Selective Label-Free Detection of Imidacloprid by a Graphene Quantum Dot Fluorescent Probe
Abstract
1. Introduction
2. Results and Discussion
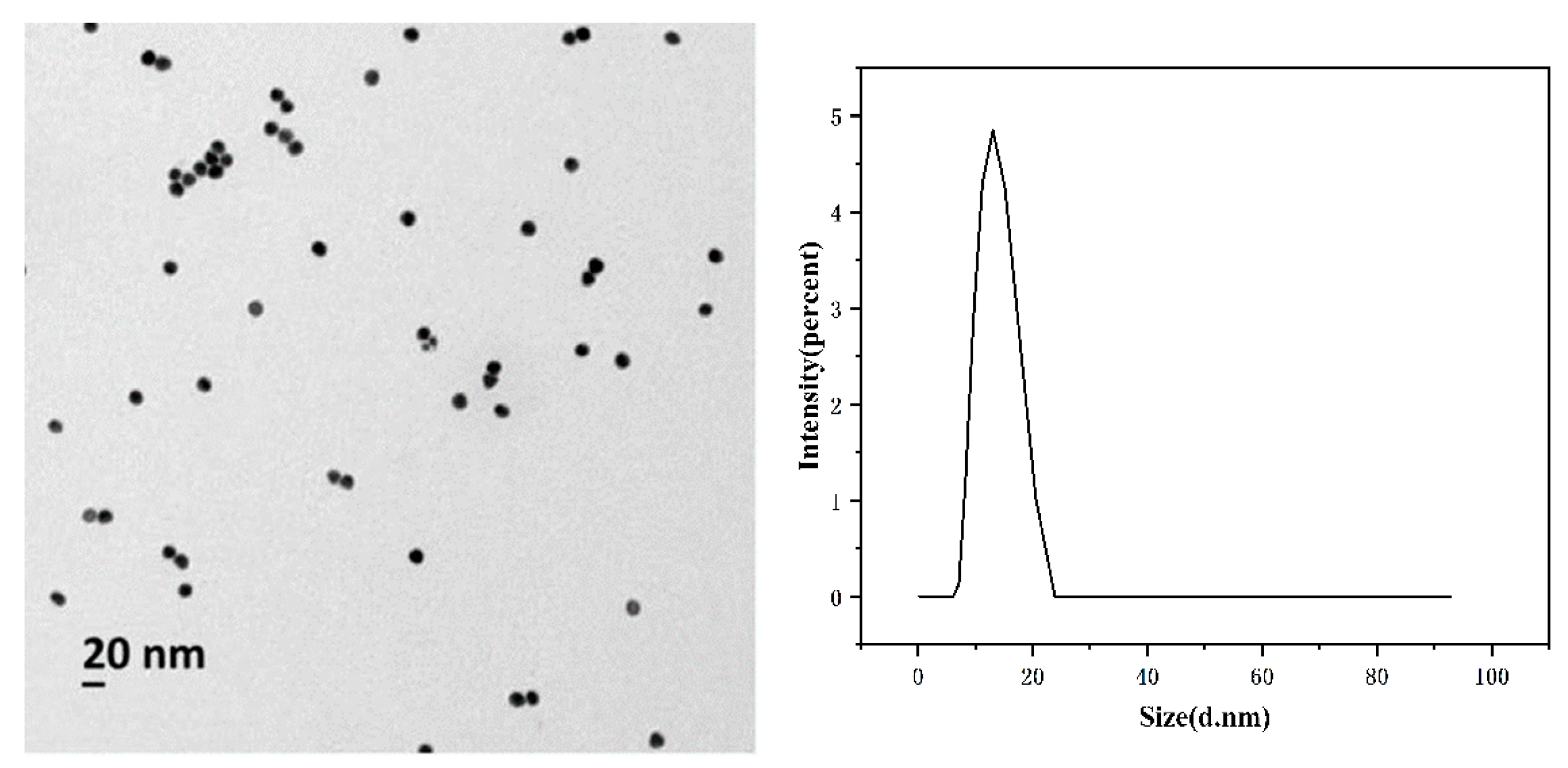
2.1. Characterization of AuNPs
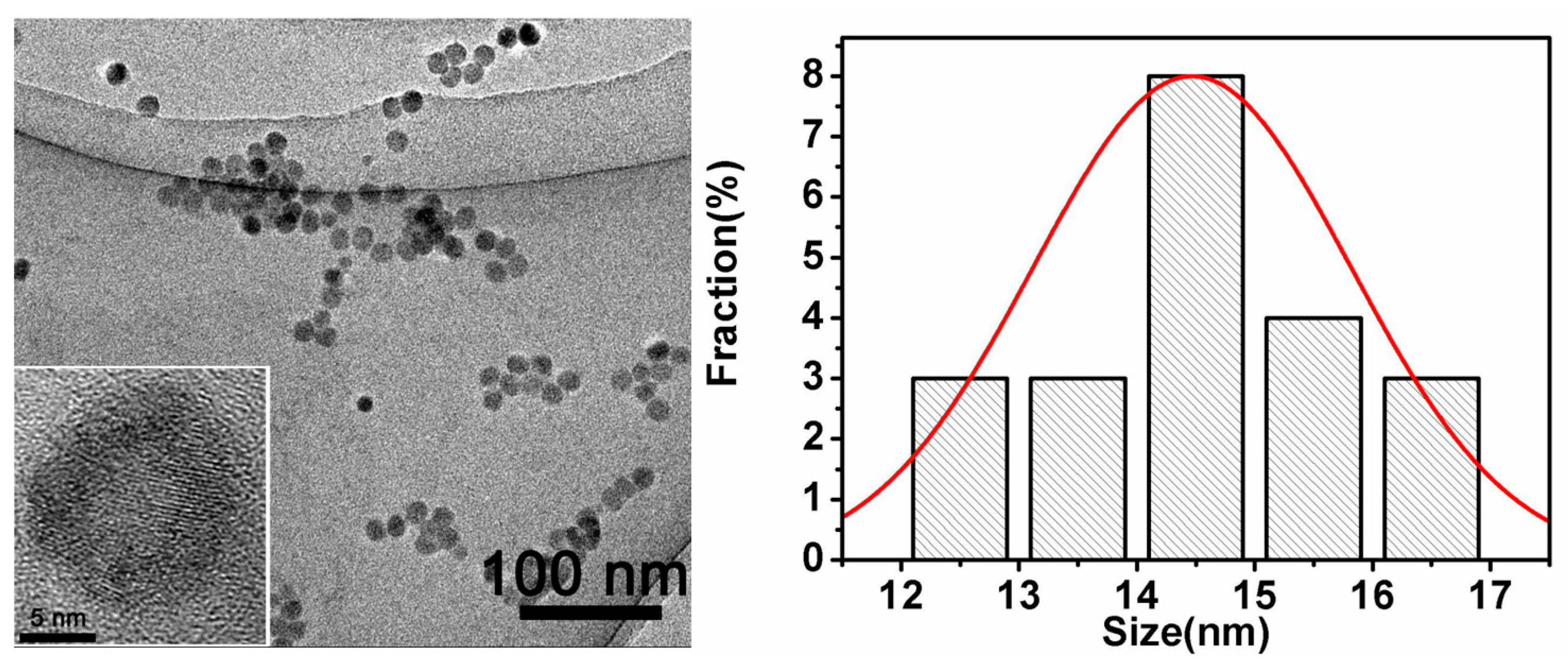
2.2. Characterization of GQDs
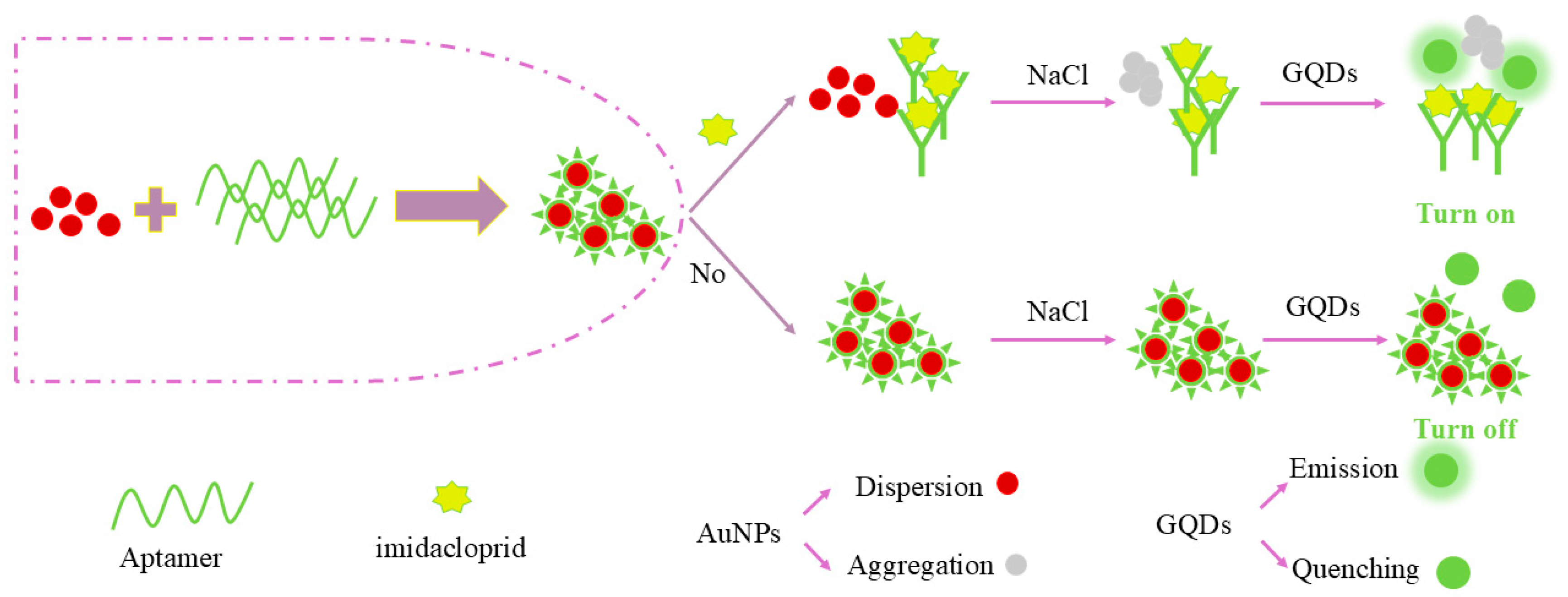
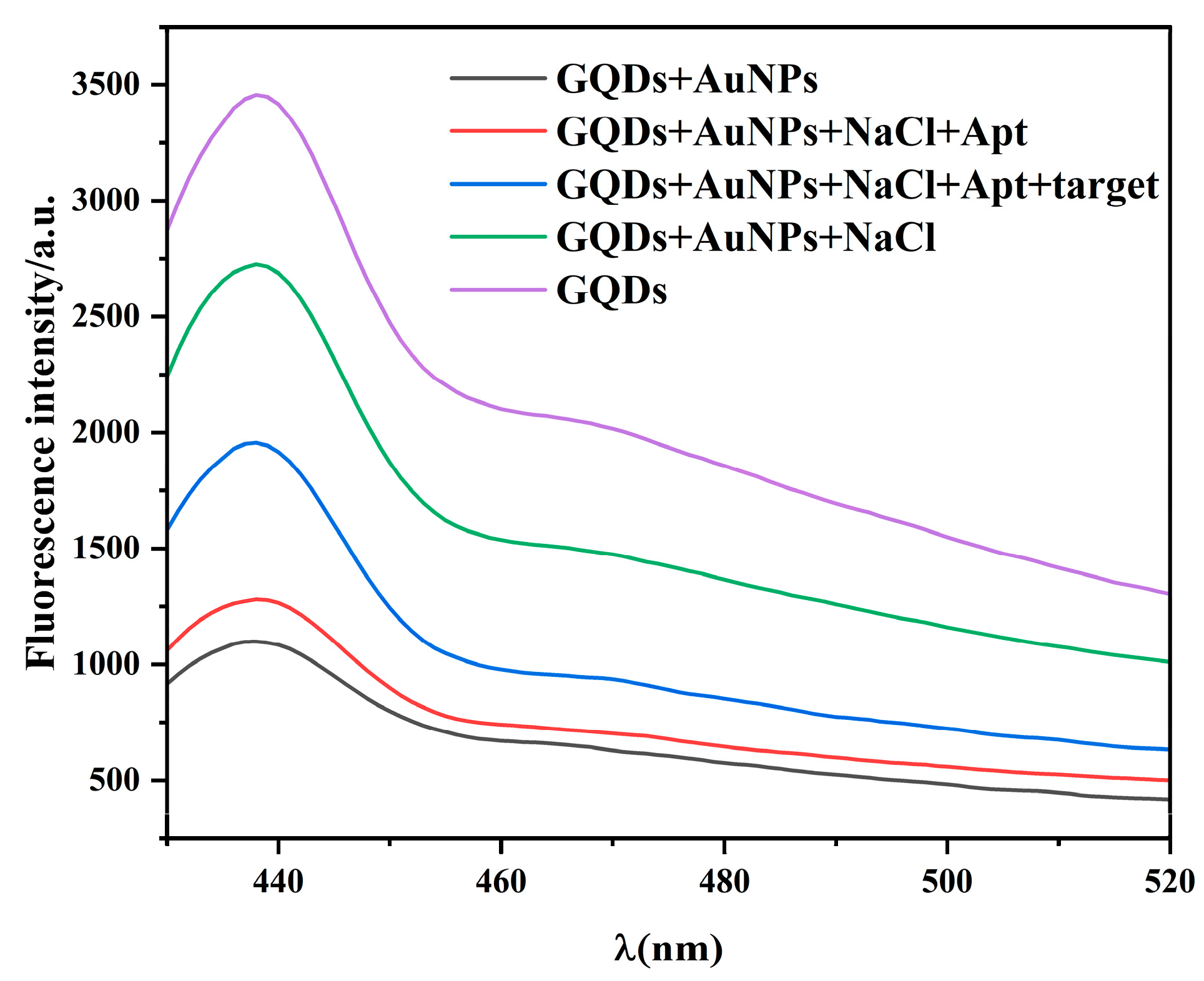
2.3. Feasibility Analysis
2.4. Optimization of Imidacloprid Detection Conditions
2.4.1. Effect of Aptamer Concentration on Biosensors
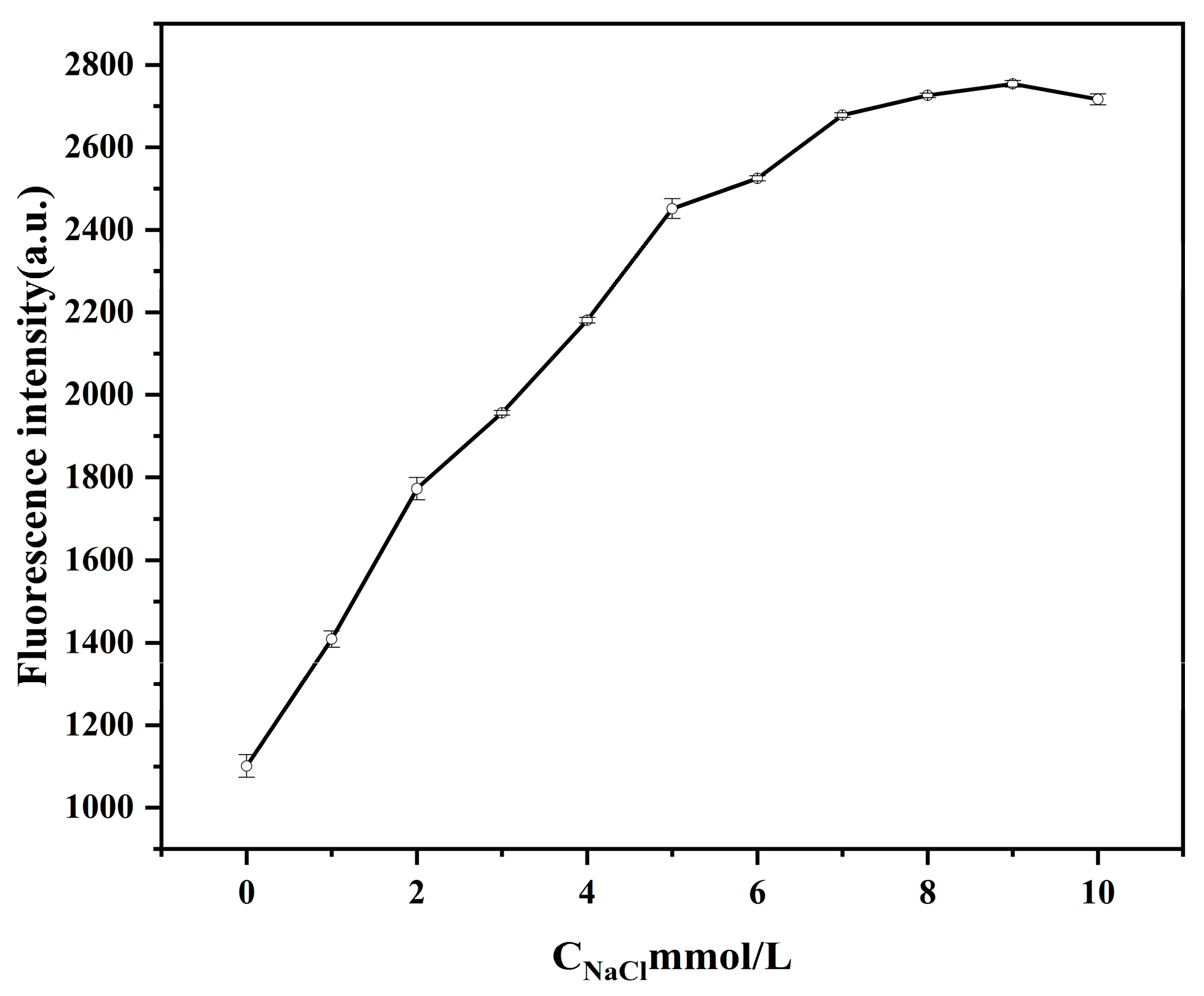
2.4.2. Effect of NaCl Concentration on the Performance of Biosensors
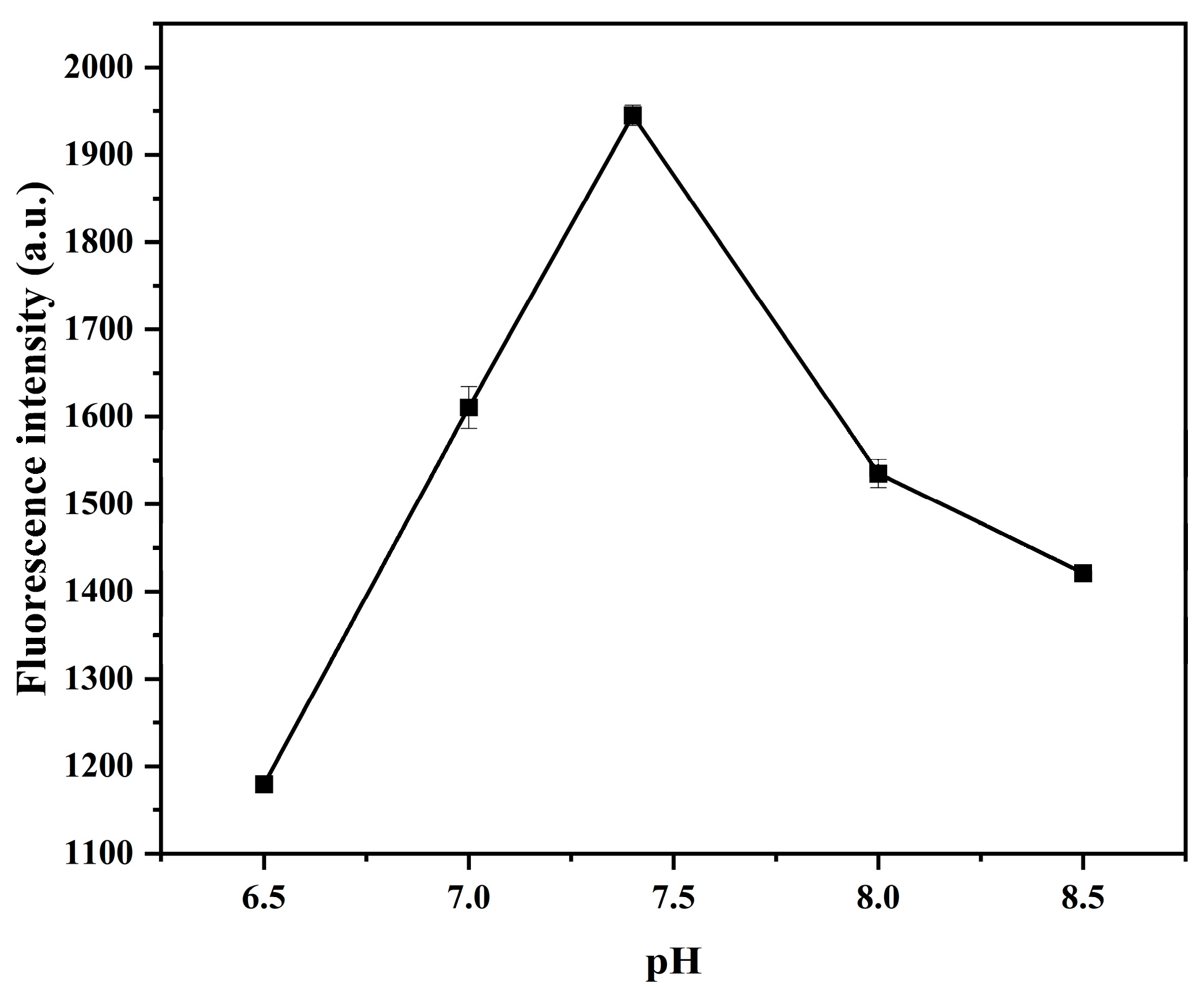
2.4.3. Study on pH of Buffer Solution
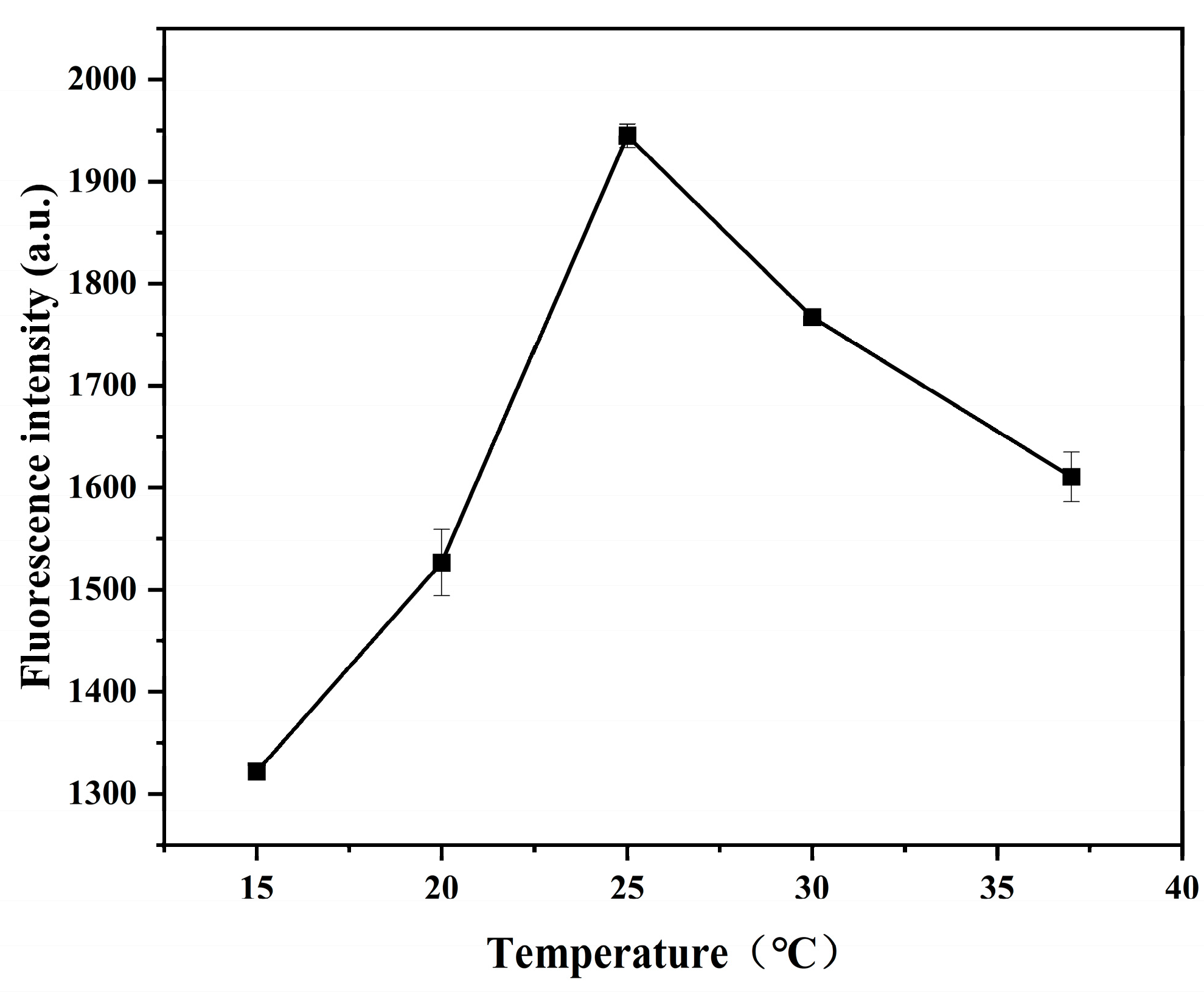
2.4.4. Influence of Temperature of the Sensing System
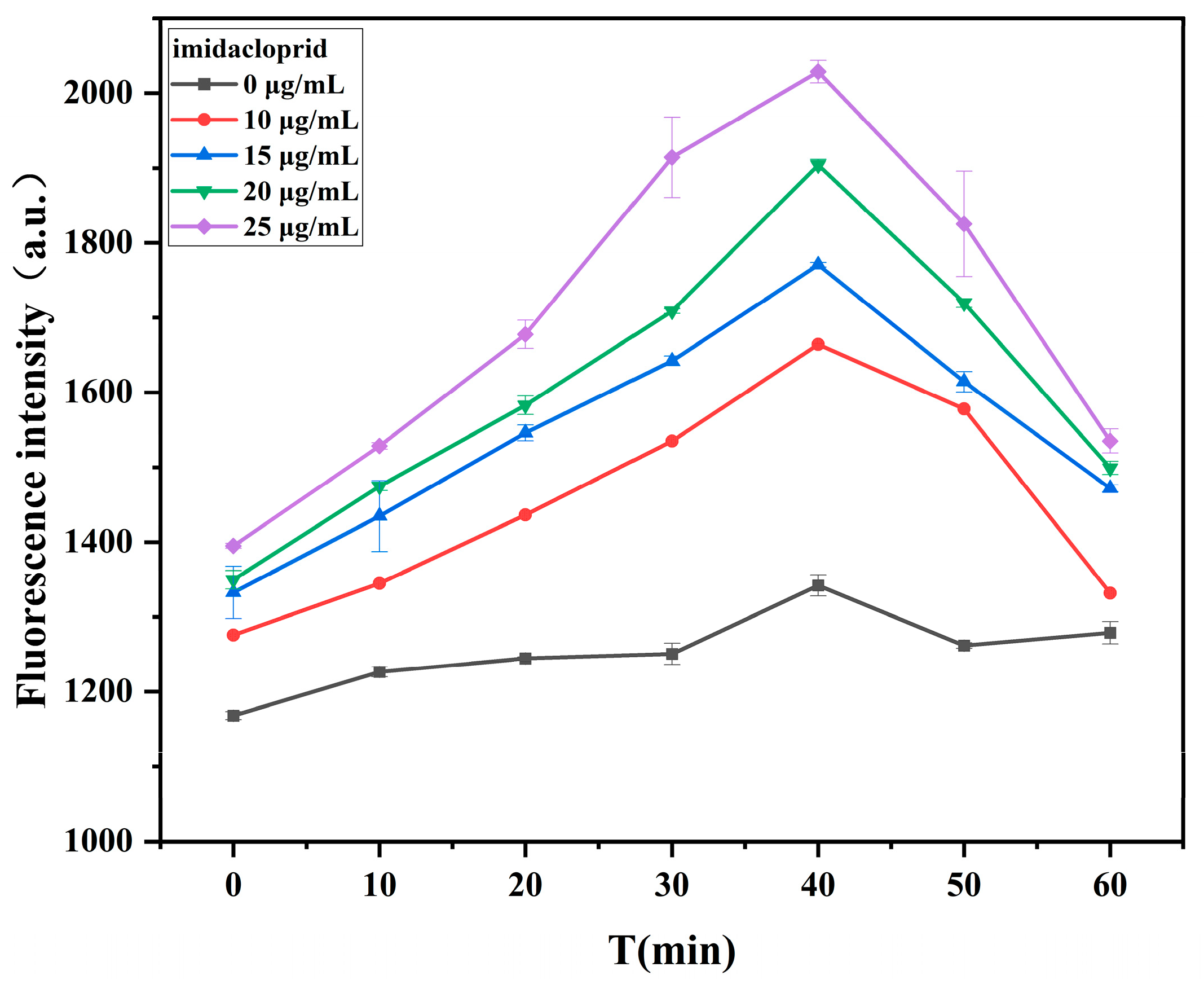
2.4.5. Effect of Incubation Time of Imidacloprid and Aptamer on Fluorescence Intensity of Biosensor
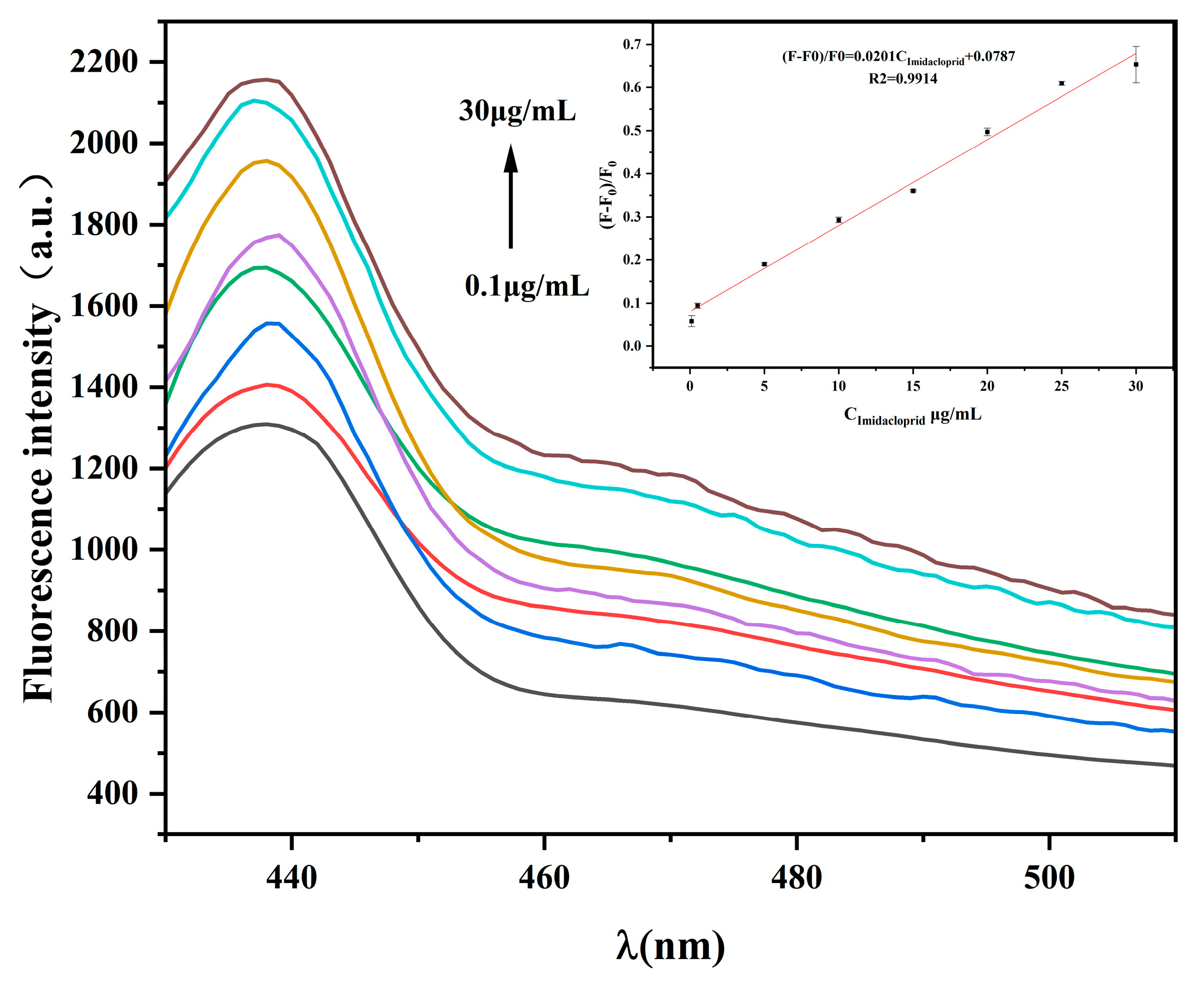
2.5. Analysis of the Detection Sensitivity of the Fluorescence Sensor
