4-Hydroxycoumarin Exhibits Antinociceptive and Anti-Inflammatory Effects Through Cytokine Modulation: An Integrated In Silico and In Vivo Study
Abstract
1. Introduction
2. Results
2.1. In Silico Tests
2.2. In Vivo Tests
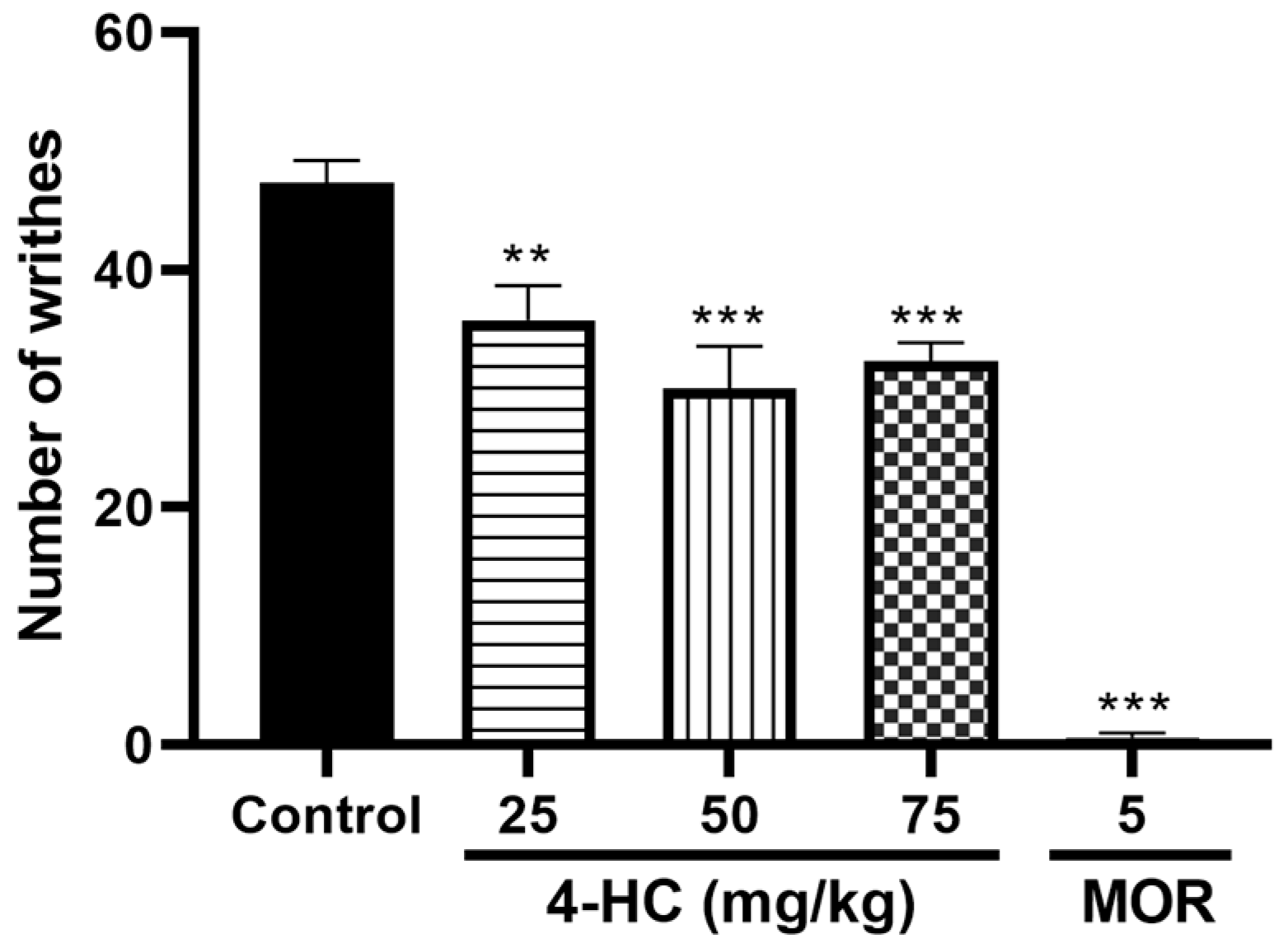
Effect of 4-HC on the Acetic Acid-Induced Writhing Protocol
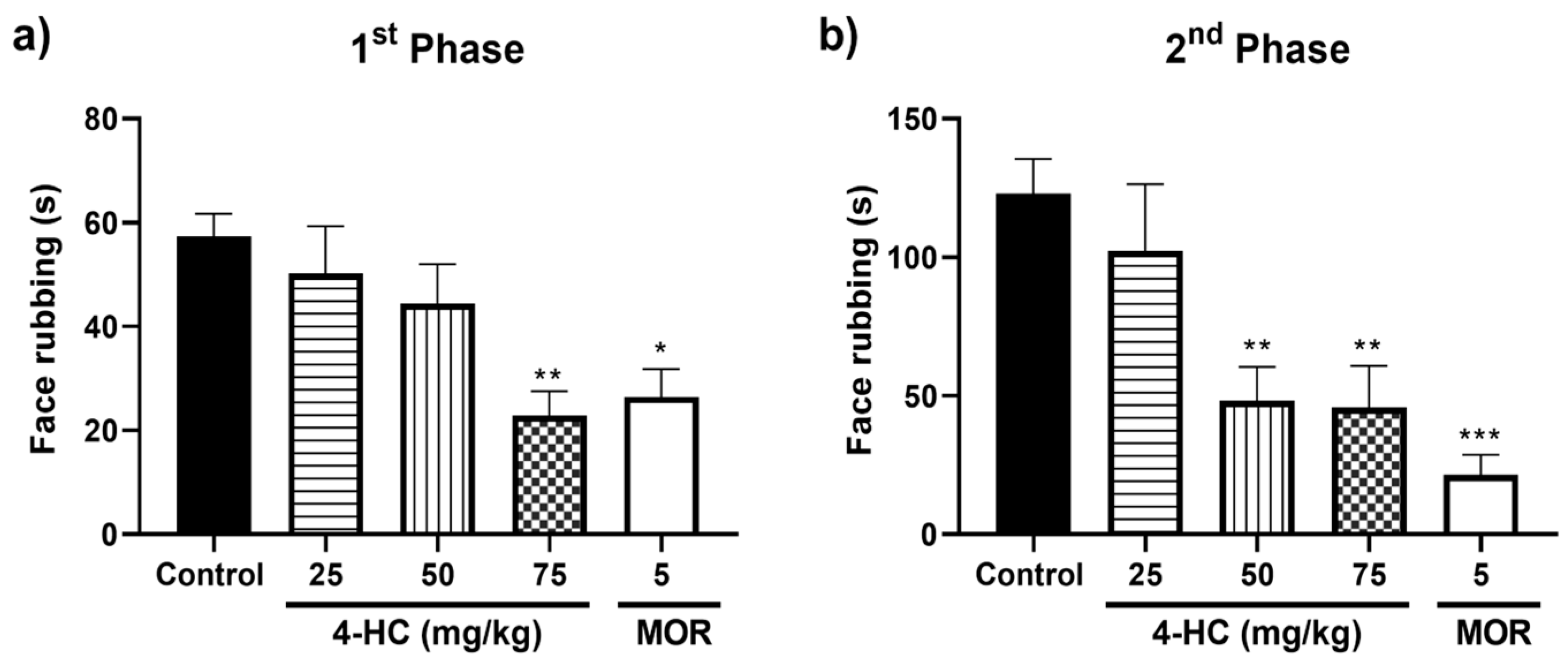
2.3. Effect of 4-HC in the Formalin-Induced Orofacial Nociception Protocol
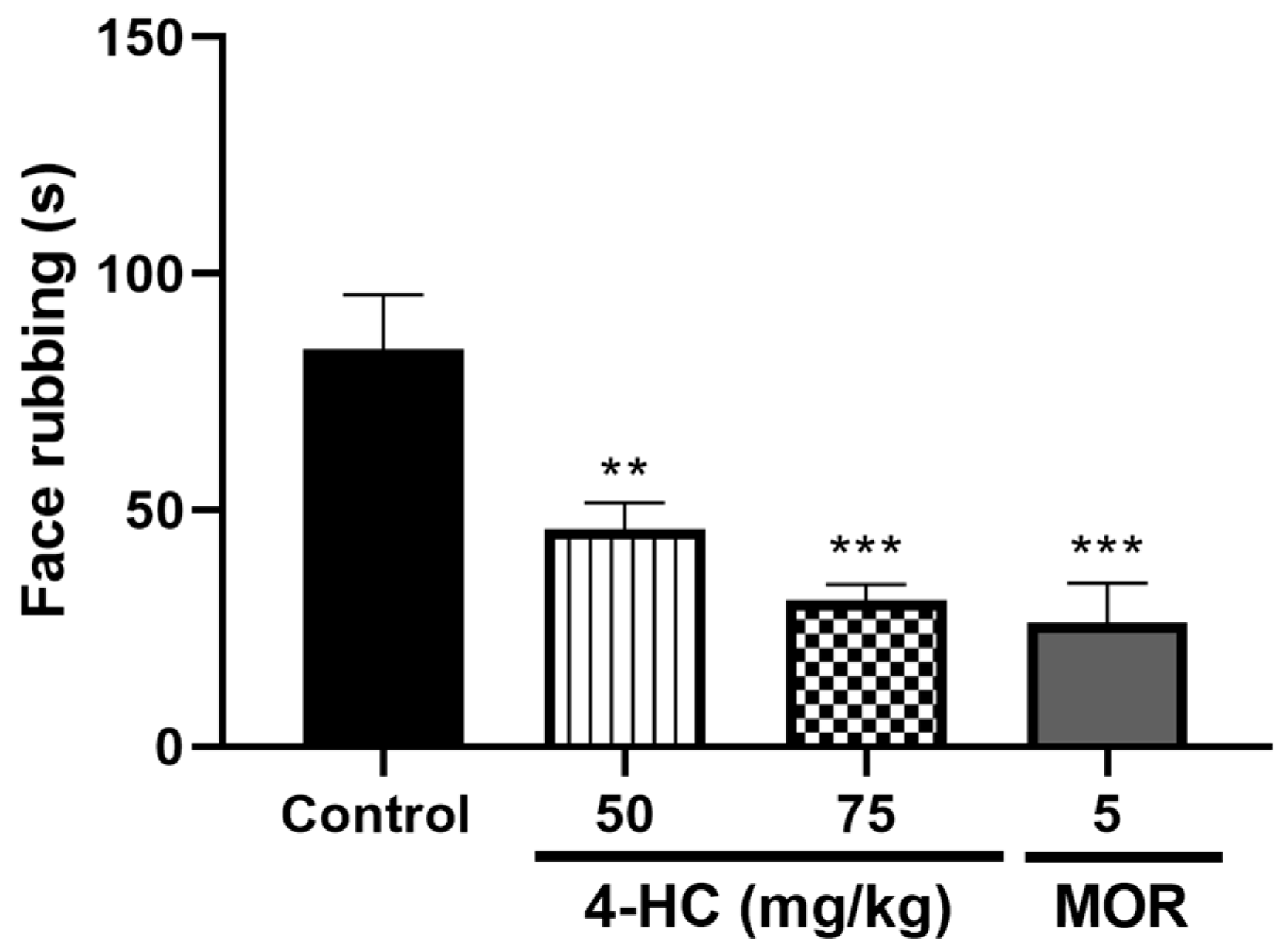
2.4. Effect of 4-HC in the Glutamate-Induced Orofacial Nociception Protocol
2.4.1. Effect of 4-HC on the Capsaicin-Induced Orofacial Nociception Protocol
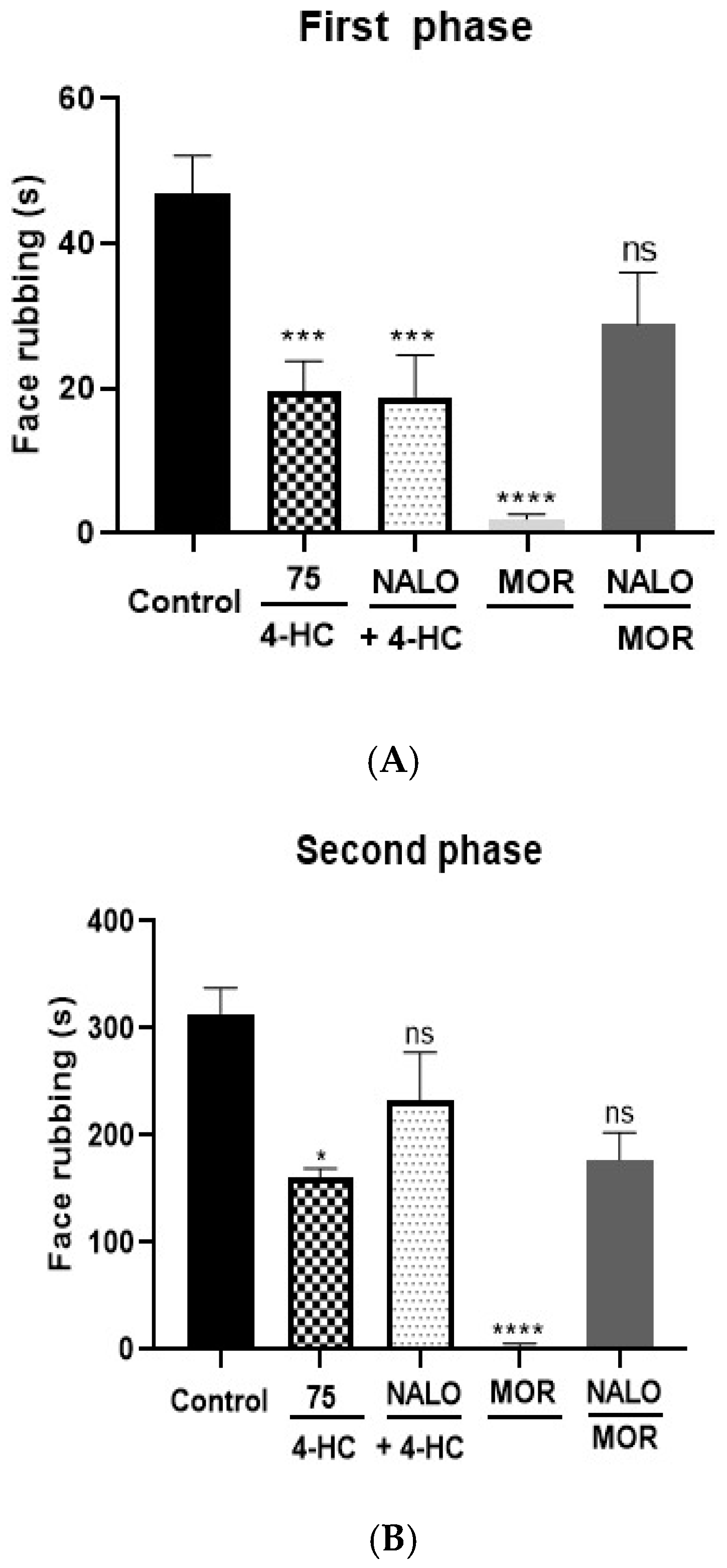
2.4.2. Opioid Receptors
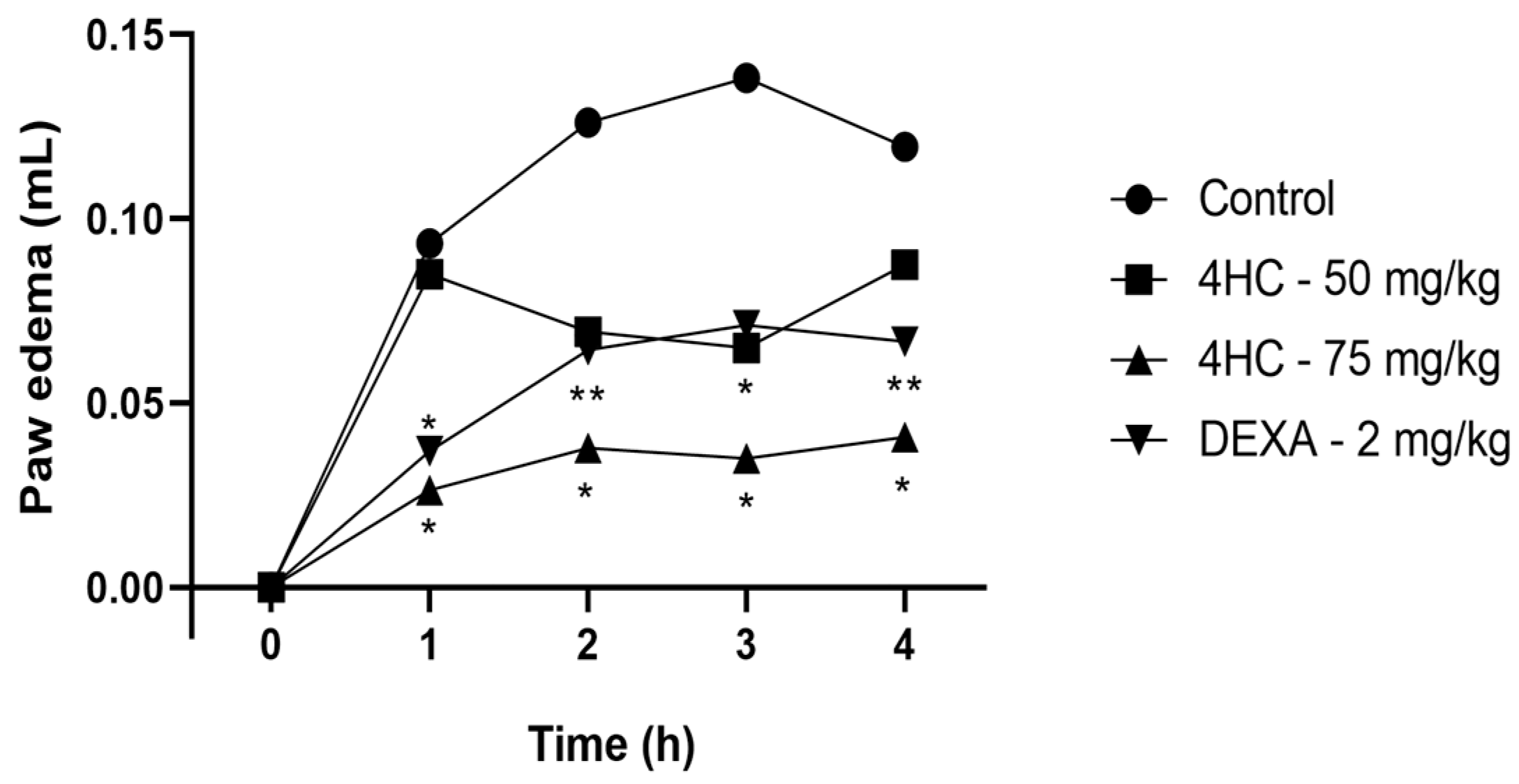
2.5. Carrageenan-Induced Paw Edema Test
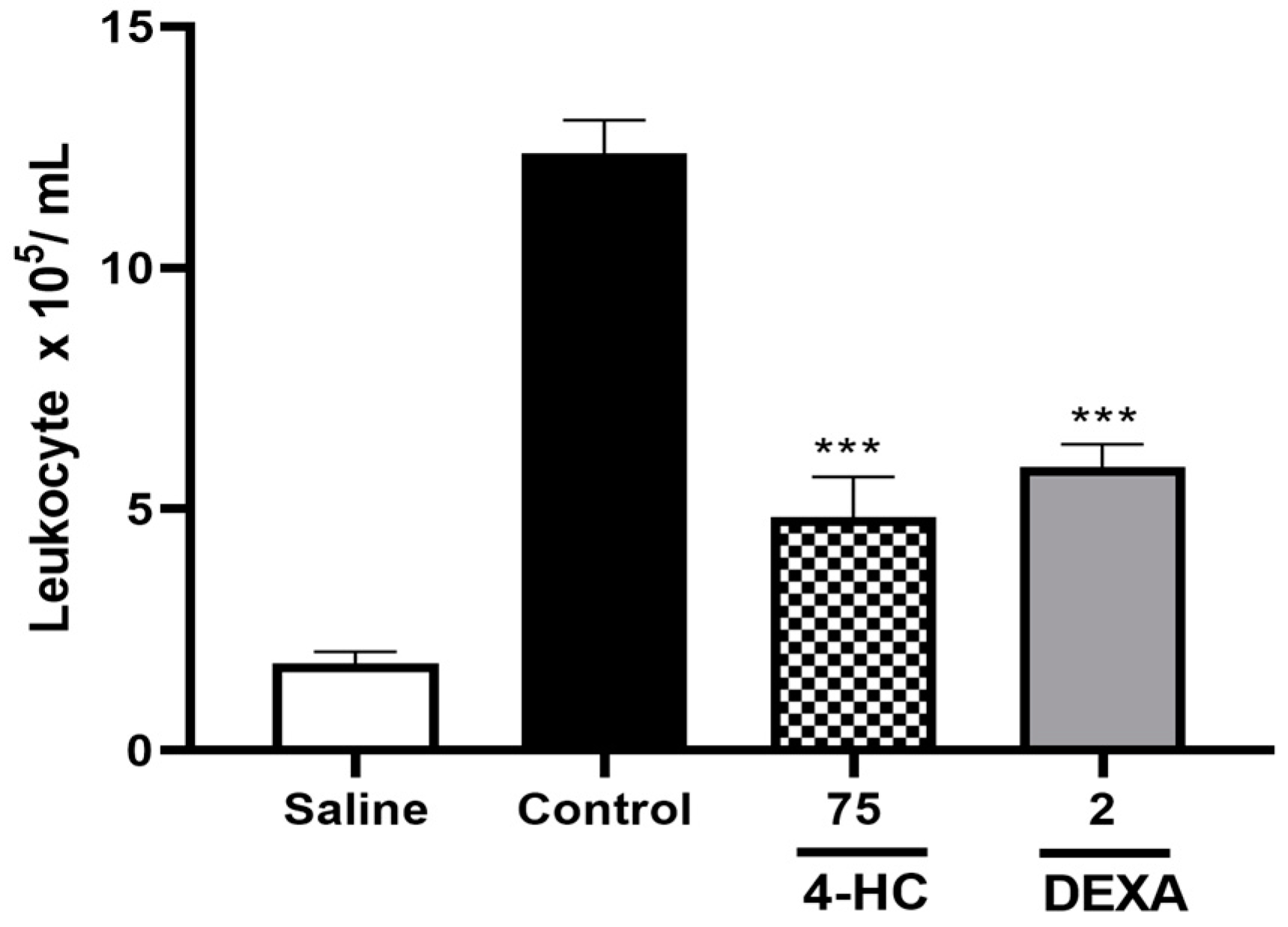
2.6. Leukocyte Count Test and Pro-Inflammatory Cytokine TNF-α Dosage
3. Discussion
4. Materials and Methods
4.1. Computational Studies
4.2. In Vivo Tests
4.2.1. Animals
4.2.2. Materials Preparation
4.2.3. Acetic Acid-Induced Abdominal Contortions Test
4.2.4. Formalin-Induced Orofacial Nociception Test
4.2.5. Glutamate-Induced Orofacial Nociception Test
4.2.6. Capsaicin-Induced Orofacial Nociception Test
4.2.7. Investigation of the Opioid System in the Orofacial Antinociceptive Activity of 4-Hydroxycoumarin
4.2.8. Evaluation of Anti-Inflammatory Activity Using the Carrageenan-Induced Paw Edema Protocol
4.2.9. Leukocyte Count Test and TNF-α Cytokine Dosage
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeSantana, J.M.; Perissinotti, D.M.N.; Oliveira Junior, J.O.D.; Correia, L.M.F.; Oliveira, C.M.D.; Fonseca, P.R.B.D. Definition of Pain Revised after Four Decades. Braz. J. Pain 2020, 3, 197–198. [Google Scholar] [CrossRef]
- Câmara-Gomes, L.F.; Dibai Filho, A.V.; Diniz, R.R.; Alvares, P.D.; Veneroso, C.E.; Cabido, C.E.T. Mechanisms of muscle stretching exercises for reduction of low back pain: Narrative review. Braz. J. Pain 2022, 5, 52–55. [Google Scholar] [CrossRef]
- Cavalcante, S.R.P.; Fernandes, H.B.; Santos, J.S. Uma análise sobre o potencial da cumarina e a sua utilização como fármaco natural. Res. Soc. Dev. 2022, 11, e269111637850. [Google Scholar] [CrossRef]
