The Clinical Role of miRNAs in the Development and Treatment of Glioblastoma
Abstract
1. Introduction
2. General Molecular Features
3. Glioblastoma Management
4. Clinical Relevance of the Molecular Profile
- Astrocytoma, IDH-mutant: Previously, IDH-mutant tumors were classified as diffuse astrocytoma, anaplastic astrocytoma, or GBM. The latest classification now consolidates these into a single type of IDH-mutant astrocytoma, graded as 2, 3, or 4.
- Grading criteria: The grading of IDH-mutant diffuse astrocytic tumors is no longer based solely on histology. It also considers the presence of the CDKN2A/B homozygous deletion mutation, which results in a CNS WHO grade of 4, even if microvascular proliferation or necrosis is absent.
- GBM, IDH-wildtype: This classification identifies specific molecular markers for this tumor, such as the presence of a TERT promoter mutation (associated with increased telomerase activity, crucial for tumor cell immortalization) or EGFR gene amplification, leading to overexpression of the receptor and the combined gain of chromosome 7 and loss of chromosome 10 (+7/−10). If any of these markers are found in an IDH-wildtype diffuse astrocytic glioma in adults, the diagnosis should be GBM, IDH-wildtype.
- Pediatric patients: The diagnostic criteria for IDH-wildtype diffuse astrocytomas differ in pediatric patients, who are diagnosed using different categories of pediatric-type gliomas [2].
- Proneural group: This group is characterized by proneural gene expression patterns and RTK I/LGm6 DNA methylation profiles. This subgroup often shows amplifications of genes such as cyclin-dependent kinase 4 (CDK4) and platelet-derived growth factor alpha (PDGFRA). It is more prevalent among younger adults.
- Classical group: This group exhibits classical gene expression patterns and classic-like RTK II DNA methylation profiles. It is marked by frequent EGFR amplifications and the loss of CDKN2A/B genes.
- Mesenchymal group: This group OS enriched for tumors with neurofibromatosis type 1 (NF1) loss and increased infiltration by macrophages [22]. This subgroup is associated with a mesenchymal or mesenchymal-like subtype.
5. microRNAs (miRNAs) and GBM
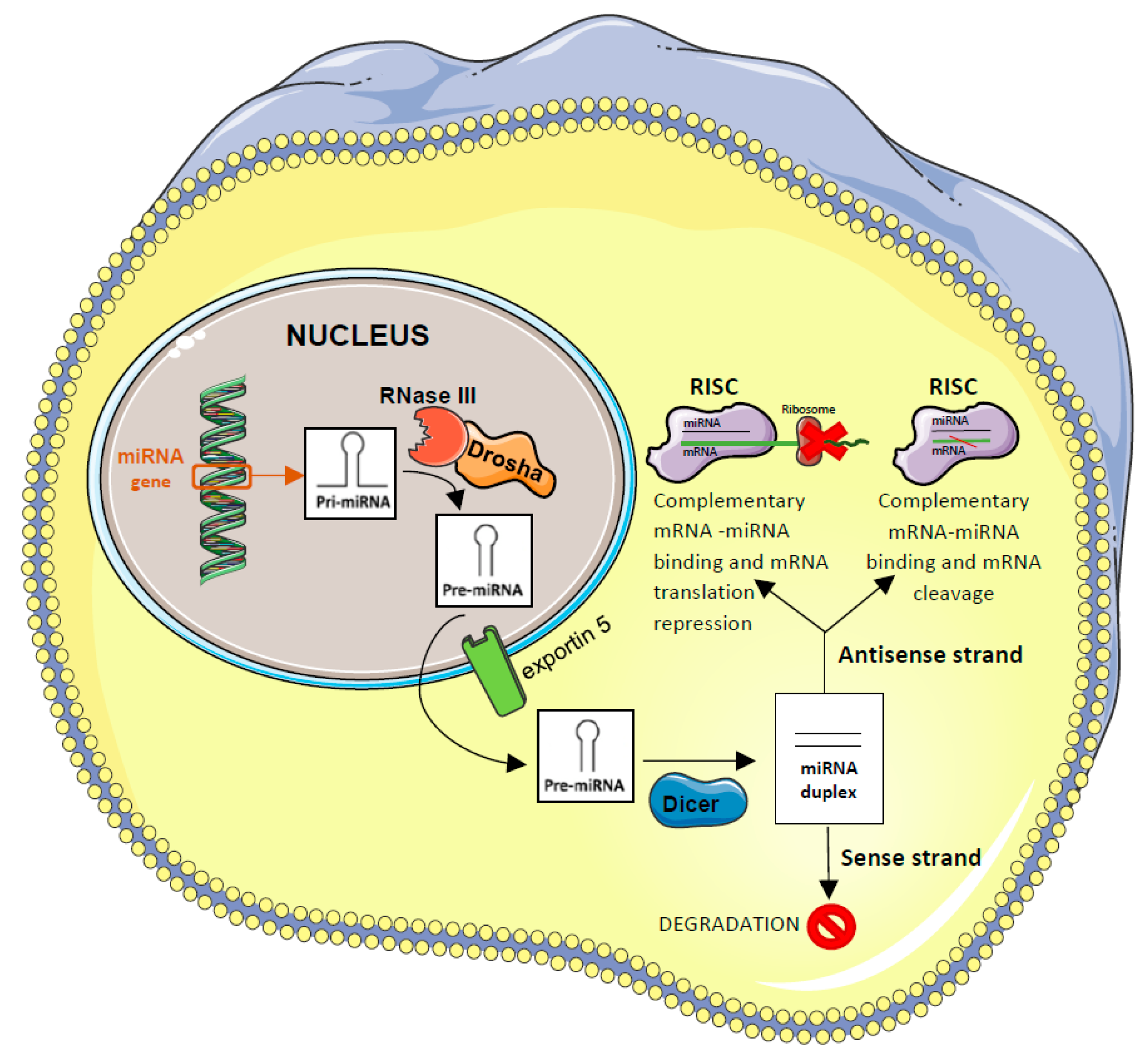
5.1. miRNAs Function and Biogenesis
5.2. miRNAs in Cancer
5.3. miRNAs in GBM
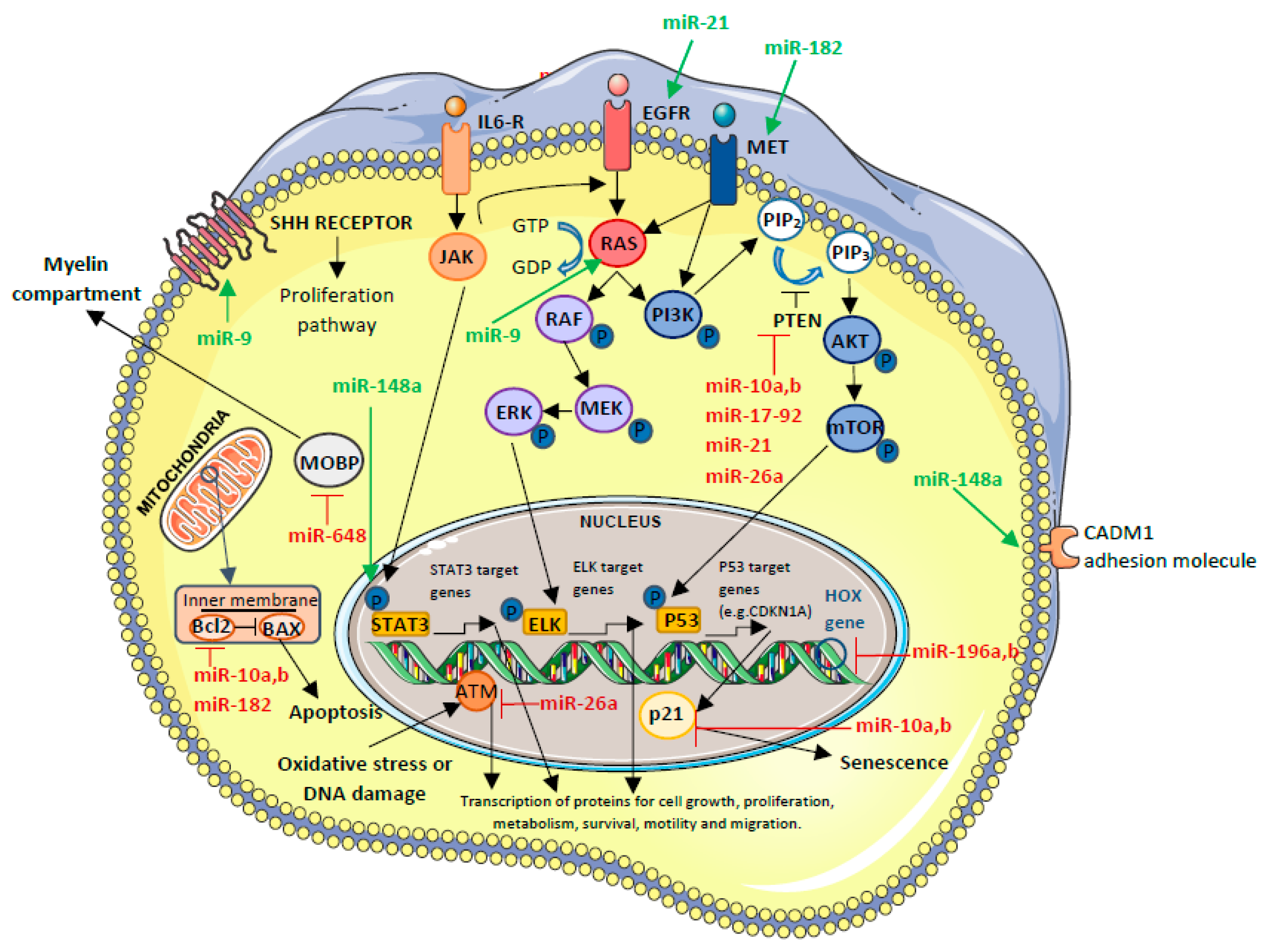
5.3.1. OncomiR-Upregulated miRNAs
OncomiR Involved in GBM Biogenesis
- miR-17-92 cluster
OncomiR Involved in GBM Prognosis
- miR-9
- miR-10a and miR-10b
- miR-148a
- miR-182
- miR-196a and miR-196b
OncomiR with Effect on Therapy Efficacy
- miR-26a
- miR-648
OncomiRs Involved in GBM Biogenesis That, in the Future, Will Have a Clinical Role Through Their Inhibition
- miR-21
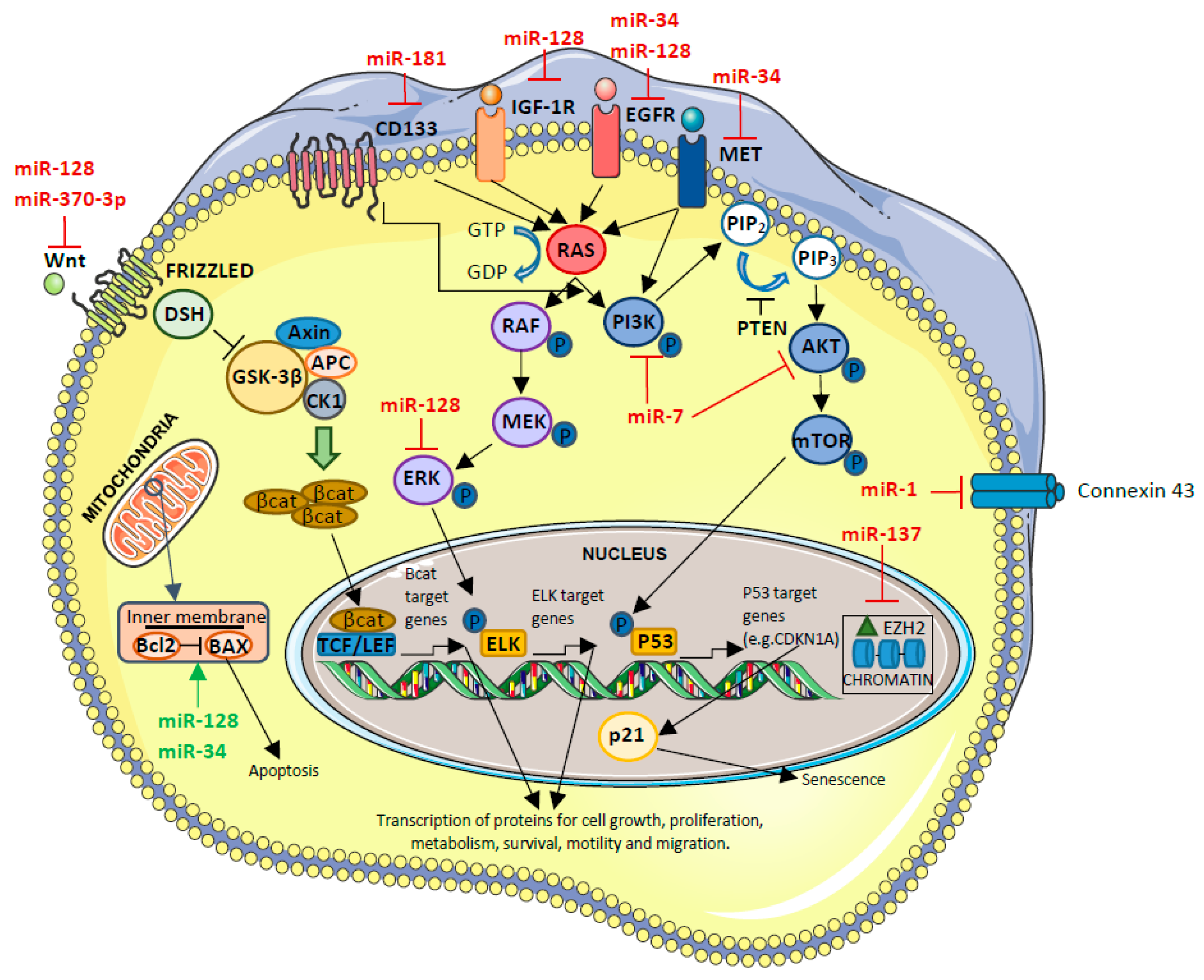
5.3.2. Tumor-Suppressor miRNAs-Downregulated miRNAs
Tumor-Suppressor miRNAs Involved in GBM Biogenesis
- miR-1
Tumor-Suppressor miRNAs Involved in GBM Prognosis
- miR-128
- miR-137
- miR-181 family
Tumor-Suppressor miRNAs with Effect on Therapy Efficacy
- miR-370-3p
Tumor-Suppressor miRNAs Involved in GBM Biogenesis That, in the Future, Will Have a Clinical Role Through Their Inhibition
- miR-7
- miR-34
| miRNA | Target | Expression in GBM | Function/Role in GBM if the miRNA is Overexpressed | Clinical Applications | References | |
|---|---|---|---|---|---|---|
| In Vitro | In Vivo | |||||
| miR-9 | -RAS and MYC -PTCH1 | ↑ | Cancer cell proliferation↑ Tumor cell transformation↑ Inflammation↑ Angiogenesis↑ Apoptosis↓ | Overexpression can enhance the resistance to TMZ in GBM cells (p < 0.05). | Associated with short-term survivors. | [51,52,53,54,55] |
| miR-10a, b | -CDKN1A, BIM, BCL2, TEAP2C, and PTEN -HOXD10 | ↑ | Cancer cell proliferation↑ Tumor cell migration↑ Invasion↑ EMT promotion↑ Apoptosis↓ GSC differentiation↑ | The expression is higher in GBM than in other gliomas. | [56,57,58,59,60,61,62] | |