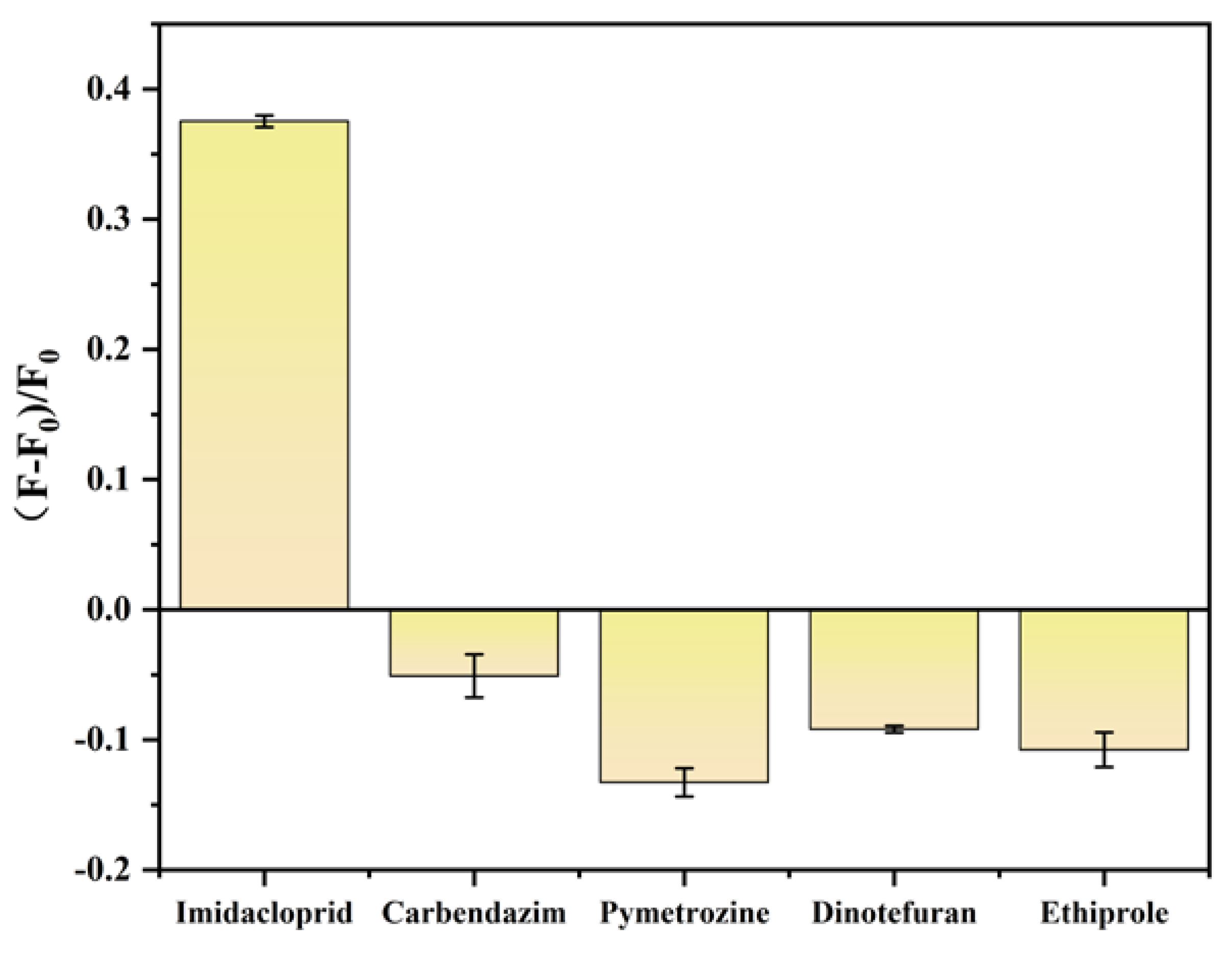
2.6. Biosensor Specificity Analysis
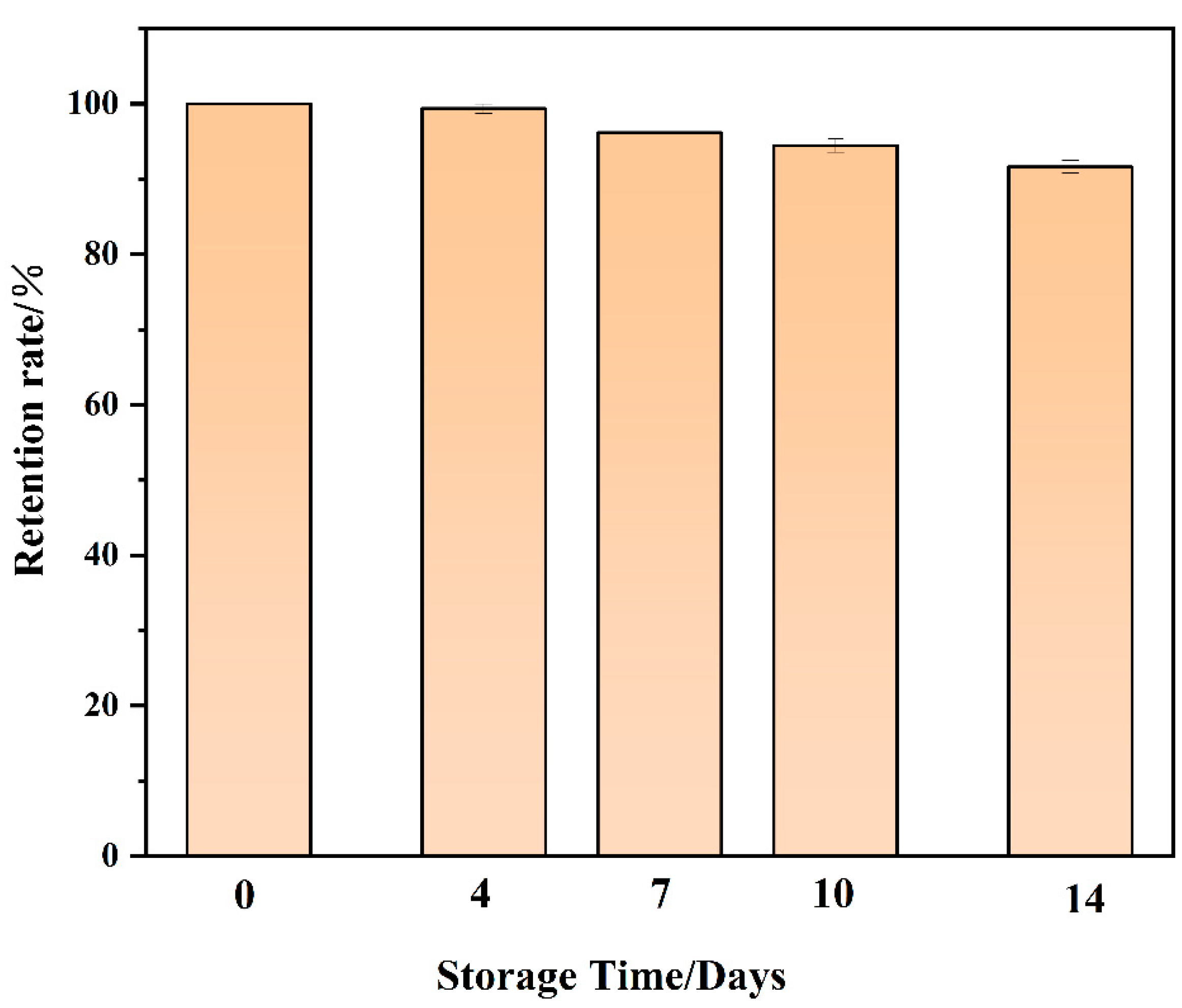
2.7. Stability Analysis of Biosensors
2.8. Testing of Chinese Medicine Samples
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Instrumentation
3.3. Optimization of Biosensors
3.3.1. Optimization of Apt Concentration
3.3.2. Optimization of NaCl Concentration
3.3.3. Optimization of Incubation Time Between Aptamer and Imidacloprid
3.4. Evaluation of the Detection Sensitivity of the Fluorescence Sensor
3.5. Identification of Imidacloprid in TCM Specimens
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gautam, P.; Pandey, A.K.; Gupta, A.; Dubey, S.K. Microcosm-omics centric investigation reveals elevated bacterial degradation of imidacloprid. Environ. Pollut. 2023, 324, 121402. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Raj, S. A rare poisoning with a still rarer presentation: Imidacloprid. Int. J. Res. Med. Sci. 2022, 11, 372. [Google Scholar] [CrossRef]
