A Benzodiazepine-Derived Molecule That Interferes with the Bio-Mechanical Properties of Glioblastoma-Astrocytoma Cells Altering Their Proliferation and Migration
Abstract
1. Introduction
2. Results
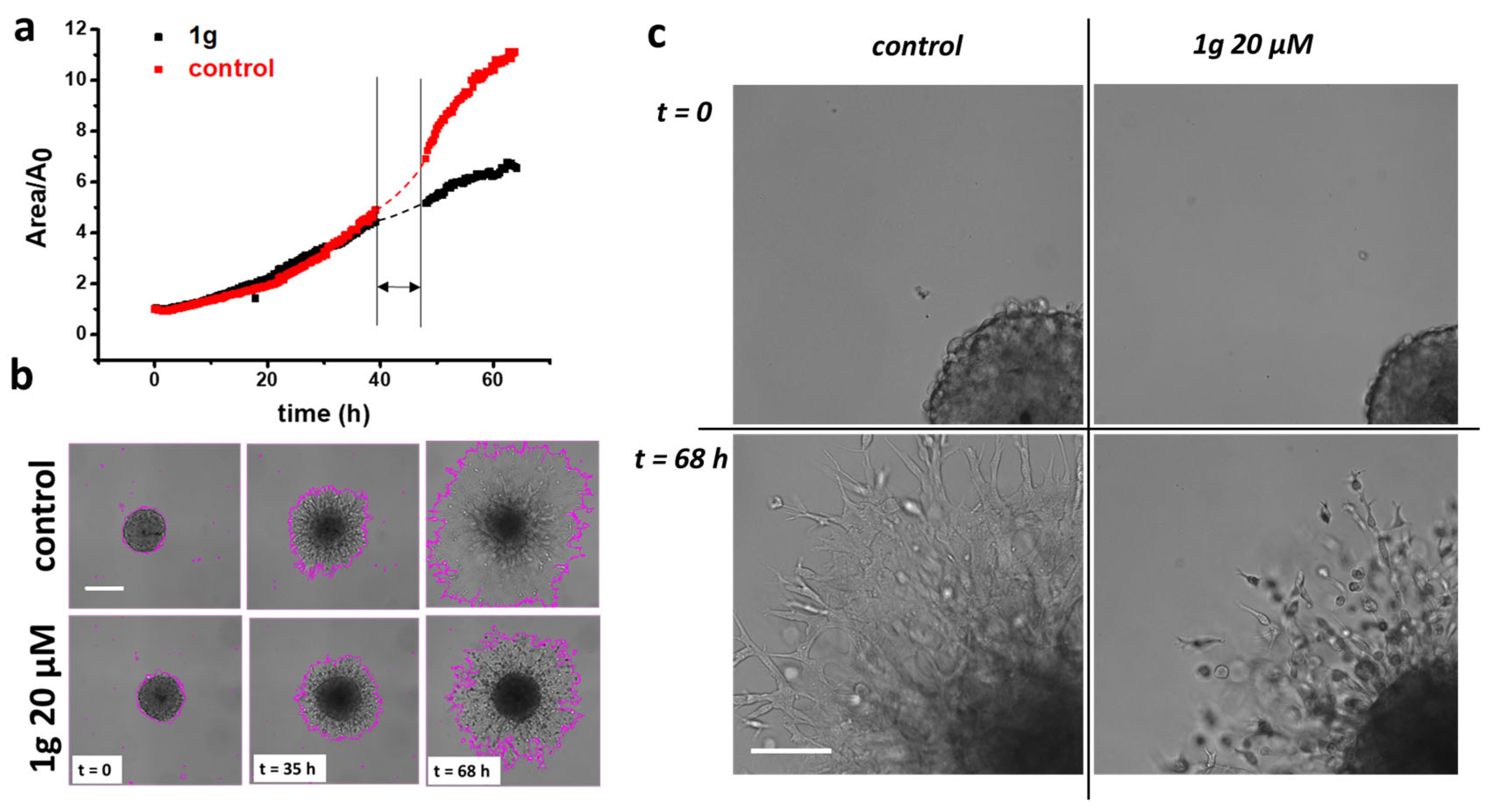
2.1. 1g Reduces U87MG Spheroid Matrix Invasion
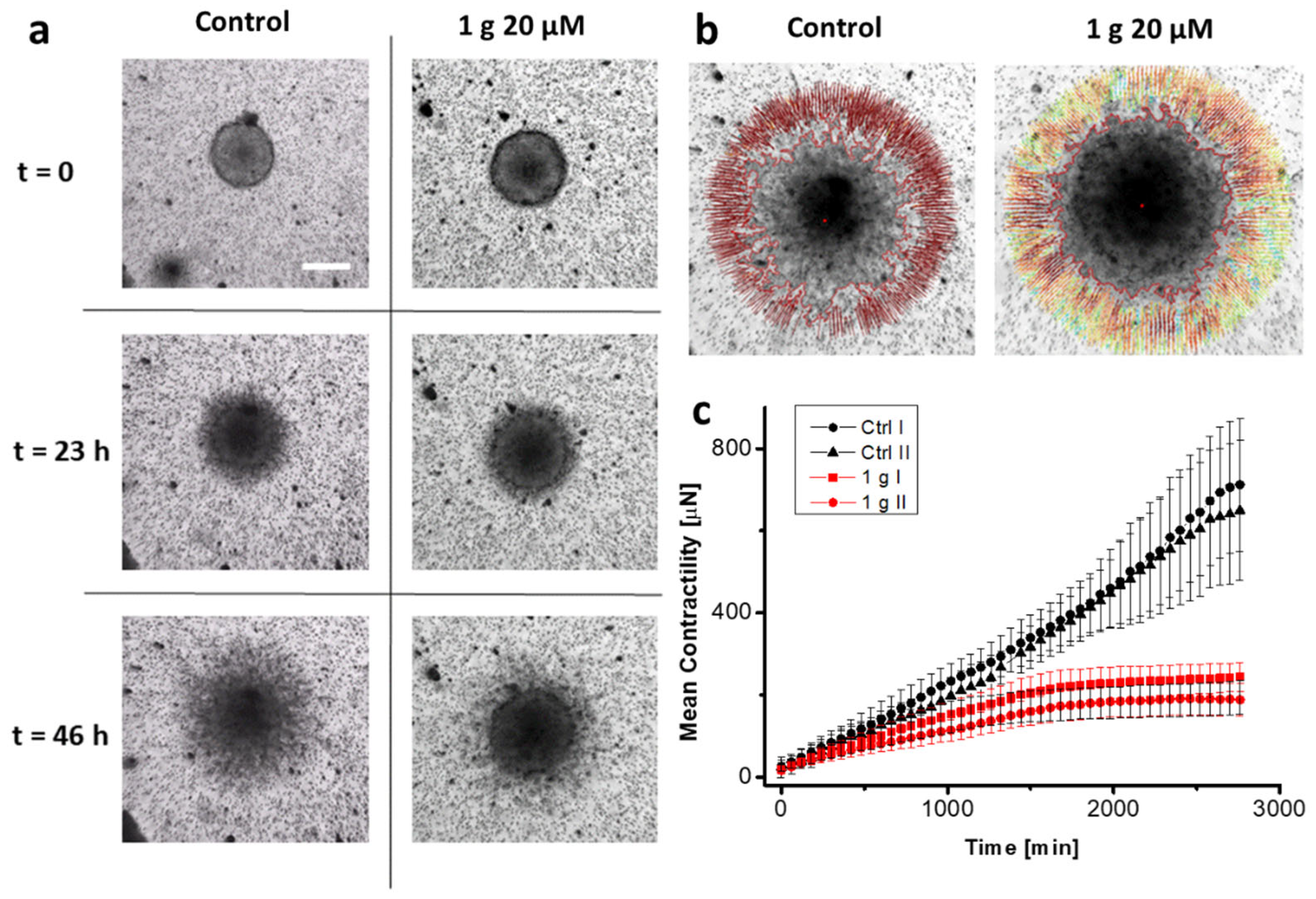
2.2. 1g Reduces the Contractility of U87MG Spheroids Embedded in Matrigel
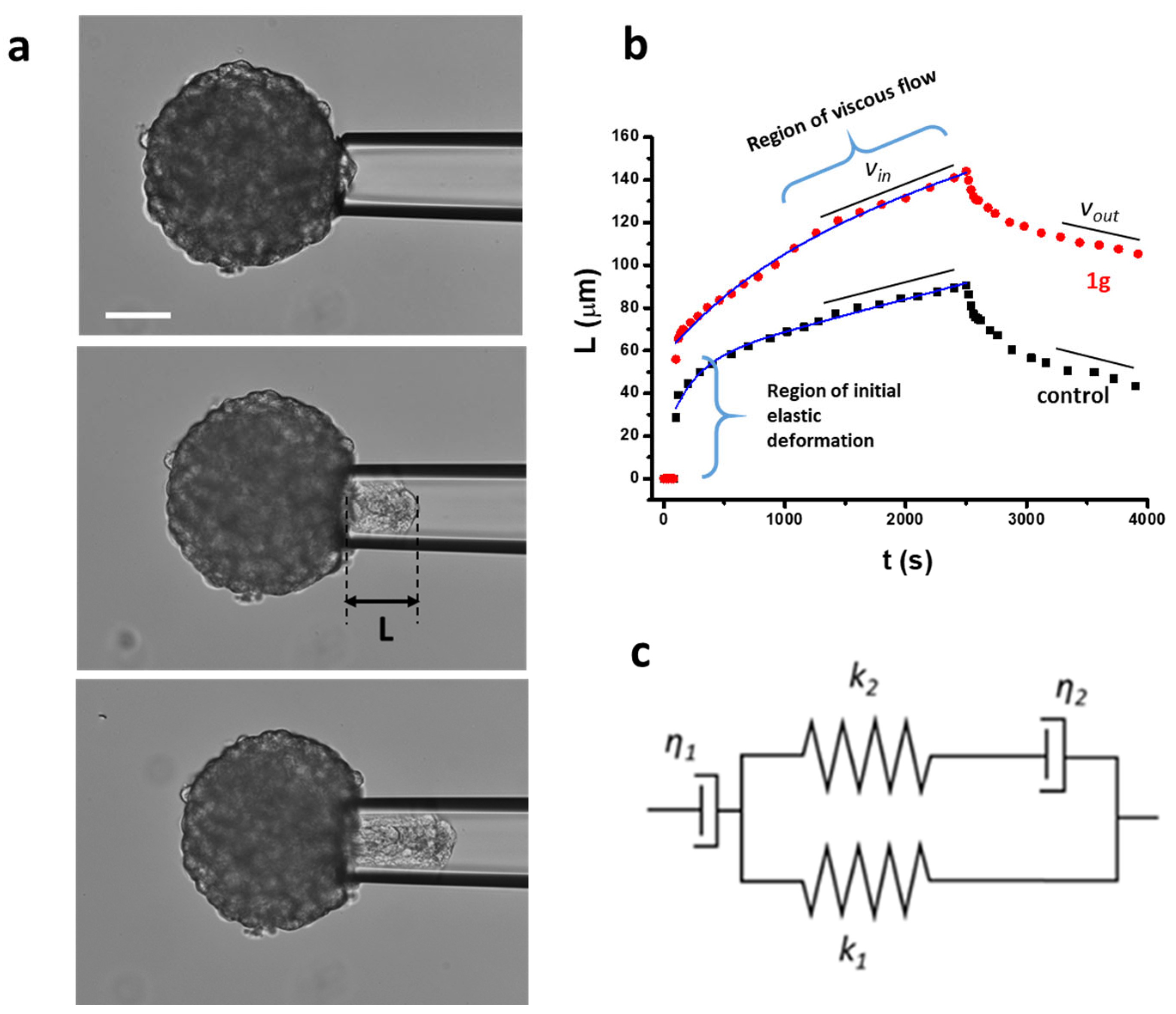
2.3. 1g Decreases the Surface Tension of U87MG Spheroids
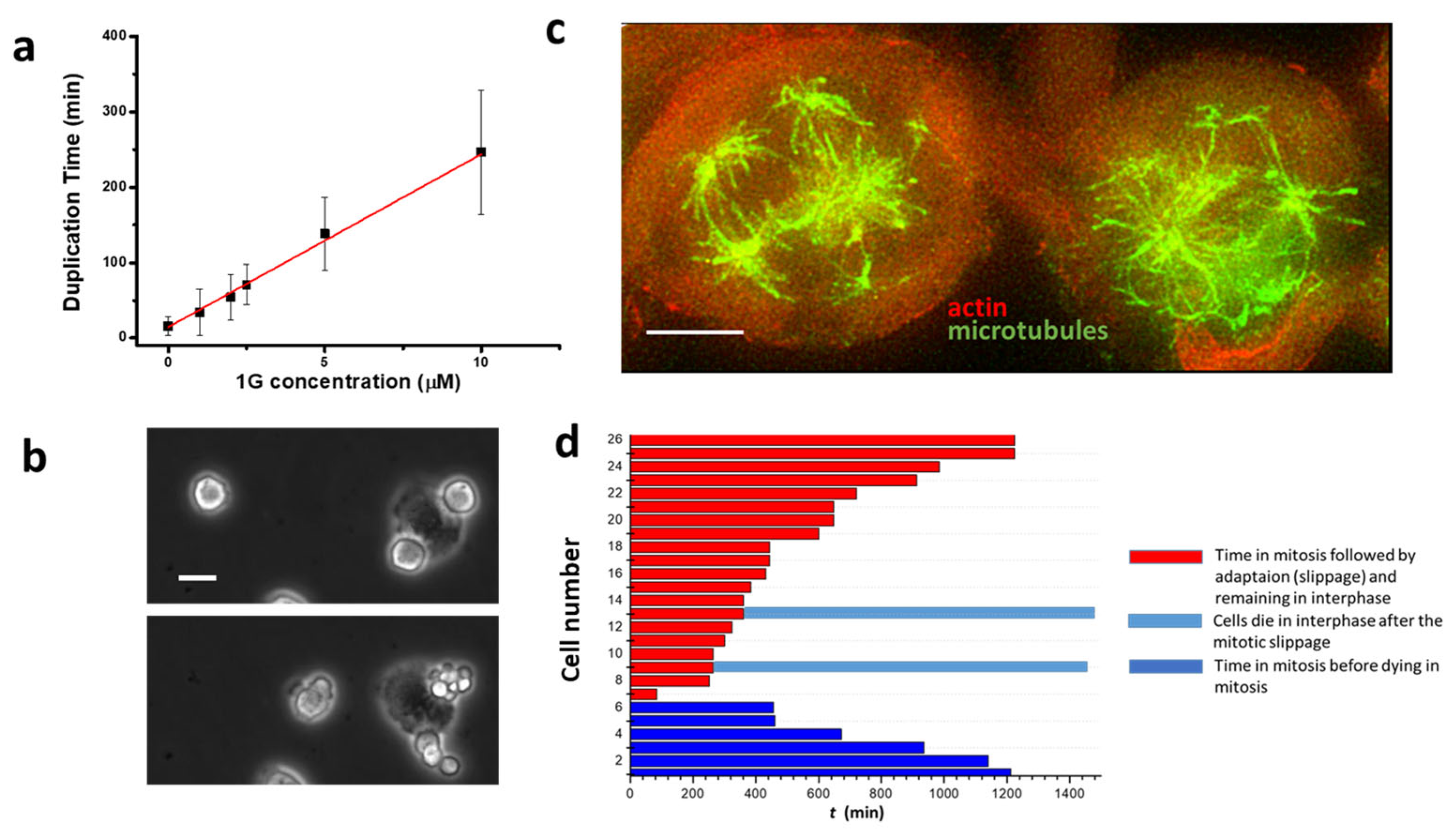
2.4. 1g Reduces Cell Proliferation in 2D Cell Cultures
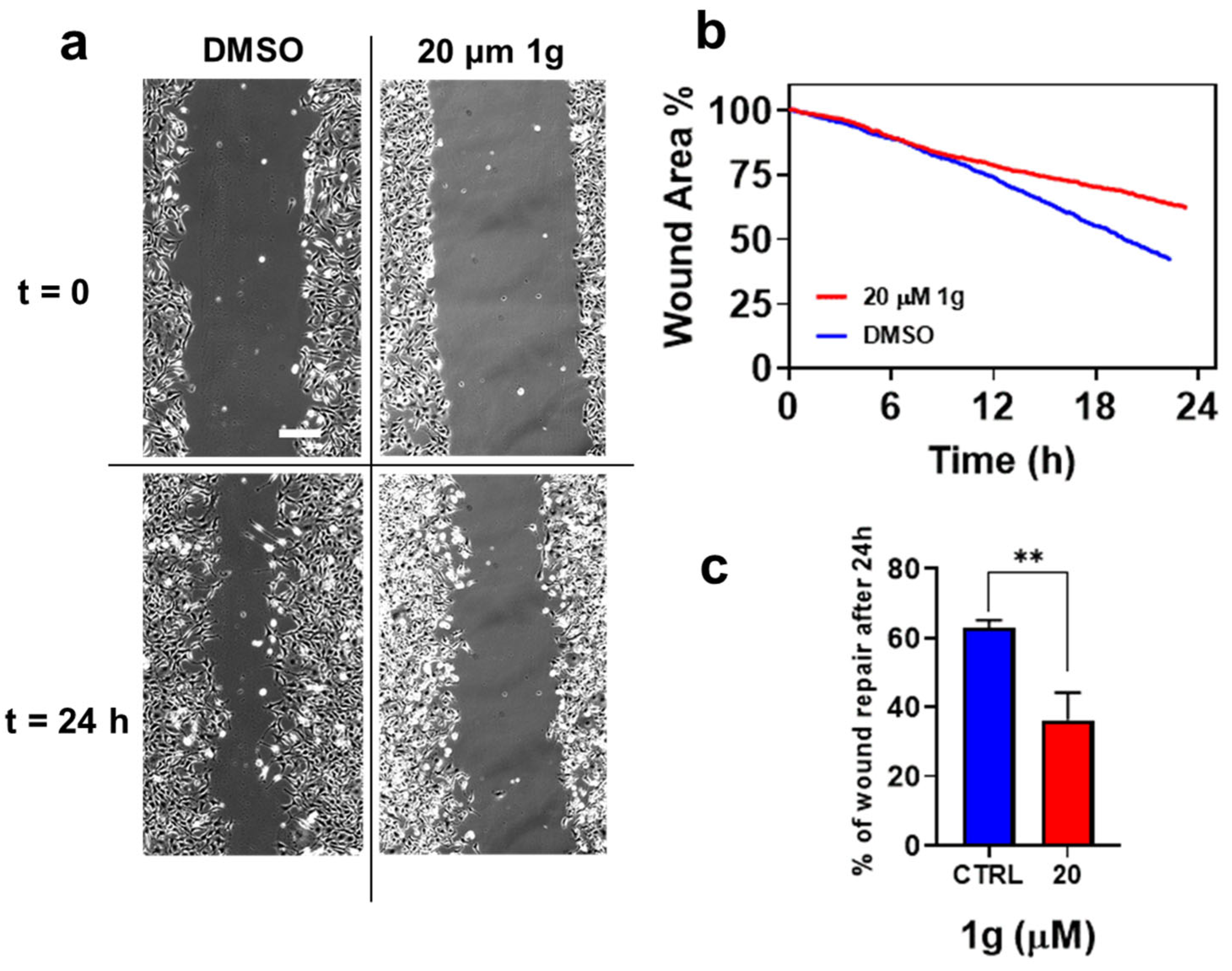
2.5. 1g Inhibits the Migration of GBM in 2D Cultures
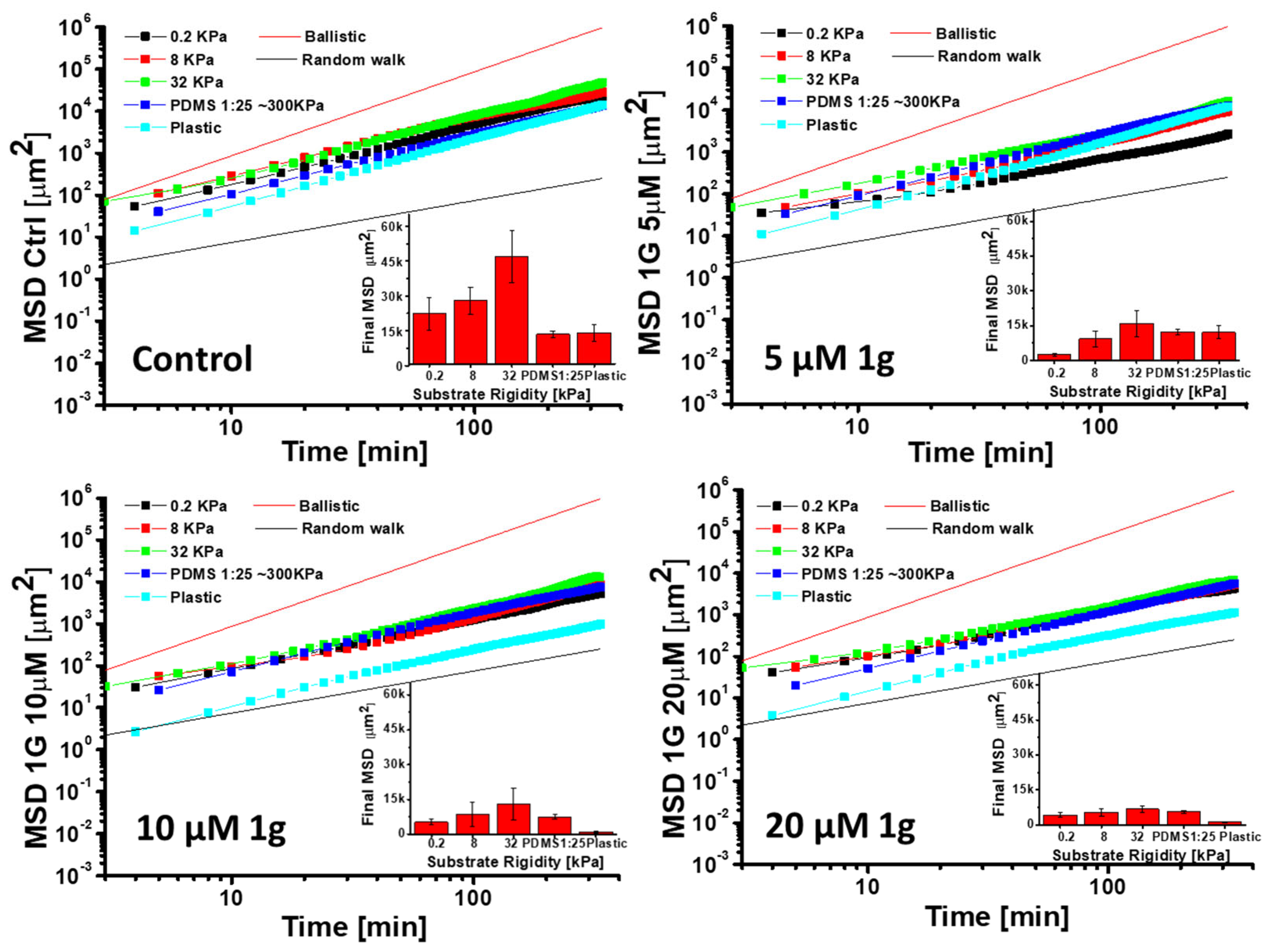
2.6. 1g Increases the Cell Stiffness of GBM Cells as Measured by Atomic Force Microscopy
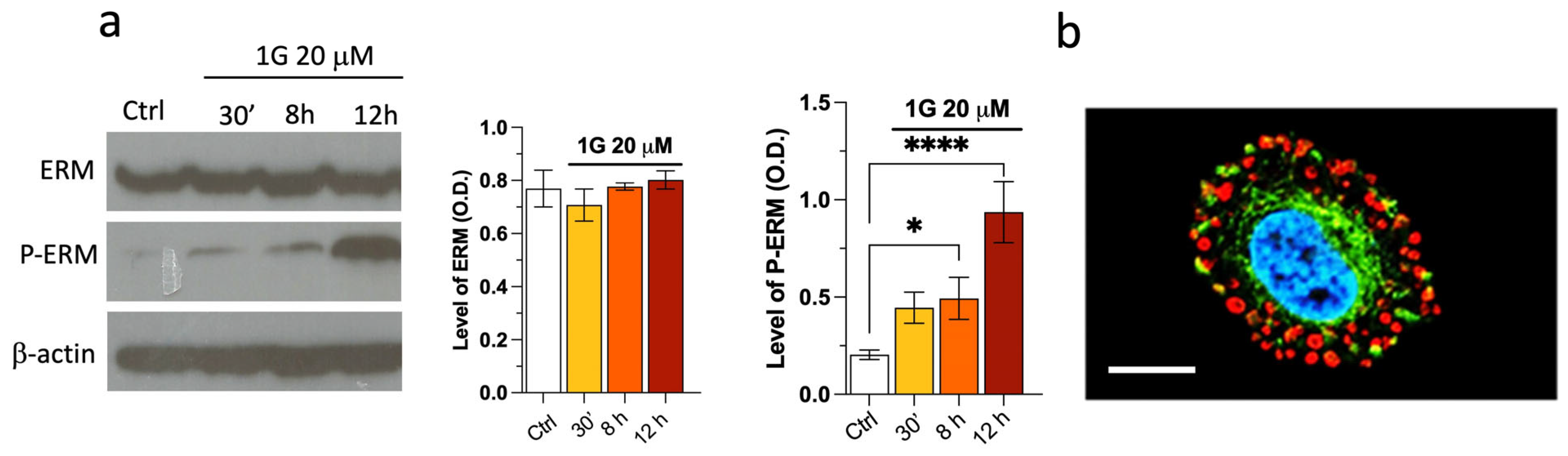
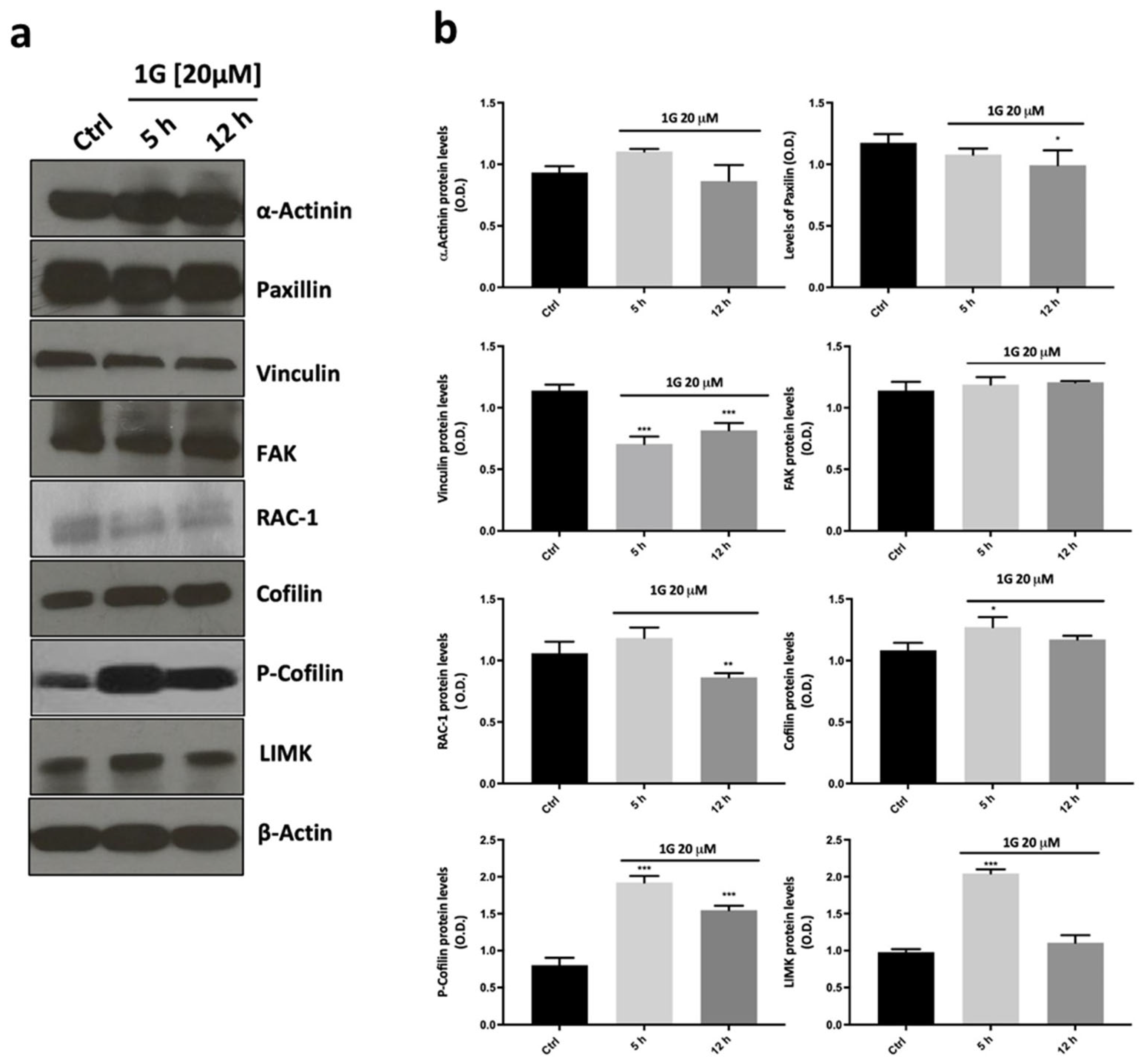
2.7. The Modulation of the Principal Pathways Involved in Cell Adhesion and Migration
3. Discussion
4. Materials and Methods
4.1. Cell Line and Treatment
4.2. 2D Cell Migration
4.3. Adhesion Assay
4.4. Western Blot
4.5. Spheroid Preparation
4.6. The 3D Traction Force Microscopy of Spheroids
4.7. Micropipette Aspiration Technique
4.8. Dynamic Mechanical Analysis by AFM
4.9. A Ting Model Analysis of Single Force Curves
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncology 2014, 16, iv1–iv63. [Google Scholar] [CrossRef] [PubMed]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Pollard, T.D.; Borisy, G.G. Cellular Motility Driven by Assembly and Disassembly of Actin Filaments. Cell 2003, 113, 549. [Google Scholar] [CrossRef]
- Nürnberg, A.; Kitzing, T.; Grosse, R. Nucleating Actin for Invasion. Nat. Rev. Cancer 2011, 11, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Condeelis, J. Regulation of the Actin Cytoskeleton in Cancer Cell Migration and Invasion. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2007, 1773, 642–652. [Google Scholar] [CrossRef]
- Nguyen, R.Y.; Xiao, H.; Gong, X.; Arroyo, A.; Cabral, A.T.; Fischer, T.T.; Flores, K.M.; Zhang, X.; Robert, M.E.; Ehrlich, B.E.; et al. Cytoskeletal Dynamics Regulates Stromal Invasion Behavior of Distinct Liver Cancer Subtypes. Commun. Biol. 2022, 5, 202. [Google Scholar] [CrossRef]
- Ridley, A.J. Rho GTPases and Cell Migration. J. Cell Sci. 2001, 114, 2713–2722. [Google Scholar] [CrossRef]
- Giese, A.; Loo, M.A.; Tran, N.; Haskett, D.; Coons, S.W.; Berens, M.E. Dichotomy of Astrocytoma Migration and Proliferation. Int. J. Cancer 1996, 67, 275–282. [Google Scholar] [CrossRef]
- Xie, Q.; Mittal, S.; Berens, M.E. Targeting Adaptive Glioblastoma: An Overview of Proliferation and Invasion. Neuro-Oncology 2014, 16, 1575–1584. [Google Scholar] [CrossRef]
- Klank, R.L.; Decker Grunke, S.A.; Bangasser, B.L.; Forster, C.L.; Price, M.A.; Odde, T.J.; SantaCruz, K.S.; Rosenfeld, S.S.; Canoll, P.; Turley, E.A.; et al. Biphasic Dependence of Glioma Survival and Cell Migration on CD44 Expression Level. Cell Rep. 2017, 18, 23–31. [Google Scholar] [CrossRef]
- Picariello, H.S.; Kenchappa, R.S.; Rai, V.; Crish, J.F.; Dovas, A.; Pogoda, K.; McMahon, M.; Bell, E.S.; Chandrasekharan, U.; Luu, A.; et al. Myosin IIA Suppresses Glioblastoma Development in a Mechanically Sensitive Manner. Proc. Natl. Acad. Sci. USA 2019, 116, 15550–15559. [Google Scholar] [CrossRef] [PubMed]
- Ivkovic, S.; Beadle, C.; Noticewala, S.; Massey, S.C.; Swanson, K.R.; Toro, L.N.; Bresnick, A.R.; Canoll, P.; Rosenfeld, S.S. Direct Inhibition of Myosin II Effectively Blocks Glioma Invasion in the Presence of Multiple Motogens. MBoC 2012, 23, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Hatzikirou, H.; Basanta, D.; Simon, M.; Schaller, K.; Deutsch, A. “Go or Grow”: The Key to the Emergence of Invasion in Tumour Progression? Math. Med. Biol. 2012, 29, 49–65. [Google Scholar] [CrossRef]