| miR-17-92 cluster | -Some cell-cycle inhibitors, such as PTEN and CDKN1A | ↑ | Cancer cell proliferation↑ Apoptosis↓ GSC differentiation↑ | This cluster is associated with high aggressiveness, higher invasion, and replication capability. | [48,49,50] | |
| miR-21 | -PTEN, p53 -EGFR, Cyclin D1, and AKT2 -SPOCK1 -RECK and TIMP3 | ↑ | Cancer cell proliferation↑ Tumor cell migration↑ Invasion↑ | Hypothetically that silencing of this miRNA can be used, in the future, as a therapy in the treatment of GBM. | [35,48,56,59,86] | |
| miR-26a | -PTEN, ATM | ↑ | Cancer cell proliferation↑ Invasion↑ | Overexpression, reducing DNA repair ability and enhancing radio sensitivity to radiotherapy. | [57,80,81,82] | |
| miR-148a | -CADM1 -FIH1 | ↑ | Angiogenesis↑ Invasion↑ | Overexpressed in the plasma from the serum of GBM patients if compared to healthy cases. -Expressed in the high-risk group (i.e., patients characterized by low survival). | [56,64,65,66] | |
| miR-182 | -BCL2L12 -HIF2A -MET -CYLD -LRRC4 | ↑ | Uncontrolled cell proliferation↑ Apoptosis↑ GSC differentiation↑ | Correlates with better response to TMZ based chemotherapy and with better survival (p = 0.01/p = 0.04). | [68,69,70] | |
| miR-196a, b | -HOXB8, HOXC8, HOXD8, HOXA7, HOXB7 -ERG -HMGA2 -ANXA1 | ↑ | Cancer cell proliferation↑ Apoptosis↓ | Overexpression favors cells’ proliferation. | miR-196b expression correlated with OS (p = 0.01). | [75,76,77,78,79] |
| miR-648 | -MOBP | ↑ | Cancer cell proliferation↑ Invasion↑ | The expression by transfection enhanced responsivity of TMZ in MGMT-expressing T98G glioma cells. | Correlation between OS and miR-648 expression. | [79,83,84,85] |
| miRNA | Target | Expression in GBM | Function/Role in GBM if the miRNA Expression is Inhibited | Clinical Applications | References | |
|---|---|---|---|---|---|---|
| In Vitro | ||||||
| miR-1 | -Connexin-43 -G6PD | ↓ | Cancer cell proliferation↑ Tumor cell migration↑ Apoptosis↓ | Inhibition can enhance the cells proliferation and the sensitivity of GBM cells towards TMZ. | [56,87,88,89] | |
| miR-7 | -EGFR, AKT/PI3K pathway -PKM2 | ↓ | Cancer cell proliferation↑ GSC differentiation↑ | Transfection in U373-MG GBM cell line resulted in significant suppression of EGFR mRNA and protein, leading to the inhibition of cells’ duplication. | [35,97,98] | |
| miR-34 | -Bcl2, NOTCH, NUMB -CDK6 -EGFR -c-Met | ↓ | Cancer cell proliferation↑ Apoptosis↓ Invasion↑ | -In GSC cultures, the infection by ZIKV induced miR-34 expression, inhibiting the anti-apoptotic protein Bcl-2 and Numb, involved in GSC invasion. -In mouse models, ZIKV reduced brain tumor size and metastasis. | [99,100,101,102,103,104,105,106] | |
| miR-128 | -WNT -ERK -EGFR -IGF1R -Bcl2 -PDGFRA -Caspase | ↓ | Cancer cell proliferation↑ Apoptosis↓ | The low expression can be associated with high-grade glioma cell lines and, consequently, a worse prognosis. | [56,90,91] | |
| miR-137 | -EZH2 | ↓ | Cancer cell proliferation↑ Apoptosis↓ Angiogenesis↑ | Expression level of miR-137 was downregulated in GBM cells. | The low level of this miRNA was related to poor prognosis in GBM patients. | [56,92,93] |
| miR-181 family | -CD133 and BMI1 CCN1 | ↓ | Cancer cell proliferation↑ GSC differentiation↑ Apoptosis↓ Invasion↑ | -Low level of expression was related to poor prognosis in GBM patients. -Low level of expression of miR-181c or low expression of miR-181d, in combination with expression of miR-648, predicts the worst prognosis. | [78,84,87,94] | |
| miR-370-3p | -WNT -FOX01, FOXM1 and TGFβ. | ↓ | Cancer cell proliferation↑ Invasion↑ | When miR-370-3p is upregulated, GBM growth is inhibited. | [95,96] | |
5.4. Exosomal miRNAs and Cancer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncology 2020, 22, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23 (Suppl. S2), iii1–iii105. [Google Scholar] [CrossRef]
- Vijapura, C.; Saad Aldin, E.; Capizzano, A.A.; Policeni, B.; Sato, Y.; Moritani, T. Genetic Syndromes Associated with Central Nervous System Tumors. Radiographics 2017, 37, 258–280. [Google Scholar] [CrossRef]
