 588–597. [Google Scholar] [CrossRef]
- Ghanavatinas, Y.; Salati, A.P.; Movahedini, A.; Shahriari, A. Changes in gill antioxidant status in Acanthopagrus sheim exposed to different environmental salinities. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 1479–1483. [Google Scholar] [CrossRef]
- Long, X.W.; Wu, X.G.; Zhao, L.; Liu, J.G.; Cheng, Y.G. Effects of dietary supplementation with Haematococcus pluvialis cell powder on coloration, ovarian development and antioxidation capacity of adult female Chinese mitten crab, Eriocheir sinensis. Aquaculture 2017, 473, 545–553. [Google Scholar] [CrossRef]
- Zhu, H.P.; Liu, Z.G.; Gao, F.Y.; Lu, M.X.; Liu, Y.J.; Su, H.H.; Ma, D.M.; Ke, X.L.; Wang, M.; Cao, J.M.; et al. Characterization and expression of Na+/K+-ATPase in gills and kidneys of the Teleost fish Oreochromis mossambicus, Oreochromis urolepis hornorum and their hybrids in response to salinity challenge. Comp. Biochem. Physiol. Part A 2018, 224, 1–10. [Google Scholar] [CrossRef]
- Bu, X.Y.; Zhu, J.H.; Liu, S.B.; Wang, C.L.; Xiao, S.S.; Lu, M.X.; Li, E.C.; Wang, X.D.; Qin, J.G.; Chen, L.Q. Growth, osmotic response and transcriptome response of the euryhaline teleost, Oreochromis mossambicus fed different myo-inositol levels under long-term salinity stress. Aquaculture 2021, 534, 736294. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Li, Y.M.; Sepúlveda, M.S.; Jiang, Q.C.; Jiao, Y.; Chen, Q.; Huang, Y.Y.; Tian, J.T.; Zhao, Y.L. Development of an adverse outcome pathway for nanoplastic toxicity in Daphnia pulex using proteomics. Sci. Total Environ. 2021, 766, 144249. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, X.Y.; Hu, Y.D.; Xu, J.J.; Wang, T.; Yin, S.W. iTRAQ-based quantitative proteomic analysis of Takifugu fasciatus liver in response to low-temperature stress. J. Proteom. 2019, 201, 27–36. [Google Scholar] [CrossRef]
- Ghisaura, S.; Pagnozzi, D.; Melis, R.; Biosa, G.; Slawski, H.; Uzzau, S.; Anedda, R.; Addis, M.F. Liver proteomics of gilthead sea bream (Sparus aurata) exposed to cold stress. J. Therm. Biol. 2019, 82, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Magouz, F.I.; Mahmoud, S.A.; El-Morsy, R.A.; Paray, B.A.; Soliman, A.A.; Zaineldin, A.I.; Dawood, M. Dietary menthol essential oil enhanced the growth performance, digestive enzyme activity, immune-related genes, and resistance against acute ammonia exposure in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 530, 735944. [Google Scholar] [CrossRef]
- Li, S.; Wang, R.; Dai, Z.; Wang, C.; Wu, Z. Dietary supplementation with Yucca schidigera extract alleviated heat stress-induced unfolded protein response and oxidative stress in the intestine of Nile tilapia (Oreochromis niloticus). Ecotoxicol. Environ. Saf. 2021, 219, 112299. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Pena, E.; Martínez, R.; Camarillo, S.; Burggren, W.; Alvarez, A. Genomic profiling in non-small-cell lung cancer in young patients. A systematic review. ESMO Open 2021, 6, 100045. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Metwally, A.E.S.; El-Sharawy, M.E.; Ghozlan, A.M.; Abdel-Latif, H.M.R.; Van Doan, H.; Ali, M.A.M. The influences of ferulic acid on the growth performance, haemato-immunological responses, and immune-related genes of Nile tilapia (Oreochromis niloticus) exposed to heat stress. Aquaculture 2020, 525, 735320. [Google Scholar] [CrossRef]