- Giese, A.; Bjerkvig, R.; Berens, M.E.; Westphal, M. Cost of Migration: Invasion of Malignant Gliomas and Implications for Treatment. JCO 2003, 21, 1624–1636. [Google Scholar] [CrossRef]
- Dhruv, H.D.; McDonough Winslow, W.S.; Armstrong, B.; Tuncali, S.; Eschbacher, J.; Kislin, K.; Loftus, J.C.; Tran, N.L.; Berens, M.E. Reciprocal Activation of Transcription Factors Underlies the Dichotomy between Proliferation and Invasion of Glioma Cells. PLoS ONE 2013, 8, e72134. [Google Scholar] [CrossRef]
- Ulrich, T.A.; De Juan Pardo, E.M.; Kumar, S. The Mechanical Rigidity of the Extracellular Matrix Regulates the Structure, Motility, and Proliferation of Glioma Cells. Cancer Res. 2009, 69, 4167–4174. [Google Scholar] [CrossRef]
- Pérez-González, A.; Bévant, K.; Blanpain, C. Cancer Cell Plasticity during Tumor Progression, Metastasis and Response to Therapy. Nat Cancer 2023, 4, 1063–1082. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, J.; Chen, B.; Wang, K.; Tang, Y.; Liang, X. Plasticity of Cancer Cell Invasion: Patterns and Mechanisms. Transl. Oncol. 2021, 14, 100899. [Google Scholar] [CrossRef]
- Omuro, A.M.P.; Faivre, S.; Raymond, E. Lessons Learned in the Development of Targeted Therapy for Malignant Gliomas. Mol. Cancer Ther. 2007, 6, 1909–1919. [Google Scholar] [CrossRef]
- Woehrer, A.; Bauchet, L.; Barnholtz-Sloan, J.S. Glioblastoma Survival: Has It Improved? Evidence from Population-Based Studies. Curr. Opin. Neurol. 2014, 27, 666–674. [Google Scholar] [CrossRef]
- Nakada, M.; Nakada, S.; Demuth, T.; Tran, N.L.; Hoelzinger, D.B.; Berens, M.E. Molecular Targets of Glioma Invasion. Cell. Mol. Life Sci. 2007, 64, 458–478. [Google Scholar] [CrossRef] [PubMed]
- Beadle, C.; Assanah, M.C.; Monzo, P.; Vallee, R.; Rosenfeld, S.S.; Canoll, P. The Role of Myosin II in Glioma Invasion of the Brain. MBoC 2008, 19, 3357–3368. [Google Scholar] [CrossRef]
- Manning, T.J.; Parker, J.C.; Sontheimer, H. Role of Lysophosphatidic Acid and Rho in Glioma Cell Motility. Cell Motil. Cytoskelet. 2000, 45, 185–199. [Google Scholar] [CrossRef]
- Salhia, B.; Rutten, F.; Nakada, M.; Beaudry, C.; Berens, M.; Kwan, A.; Rutka, J.T. Inhibition of Rho-Kinase Affects Astrocytoma Morphology, Motility, and Invasion through Activation of Rac1. Cancer Res. 2005, 65, 8792–8800. [Google Scholar] [CrossRef] [PubMed]
- Liew, H.Y.; Liew, X.H.; Lin, W.X.; Lee, Y.Z.; Ong, Y.S.; Ogawa, S.; Chong, L.H. Cellular Traction Force Holds the Potential as a Drug Testing Readout for In Vitro Cancer Metastasis. Cel. Mol. Bioeng. 2024, 17, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, X.; Zhao, T.; Huang, C.; Wei, Q.; Tang, X.; Santy, L.C.; Saif, M.T.A.; Zhang, S. A Traction Force Threshold Signifies Metastatic Phenotypic Change in Multicellular Epithelia. Soft Matter. 2019, 15, 7203–7210. [Google Scholar] [CrossRef]
- Vining, K.H.; Mooney, D.J. Mechanical Forces Direct Stem Cell Behaviour in Development and Regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- Hall, A. Rho GTPases and the Actin Cytoskeleton. Science 1998, 279, 509–514. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Keely, P.J. Mechanical Signaling through the Cytoskeleton Regulates Cell Proliferation by Coordinated Focal Adhesion and Rho GTPase Signaling. J. Cell Sci. 2011, 124, 1195–1205. [Google Scholar] [CrossRef]
- Bighi, B.; Ragazzini, G.; Gallerani, A.; Mescola, A.; Scagliarini, C.; Marcuzzi, M.; Cavallini, C.; Tassinari, R.; Bianchi, M.; Ventura, C.; et al. Cell Stretching Devices Integrated with Live Cell Imaging: A Powerful Approach to Study How Cells React to Mechanical Cues. Prog. Biomed. Eng. 2024, 7, 012005. [Google Scholar] [CrossRef]