- Jonsson, P.; Lin, A.L.; Young, R.J.; DiStefano, N.M.; Hyman, D.M.; Li, B.T.; Berger, M.F.; Zehir, A.; Ladanyi, M.; Solit, D.B.; et al. Genomic Correlates of Disease Progression and Treatment Response in Prospectively Characterized Gliomas. Clin. Cancer Res. 2019, 25, 5537–5547. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Hanna, C.; Kurian, K.M.; Williams, K.; Watts, C.; Jackson, A.; Carruthers, R.; Strathdee, K.; Cruickshank, G.; Dunn, L.; Erridge, S.; et al. Pharmacokinetics, safety, and tolerability of olaparib and temozolomide for recurrent glioblastoma: Results of the phase I OPARATIC trial. Neuro-Oncology 2020, 22, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Hau, E.; Shen, H.; Clark, C.; Graham, P.H.; Koh, E.S.; Mc Donald, K.L. The evolving roles and controversies of radiotherapy in the treatment of glioblastoma. J. Med. Radiat. Sci. 2016, 63, 114–123. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316, Erratum in JAMA 2018, 319, 1824. https://doi.org/10.1001/jama.2018.3431. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477, Erratum in Cell 2014, 157, 753. [Google Scholar] [CrossRef]
- Lin, Y.J.; Mashouf, L.A.; Lim, M. CAR T Cell Therapy in Primary Brain Tumors: Current Investigations and the Future. Front. Immunol. 2022, 13, 817296. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Gorlia, T.; Bendszus, M.; Taphoorn, M.; Sahm, F.; Harting, I.; Brandes, A.A.; Taal, W.; Domont, J.; Idbaih, A.; et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N. Engl. J. Med. 2017, 377, 1954–1963. [Google Scholar] [CrossRef]
- Louis, D.N.; Aldape, K.; Brat, D.J.; Capper, D.; Ellison, D.W.; Hawkins, C.; Paulus, W.; Perry, A.; Reifenberger, G.; Figarella-Branger, D.; et al. Announcing cIMPACT-NOW: The Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy. Acta Neuropathol. 2017, 133, 1–3. [Google Scholar] [CrossRef]
- Leske, H.; Dalgleish, R.; Lazar, A.J.; Reifenberger, G.; Cree, I.A. A common classification framework for histone sequence alterations in tumours: An expert consensus proposal. J. Pathol. 2021, 254, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Voronovich, Z.; Clark, K.; Hands, I.; Mannas, J.; Walsh, M.; Nikiforova, M.N.; Durbin, E.B.; Weiss, H.; Horbinski, C. Predicting the likelihood of an isocitrate dehydrogenase 1 or 2 mutation in diagnoses of infiltrative glioma. Neuro-Oncology 2014, 16, 1478–1483. [Google Scholar] [CrossRef]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Louis, D.N.; Wesseling, P.; Aldape, K.; Brat, D.J.; Capper, D.; Cree, I.A.; Eberhart, C.; Figarella-Branger, D.; Fouladi, M.; Fuller, G.N.; et al. cIMPACT-NOW update 6: New entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020, 30, 844–856. [Google Scholar] [CrossRef]
- Capper, D.; Stichel, D.; Sahm, F.; Jones, D.T.W.; Schrimpf, D.; Sill, M.; Schmid, S.; Hovestadt, V.; Reuss, D.E.; Koelsche, C.; et al. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: The Heidelberg experience. Acta Neuropathol. 2018, 136, 181–210. [Google Scholar] [CrossRef]
- Korshunov, A.; Schrimpf, D.; Ryzhova, M.; Sturm, D.; Chavez, L.; Hovestadt, V.; Sharma, T.; Habel, A.; Burford, A.; Jones, C.; et al. H3-/IDH-wild type pediatric glioblastoma is comprised of molecularly and prognostically distinct subtypes with associated oncogenic drivers. Acta Neuropathol. 2017, 134, 507–516. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; deCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56.e6, Erratum in Cancer Cell 2018, 33, 152. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Reifenberger, G.; Weber, R.G.; Riehmer, V.; Kaulich, K.; Willscher, E.; Wirth, H.; Gietzelt, J.; Hentschel, B.; Westphal, M.; Simon, M.; et al. Molecular characterization of long-term survivors of glioblastoma using genome- and transcriptome-wide profiling. Int. J. Cancer 2014, 135, 1822–1831. [Google Scholar] [CrossRef]