- Sachi, I.; Bonomo, M.M.; Sakuragui, M.M.; Modena, P.Z.; Paulino, M.G.; Carlos, R.M.; Fernandes, J.B.; Fernandes, M.N. Biochemical and morphological biomarker responses in the gills of a Neotropical fish exposed to a new flavonoid metalinsecticide. Ecotoxicol. Environ. Saf. 2021, 208, 111459. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, X.Q.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Feng, L. Dietary phosphorus deficiency caused alteration of gill immune and physical barrier function in the grass carp (Ctenopharyngodon idella) after infection with Flavobacterium columnare. Aquaculture 2019, 506, 1–13. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Noreldin, A.E.; Sewilam, H. Long term salinity disrupts the hepatic function, intestinal health, and gills antioxidative status in Nile tilapia stressed with hypoxia. Ecotoxicol. Environ. Saf. 2021, 220, 112412. [Google Scholar] [CrossRef]
- Harper, C.; Wolf, J.C. Morphologic effects of the stress response in fish. ILAR J. 2009, 50, 387–396. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, H.; Wang, H.; Ren, Y.; Zhao, J.; Li, Y. RNA-Seq analysis of salinity stress-responsive transcriptome in the liver of spotted sea bass (Lateolabrax maculatus). PLoS ONE 2017, 12, e0173238. [Google Scholar] [CrossRef]
- Si, Y.F.; Wen, H.S.; Li, Y.; He, F.; Li, J.F.; Li, S.P.; He, H.W. Liver transcriptome analysis reveals extensive transcriptional plasticity during acclimation to low salinity in Cynoglossus semilaevis. BMC Genom. 2018, 19, 464. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, J.; Yan, M.; Tang, S.; Wang, X.; Qin, J.G.; Chen, L.; Li, E. Inulin alleviates hypersaline-stress induced oxidative stress and dysbiosis of gut microbiota in Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 529, 735681. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Saad, M.F.; Shukry, M.; El-Keredy, A.M.; Nasif, O.; Van Doan, H.; Dawood, M.A. Physiological and ion changes of Nile tilapia (Oreochromis niloticus) under the effect of salinity stress. Aquac. Rep. 2021, 19, 100567. [Google Scholar] [CrossRef]
- Sun, J.L.; Zhao, L.L.; Liao, L.; Tang, X.H.; Cui, C.; Liu, Q.; He, K.; Ma, J.D.; Jin, L.; Yan, T.; et al. Interactive effect of thermal and hypoxia on largemouth bass (Micropterus salmoides) gill and liver: Aggravation of oxidative stress, inhibition of immunity and promotion of cell apoptosis. Fish. Shellfish Immunol. 2020, 98, 923–936. [Google Scholar] [CrossRef]
- Li, H.L.; Lin, H.R.; Xia, J.H. Differential gene expression profiles and alternative isoform regulations in gill of Nile tilapia in response to acute hypoxia. Mar. Biotechnol. 2017, 19, 551–562. [Google Scholar] [CrossRef]
- Xu, C.; Li, E.; Suo, Y.; Su, Y.; Lu, M.; Zhao, Q.; Qin, J.G.; Chen, L. Histological and transcriptomic responses of two immune organs, the spleen and head kidney, in Nile tilapia (Oreochromis niloticus) to long-term hypersaline stress. Fish Shellfish Immunol. 2018, 76, 48–57. [Google Scholar] [CrossRef]
- Campa, C.; Hernandez, S.; Ascencio, F. Superoxide dismutase as modulator of immune function in American white shrimp (Litopenaeus vannamei). Comp. Biochem. Physiol. 2002, 133, 557–565. [Google Scholar] [CrossRef]