- McLuckie, C.; Moltschaniwskyj, N.; Gaston, T.; Dunstan, R.H.; Crompton, M.; Butcherine, P.; Benkendorff, K.; Taylor, M.D. Lethal and sub-lethal effects of environmentally relevant levels of imidacloprid pesticide to Eastern School Prawn, Metapenaeus macleayi. Sci. Total Environ. 2020, 742, 140449. [Google Scholar] [CrossRef] [PubMed]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2014, 22, 5–34. [Google Scholar] [CrossRef]
- Ramya, N.; Pasupuleti, B. Advantages of Bio-insecticides in Relation to Soil Quality Improvement for Agriculture. Int. J. Agro Nutr. Pract. 2021, 1, 1–4. [Google Scholar] [CrossRef]
- Samnani, P.; Vishwakarma, K.; Pandey, S.Y. Simple and sensitive method for determination of imidacloprid residue in soil and water by HPLC. Bull. Environ. Contam. Toxicol. 2011, 86, 554–558. [Google Scholar] [CrossRef]
- Buchweitz, J.P.; Viner, T.C.; Lehner, A.F. Qualitative identification of imidacloprid in postmortem animal tissue by gas chromatography-tandem mass spectrometry. Toxicol. Mech. Methods 2019, 29, 511–517. [Google Scholar] [CrossRef]