- Weller, M.; Felsberg, J.; Hartmann, C.; Berger, H.; Steinbach, J.P.; Schramm, J.; Westphal, M.; Schackert, G.; Simon, M.; Tonn, J.C.; et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: A prospective translational study of the German Glioma Network. J. Clin. Oncol. 2009, 27, 5743–5750. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Tabatabai, G.; Kästner, B.; Felsberg, J.; Steinbach, J.P.; Wick, A.; Schnell, O.; Hau, P.; Herrlinger, U.; Sabel, M.C.; et al. MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The DIRECTOR Trial. Clin. Cancer Res. 2015, 21, 2057–2064. [Google Scholar] [CrossRef]
- Malmström, A.; Grønberg, B.H.; Marosi, C.; Stupp, R.; Frappaz, D.; Schultz, H.; Abacioglu, U.; Tavelin, B.; Lhermitte, B.; Hegi, M.E.; et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012, 13, 916–926. [Google Scholar] [CrossRef]
- Tandel, S.G.; Biswas, M.; Kakde, G.O.; Tiwari, A.; Suri, S.H.; Turk, M.; Laird, J.R.; Asare, C.K.; Ankrah, A.A.; Khanna, N.N. A Review on a Deep Learning Perspective in Brain Cancer Classification. Cancers 2019, 11, 111. [Google Scholar] [CrossRef]
- DeWitt, J.C.; Jordan, J.T.; Frosch, M.P.; Samore, W.R.; Iafrate, A.J.; Louis, D.N.; Lennerz, J.K. Cost-effectiveness of IDH testing in diffuse gliomas according to the 2016 WHO classification of tumors of the central nervous system recommendations. Neuro-Oncology 2017, 19, 1640–1650. [Google Scholar] [CrossRef]
- Korshunov, A.; Chavez, L.; Sharma, T.; Ryzhova, M.; Schrimpf, D.; Stichel, D.; Capper, D.; Sturm, D.; Kool, M.; Habel, A.; et al. Epithelioid glioblastomas stratify into established diagnostic subsets upon integrated molecular analysis. Brain Pathol. 2018, 28, 656–662. [Google Scholar] [CrossRef]
- Körber, V.; Yang, J.; Barah, P.; Wu, Y.; Stichel, D.; Gu, Z.; Fletcher, M.N.C.; Jones, D.; Hentschel, B.; Lamszus, K.; et al. Evolutionary Trajectories of IDHWT Glioblastomas Reveal a Common Path of Early Tumorigenesis Instigated Years ahead of Initial Diagnosis. Cancer Cell 2019, 35, 692–704.e12. [Google Scholar] [CrossRef]
- Touat, M.; Li, Y.Y.; Boynton, A.N.; Spurr, L.F.; Iorgulescu, J.B.; Bohrson, C.L.; Cortes-Ciriano, I.; Birzu, C.; Geduldig, J.E.; Pelton, K.; et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 2020, 580, 517–523. [Google Scholar] [CrossRef]
- Barthel, F.P.; Johnson, K.C.; Varn, F.S.; Moskalik, A.D.; Tanner, G.; Kocakavuk, E.; Anderson, K.J.; Abiola, O.; Aldape, K.; Alfaro, K.D.; et al. Longitudinal molecular trajectories of diffuse glioma in adults. Nature 2019, 576, 112–120. [Google Scholar] [CrossRef]
- Hasan, H.; Afzal, M.; Castresana, J.S.; Shahi, M.H. A Comprehensive Review of miRNAs and Their Epigenetic Effects in Glioblastoma. Cells 2023, 12, 1578. [Google Scholar] [CrossRef]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef]
- Ouellet, D.L.; Perron, M.P.; Gobeil, L.A.; Plante, P.; Provost, P. MicroRNAs in gene regulation: When the smallest governs it all. J. Biomed. Biotechnol. 2006, 2006, 69616. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Leonov, G.; Shah, K.; Yee, D.; Timmis, J.; Sharp, T.V.; Lagos, D. Suppression of AGO2 by miR-132 as a determinant of miRNA-mediated silencing in human primary endothelial cells. Int. J. Biochem. Cell Biol. 2015, 69, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, X.; Cobb, G.P.; Anderson, T.A. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007, 302, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sempere, L.F.; Azmi, A.S.; Moore, A. microRNA-based diagnostic and therapeutic applications in cancer medicine. Wiley Interdiscip. Rev. RNA 2021, 12, e1662. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Hou, W. Regulation of angiogenesis by microRNAs in cancer. Mol. Med. Rep. 2021, 24, 583. [Google Scholar] [CrossRef]
- Uzuner, E.; Ulu, G.T.; Gürler, S.B.; Baran, Y. The Role of miRNA in Cancer: Pathogenesis, Diagnosis, and Treatment. Methods Mol. Biol. 2022, 2257, 375–422. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Horton, S.; Saldivar, J.C.; Miuma, S.; Stampfer, M.R.; Heerema, N.A.; Huebner, K. Common chromosome fragile sites in human and murine epithelial cells and FHIT/FRA3B loss-induced global genome instability. Genes Chromosomes Cancer 2013, 52, 1017–1029, Erratum in Genes Chromosomes Cancer 2019, 58, 824. https://doi.org/10.1002/gcc.22797. [Google Scholar] [CrossRef]