- Jia, R.; Li, Y.; Cao, L.; Du, J.; Zheng, T.; Qian, H.; Gu, Z.; Jeney, G.; Xu, P.; Yin, G. Antioxidative, anti-inflammatory and hepatoprotective effects of resveratrol on oxidative stress-induced liver damage in tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 215, 56–66. [Google Scholar] [CrossRef]
- Xie, J.; Dong, W.; Liu, R.; Wang, Y.; Li, Y. Research on the hepatotoxicity mechanism of citrate-modified silver nanoparticles based on metabolomics and proteomics. Nanotoxicology 2018, 12, 18–31. [Google Scholar] [CrossRef]
- Xiang, Q.Q.; Gao, Y.; Li, Q.Q.; Ling, J.; Chen, L.Q. Proteomic profiling reveals the differential toxic responses of gills of common carp exposed to nanosilver and silver nitrate. J. Hazard. Mater. 2020, 394, 122562. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Wang, L.; Zhou, J.; Zou, J.X.; Fan, L.F. New insights into the immune regulation and tissue repair of Litopenaeus vannamei during temperature fluctuation using TMT-based proteomics. Fish Shellfish Immunol. 2020, 106, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Lambert, I.H.; Hoffmann, E.K.; Pedersen, S.F. Cell volume regulation: Physiology and pathophysiology. Acta Physiol. 2008, 194, 255–282. [Google Scholar] [CrossRef]
- Jakab, R.L.; Collaco, A.M.; Ameen, N.A. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G82–G98. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.P.; Cramb, G. Branchial expression of an aquaporin 3 (AQP-3) homologue is downregulated in the European eel Anguilla anguilla following seawater acclimation. J. Exp. Biol. 2002, 205, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.R.; Richards, J.G.; Forbush, B.; Isenring, P.; Schulte, P.M. Changes in gene expression in gills of the euryhaline killifish Fundulus heteroclitus after abrupt salinity transfer. Am. J. Phys. 2004, 287, 300–309. [Google Scholar] [CrossRef]
- Tipsmark, C.K.; Madsen, S.S.; Borski, R.J. Effect of salinity on expression of branchial ion transporters in striped bass (Morone saxatilis). J. Exp. Zool. 2004, 301A, 979–991. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Z.; Wang, C.; Li, E.; Qin, J.G.; Chen, L. Dietary supplementation of selenium yeast enhances the antioxidant capacity and immune response of juvenile Eriocheir Sinensis under nitrite stress. Fish Shellfish Immunol. 2019, 87, 22–31. [Google Scholar] [CrossRef]
- Ai, L.; Shit, C.; Wai, W.; Siew, L.; Yuen, I. Both seawater acclimation and environmental ammonia exposure lead to increases in mRNA expression and protein abundance of Na:K:2Cl cotransporter in the gills of the climbing perch, Anabas testudineus. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2012, 182, 491–506. [Google Scholar] [CrossRef]
- Flemmer, A.W.; Monette, M.Y.; Djurisic, M.; Dowd, B.; Darman, R.; Gimenez, I.; Forbush, B. Phosphorylation state of the Na-K-Cl- cotransporter (NKCC1) in the gills of Atlantic killifish (Fundulus heteroclitus) during acclimation to water of varying salinity. J. Exp. Biol. 2010, 213, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, C.; McCormick, S.D. Functional characterization and osmoregulatory role of the Na-K-2Cl cotransporter in the gill of sea lamprey (Petromyzon marinus), a basal vertebrate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R17–R29. [Google Scholar] [CrossRef]