- Kumar, S.; Weaver, V.M. Mechanics, Malignancy, and Metastasis: The Force Journey of a Tumor Cell. Cancer Metastasis Rev. 2009, 28, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wong, I.Y.; Guo, M. Reciprocity of Cell Mechanics with Extracellular Stimuli: Emerging Opportunities for Translational Medicine. Small 2022, 18, 2107305. [Google Scholar] [CrossRef]
- Chen, C.S. Mechanotransduction—A Field Pulling Together? J. Cell Sci. 2008, 121, 3285–3292. [Google Scholar] [CrossRef]
- Vogel, V.; Sheetz, M. Local Force and Geometry Sensing Regulate Cell Functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional Homeostasis and the Malignant Phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Hemmat, M.; Castle, B.T.; Odde, D.J. Microtubule Dynamics: Moving toward a Multi-Scale Approach. Curr. Opin. Cell Biol. 2018, 50, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Akhmanova, A.; Steinmetz, M.O. Control of Microtubule Organization and Dynamics: Two Ends in the Limelight. Nat. Rev. Mol. Cell Biol. 2015, 16, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, S.; Kumar, K.; Das, R.; Negi, A.S. Microtubule Associated Proteins as Targets for Anticancer Drug Development. Bioorg. Chem. 2021, 116, 105320. [Google Scholar] [CrossRef]
- Wattanathamsan, O.; Pongrakhananon, V. Emerging Role of Microtubule-Associated Proteins on Cancer Metastasis. Front. Pharmacol. 2022, 13, 935493. [Google Scholar] [CrossRef]
- Pirani, V.; Métivier, M.; Gallaud, E.; Thomas, A.; Ku, S.; Chretien, D.; Ettari, R.; Giet, R.; Corsi, L.; Benaud, C. A Novel Benzodiazepine Derivative That Suppresses Microtubule Dynamics and Impairs Mitotic Progression. J. Cell Sci. 2020, 133, jcs239244. [Google Scholar] [CrossRef]
- Parenti, S.; Casagrande, G.; Montanari, M.; Espahbodinia, M.; Ettari, R.; Grande, A.; Corsi, L. A Novel 2,3-Benzodiazepine-4-One Derivative AMPA Antagonist Inhibits G2/M Transition and Induces Apoptosis in Human Leukemia Jurkat T Cell Line. Life Sci. 2016, 152, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Micale, N.; Colleoni, S.; Postorino, G.; Pellicanò, A.; Zappalà, M.; Lazzaro, J.; Diana, V.; Cagnotto, A.; Mennini, T.; Grasso, S. Structure–Activity Study of 2,3-Benzodiazepin-4-Ones Noncompetitive AMPAR Antagonists: Identification of the 1-(4-Amino-3-Methylphenyl)-3,5-Dihydro-7,8-Ethylenedioxy-4H-2,3-Benzodiazepin-4-One as Neuroprotective Agent. Bioorg. Med. Chem. 2008, 16, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Boot, R.C.; Koenderink, G.H.; Boukany, P.E. Spheroid Mechanics and Implications for Cell Invasion. Adv. Phys. X 2021, 6, 1978316. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D Tumor Spheroid Models for in Vitro Therapeutic Screening: A Systematic Approach to Enhance the Biological Relevance of Data Obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of Applying Multicellular Tumor Spheroids in Preclinical Phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Jain, N. Multicellular Tumor Spheroids: A Convenient in Vitro Model for Translational Cancer Research. Life Sci. 2024, 358, 123184. [Google Scholar] [CrossRef]
- Tevis, K.M.; Colson, Y.L.; Grinstaff, M.W. Embedded Spheroids as Models of the Cancer Microenvironment. Adv. Biosys. 2017, 1, 1700083. [Google Scholar] [CrossRef]
- Mark, C.; Grundy, T.J.; Strissel, P.L.; Böhringer, D.; Grummel, N.; Gerum, R.; Steinwachs, J.; Hack, C.C.; Beckmann, M.W.; Eckstein, M.; et al. Collective Forces of Tumor Spheroids in Three-Dimensional Biopolymer Networks. eLife 2020, 9, e51912. [Google Scholar] [CrossRef]
- Koch, T.M.; Münster, S.; Bonakdar, N.; Butler, J.P.; Fabry, B. 3D Traction Forces in Cancer Cell Invasion. PLoS ONE 2012, 7, e33476. [Google Scholar] [CrossRef]
- Kopanska, K.S.; Alcheikh, Y.; Staneva, R.; Vignjevic, D.; Betz, T. Tensile Forces Originating from Cancer Spheroids Facilitate Tumor Invasion. PLoS ONE 2016, 11, e0156442. [Google Scholar] [CrossRef]