- Lamontain, V.; Schmid, T.; Weber-Steffens, D.; Zeller, D.; Jenei-Lanzl, Z.; Wajant, H.; Straub, R.H.; Männel, D.N. Stimulation of TNF Receptor Type 2 Expands Regulatory T Cells and Ameliorates Established Collagen-Induced Arthritis in Mice. Cell. Mol. Immunol. 2019, 16, 65–74. [Google Scholar] [CrossRef]
- Cioce, A.; Cavani, A.; Cattani, C.; Scopelliti, F. Role of the Skin Immune System in Wound Healing. Cells 2024, 13, 624. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.J.G.; Cardoso, I.P.; Oliveira, I.S.; Chaves, M.D.G.A.M.; Fabri, G.M.C. The Expression of the Nitric Oxide Synthase Enzyme in Periodontal Disease and Orofacial Pain: Systematic Review. Braz. J. Pain 2021, 4, 362–368. [Google Scholar] [CrossRef]
- Ferreira, J.M.T.; Vidoti, V.A.; Simm, W.; Lopes, K.C. O impacto da disfunção temporomandibular e da dor orofacial na qualidade de vida. Braz. J. Surg. Clin. Res. 2019, 12, 7–12. [Google Scholar]
- Franco, D.; Pereira, T.; Vitorio, F.; Nadur, N.; Lacerda, R.; Kümmerle, A. A Importância das Cumarinas para a Química Medicinal e o Desenvolvimento de Compostos Bioativos nos Últimos Anos. Quím. Nova 2020, 44, 180–197. [Google Scholar] [CrossRef]
- Gouda, M.A.; Hussein, B.H.M.; El-Demerdash, A.; Ibrahim, M.E.; Salem, M.A.; Helal, M.H.; Hamama, W.S. A Review: Synthesis and Medicinal Importance of Coumarins and Their Analogues (Part II). Curr. Bioact. Compd. 2020, 16, 993–1008. [Google Scholar] [CrossRef]
- Kumar, J.A.; Saidachary, G.; Mallesham, G.; Sridhar, B.; Jain, N.; Kalivendi, S.V.; Rao, V.J.; Raju, B.C. Synthesis, Anticancer Activity and Photophysical Properties of Novel Substituted 2-Oxo-2H-Chromenylpyrazolecarboxylates. Eur. J. Med. Chem. 2013, 65, 389–402. [Google Scholar] [CrossRef]
- Abdou, M.M.; El-Saeed, R.A.; Bondock, S. Recent Advances in 4-Hydroxycoumarin Chemistry. Part 1: Synthesis and Reactions. Arab. J. Chem. 2019, 12, 88–121. [Google Scholar] [CrossRef]
- Chanda, C.; Aluru, R.R. Anticuagulants: An Overview of Natural and Synthetic Therapeutic Anticoagulants. J. Biochem. Technol. 2021, 12, 17–21. [Google Scholar] [CrossRef]
- Ganguly, N.C.; Mondal, P.; Roy, S. A Mild Efficient Iodine-Catalyzed Synthesis of Novel Anticoagulants with 2,8-Dioxabicyclo[3.3.1]Nonane Core. Tetrahedron Lett. 2013, 54, 2386–2390. [Google Scholar] [CrossRef]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic Potential of Coumarins as Antiviral Agents. Eur. J. Med. Chem. 2016, 123, 236–255. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sharma, S.; Sahu, D. An Insight into the Therapeutic Applications of Coumarin Compounds and Their Mechanisms of Action. Eur. J. Pharm. Sci. 2020, 152, 105424. [Google Scholar] [CrossRef] [PubMed]