- Tipsmark, C.K.; Madsen, S.S.; Ceidelin, M.; Christensen, A.S.; Cutler, C.P.; Cramb, G. Dynamics of Na+, K+, 2Cl− cotransporter and Na+, K+-ATPase expression in the branchial epithelium of brown trout (Salmo trutta) and Atlantic salmon (Salmo salar). J Exp Zool. 2002, 293, 106–118. [Google Scholar] [CrossRef]
- Seale, A.P.; Watanabe, S.; Grau, E.G. Osmoreception: Perspectives on signal transduction and environmental modulation. Gen. Comp. Endocrinol. 2012, 176, 354–360. [Google Scholar] [CrossRef]
- Giffard-Mena, I.; Boulo, V.; Aujoulat, F.; Fowden, H.; Castille, R.; Charmantier, G.; Cramb, G. Aquaporin molecular characterization in the sea-bass (Dicentrarchus labrax): The effect of salinity on AQP1 and AQP3 expression. Comp. Biochem. Physiol. A 2007, 148, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Tse, W.K.F.; Au, D.W.T.; Wong, C.K.C. Characterization of ion channel and transporter mRNA expressions in isolated gill chloride and pavement cells of seawater acclimating eels. Biochem. Biophys. Res. Commun. 2006, 346, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Tipsmark, C.K.; Sørensen, K.J.; Madsen, S.S. Aquaporin expression dynamics in osmoregulatory tissues of Atlantic salmon during smoltification and seawater acclimation. J. Exp. Biol. 2010, 213, 368–379. [Google Scholar] [CrossRef]
- Mobasheri, A.; Avila, J.; Cozar-Castellano, I.; Brownleader, M.D.; Trevan, M.; Francis, M.J.; Lamb, J.F.; Martin-Vasallo, P. Na+, K + -ATPase isozyme diversity; comparative biochemistry and physiological implications of novel functional interactions. Biosci. Rep. 2000, 20, 51–91. [Google Scholar] [CrossRef]
- Horisberger, J.D.; Kharoubi-Hess, S. Functional differences between a subunit isoforms of the rat Na,K-ATPase expressed in Xenopus oocytes. J. Physiol. 2002, 539, 669–680. [Google Scholar] [CrossRef]
- Feng, S.H.; Leu, J.H.; Yang, C.H.; Fang, M.J.; Huang, C.J.; Hwang, P.P. Gene expression of Na+-K+-ATPase a1 and a3 subunits in gills of the teleost Oreochromis mossambicus, adapted to different environmental salinities. Mar. Biotechnol. 2002, 4, 379–391. [Google Scholar] [CrossRef]
- Richards, J.G.; Semple, J.W.; Bystriansky, J.S.; Schulte, P.M. Na+/K+-ATPase α-isoform switching in gills of rainbow trout (Oncorhynchus mykiss) during salinity transfer. J. Exp. Biol. 2003, 206, 4475–4486. [Google Scholar] [CrossRef]
- Kang, C.K.; Tsai, S.C.; Lee, T.H.; Hwang, P.P. Differential expression of branchial Na+/K+-ATPase of two medaka species, Oryzias latipes and Oryzias dancena, with different salinity tolerances acclimated to fresh water, brackish water and seawater. Comp. Biochem. Physiol. A 2008, 151, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, T.O.; Ebbesson, L.O.E.; Madsen, S.S.; McCormick, S.D.; Andresson, E.; Björnsson, B.T.; Prunet, P.; Stefansson, S.O. Differential expression of gill Na+, K+−ATPase alpha- and beta-subunits, Na+, K+, 2Cl− cotransporter and CFTR anion channel in juvenile anadromous and landlocked Atlantic salmon Salmo salar. J. Exp. Biol. 2007, 210, 2885–2896. [Google Scholar] [CrossRef] [PubMed]
- Keinan, N.; Pahima, H.; Ben-Hail, D.; Varda, S.B. The role of Calcium in VDAC1 oligomerization and mitochondria-mediated apoptosis. Biochim. Biophys. Acta 2013, 1833, 1745–1754. [Google Scholar] [CrossRef]