- Steinwachs, J.; Metzner, C.; Skodzek, K.; Lang, N.; Thievessen, I.; Mark, C.; Münster, S.; Aifantis, K.E.; Fabry, B. Three-Dimensional Force Microscopy of Cells in Biopolymer Networks. Nat. Methods 2016, 13, 171–176. [Google Scholar] [CrossRef]
- Buchmann, B.; Fernández, P.; Bausch, A.R. The Role of Nonlinear Mechanical Properties of Biomimetic Hydrogels for Organoid Growth. Biophys. Rev. 2021, 2, 021401. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, D.; Guevorkian, K.; Douezan, S.; Brochard-Wyart, F. Soft Matter Models of Developing Tissues and Tumors. Science 2012, 338, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Ehrig, S.; Schamberger, B.; Bidan, C.M.; West, A.; Jacobi, C.; Lam, K.; Kollmannsberger, P.; Petersen, A.; Tomancak, P.; Kommareddy, K.; et al. Surface Tension Determines Tissue Shape and Growth Kinetics. Sci. Adv. 2019, 5, eaav9394. [Google Scholar] [CrossRef] [PubMed]
- Nagle, I.; Richert, A.; Quinteros, M.; Janel, S.; Buysschaert, E.; Luciani, N.; Debost, H.; Thevenet, V.; Wilhelm, C.; Prunier, C.; et al. Surface Tension of Model Tissues during Malignant Transformation and Epithelial–Mesenchymal Transition. Front. Cell Dev. Biol. 2022, 10, 926322. [Google Scholar] [CrossRef] [PubMed]
- Guevorkian, K.; Colbert, M.-J.; Durth, M.; Dufour, S.; Brochard-Wyart, F. Aspiration of Biological Viscoelastic Drops. Phys. Rev. Lett. 2010, 104, 218101. [Google Scholar] [CrossRef]
- Guevorkian, K.; Maître, J.-L. Micropipette Aspiration. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 139, pp. 187–201. ISBN 978-0-12-809373-3. [Google Scholar]
- Boot, R.C.; Roscani, A.; Van Buren, L.; Maity, S.; Koenderink, G.H.; Boukany, P.E. High-Throughput Mechanophenotyping of Multicellular Spheroids Using a Microfluidic Micropipette Aspiration Chip. Lab Chip 2023, 23, 1768–1778. [Google Scholar] [CrossRef]
- Guevorkian, K.; Gonzalez-Rodriguez, D.; Carlier, C.; Dufour, S.; Brochard-Wyart, F. Mechanosensitive Shivering of Model Tissues under Controlled Aspiration. Proc. Natl. Acad. Sci. USA 2011, 108, 13387–13392. [Google Scholar] [CrossRef]
- Ferraro, G.; Mozzicafreddo, M.; Ettari, R.; Corsi, L.; Monti, M.C. A Proteomic Platform Unveils the Brain Glycogen Phosphorylase as a Potential Therapeutic Target for Glioblastoma Multiforme. Int. J. Mol. Sci. 2022, 23, 8200. [Google Scholar] [CrossRef]
- Venere, M.; Horbinski, C.; Crish, J.F.; Jin, X.; Vasanji, A.; Major, J.; Burrows, A.C.; Chang, C.; Prokop, J.; Wu, Q.; et al. The Mitotic Kinesin KIF11 Is a Driver of Invasion, Proliferation, and Self-Renewal in Glioblastoma. Sci. Transl. Med. 2015, 7, 304ra143. [Google Scholar] [CrossRef]
- Wang, F.; Lin, S.L. Knockdown of Kinesin KIF11 Abrogates Directed Migration in Response to Epidermal Growth Factor-Mediated Chemotaxis. Biochem. Biophys. Res. Commun. 2014, 452, 642–648. [Google Scholar] [CrossRef]
- Zasadil, L.M.; Andersen, K.A.; Yeum, D.; Rocque, G.B.; Wilke, L.G.; Tevaarwerk, A.J.; Raines, R.T.; Burkard, M.E.; Weaver, B.A. Cytotoxicity of Paclitaxel in Breast Cancer Is Due to Chromosome Missegregation on Multipolar Spindles. Sci. Transl. Med. 2014, 6, 229ra43. [Google Scholar] [CrossRef] [PubMed]
- Field, J.J.; Kanakkanthara, A.; Miller, J.H. Microtubule-Targeting Agents Are Clinically Successful Due to Both Mitotic and Interphase Impairment of Microtubule Function. Bioorg. Med. Chem. 2014, 22, 5050–5059. [Google Scholar] [CrossRef] [PubMed]
- Miroshnikova, Y.A.; Mouw, J.K.; Barnes, J.M.; Pickup, M.W.; Lakins, J.N.; Kim, Y.; Lobo, K.; Persson, A.I.; Reis, G.F.; McKnight, T.R.; et al. Tissue Mechanics Promote IDH1-Dependent HIF1α–Tenascin C Feedback to Regulate Glioblastoma Aggression. Nat. Cell Biol. 2016, 18, 1336–1345. [Google Scholar] [CrossRef]