- Andrianjafy, T.M.; Ramanandraibe, V.V.; Andrianarijaona, E.T.; Ramarosandratana, N.H.; Ravaomanarivo, L.H.; Mavingui, P.; Lemaire, M. Field Assessment of 4-Hydroxycoumarin as an Attractant for Anthropophilic Anopheles Spp. Vectors of Malaria in Madagascar. Sci. Rep. 2020, 10, 3048. [Google Scholar] [CrossRef]
- Sumorek-Wiadro, J.; Zając, A.; Maciejczyk, A.; Jakubowicz-Gil, J. Furanocoumarins in Anticancer Therapy—For and Against. Fitoterapia 2020, 142, 104492. [Google Scholar] [CrossRef]
- Jin, A.; Wang, Y.; Tong, L.; Liu, G.; Feng, J.; Li, Y.; Shen, C.; Wu, W. Coumarins and Flavones from Ficus Erecta and Their Anti-Inflammatory Activity. J. Ethnopharmacol. 2024, 333, 118472. [Google Scholar] [CrossRef]
- Kumar, A.; Kini, S.G.; Rathi, E. A Recent Appraisal of Artificial Intelligence and In Silico ADMET Prediction in the Early Stages of Drug Discovery. Mini Rev. Med. Chem. 2021, 21, 2788–2800. [Google Scholar] [CrossRef]
- Prieto-Martínez, F.D.; Arciniega, M.; Medina-Franco, J.L. Acoplamiento Molecular: Avances Recientes y Retos. TIP Rev. Espec. Cienc. Quím.-Biol. 2018, 21, 65–87. [Google Scholar] [CrossRef]
- Muhammed, M.T.; Aki-Yalcin, E. Molecular Docking: Principles, Advances, and Its Applications in DrugDiscovery. Lett. Drug Des. Discov. 2024, 21, 480–495. [Google Scholar] [CrossRef]
- Cruz, J.V.; Rosa, J.M.C.; Kimani, N.M.; Giuliatti, S.; Dos Santos, C.B.R. The Role of Celecoxib as a Potential Inhibitor in the Treatment ofInflammatory Diseases—A Review. Curr. Med. Chem. 2022, 29, 3028–3049. [Google Scholar] [CrossRef] [PubMed]
- Gamal-Eltrabily, M.; Martínez-Lorenzana, G.; González-Hernández, A.; Condés-Lara, M. Cortical Modulation of Nociception. Neuroscience 2021, 458, 256–270. [Google Scholar] [CrossRef]
- Velzen, M.V.; Dahan, J.D.C.; Van Dorp, E.L.A.; Mogil, J.S.; Hooijmans, C.R.; Dahan, A. Efficacy of Ketamine in Relieving Neuropathic Pain: A Systematic Review and Meta-Analysis of Animal Studies. Pain 2021, 162, 2320–2330. [Google Scholar] [CrossRef]