- Tan, G.; Zhao, Y.; Wang, M.; Chen, X.; Wang, B.; Li, Q.X. Ultrasensitive quantitation of imidacloprid in vegetables by colloidal gold and time-resolved fluorescent nanobead traced lateral flow immunoassays. Food Chem. 2020, 311, 126055. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, J.; Chen, L.; Chen, H.; Dang, S.; Li, F. Aptamer-based assembly systems for SARS-CoV-2 detection and therapeutics. Chem. Soc. Rev. 2024, 53, 6830–6859. [Google Scholar] [CrossRef]
- Horii, K.; Omi, K.; Yoshida, Y.; Imai, Y.; Sakai, N.; Oka, A.; Masuda, H.; Furuichi, M.; Tanimoto, T.; Waga, I. Development of a sphingosylphosphorylcholine detection system using RNA aptamers. Molecules 2010, 15, 5742–5755. [Google Scholar] [CrossRef]
- Azzouz, A.; Kumar, V.; Hejji, L.; Kim, K.H. Advancements in nanomaterial-based aptasensors for the detection of emerging organic pollutants in environmental and biological samples. Biotechnol. Adv. 2023, 66, 108156. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, M.; Khezerlou, A.; Khalilzadeh, B.; Ehsani, A.; Kazemian, H. Aptamer-modified metal organic frameworks for measurement of food contaminants: A review. Mikrochim. Acta 2023, 190, 371. [Google Scholar] [CrossRef]