- Grundy, T.J.; De Leon, E.; Griffin, K.R.; Stringer, B.W.; Day, B.W.; Fabry, B.; Cooper-White, J.; O’Neill, G.M. Differential Response of Patient-Derived Primary Glioblastoma Cells to Environmental Stiffness. Sci. Rep. 2016, 6, 23353. [Google Scholar] [CrossRef]
- Zhang, C.; Tan, Y.; Feng, J.; Huang, C.; Liu, B.; Fan, Z.; Xu, B.; Lu, T. Exploration of the Effects of Substrate Stiffness on Biological Responses of Neural Cells and Their Mechanisms. ACS Omega 2020, 5, 31115–31125. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Tang, Y.; Xu, Y.; Sun, Q.; Liao, F.; Han, D. Substrate Stiffness Influences the Outcome of Antitumor Drug Screening in Vitro. Clin. Hemorheol. Microcirc. 2013, 55, 121–131. [Google Scholar] [CrossRef]
- Bangasser, B.L.; Shamsan, G.A.; Chan, C.E.; Opoku, K.N.; Tüzel, E.; Schlichtmann, B.W.; Kasim, J.A.; Fuller, B.J.; McCullough, B.R.; Rosenfeld, S.S.; et al. Shifting the Optimal Stiffness for Cell Migration. Nat. Commun. 2017, 8, 15313. [Google Scholar] [CrossRef]
- Seetharaman, S.; Vianay, B.; Roca, V.; Farrugia, A.J.; De Pascalis, C.; Boëda, B.; Dingli, F.; Loew, D.; Vassilopoulos, S.; Bershadsky, A.; et al. Microtubules Tune Mechanosensitive Cell Responses. Nat. Mater. 2022, 21, 366–377. [Google Scholar] [CrossRef]
- Suresh, S.; Spatz, J.; Mills, J.P.; Micoulet, A.; Dao, M.; Lim, C.T.; Beil, M.; Seufferlein, T. Connections between Single-Cell Biomechanics and Human Disease States: Gastrointestinal Cancer and Malaria. Acta Biomater. 2005, 1, 15–30. [Google Scholar] [CrossRef]
- The Physical Sciences—Oncology Centers Network; Agus, D.B.; Alexander, J.F.; Arap, W.; Ashili, S.; Aslan, J.E.; Austin, R.H.; Backman, V.; Bethel, K.J.; Bonneau, R.; et al. A Physical Sciences Network Characterization of Non-Tumorigenic and Metastatic Cells. Sci. Rep. 2013, 3, 1449. [Google Scholar] [CrossRef]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell Stiffness Is a Biomarker of the Metastatic Potential of Ovarian Cancer Cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Kuang, D.; Zhang, B.; Song, G. Cell Stiffness Determined by Atomic Force Microscopy and Its Correlation with Cell Motility. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, M.; Guck, J. Single-Cell Mechanics: Structural Determinants and Functional Relevance. Annu. Rev. Biophys. 2024, 53, 367–395. [Google Scholar] [CrossRef]
- Zhang, G.; Long, M.; Wu, Z.-Z.; Yu, W.-Q. Mechanical Properties of Hepatocellular Carcinoma Cells. WJG 2002, 8, 243. [Google Scholar] [CrossRef]
- Mizuno, D.; Tardin, C.; Schmidt, C.F.; MacKintosh, F.C. Nonequilibrium Mechanics of Active Cytoskeletal Networks. Science 2007, 315, 370–373. [Google Scholar] [CrossRef]
- Alcaraz, J.; Buscemi, L.; Grabulosa, M.; Trepat, X.; Fabry, B.; Farré, R.; Navajas, D. Microrheology of Human Lung Epithelial Cells Measured by Atomic Force Microscopy. Biophys. J. 2003, 84, 2071–2079. [Google Scholar] [CrossRef]
- Mahaffy, R.E.; Shih, C.K.; MacKintosh, F.C.; Käs, J. Scanning Probe-Based Frequency-Dependent Microrheology of Polymer Gels and Biological Cells. Phys. Rev. Lett. 2000, 85, 880–883. [Google Scholar] [CrossRef]
- Bershadsky, A.; Chausovsky, A.; Becker, E.; Lyubimova, A.; Geiger, B. Involvement of Microtubules in the Control of Adhesion-Dependent Signal Transduction. Curr. Biol. 1996, 6, 1279–1289. [Google Scholar] [CrossRef]
- Liu, B.P.; Chrzanowska-Wodnicka, M.; Burridge, K. Microtubule Depolymerization Induces Stress Fibers, Focal Adhesions, and DNA Synthesis via the GTP-Binding Protein Rho. Cell Adhes. Commun. 1998, 5, 249–255. [Google Scholar] [CrossRef]