- Husain, A.; Makadia, V.; Valicherla, G.R.; Riyazuddin, M.; Gayen, J.R. Approaches to Minimize the Effects of P-glycoprotein in Drug Transport: A Review. Drug Dev. Res. 2022, 83, 825–841. [Google Scholar] [CrossRef]
- Fanni, D.; Pinna, F.; Gerosa, C.; Paribello, P.; Carpiniello, B.; Faa, G.; Manchia, M. Anatomical Distribution and Expression of CYP in Humans: Neuropharmacological Implications. Drug Dev. Res. 2021, 82, 628–667. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef]
- Olonode, E.T.; Aderibigbe, A.O.; Bakre, A.G. Anti-Nociceptive Activity of the Crude Extract of Myrianthus Arboreus P. Beauv (Cecropiaceae) in Mice. J. Ethnopharmacol. 2015, 171, 94–98. [Google Scholar] [CrossRef]
- Martins, R.T.; Almeida, D.B.D.; Monteiro, F.M.D.R.; Kowacs, P.A.; Ramina, R. Receptores opioides até o contexto atual. Rev. Dor 2012, 13, 75–79. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, A.; Kaur, J.; Bhatti, M.S.; Singh, P.; Bhatti, R. Bergapten Inhibits Chemically Induced Nociceptive Behavior and Inflammation in Mice by Decreasing the Expression of Spinal PARP, iNOS, COX-2 and Inflammatory Cytokines. Inflammopharmacology 2019, 27, 749–760. [Google Scholar] [CrossRef]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal Models of Nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [CrossRef] [PubMed]
- Gripp, E.L.O.; Carneiro, L.U.; Pereira, I.D.S.P.; Vega, M.R.G.; Marinho, B.G. Avaliação das propriedades analgésicas de Anaxagorea dolichocarpa Sprague and Sandwith LC. Braz. J. Health Rev. 2020, 3, 382–395. [Google Scholar] [CrossRef]
- Cheriyan, B.V., Sr.; Kadhirvelu, P., Sr.; Nadipelly, J., Jr.; Shanmugasundaram, J.; Sayeli, V., Sr.; Subramanian, V., Sr. Anti-nociceptive Effect of 7-methoxy Coumarin from Eupatorium Triplinerve Vahl (Asteraceae). Pharmacogn. Mag. 2017, 13, 81–84. [Google Scholar] [PubMed]
- Xu, Y.; Yu, Y.; Wang, Q.; Li, W.; Zhang, S.; Liao, X.; Liu, Y.; Su, Y.; Zhao, M.; Zhang, J. Active Components of Bupleurum Chinense and Angelica Biserrata Showed Analgesic Effects in Formalin Induced Pain by Acting on Nav1.7. J. Ethnopharmacol. 2021, 269, 113736. [Google Scholar] [CrossRef] [PubMed]
- Beirith, A.; Santos, A.R.S.; Calixto, J.B. Mechanisms Underlying the Nociception and Paw Oedema Caused by Injection of Glutamate into the Mouse Paw. Brain Res. 2002, 924, 219–228. [Google Scholar] [CrossRef]