- Hsing, L.C.; Rudensky, A.Y. The lysosomal cysteine proteases in MHC clas Iantigen presentation. Immunol. Rev. 2005, 207, 229–241. [Google Scholar] [CrossRef]
- Cai, X.; Gao, C.B.; Song, H.H.; Yang, N.; Fu, Q.; Tan, F.H.; Li, C. Characterization, expression profiling and functional characterization of cathepsin Z (CTSZ) in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2019, 84, 599–608. [Google Scholar] [CrossRef]
- Choi, K.M.; Joo, M.S.; Cho, D.H.; Hyun-Ja, H.; Myoung, S.K.; Mi, Y.C.; Sung, H.; Do-Hyung, K.; Chan-Il, P. Functional analysis and gene expression profiling of extracellular cathepsin Z in red sea bream, Pagrus major. Fish Shellfish Immunol. 2019, 93, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.D.; Zhang, J.; Ji, X.S.; Zhou, F.N.; Fu, Y. Molecular cloning, characterization and expression of cathepsin D from grass carp (Ctenopharyn godonidella). Fish Shellfish Immunol. 2012, 33, 1207–1214. [Google Scholar] [CrossRef]
- Kao, C.M.; Huang, F.L. Cloning and expression of carp cathepsin Z: Possible involvement in yolk metabolism. Comp. Biochem. Physiol. B 2008, 149, 541–551. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Breves, J.P.; Haws, M.C.; Lerner, D.T.; Grau, E.G.; Seale, A.P. Acute salinity tolerance and the control of two prolactins and their receptors in the Nile tilapia (Oreochromis niloticus) and Mozambique tilapia (O. mossambicus): A comparative study. Gen. Comp. Endocrinol. 2018, 257, 168–176. [Google Scholar] [CrossRef]
- Lockwood, B.L.; Somero, G.N. Transcriptomic responses to salinity stress in invasive and native blue mussels (genus mytilus). Mol. Ecol. 2011, 20, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, H.; Kong, L.; Liu, S.; Li, Q. High throughput sequencing of small RNAs transcriptomes in two Crassostrea oysters identifies microRNAs involved in osmotic stress response. Sci. Rep. 2016, 6, 22687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, H.; Kong, L.; Li, Q. Transcriptomic responses to salinity stress in the pacific oyster Crassostrea gigas. PLoS ONE 2012, 7, e46244. [Google Scholar] [CrossRef]
- Alexander, R.T.; Dimke, H.; Cordat, E. Proximal tubular NHEs: Sodium, protons and calcium? Ren. Physiol. 2013, 305, F229–F236. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.T.; Jiang, L.W.; Chen, P.; Huo, Z.M.; Yang, F.; Yan, X.W. High throughput sequencing of RNA transcriptomes in Ruditapes philippinarum identifies genes involved in osmotic stress response. Sci. Rep. 2017, 7, 4953. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Fiol, D.F.; Kültz, D. Osmotic stress sensing and signaling in fishes. FEBS J. 2007, 274, 5790–5798. [Google Scholar] [CrossRef]
- Kültz, D.; Avila, K. Mitogen-activated protein kinases are in vivo transducers of osmosensory signals in fish gill cells. Comp. Biochem. Physiol. A 2001, 129, 821–829. [Google Scholar] [CrossRef]
- Evans, T.E.; Somero, G.N. Phosphorylation events catalyzed by major cell signaling proteins differ in response to thermal and osmotic stress among native (Mytilus californianus and Mytilus trossulus) and invasive (Mytilus galloprovincialis) species of mussels. Physiol. Biochem. Zool. 2010, 83, 984–996. [Google Scholar] [CrossRef]