- Takahashi, R.; Okajima, T. Mapping Power-Law Rheology of Living Cells Using Multi-Frequency Force Modulation Atomic Force Microscopy. Appl. Phys. Lett. 2015, 107, 173702. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Wang, W.-H.; Hardy, S.D.; Geahlen, R.L.; Raman, A. Measuring Nanoscale Viscoelastic Parameters of Cells Directly from AFM Force-Displacement Curves. Sci. Rep. 2017, 7, 1541. [Google Scholar] [CrossRef] [PubMed]
- Areti, A.; Komirishetty, P.; Zochodne, D.W. Collaborative Roles for RAC1, ERM Proteins and PTEN During Adult Sensory Axon Regeneration. Mol. Neurobiol. 2024, 62, 786–805. [Google Scholar] [CrossRef]
- Schneider, I.C.; Hays, C.K.; Waterman, C.M. Epidermal Growth Factor–Induced Contraction Regulates Paxillin Phosphorylation to Temporally Separate Traction Generation from De-Adhesion. MBoC 2009, 20, 3155–3167. [Google Scholar] [CrossRef] [PubMed]
- Panopoulos, A.; Howell, M.; Fotedar, R.; Margolis, R.L. Glioblastoma Motility Occurs in the Absence of Actin Polymer. MBoC 2011, 22, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, J.; Cramer, L.P.; Baum, B.; McGee, K.M. Myosin II-Dependent Cortical Movement Is Required for Centrosome Separation and Positioning during Mitotic Spindle Assembly. Cell 2004, 117, 361–372. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Nalbant, P.; Birkenfeld, J.; Chang, Z.-F.; Bokoch, G.M. GEF-H1 Couples Nocodazole-Induced Microtubule Disassembly to Cell Contractility via RhoA. MBoC 2008, 19, 2147–2153. [Google Scholar] [CrossRef]
- Lv, S.; Chen, Z.; Mi, H.; Yu, X. Cofilin Acts as a Booster for Progression of Malignant Tumors Represented by Glioma. CMAR 2022, 14, 3245–3269. [Google Scholar] [CrossRef]
- Chignola, R.; Schenetti, A.; Andrighetto, G.; Chiesa, E.; Foroni, R.; Sartoris, S.; Tridente, G.; Liberati, D. Forecasting the Growth of Multicell Tumour Spheroids: Implications for the Dynamic Growth of Solid Tumours. Cell Prolif. 2000, 33, 219–229. [Google Scholar] [CrossRef]
- Marušić, M.; Bajzer, Ž.; Freyer, J.P.; Vuk-Pavlović, S. Analysis of Growth of Multicellular Tumour Spheroids by Mathematical Models. Cell Prolif. 1994, 27, 73–94. [Google Scholar] [CrossRef]
- Bangasser, B.L.; Rosenfeld, S.S.; Odde, D.J. Determinants of Maximal Force Transmission in a Motor-Clutch Model of Cell Traction in a Compliant Microenvironment. Biophys. J. 2013, 105, 581–592. [Google Scholar] [CrossRef]
- Stroka, K.M.; Aranda-Espinoza, H. Neutrophils Display Biphasic Relationship between Migration and Substrate Stiffness. Cell Motil. Cytoskelet. 2009, 66, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Peyton, S.R.; Putnam, A.J. Extracellular Matrix Rigidity Governs Smooth Muscle Cell Motility in a Biphasic Fashion. J. Cell. Physiol. 2005, 204, 198–209. [Google Scholar] [CrossRef] [PubMed]
- DiMilla, P.A.; Barbee, K.; Lauffenburger, D.A. Mathematical Model for the Effects of Adhesion and Mechanics on Cell Migration Speed. Biophys. J. 1991, 60, 15–37. [Google Scholar] [CrossRef] [PubMed]
- DiMilla, P.; Stone, J.; Quinn, J.; Albelda, S.; Lauffenburger, D. Maximal Migration of Human Smooth Muscle Cells on Fibronectin and Type IV Collagen Occurs at an Intermediate Attachment Strength. J. Cell Biol. 1993, 122, 729–737. [Google Scholar] [CrossRef]
- Prahl, L.S.; Odde, D.J. Modeling Cell Migration Mechanics. In Biomechanics in Oncology; Advances in Experimental Medicine and Biology; Dong, C., Zahir, N., Konstantopoulos, K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 1092, pp. 159–187. ISBN 978-3-319-95293-2. [Google Scholar]
- Kamath, K.; Smiyun, G.; Wilson, L.; Jordan, M.A. Mechanisms of Inhibition of Endothelial Cell Migration by Taxanes. Cytoskeleton 2014, 71, 46–60. [Google Scholar] [CrossRef]
- Honoré, S.; Pagano, A.; Gauthier, G.; Bourgarel-Rey, V.; Verdier-Pinard, P.; Civiletti, K.; Kruczynski, A.; Braguer, D. Antiangiogenic Vinflunine Affects EB1 Localization and Microtubule Targeting to Adhesion Sites. Mol. Cancer Ther. 2008, 7, 2080–2089. [Google Scholar] [CrossRef]