- Melo, A.P.D.; Fujii, Y.W.H.; Rangel, M.P.; Nishida, F.S. Retirada de opioides: Uma revisão bibliográfica/opioid withdrawal: A literature review. Braz. J. Dev. 2020, 6, 67098–67112. [Google Scholar] [CrossRef]
- Park, S.-H.; Sim, Y.-B.; Kang, Y.-J.; Kim, S.-S.; Kim, C.-H.; Kim, S.-J.; Lim, S.-M.; Suh, H.-W. Antinociceptive Profiles and Mechanisms of Orally Administered Coumarin in Mice. Biol. Pharm. Bull. 2013, 36, 925–930. [Google Scholar] [CrossRef]
- Farsam, H.; Amanlou, M.; Reza Dehpour, A.; Jahaniani, F. Anti-Inflammatory and Analgesic Activity of Biebersteinia Multifida DC. Root Extract. J. Ethnopharmacol. 2000, 71, 443–447. [Google Scholar] [CrossRef]
- Guay, J.; Bateman, K.; Gordon, R.; Mancini, J.; Riendeau, D. Carrageenan-Induced Paw Edema in Rat Elicits a Predominant Prostaglandin E2 (PGE2) Response in the Central Nervous System Associated with the Induction of Microsomal PGE2 Synthase-1. J. Biol. Chem. 2004, 279, 24866–24872. [Google Scholar] [CrossRef]
- Liang, C.; Chen, Z.-H.; Huang, Z.-Y.; Zu, F.-Q. Optimizing Microstructures and Mechanical Properties of Hypereutectic Al-18%Si Alloy via Manipulating Its Parent Liquid State. Mater. Sci. Eng. A 2017, 690, 387–392. [Google Scholar] [CrossRef]
- Ju, S.; Tan, Y.; Wang, Q.; Zhou, L.; Wang, K.; Wen, C.; Wang, M. Antioxidant and Anti-inflammatory Effects of Esculin and Esculetin (Review). Exp. Ther. Med. 2024, 27, 248. [Google Scholar] [CrossRef]
- Antika, L.D.; Tasfiyati, A.N.; Hikmat, H.; Septama, A.W. Scopoletin: A Review of Its Source, Biosynthesis, Methods of Extraction, and Pharmacological Activities. Z. Naturforschung C 2022, 77, 303–316. [Google Scholar] [CrossRef]
- Myung, Y.; de Sá, A.G.C.; Ascher, D.B. Deep-PK: Deep Learning for Small Molecule Pharmacokinetic and Toxicity Prediction. Nucleic Acids Res. 2024, 52, W469–W475. [Google Scholar] [CrossRef] [PubMed]
- Piccagli, L.; Fabbri, E.; Borgatti, M.; Bezzerri, V.; Mancini, I.; Nicolis, E.; Dechecchi, M.C.; Lampronti, I.; Cabrini, G.; Gambari, R. Docking of Molecules Identified in Bioactive Medicinal Plants Extracts into the P50 NF-kappaB Transcription Factor: Correlation with Inhibition of NF-kappaB/DNA Interactions and Inhibitory Effects on IL-8 Gene Expression. BMC Struct. Biol. 2008, 8, 38. [Google Scholar] [CrossRef]