- Behrooz, A.B.; Latifi-Navid, H.; Nezhadi, A.; Świat, M.; Los, M.; Jamalpoor, Z.; Ghavami, S. Molecular mechanisms of microRNAs in glioblastoma pathogenesis. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119482. [Google Scholar] [CrossRef]
- Makowska, M.; Smolarz, B.; Romanowicz, H. microRNAs (miRNAs) in Glioblastoma Multiforme (GBM)-Recent Literature Review. Int. J. Mol. Sci. 2023, 24, 3521. [Google Scholar] [CrossRef]
- Barciszewska, A.M. MicroRNAs as efficient biomarkers in high-grade gliomas. Folia Neuropathol. 2016, 54, 369–374. [Google Scholar] [CrossRef]
- Regazzo, G.; Terrenato, I.; Spagnuolo, M.; Carosi, M.; Cognetti, G.; Cicchillitti, L.; Sperati, F.; Villani, V.; Carapella, C.; Piaggio, G.; et al. A restricted signature of serum miRNAs distinguishes glioblastoma from lower grade gliomas. J. Exp. Clin. Cancer Res. 2016, 35, 124. [Google Scholar] [CrossRef]
- Evers, L.; Schäfer, A.; Pini, R.; Zhao, K.; Stei, S.; Nimsky, C.; Bartsch, J.W. Identification of Dysregulated microRNAs in Glioblastoma Stem-like Cells. Brain Sci. 2023, 13, 350. [Google Scholar] [CrossRef]
- Coolen, M.; Katz, S.; Bally-Cuif, L. miR-9: A versatile regulator of neurogenesis. Front. Cell. Neurosci. 2013, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Cui, H. Epigenetic modulation of metabolism in glioblastoma. Semin. Cancer Biol. 2019, 57, 45–51. [Google Scholar] [CrossRef]
- Munoz, J.L.; Rodriguez-Cruz, V.; Rameshwar, P. High expression of miR-9 in CD133+ glioblastoma cells in chemoresistance to temozolomide. J. Cancer Stem Cell Res. 2015, 3, e1003. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.Q.; Wei, N.L.; Mu, L.Y.; Wang, X.Q.; Zhang, Y.N.; Zhou, W.N.; Pan, Y.W. A 4-miRNAs signature predicts survival in glioblastoma multiforme patients. Cancer Biomark. Sect. A Dis. Markers 2017, 20, 443–452. [Google Scholar] [CrossRef]
- Geng, L.; Xu, J.; Zhu, Y.; Hu, X.; Liu, Y.; Yang, K.; Xiao, H.; Zou, Y.; Liu, H.; Ji, J.; et al. Targeting miR-9 in Glioma Stem Cell-Derived Extracellular Vesicles: A Novel Diagnostic and Therapeutic Biomarker. Transl. Oncol. 2022, 22, 101451. [Google Scholar] [CrossRef]
- Rezaei, O.; Honarmand, K.; Nateghinia, S.; Taheri, M.; Ghafouri-Fard, S. miRNA signature in glioblastoma: Potential biomarkers and therapeutic targets. Exp. Mol. Pathol. 2020, 117, 104550. [Google Scholar] [CrossRef]
- Song, Z.; Xue, Z.; Wang, Y.; Imran, M.; Assiri, M.; Fahad, S. Insights into the roles of non-coding RNAs and angiogenesis in glioblastoma: An overview of current research and future perspectives. Biochim. Biophys. Acta Gen. Subj. 2024, 1868, 130567. [Google Scholar] [CrossRef] [PubMed]
- Santolini, E.; Puri, C.; Salcini, A.E.; Gagliani, M.C.; Pelicci, P.G.; Tacchetti, C.; Di Fiore, P.P. Numb is an endocytic protein. J. Cell Biol. 2000, 151, 1345–1352. [Google Scholar] [CrossRef]
- de Menezes, M.R.; Acioli, M.E.A.; da Trindade, A.C.L.; da Silva, S.P.; de Lima, R.E.; da Silva Teixeira, V.G.; Vasconcelos, L.R.S. Potential role of microRNAs as biomarkers in human glioblastoma: A mini systematic review from 2015 to 2020. Mol. Biol. Rep. 2021, 48, 4647–4658. [Google Scholar] [CrossRef]
- Junior, L.G.D.; Baroni, M.; Lira, R.C.P.; Teixeira, S.; Fedatto, P.F.; Silveira, V.S.; Suazo, V.K.; Veronez, L.C.; Panepucci, R.A.; Antônio, D.S.M.; et al. High-throughput microRNA profile in adult and pediatric primary glioblastomas: The role of miR-10b-5p and miR-630 in the tumor aggressiveness. Mol. Biol. Rep. 2020, 47, 6949–6959. [Google Scholar] [CrossRef]
- Gabriely, G.; Yi, M.; Narayan, R.S.; Niers, J.M.; Wurdinger, T.; Imitola, J.; Ligon, K.L.; Kesari, S.; Esau, C.; Stephens, R.M.; et al. Human glioma growth is controlled by microRNA-10b. Cancer Res. 2011, 71, 3563–3572. [Google Scholar] [CrossRef]
