Lymphomonocytic Extracellular Vesicles Influence Fibroblast Proliferation and Collagen Production in Systemic Sclerosis
Abstract
1. Introduction
2. Results
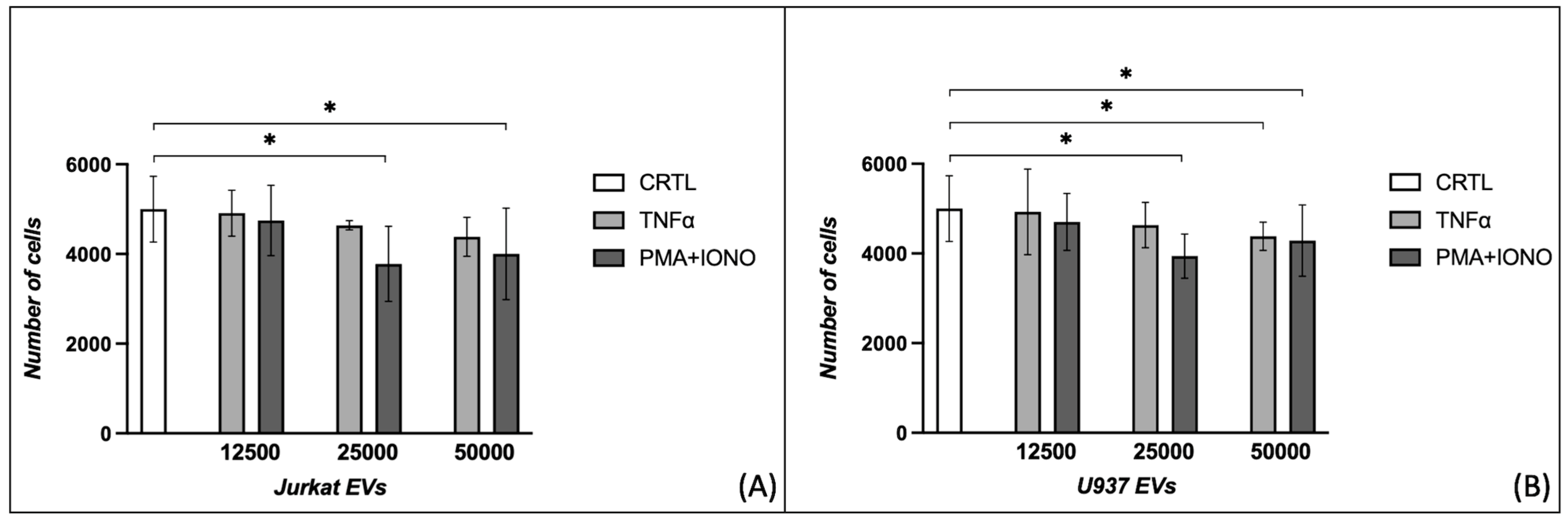
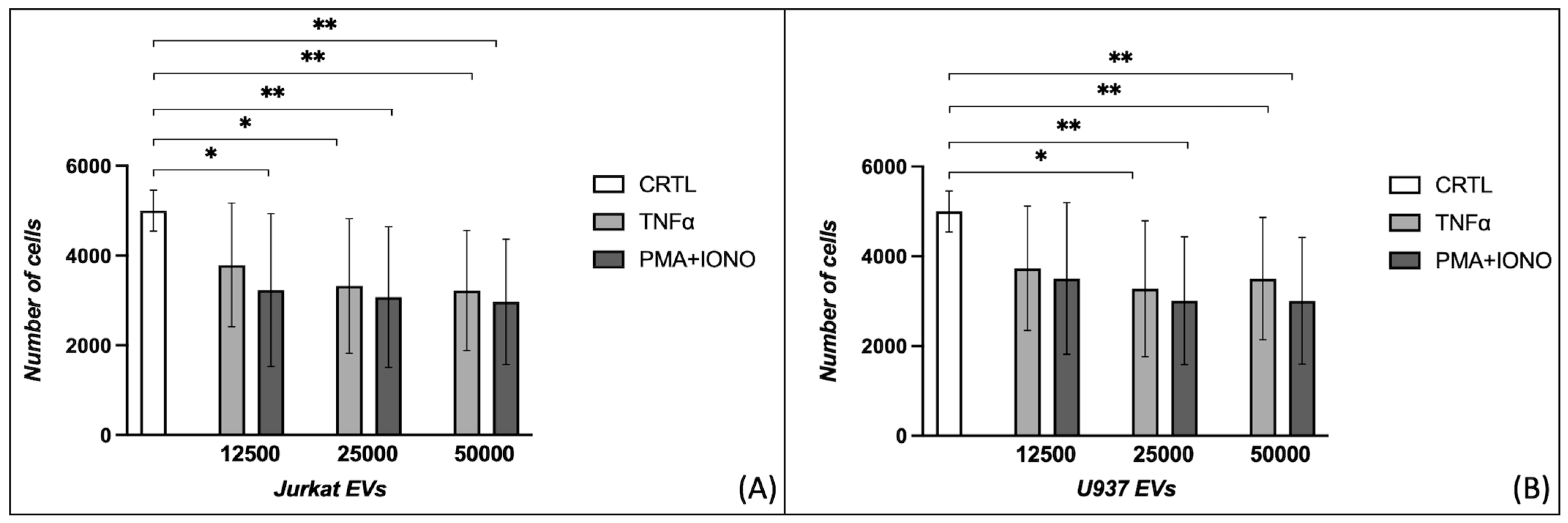
2.1. Effects of Extracellular Vesicles on Fibroblast Proliferation
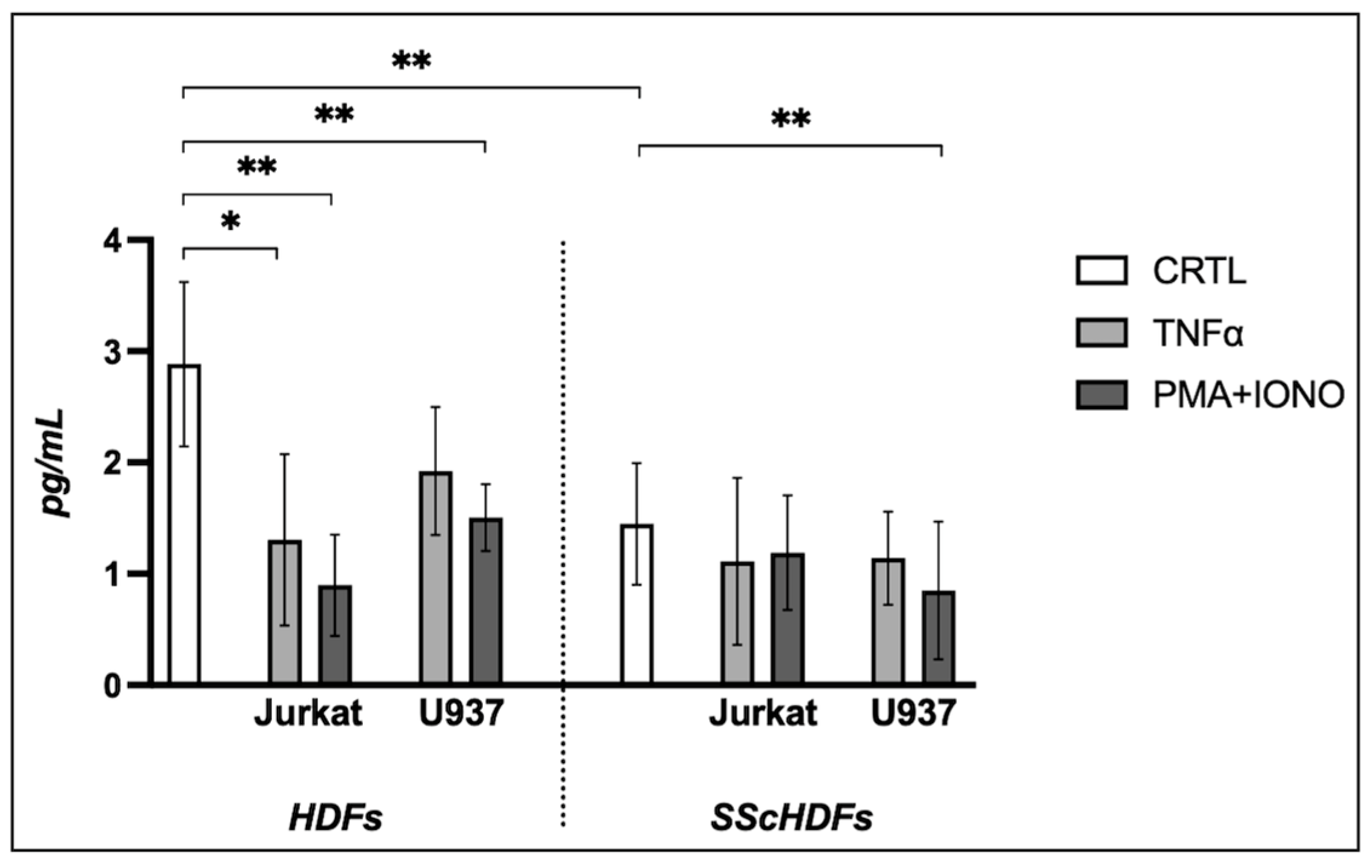
2.2. Effects of Extracellular Vesicles on Collagen Production
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Treatments
4.2. Patients and Healthy Donors
4.3. Extracellular Vesicle Production and Isolation
4.4. Proliferation Assay
4.5. Collagen Production
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volkmann, E.R.; Andréasson, K.; Smith, V. Systemic Sclerosis. Lancet 2023, 401, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Noviani, M.; Chellamuthu, V.R.; Albani, S.; Low, A.H.L. The Pathogenesis of Systemic Sclerosis: The Origin of Fibrosis and Interlink with Vasculopathy and Autoimmunity. Int. J. Mol. Sci. 2023, 24, 14287. [Google Scholar] [CrossRef] [PubMed]
- Mostmans, Y.; Cutolo, M.; Giddelo, C.; Decuman, S.; Melsens, K.; Declercq, H.; Vandecasteele, E.; De Keyser, F.; Distler, O.; Gutermuth, J.; et al. The Role of Endothelial Cells in the Vasculopathy of Systemic Sclerosis: A Systematic Review. Autoimmun. Rev. 2017, 16, 774–786. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Wakabayashi, I.; Kimuro, S.; Takahashi, N.; Takahashi, K.; Kobayashi, M.; Maishi, N.; Podyma-Inoue, K.A.; Hida, K.; Miyazono, K.; et al. TNF-α Enhances TGF-β-Induced Endothelial-to-Mesenchymal Transition via TGF-β Signal Augmentation. Cancer Sci. 2020, 111, 2385–2399. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Jungel, A.; Huber, L.C.; Seemayer, C.A.; Reich, C.F.; Gay, R.E.; Michel, B.A.; Fontana, A.; Gay, S.; Pisetsky, D.S.; et al. The Induction of Matrix Metalloproteinase and Cytokine Expression in Synovial Fibroblasts Stimulated with Immune Cell Microparticles. Proc. Natl. Acad. Sci. USA 2005, 102, 2892–2897. [Google Scholar] [CrossRef]
- De Lorenzis, E.; Rindone, A.; Di Donato, S.; Del Galdo, F. Circulating Extracellular Vesicles in the Context of Interstitial Lung Disease Related to Systemic Sclerosis: A Scoping Literature Review. Autoimmun. Rev. 2023, 22, 103401. [Google Scholar] [CrossRef]
- Colic, J.; Campochiaro, C.; Matucci-Cerinic, M. Extracellular Vesicles and Interstitial Lung Disease in Systemic Sclerosis: State of the Art! Rheumatol. Immunol. Res. 2024, 5, 136–140. [Google Scholar] [CrossRef]
- Iversen, L.; Ostergaard, O.; Ullman, S.; Nielsen, C.; Halberg, P.; Karlsmark, T.; Heegaard, N.; Jacobsen, S. Circulating Microparticles and Plasma Levels of Soluble E- and P-Selectins in Patients with Systemic Sclerosis. Scand. J. Rheumatol. 2013, 42, 473–482. [Google Scholar] [CrossRef]
- Jung, C.; Drummer, K.; Oelzner, P.; Figulla, H.R.; Boettcher, J.; Franz, M.; Betge, S.; Foerster, M.; Wolf, G.; Pfeil, A. The Association between Endothelial Microparticles and Inflammation in Patients with Systemic Sclerosis and Raynaud’s Phenomenon as Detected by Functional Imaging. Clin. Hemorheol. Microcirc. 2016, 61, 549–557. [Google Scholar] [CrossRef]