- Senior, A.E. ATP synthesis by oxidative phosphorylation. Physiol. Rev. 1988, 68, 177e231. [Google Scholar] [CrossRef]
- Guzy, R.D.; Hoyos, B.; Robin, E.; Chen, H.; Liu, L.P.; Mansfield, K.D.; Celeste Simon, M.; Hammerling, U.; Schumacker, P.T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metabol. 2005, 1, 401e408. [Google Scholar] [CrossRef] [PubMed]
- Renate, P.; Barat, I.; Anna, G.; Faulhammer, P.; Rose, F.; Weissmann, N.; Braun-Dullaeus, R.C.; Kummer, W. Essential role of complex II of the respiratory chain in hypoxia-induced ROS generation in the pulmonary vasculature. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L710eL719. [Google Scholar] [CrossRef]
- Daichi, O.; Ryota, I.; Hiroyuki, N. Rotation and structure of FoF1-ATP synthase. J. Biochem. 2011, 149, 655–664. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; He, Y.; Wang, L. Mitochondrial energy metabolism in the hepatopancreas of freshwater crabs (Sinopotamon henanense) after cadmium exposure. Environ. Sci. Process. Impacts 2015, 17, 156e165. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Yang, F.F.; Ling, R.Z.; Liao, S.A.; Miao, Y.T.; Ye, C.X.; Wang, A.L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Xu, Z.E.; Zhang, X.Y.; Zhu, C.C.; Wang, Y.N.; Min, W.P. Cadmium triggers kidney cell apoptosis of purse red common carp (Cyprinus carpio) without caspase-8 activation. Dev. Comp. Immunol. 2013, 41, 728e737. [Google Scholar] [CrossRef]
- Yang, C.; Lin, X.W.; Xu, W.H. Cathepsin L participates in the remodeling of the midgut through dissociation of midgut cells and activation of apoptosis via caspase-1. Insect Biochem. Mol. Biol. 2017, 82, 21e30. [Google Scholar] [CrossRef]
- Ye, L.; Jiang, S.; Zhu, X.; Yang, Q.; Wen, W.; Wu, K. Effects of salinity on growth and energy budget of juvenile Penaeus monodon. Aquaculture 2009, 290, 140–144. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Hwang, P.P. Some insights into energy metabolism for osmoregulation in fish. Comp. Biochem. Physiol. C 2008, 148, 419–429. [Google Scholar] [CrossRef]
- Setiarto, A.; Augusto Strüssmann, C.; Takashima, F.; Watanabe, S.; Yokota, M. Short term responses of adult kuruma shrimp Marsupenaeus japonicus (Bate) to environmental salinity: Osmotic regulation, oxygen consumption and ammonia excretion. Aquac. Res. 2004, 35, 669–677. [Google Scholar] [CrossRef]
- Prangnell, D.I.; Fotedar, R. Effect of sudden change in potassium concentration on Penaeus latisulcatus Kishinouye survival, osmolality and health in inland saline water cultures. Hydrobiologia 2009, 626, 145–153. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Osmoregulation in decapod crustaceans: Implications to aquaculture productivity, methods for potential improvement and interactions with elevated ammonia exposure. Aquaculture 2012, 334, 12–23. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional sh gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.P.; Lee, T.H. New insights into sh ion regulation and mitochondrion-rich cells. Comp. Biochem. Physiol. A 2007, 148, 479–497. [Google Scholar] [CrossRef]
- Su, H.H.; Li, Y.Y.; Ma, D.M.; Fan, J.J.; Zhong, Z.X.; Zhu, H.P. Metabolism responses in the intestine of Oreochromis mossambicus exposed to salinity, alkalinity and salt-alkalinity stress using LC-MS/MS-based metabolomics. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 45, 101044. [Google Scholar] [CrossRef]