- Sun, L.; Yan, W.; Wang, Y.; Sun, G.; Luo, H.; Zhang, J.; Wang, X.; You, Y.; Yang, Z.; Liu, N. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011, 1389, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhao, X.; Ming, J.; Liu, X.; Liu, D.; Jiang, C. Stepwise detection and evaluation reveal miR-10b and miR-222 as a remarkable prognostic pair for glioblastoma. Oncogene 2019, 38, 6142–6157. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Zhu, A.; Gong, L. Exosomes of glioma cells deliver miR-148a to promote proliferation and metastasis of glioblastoma via targeting CADM1. Bull. Du Cancer 2018, 105, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Patric, I.R.; Somasundaram, K. A ten-microRNA expression signature predicts survival in glioblastoma. PLoS ONE 2011, 6, e17438. [Google Scholar] [CrossRef]
- Vo, D.T.; Qiao, M.; Smith, A.D.; Burns, S.C.; Brenner, A.J.; Penalva, L.O. The oncogenic RNA-binding protein Musashi1 is regulated by tumor suppressor miRNAs. RNA Biol. 2011, 8, 817–828. [Google Scholar] [CrossRef]
- Shea, A.; Harish, V.; Afzal, Z.; Chijioke, J.; Kedir, H.; Dusmatova, S.; Roy, A.; Ramalinga, M.; Harris, B.; Blancato, J.; et al. MicroRNAs in glioblastoma multiforme pathogenesis and therapeutics. Cancer Med. 2016, 5, 1917–1946. [Google Scholar] [CrossRef]
- Dambal, S.; Shah, M.; Mihelich, B.; Nonn, L. The microRNA-183 cluster: The family that plays together stays together. Nucleic Acids Res. 2015, 43, 7173–7188. [Google Scholar] [CrossRef]
- Schneider, B.; William, D.; Lamp, N.; Zimpfer, A.; Henker, C.; Classen, C.F.; Erbersdobler, A. The miR-183/96/182 cluster is upregulated in glioblastoma carrying EGFR amplification. Mol. Cell. Biochem. 2022, 477, 2297–2307. [Google Scholar] [CrossRef]
- Vilar, J.B.; Christmann, M.; Tomicic, M.T. Alterations in Molecular Profiles Affecting Glioblastoma Resistance to Radiochemotherapy: Where Does the Good Go? Cancers 2022, 14, 2416. [Google Scholar] [CrossRef]
- Kouri, F.M.; Ritner, C.; Stegh, A.H. miRNA-182 and the regulation of the glioblastoma phenotype—Toward miRNA-based precision therapeutics. Cell Cycle 2015, 14, 3794–3800. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shen, J.; Hodges, T.R.; Song, R.; Fuller, G.N.; Heimberger, A.B. Serum microRNA profiling in patients with glioblastoma: A survival analysis. Mol. Cancer 2017, 16, 59. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, L.; Song, Z.; Guo, C.; Zhu, J.; Li, Z.; Zhu, S. Potential Diagnostic and Prognostic Value of Plasma Circulating MicroRNA-182 in Human Glioma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 855–862. [Google Scholar] [CrossRef]
- Jiang, L.; Mao, P.; Song, L.; Wu, J.; Huang, J.; Lin, C.; Yuan, J.; Qu, L.; Cheng, S.Y.; Li, J. miR-182 as a prognostic marker for glioma progression and patient survival. Am. J. Pathol. 2010, 177, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Zhang, L.; Weakley, S.M.; Yao, Q. MicroRNA-196: Critical roles and clinical applications in development and cancer. J. Cell Mol. Med. 2011, 15, 14–23. [Google Scholar] [CrossRef]
- Takkar, S.; Sharma, V.; Ghosh, S.; Suri, A.; Sarkar, C.; Kulshreshtha, R. Hypoxia-inducible miR-196a modulates glioblastoma cell proliferation and migration through complex regulation of NRAS. Cell. Oncol. 2021, 44, 433–451. [Google Scholar] [CrossRef]
- Hassan, A.; Mosley, J.; Singh, S.; Zinn, P.O. A Comprehensive Review of Genomics and Noncoding RNA in Gliomas. Top. Magn. Reson. Imaging 2017, 26, 3–14. [Google Scholar] [CrossRef]
- Lakomy, R.; Sana, J.; Hankeova, S.; Fadrus, P.; Kren, L.; Lzicarova, E.; Svoboda, M.; Dolezelova, H.; Smrcka, M.; Vyzula, R.; et al. miR-195, miR-196b, miR-181c, miR-21 expression levels and O-6-methylguanine-DNA methyltransferase methylation status are associated with clinical outcome in glioblastoma patients. Cancer Sci. 2011, 102, 2186–2190. [Google Scholar] [CrossRef]
- Cardia, A.; Epistolio, S.; Zaed, I.; Sahnane, N.; Cerutti, R.; Cipriani, D.; Barizzi, J.; Spina, P.; Stefanini, F.M.; Cerati, M.; et al. Identification of MGMT Downregulation Induced by miRNA in Glioblastoma and Possible Effect on Temozolomide Sensitivity. J. Clin. Med. 2023, 12, 2061. [Google Scholar] [CrossRef]