- Wu, B.; Ga, L.; Wang, Y.; Ai, J. Recent Advances in the Application of Bionanosensors for the Analysis of Heavy Metals in Aquatic Environments. Molecules 2023, 29, 34. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, T.; Zhong, Y.; Li, R.; Deng, W.; Xiao, X.; Xu, Y.; Zhang, J.; Hu, X.; Wang, Y. A review of nanomaterials for biosensing applications. J. Mater. Chem. B 2024, 12, 1168–1193. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent Progress in Nanomaterial-Based Optical Aptamer Assay for the Detection of Food Chemical Contaminants. ACS Appl. Mater. Interfaces 2017, 9, 23287–23301. [Google Scholar] [CrossRef]
- Sun, H.J.; Ren, J.S.; Qu, X.G. Carbon Nanomaterials and DNA: From Molecular Recognition to Applications. Acc. Chem. Res. 2016, 49, 461–470. [Google Scholar] [CrossRef]
- Kamel, A.H.; Abd-Rabboh, H.S.M. Imprinted polymer/reduced graphene oxide-modified glassy carbon electrode-based highly sensitive potentiometric sensing module for imidacloprid detection. Microchem. J. 2024, 197, 109789. [Google Scholar] [CrossRef]
- Renu; Kau, J.; Garg, T.; Aggarwal, D.; Kumar, V.; Tikoo, K.; Kaushik, A.; Singhal, S. Synthesis of novel α-cyclodextrin conjugated ZnFe2O4-GO nanocomposites for electrochemical detection of hazardous imidacloprid insecticide and enhanced photodegradation of levofloxacin drug. J. Electroanal. Chem. 2024, 956, 118074. [Google Scholar] [CrossRef]
- Pfaffen, V.; Betancourth, J.M. Electrochemical Detection of Imidacloprid Using Antimony Film Electrodes: A Chemometric Approach. Microchem. J. 2024, 201, 110562. [Google Scholar] [CrossRef]
- Guo, J.W.; Liu, X.L.; Wang, A.Z.; Yu, X.; Ding, L.H. Antifouling electrochemical sensor-based on mesoporous silica film for imidacloprid detection in Traditional Chinese medicine. Microchem. J. 2022, 183, 107964. [Google Scholar] [CrossRef]