- Rafiq, N.B.M.; Nishimura, Y.; Plotnikov, S.V.; Thiagarajan, V.; Zhang, Z.; Shi, S.; Natarajan, M.; Viasnoff, V.; Kanchanawong, P.; Jones, G.E.; et al. A Mechano-Signalling Network Linking Microtubules, Myosin IIA Filaments and Integrin-Based Adhesions. Nat. Mater. 2019, 18, 638–649. [Google Scholar] [CrossRef]
- Prahl, L.S.; Bangasser, P.F.; Stopfer, L.E.; Hemmat, M.; White, F.M.; Rosenfeld, S.S.; Odde, D.J. Microtubule-Based Control of Motor-Clutch System Mechanics in Glioma Cell Migration. Cell Rep. 2018, 25, 2591–2604.e8. [Google Scholar] [CrossRef]
- Krylyshkina, O.; Anderson, K.I.; Kaverina, I.; Upmann, I.; Manstein, D.J.; Small, J.V.; Toomre, D.K. Nanometer Targeting of Microtubules to Focal Adhesions. J. Cell Biol. 2003, 161, 853–859. [Google Scholar] [CrossRef]
- Bouchet, B.P.; Gough, R.E.; Ammon, Y.-C.; Van De Willige, D.; Post, H.; Jacquemet, G.; Altelaar, A.M.; Heck, A.J.; Goult, B.T.; Akhmanova, A. Talin-KANK1 Interaction Controls the Recruitment of Cortical Microtubule Stabilizing Complexes to Focal Adhesions. eLife 2016, 5, e18124. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Godinho, S.A.; Chandhok, N.S.; Ganem, N.J.; Azioune, A.; Thery, M.; Pellman, D. Mechanisms to Suppress Multipolar Divisions in Cancer Cells with Extra Centrosomes. Genes Dev. 2008, 22, 2189–2203. [Google Scholar] [CrossRef]
- Bates, D.; Eastman, A. Microtubule Destabilising Agents: Far More than Just Antimitotic Anticancer Drugs. Brit. J. Clin. Pharma. 2017, 83, 255–268. [Google Scholar] [CrossRef]
- Chen, Y.; Chou, W.-C.; Ding, Y.-M.; Wu, Y.-C. Caffeine Inhibits Migration in Glioma Cells through the ROCK-FAK Pathway. Cell. Physiol. Biochem. 2014, 33, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Pardi, R. Signal Transduction, Adhesion Receptors. Nat. Educ. 2010, 3, 38. [Google Scholar]
- Kim, Y.-B.; Choi, S.; Choi, M.-C.; Oh, M.-A.; Lee, S.-A.; Cho, M.; Mizuno, K.; Kim, S.-H.; Lee, J.W. Cell Adhesion-Dependent Cofilin Serine 3 Phosphorylation by the Integrin-Linked Kinase·c-Src Complex. J. Biol. Chem. 2008, 283, 10089–10096. [Google Scholar] [CrossRef] [PubMed]
- Beri, P.; Popravko, A.; Yeoman, B.; Kumar, A.; Chen, K.; Hodzic, E.; Chiang, A.; Banisadr, A.; Placone, J.K.; Carter, H.; et al. Cell Adhesiveness Serves as a Biophysical Marker for Metastatic Potential. Cancer Res. 2020, 80, 901–911. [Google Scholar] [CrossRef]
- Leguay, K.; Decelle, B.; Elkholi, I.E.; Bouvier, M.; Côté, J.-F.; Carréno, S. Interphase Microtubule Disassembly Is a Signaling Cue That Drives Cell Rounding at Mitotic Entry. J. Cell Biol. 2022, 221, e202109065. [Google Scholar] [CrossRef]
- Solinet, S.; Mahmud, K.; Stewman, S.F.; Ben El Kadhi, K.; Decelle, B.; Talje, L.; Ma, A.; Kwok, B.H.; Carreno, S. The Actin-Binding ERM Protein Moesin Binds to and Stabilizes Microtubules at the Cell Cortex. J. Cell Biol. 2013, 202, 251–260. [Google Scholar] [CrossRef]
- Fievet, B.T.; Gautreau, A.; Roy, C.; Del Maestro, L.; Mangeat, P.; Louvard, D.; Arpin, M. Phosphoinositide Binding and Phosphorylation Act Sequentially in the Activation Mechanism of Ezrin. J. Cell Biol. 2004, 164, 653–659. [Google Scholar] [CrossRef]
- Tachibana, K.; Haghparast, S.M.A.; Miyake, J. Inhibition of Cell Adhesion by Phosphorylated Ezrin/Radixin/Moesin. Cell Adhes. Migr. 2015, 9, 502–512. [Google Scholar] [CrossRef]
- Fukata, M.; Watanabe, T.; Noritake, J.; Nakagawa, M.; Yamaga, M.; Kuroda, S.; Matsuura, Y.; Iwamatsu, A.; Perez, F.; Kaibuchi, K. Rac1 and Cdc42 Capture Microtubules through IQGAP1 and CLIP-170. Cell 2002, 109, 873–885. [Google Scholar] [CrossRef]
- Michaelson, D.; Abidi, W.; Guardavaccaro, D.; Zhou, M.; Ahearn, I.; Pagano, M.; Philips, M.R. Rac1 Accumulates in the Nucleus during the G2 Phase of the Cell Cycle and Promotes Cell Division. J. Cell Biol. 2008, 181, 485–496. [Google Scholar] [CrossRef]