- Wang, J.L.; Limburg, D.; Graneto, M.J.; Springer, J.; Hamper, J.R.B.; Liao, S.; Pawlitz, J.L.; Kurumbail, R.G.; Maziasz, T.; Talley, J.J.; et al. The Novel Benzopyran Class of Selective Cyclooxygenase-2 Inhibitors. Part 2: The Second Clinical Candidate Having a Shorter and Favorable Human Half-Life. Bioorganic Med. Chem. Lett. 2010, 20, 7159–7163. [Google Scholar] [CrossRef]
- Kim, J.J.; Gharpure, A.; Teng, J.; Zhuang, Y.; Howard, R.J.; Zhu, S.; Noviello, C.M.; Walsh, R.M.; Lindahl, E.; Hibbs, R.E. Shared Structural Mechanisms of General Anaesthetics and Benzodiazepines. Nature 2020, 585, 303–308. [Google Scholar] [CrossRef]
- Rosenfeld, R.J.; Garcin, E.D.; Panda, K.; Andersson, G.; Åberg, A.; Wallace, A.V.; Morris, G.M.; Olson, A.J.; Stuehr, D.J.; Tainer, J.A.; et al. Conformational Changes in Nitric Oxide Synthases Induced by Chlorzoxazone and Nitroindazoles: Crystallographic and Computational Analyses of Inhibitor Potency. Biochemistry 2002, 41, 13915–13925. [Google Scholar] [CrossRef]
- Ghosh, G.; Duyne, G.V.; Ghosh, S. Structure of NF-KB P50 Homodimer Bound to a KB Site. Nature 1995, 373, 303–310. [Google Scholar] [PubMed]
- Wang, H.; Lv, S.; Stroebel, D.; Zhang, J.; Pan, Y.; Huang, X.; Zhang, X.; Paoletti, P.; Zhu, S. Gating Mechanism and a Modulatory Niche of Human GluN1-GluN2A NMDA Receptors. Neuron 2021, 109, 2443–2456.e5. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, Y.; He, B.; He, X.; Zhou, X.E.; Guo, S.; Rao, Q.; Yang, J.; Liu, J.; Zhou, Q.; et al. Molecular Recognition of Morphine and Fentanyl by the Human μ-Opioid Receptor. Cell 2022, 185, 4361–4375.e19. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, E.; Julius, D.; Cheng, Y. TRPV1 Structures in Nanodiscs Reveal Mechanisms of Ligand and Lipid Action. Nature 2016, 534, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Bastos, G.N.T.; Santos, A.R.S.; Ferreira, V.M.M.; Costa, A.M.R.; Bispo, C.I.; Silveira, A.J.A.; Do Nascimento, J.L.M. Antinociceptive Effect of the Aqueous Extract Obtained from Roots of Physalis Angulata L. on Mice. J. Ethnopharmacol. 2006, 103, 241–245. [Google Scholar] [CrossRef]