- Brahim, M.B.; Ammar, H.B.; Abdelhédi, R.; Samet, Y. Electrochemical behavior and analytical detection of Imidacloprid insecticide on a BDD electrode using square-wave voltammetric method. Chin. Chem. Lett. 2016, 27, 666–672. [Google Scholar] [CrossRef]

| Methods | Detection Limit (ng/mL) | Linear Range (ng/mL) | References |
|---|---|---|---|
| Imprinted polymer/reduced graphene oxide-modified glassy carbon electrode-based | 51.1 | 1.278 × 102–2.5566 × 105 | [17] |
| Electrochemical detection | 66.47 | 1.0226 × 103–1.0226 × 105 | [18] |
| Electrochemical | 1.892 × 103 | 2.3009 × 103–1.2783 × 104 | [19] |
| Antifouling electro- chemical sensor | 300 | 1 × 103–4 × 105 | [20] |
| Electrochemical | 2.1987 × 103 | 7.6698 × 103–5.1132 × 104 | [21] |
| The current method | 52.42 | 100–3 × 104 | This work |
| Sample | Added (μg/mL) | Found (μg/mL) | Recovery (%) | RSD (%) (n = 3) |
|---|---|---|---|---|
| Yam | 5 | 4.66 | 93.13 | 3.77 |
| 10 | 9.23 | 92.27 | 2.18 | |
| 15 | 14.14 | 94.30 | 0.69 | |
| Matrine | 5 | 5.04 | 100.82 | 4.14 |
| 10 | 9.93 | 99.36 | 3.93 | |
| 15 | 14.06 | 93.73 | 0.45 | |
| Aloe leaf | 5 | 4.98 | 99.54 | 2.64 |