- Su, H.H.; Ma, D.M.; Fan, J.J.; Zhong, Z.X.; Li, Y.Y.; Zhu, H.P. Metabolism response mechanism in the gill of Oreochromis mossambicus under salinity, alkalinity and saline-alkalinity stresses. Ecotoxicol. Environ. Saf. 2023, 251, 114523. [Google Scholar] [CrossRef]
- Xu, C.; Li, E.; Liu, Y.; Wang, X.; Qin, J.G.; Chen, L. Comparative proteome analysis of the hepatopancreas from the Pacific white shrimp Litopenaeus vannamei under long-term low salinity stress. J. Proteome 2017, 162, 1–10. [Google Scholar] [CrossRef]
- Zhang, S.F.; Han, B.B.; Wu, F.X.; Huang, H.H. Quantitative proteomic analysis provides insights into the algicidal mechanism of Halobacillus sp. P1 against the marine diatom Skeletonema costatum. Sci. Total Environ. 2020, 717, 137048. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.Z.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2008, 37, 1–13. [Google Scholar] [CrossRef]









| Gene | GenBank Cod | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) | Length (bp) | Annealing Tm °C |
|---|---|---|---|---|---|
| Slc25a6 | XM_003445230.5 | GCGGGTAACCTGGCATCTGG | ATCAGCGGCGAGACGTGTTC | 93 | 60 |
| IDH2 | DQ465384.1 | ACAGACTTCCTCGACGCCAT | AGTGACATTGCCATCTTCGTGCT | 94 | 60 |
| ATP1α | XM_025903006.1 | ACTGTCATGGGCCGTATCGC | CCAGGAAGACGGCCACTCCA | 115 | 60 |
| ATP1β | XM_003454723.5 | TCTGGCTTGGAGGACACCGA | GATCACGTTGGGCTGCACCT | 153 | 60 |
| AQP3 | XM_013264600.3 | CCATTCCTTGGCGCCATTCT | CCTGCTTCTTGTCACGTGCT | 88 | 60 |
| Slc12a2 | XM_003444449.5 | GAGCCCGACAGTCCCTCTGA | CATCGCTCTGCGGTCGTGAT | 232 | 60 |
| CTSZ | XM_003441502.5 | GCTCATGCCACGGAGGAGAC | GTGGTGCAGGTGCCACATTG | 136 | 60 |
| CTSA | NM_001311320.1 | CGCTCGCTGAGAGGGTGATG | AGGCCGTGGTAGTAGGCGAA | 124 | 60 |
| VDAC2 | XM_003451964.5 | CCATCCACGGAGCTGCTGTC | ATGGAGCCGCCAAACTCTGC | 172 | 60 |
| VDAC1 | XM_003451434.5 | GGCTGGCTGGCTACCAGATG | CAGTCCAGGCCAGGTTGACG | 189 | 60 |
| CTSD | XM_003452585.5 | CGAGCATCTCCGTGGATGGG | CAGGAGCAGCTCACCACCAG | 131 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.; Ma, D.; Fan, J.; Zhong, Z.; Tian, Y.; Zhu, H. A TMT-Based Proteomic Analysis of Osmoregulation in the Gills of Oreochromis mossambicus Exposed to Three Osmotic Stresses. Int. J. Mol. Sci. 2025, 26, 2791. https://doi.org/10.3390/ijms26062791
Su H, Ma D, Fan J, Zhong Z, Tian Y, Zhu H. A TMT-Based Proteomic Analysis of Osmoregulation in the Gills of Oreochromis mossambicus Exposed to Three Osmotic Stresses. International Journal of Molecular Sciences. 2025; 26(6):2791. https://doi.org/10.3390/ijms26062791
Chicago/Turabian StyleSu, Huanhuan, Dongmei Ma, Jiajia Fan, Zaixuan Zhong, Yuanyuan Tian, and Huaping Zhu. 2025. "A TMT-Based Proteomic Analysis of Osmoregulation in the Gills of Oreochromis mossambicus Exposed to Three Osmotic Stresses" International Journal of Molecular Sciences 26, no. 6: 2791. https://doi.org/10.3390/ijms26062791
APA StyleSu, H., Ma, D., Fan, J., Zhong, Z., Tian, Y., & Zhu, H. (2025). A TMT-Based Proteomic Analysis of Osmoregulation in the Gills of Oreochromis mossambicus Exposed to Three Osmotic Stresses. International Journal of Molecular Sciences, 26(6), 2791. https://doi.org/10.3390/ijms26062791