- Siqueira-Lima, P.S.; Araújo, A.A.S.; Lucchese, A.M.; Quintans, J.S.S.; Menezes, P.P.; Alves, P.B.; De Lucca Júnior, W.; Santos, M.R.V.; Bonjardim, L.R.; Quintans-Júnior, L.J. β-Cyclodextrin Complex Containing Lippia grata Leaf Essential Oil Reduces Orofacial Nociception in Mice—Evidence of Possible Involvement of Descending Inhibitory Pain Modulation Pathway. Basic Clin. Pharmacol. Toxicol. 2014, 114, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P.; Wang, K.; Cairns, B.E.; Svensson, P.; Arendt-Nielsen, L. Effects of Subcutaneous Administration of Glutamate on Pain, Sensitization and Vasomotor Responses in Healthy Men and Women. Pain 2006, 124, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.R.; Do Espírito Santo, R.F.; Melo, C.D.O.; Pachú Cavalcante, F.E.; Costa, T.B.; Barbosa, Y.V.; E Silva, Y.M.S.D.M.; De Sousa, N.F.; Villarreal, C.F.; De Moura, R.O.; et al. The Compound (E)-2-Cyano-N,3-Diphenylacrylamide (JMPR-01): A Potential Drug for Treatment of Inflammatory Diseases. Pharmaceutics 2022, 14, 188. [Google Scholar] [CrossRef]
- Rodrigues, L.C.C.; Meyer, T.N.; Pereira, J.B.B.; Silva, G.R.D. Avaliação da atividade anti-inflamatória do extrato hidroalcoólico do caroço de abacate sobre a peritonite induzida pela carragenina em ratos. Rev. Univ. Val. Rio Verde 2015, 13, 367–376. [Google Scholar] [CrossRef]
- Pelissier, T.; Pajot, J.; Dallel, R. The Orofacial Capsaicin Test in Rats: Effects of Different Capsaicin Concentrations and Morphine. Pain 2002, 96, 81–87. [Google Scholar] [CrossRef]


| Absorption | |
|---|---|
| P-Glycoprotein Inhibitor | Non-Inhibitor |
| P-Glycoprotein Substrate | Non-Substrate |
| Human Intestinal Absorption | Absorbed |
| Distribution | |
| BBB | Penetrable |
| Excretion | |
| Clearance | 5.76 |
| Half-Life of Drug | Half-Life < 3 h |
| Metabolism | |
| CYP 1A2 Inhibitor/Substrate | Non-Inhibitor/Substrate |
| CYP 2C19 Inhibitor/Substrate | Non-Inhibitor/Non-Substrate |
| CYP 2C9 Inhibitor/Substrate | Non-Inhibitor/Non-Substrate |
| CYP 2D6 Inhibitor/Substrate | Non-Inhibitor/Substrate |
| CYP 3A4 Inhibitor/Substrate | Non-Inhibitor/Non-Substrate |
| Toxicity | |
| Biodegradation | Safe |
| Carcinogenesis | Safe |
| Ames Mutagenesis | Safe |
| Target | Ligands | Escore MolDock | RMSD |
|---|---|---|---|
| COX-2 | 4-HC | −54.84 | 0.27 |
| Celecoxib | −158.60 | ||
| GABAA | 4-HC | −50.64 | 0.08 |
| Bicuculline | −146.56 | ||
| iNOS | 4-HC | −106.52 | 0.19 |
| 7-Nitroindazole | −103.34 | ||
| NFκB | 4-HC | −58.71 | - |
| Dexamethasone | −91.38 | ||
| NMDAR | 4-HC | −56.12 | - |
| Sketamine | −51.11 | ||
| µ-opioid receptor | 4-HC | −28.36 | 0.11 |
| Morphine | −78.23 | ||