| 10 | 10.17 | 101.7 | 3.02 | |
| 15 | 14.26 | 100.17 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Liu, X.; Feng, T.; Wang, X. Selective Label-Free Detection of Imidacloprid by a Graphene Quantum Dot Fluorescent Probe. Int. J. Mol. Sci. 2025, 26, 2714. https://doi.org/10.3390/ijms26062714
Huang Y, Liu X, Feng T, Wang X. Selective Label-Free Detection of Imidacloprid by a Graphene Quantum Dot Fluorescent Probe. International Journal of Molecular Sciences. 2025; 26(6):2714. https://doi.org/10.3390/ijms26062714
Chicago/Turabian StyleHuang, Yu, Xiaochen Liu, Tingting Feng, and Xiaohua Wang. 2025. "Selective Label-Free Detection of Imidacloprid by a Graphene Quantum Dot Fluorescent Probe" International Journal of Molecular Sciences 26, no. 6: 2714. https://doi.org/10.3390/ijms26062714
APA StyleHuang, Y., Liu, X., Feng, T., & Wang, X. (2025). Selective Label-Free Detection of Imidacloprid by a Graphene Quantum Dot Fluorescent Probe. International Journal of Molecular Sciences, 26(6), 2714. https://doi.org/10.3390/ijms26062714