- Huse, J.T.; Brennan, C.; Hambardzumyan, D.; Wee, B.; Pena, J.; Rouhanifard, S.H.; Sohn-Lee, C.; le Sage, C.; Agami, R.; Tuschl, T.; et al. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009, 23, 1327–1337. [Google Scholar] [CrossRef]
- Guo, P.; Lan, J.; Ge, J.; Nie, Q.; Guo, L.; Qiu, Y.; Mao, Q. miR-26a enhances the radiosensitivity of glioblastoma multiforme cells through targeting of ataxia-telangiectasia mutated. Exp. Cell Res. 2014, 320, 200–208. [Google Scholar] [CrossRef]
- ParvizHamidi, M.; Haddad, G.; Ostadrahimi, S.; Ostadrahimi, N.; Sadeghi, S.; Fayaz, S.; Fard-Esfahani, P. Circulating miR-26a and miR-21 as biomarkers for glioblastoma multiform. Biotechnol. Appl. Biochem. 2019, 66, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Siegel, S.R.; Mackenzie, J.; Chaplin, G.; Jablonski, N.G.; Griffiths, L. Circulating microRNAs involved in multiple sclerosis. Mol. Biol. Rep. 2012, 39, 6219–6225. [Google Scholar] [CrossRef] [PubMed]
- Epistolio, S.; Dazio, G.; Zaed, I.; Sahnane, N.; Cipriani, D.; Polinelli, F.; Barizzi, J.; Spina, P.; Stefanini, F.M.; Cerati, M.; et al. Clinical Relevance and Interplay between miRNAs in Influencing Glioblastoma Multiforme Prognosis. Cells 2024, 13, 276. [Google Scholar] [CrossRef]
- Kreth, S.; Limbeck, E.; Hinske, L.C.; Schütz, S.V.; Thon, N.; Hoefig, K.; Egensperger, R.; Kreth, F.W. In human glioblastomas transcript elongation by alternative polyadenylation and miRNA targeting is a potent mechanism of MGMT silencing. Acta Neuropathol. 2013, 125, 671–681. [Google Scholar] [CrossRef]
- Shaikh, M.A.J.; Altamimi, A.S.A.; Afzal, M.; Gupta, G.; Singla, N.; Gilhotra, R.; Kazmi, I.; Alzarea, S.I.; Prasher, P.; Singh, S.K.; et al. Unraveling the impact of miR-21 on apoptosis regulation in glioblastoma. Pathol. Res. Pract. 2024, 254, 155121. [Google Scholar] [CrossRef]
- Kalkan, R.; Atli, E.İ. The Impacts of miRNAs in Glioblastoma Progression. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 137–142. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, Y.; Sims, M.; Cai, C.; Pfeffer, L.M. MicroRNA-1 suppresses glioblastoma in preclinical models by targeting fibronectin. Cancer Lett. 2019, 465, 59–67. [Google Scholar] [CrossRef]
- Liu, C.; Ge, Y.Y.; Xie, X.X.; Luo, B.; Shen, N.; Liao, X.S.; Bi, S.Q.; Xu, T.; Xiao, S.W.; Zhang, Q.M. Identification of Dysregulated microRNAs in Glioma Using RNA-sequencing. Curr. Med. Sci. 2021, 41, 356–367. [Google Scholar] [CrossRef]
- Marumoto, T.; Saya, H. Molecular biology of glioma. Adv. Exp. Med. Biol. 2012, 746, 2–11. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Morais, C.M.; Pena, F.; Marante, T.; Cunha, P.P.; Jurado, A.S.; Pedroso de Lima, M.C. Differentiation of glioblastoma stem cells promoted by miR-128 or miR-302a overexpression enhances senescence-associated cytotoxicity of axitinib. Hum. Mol. Genet. 2021, 30, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Balandeh, E.; Mohammadshafie, K.; Mahmoudi, Y.; Hossein Pourhanifeh, M.; Rajabi, A.; Bahabadi, Z.R.; Mohammadi, A.H.; Rahimian, N.; Hamblin, M.R.; Mirzaei, H. Roles of Non-coding RNAs and Angiogenesis in Glioblastoma. Front. Cell Dev. Biol. 2021, 9, 716462. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, G.; Gu, Z.; Guo, Z. miR-137 inhibits proliferation and angiogenesis of human glioblastoma cells by targeting EZH2. J. Neuro-Oncol. 2015, 122, 481–489. [Google Scholar] [CrossRef]
- Li, Y.; Fan, S.; Xia, W.; Qiao, B.; Huang, K.; Zhou, J.; Liang, M. miR-181b suppresses angiogenesis by directly targeting cellular communication network factor 1. Lab. Investig. J. Tech. Methods Pathol. 2021, 101, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Nadaradjane, A.; Briand, J.; Bougras-Cartron, G.; Disdero, V.; Vallette, F.M.; Frenel, J.S.; Cartron, P.F. miR-370-3p Is a Therapeutic Tool in Anti-glioblastoma Therapy but Is Not an Intratumoral or Cell-free Circulating Biomarker. Mol. Ther. Nucleic Acids 2018, 13, 642–650. [Google Scholar] [CrossRef]