| TRPV1 | 4-HC | −62.20 | 0.66 |
| Capsazepine | −87.79 |
| Target | PDB (ID) | Resolution | Ligand |
|---|---|---|---|
| COX-2 | 3LN1 [48] | 2.40 Å | Celecoxib |
| GABAA | 6X3S [49] | 3.12 Å | Bicuculline |
| iNOS | 1M8E [50] | 2.90 Å | 7-Nitroindazole |
| NFκB | 1NFK [51] | 2.30 Å | Dexamethasone |
| NMDAR | 7EOQ [52] | 3.50 Å | Sketamine |
| µ-opioid receptor | 8EF6 [53] | 3.20 Å | Morphine |
| TRPV1 | 5IS0 [54] | 3.43 Å | Capsazepine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Fonsêca, D.V.; Rocha, J.S.; da Silva, P.R.; de Sá Novaes Pereira, H.N.; dos Santos, L.V.N.; de Santana, M.A.D.; Alves, A.F.; Pontes, A.H.O.; de Souza Gomes, J.; Felipe, C.F.B.; et al. 4-Hydroxycoumarin Exhibits Antinociceptive and Anti-Inflammatory Effects Through Cytokine Modulation: An Integrated In Silico and In Vivo Study. Int. J. Mol. Sci. 2025, 26, 2788. https://doi.org/10.3390/ijms26062788
da Fonsêca DV, Rocha JS, da Silva PR, de Sá Novaes Pereira HN, dos Santos LVN, de Santana MAD, Alves AF, Pontes AHO, de Souza Gomes J, Felipe CFB, et al. 4-Hydroxycoumarin Exhibits Antinociceptive and Anti-Inflammatory Effects Through Cytokine Modulation: An Integrated In Silico and In Vivo Study. International Journal of Molecular Sciences. 2025; 26(6):2788. https://doi.org/10.3390/ijms26062788
Chicago/Turabian Styleda Fonsêca, Diogo Vilar, Juliana Sousa Rocha, Pablo R. da Silva, Hugo Natan de Sá Novaes Pereira, Lucas Vinicius Novaes dos Santos, Melquisedec Abiaré Dantas de Santana, Alan F. Alves, Adiel H. O. Pontes, Joás de Souza Gomes, Cícero F. Bezerra Felipe, and et al. 2025. "4-Hydroxycoumarin Exhibits Antinociceptive and Anti-Inflammatory Effects Through Cytokine Modulation: An Integrated In Silico and In Vivo Study" International Journal of Molecular Sciences 26, no. 6: 2788. https://doi.org/10.3390/ijms26062788
APA Styleda Fonsêca, D. V., Rocha, J. S., da Silva, P. R., de Sá Novaes Pereira, H. N., dos Santos, L. V. N., de Santana, M. A. D., Alves, A. F., Pontes, A. H. O., de Souza Gomes, J., Felipe, C. F. B., de Sousa, D. P., Scotti, M. T., & Scotti, L. (2025). 4-Hydroxycoumarin Exhibits Antinociceptive and Anti-Inflammatory Effects Through Cytokine Modulation: An Integrated In Silico and In Vivo Study. International Journal of Molecular Sciences, 26(6), 2788. https://doi.org/10.3390/ijms26062788


_Kim.png)