- Stehbens, S.J.; Paszek, M.; Pemble, H.; Ettinger, A.; Gierke, S.; Wittmann, T. CLASPs Link Focal-Adhesion-Associated Microtubule Capture to Localized Exocytosis and Adhesion Site Turnover. Nat. Cell Biol. 2014, 16, 558–570. [Google Scholar] [CrossRef]
- Grill, S.W.; Howard, J.; Schäffer, E.; Stelzer, E.H.K.; Hyman, A.A. The Distribution of Active Force Generators Controls Mitotic Spindle Position. Science 2003, 301, 518–521. [Google Scholar] [CrossRef]
- Zappalà, M.; Pellicanò, A.; Micale, N.; Menniti, F.S.; Ferreri, G.; De Sarro, G.; Grasso, S.; De Micheli, C. New 7,8-Ethylenedioxy-2,3-Benzodiazepines as Noncompetitive AMPA Receptor Antagonists. Bioorg. Med. Chem. Lett. 2006, 16, 167–170. [Google Scholar] [CrossRef]
- Grasso, S.; De Sarro, G.; De Sarro, A.; Micale, N.; Zappalà, M.; Puia, G.; Baraldi, M.; De Micheli, C. Synthesis and Anticonvulsant Activity of Novel and Potent 2,3-Benzodiazepine AMPA/Kainate Receptor Antagonists. J. Med. Chem. 1999, 42, 4414–4421. [Google Scholar] [CrossRef]
- Ragazzini, G.; Mescola, A.; Corsi, L.; Alessandrini, A. Fabrication of a Low-Cost on-Stage Cell Incubator with Full Automation. J. Biol. Educ. 2019, 53, 165–173. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Rubino, V.; Morelli, F.; Ruggiero, G.; Landi, R.; Guidetti, G.; Canello, S.; Terrazzano, G.; Alessandrini, A. Mechanical Phenotyping of K562 Cells by the Micropipette Aspiration Technique Allows Identifying Mechanical Changes Induced by Drugs. Sci. Rep. 2018, 8, 1219. [Google Scholar] [CrossRef]
- Hans-Jurgen, B.; Jaschke, M. I Calculation of Thermal Noise in Atomic Force Microscopy. Nanotechnology 1995, 65, 1. [Google Scholar] [CrossRef]
- Alcaraz, J.; Buscemi, L.; Puig-de-Morales, M.; Colchero, J.; Baró, A.; Navajas, D. Correction of Microrheological Measurements of Soft Samples with Atomic Force Microscopy for the Hydrodynamic Drag on the Cantilever. Langmuir 2002, 18, 716–721. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragazzini, G.; Mescola, A.; Tassinari, R.; Gallerani, A.; Zannini, C.; Di Rosa, D.; Cavallini, C.; Marcuzzi, M.; Taglioli, V.; Bighi, B.; et al. A Benzodiazepine-Derived Molecule That Interferes with the Bio-Mechanical Properties of Glioblastoma-Astrocytoma Cells Altering Their Proliferation and Migration. Int. J. Mol. Sci. 2025, 26, 2767. https://doi.org/10.3390/ijms26062767
Ragazzini G, Mescola A, Tassinari R, Gallerani A, Zannini C, Di Rosa D, Cavallini C, Marcuzzi M, Taglioli V, Bighi B, et al. A Benzodiazepine-Derived Molecule That Interferes with the Bio-Mechanical Properties of Glioblastoma-Astrocytoma Cells Altering Their Proliferation and Migration. International Journal of Molecular Sciences. 2025; 26(6):2767. https://doi.org/10.3390/ijms26062767
Chicago/Turabian StyleRagazzini, Gregorio, Andrea Mescola, Riccardo Tassinari, Alessia Gallerani, Chiara Zannini, Domenico Di Rosa, Claudia Cavallini, Martina Marcuzzi, Valentina Taglioli, Beatrice Bighi, and et al. 2025. "A Benzodiazepine-Derived Molecule That Interferes with the Bio-Mechanical Properties of Glioblastoma-Astrocytoma Cells Altering Their Proliferation and Migration" International Journal of Molecular Sciences 26, no. 6: 2767. https://doi.org/10.3390/ijms26062767
APA StyleRagazzini, G., Mescola, A., Tassinari, R., Gallerani, A., Zannini, C., Di Rosa, D., Cavallini, C., Marcuzzi, M., Taglioli, V., Bighi, B., Ettari, R., Zappavigna, V., Ventura, C., Alessandrini, A., & Corsi, L. (2025). A Benzodiazepine-Derived Molecule That Interferes with the Bio-Mechanical Properties of Glioblastoma-Astrocytoma Cells Altering Their Proliferation and Migration. International Journal of Molecular Sciences, 26(6), 2767. https://doi.org/10.3390/ijms26062767