- Kirstein, A.; Schmid, T.E.; Combs, S.E. The Role of miRNA for the Treatment of MGMT Unmethylated Glioblastoma Multiforme. Cancers 2020, 12, 1099. [Google Scholar] [CrossRef]
- Morales-Martínez, M.; Vega, M.I. Role of MicroRNA-7 (miR-7) in Cancer Physiopathology. Int. J. Mol. Sci. 2022, 23, 9091. [Google Scholar] [CrossRef]
- Alamdari-Palangi, V.; Amini, R.; Karami, H. miRNA-7 enhances erlotinib sensitivity of glioblastoma cells by blocking the IRS-1 and IRS-2 expression. J. Pharm. Pharmacol. 2020, 72, 531–538. [Google Scholar] [CrossRef]
- Li, Y.; Guessous, F.; Zhang, Y.; Dipierro, C.; Kefas, B.; Johnson, E.; Marcinkiewicz, L.; Jiang, J.; Yang, Y.; Schmittgen, T.D.; et al. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009, 69, 7569–7576. [Google Scholar] [CrossRef]
- Bazzoni, R.; Bentivegna, A. Role of Notch Signaling Pathway in Glioblastoma Pathogenesis. Cancers 2019, 11, 292. [Google Scholar] [CrossRef]
- Janaki Ramaiah, M.; Divyapriya, K.; Kartik Kumar, S.; Rajesh, Y.B.R.D. Drug-induced modifications and modulations of microRNAs and long non-coding RNAs for future therapy against Glioblastoma Multiforme. Gene 2020, 723, 144126. [Google Scholar] [CrossRef]
- Francipane, M.G.; Douradinha, B.; Chinnici, C.M.; Russelli, G.; Conaldi, P.G.; Iannolo, G. Zika Virus: A New Therapeutic Candidate for Glioblastoma Treatment. Int. J. Mol. Sci. 2021, 22, 10996. [Google Scholar] [CrossRef]
- Zhu, Z.; Gorman, M.J.; McKenzie, L.D.; Chai, J.N.; Hubert, C.G.; Prager, B.C.; Fernandez, E.; Richner, J.M.; Zhang, R.; Shan, C.; et al. Zika virus has oncolytic activity against glioblastoma stem cells. J. Exp. Med. 2017, 214, 2843–2857. [Google Scholar] [CrossRef]
- Lubin, J.; Zhang, R.R.; Kuo, J. Zika Virus has Oncolytic Activity Against Glioblastoma Stem Cells. Neurosurgery 2018, 82, E113–E114. [Google Scholar] [CrossRef] [PubMed]
- Kaid, C.; Goulart, E.; Caires-Júnior, L.C.; Araujo, B.H.S.; Schanoski, A.S.; Bueno, H.; Silva, K.A.T.; Astray, R.M.; Assoni, A.F.; Júnior, A.F.R.; et al. Zika Virus Selectively Kills Aggressive Human Embryonal CNS Tumor Cells In Vitro and In Vivo. Cancer Res. 2018, 78, 3363–3374. [Google Scholar] [CrossRef] [PubMed]
- Baronti, C.; Piorkowski, G.; Charrel, R.N.; Boubis, L.; Leparc-Goffart, I.; de Lamballerie, X. Complete Coding Sequence of Zika Virus from a French Polynesia Outbreak in 2013. Genome Announc. 2014, 2, e00500-14. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Guduric-Fuchs, J.; O’Connor, A.; Camp, B.; O’Neill, C.L.; Medina, R.J.; Simpson, D.A. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genom. 2012, 13, 357. [Google Scholar] [CrossRef]
- Ohshima, K.; Inoue, K.; Fujiwara, A.; Hatakeyama, K.; Kanto, K.; Watanabe, Y.; Muramatsu, K.; Fukuda, Y.; Ogura, S.I.; Yamaguchi, K.; et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS ONE 2010, 5, e13247. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epistolio, S.; Spina, P.; Zaed, I.; Cardia, A.; Marchi, F.; Frattini, M. The Clinical Role of miRNAs in the Development and Treatment of Glioblastoma. Int. J. Mol. Sci. 2025, 26, 2723. https://doi.org/10.3390/ijms26062723
Epistolio S, Spina P, Zaed I, Cardia A, Marchi F, Frattini M. The Clinical Role of miRNAs in the Development and Treatment of Glioblastoma. International Journal of Molecular Sciences. 2025; 26(6):2723. https://doi.org/10.3390/ijms26062723
Chicago/Turabian StyleEpistolio, Samantha, Paolo Spina, Ismail Zaed, Andrea Cardia, Francesco Marchi, and Milo Frattini. 2025. "The Clinical Role of miRNAs in the Development and Treatment of Glioblastoma" International Journal of Molecular Sciences 26, no. 6: 2723. https://doi.org/10.3390/ijms26062723
APA StyleEpistolio, S., Spina, P., Zaed, I., Cardia, A., Marchi, F., & Frattini, M. (2025). The Clinical Role of miRNAs in the Development and Treatment of Glioblastoma. International Journal of Molecular Sciences, 26(6), 2723. https://doi.org/10.3390/ijms26062723