- De Oliveira, S.M.; de Azevedo Teixeira, I.L.; França, C.N.; de Oliveira Izar, M.C.; Kayser, C. Microparticles: Potential New Contributors to the Pathogenesis of Systemic Sclerosis? Adv. Rheumatol. 2023, 63, 19. [Google Scholar] [CrossRef]
- Argentino, G.; Olivieri, B.; Barbieri, A.; Beri, R.; Bason, C.; Friso, S.; Tinazzi, E. Exploring the Utility of Circulating Endothelial Cell-Derived Extracellular Vesicles as Markers of Health and Damage of Vasal Endothelium in Systemic Sclerosis Patients Treated with Iloprost. Biomedicines 2024, 12, 295. [Google Scholar] [CrossRef] [PubMed]
- Tonello, S.; D’Onghia, D.; Di Ruscio, A.; Mora, S.M.; Vincenzi, F.; Caria, G.; Fracchia, A.; Vercellino, N.; Bussolati, B.; Tanzi, A.; et al. Extracellular Vesicles as a Potential Biomarker of Pulmonary Arterial Hypertension in Systemic Sclerosis. Pharmaceuticals 2025, 18, 259. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The Roles of Extracellular Vesicles in the Immune System. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, S.A.; Piera-Velazquez, S. Probable Role of Exosomes in the Extension of Fibrotic Alterations from Affected to Normal Cells in Systemic Sclerosis. Rheumatology 2023, 62, 999–1008. [Google Scholar] [CrossRef]
- Mouawad, J.E.; Sanderson, M.; Sharma, S.; Helke, K.L.; Pilewski, J.M.; Nadig, S.N.; Feghali-Bostwick, C. Role of Extracellular Vesicles in the Propagation of Lung Fibrosis in Systemic Sclerosis. Arthritis Rheumatol. 2023, 75, 2228–2239. [Google Scholar] [CrossRef]
- Yin, W.; Ouyang, S.; Li, Y.; Xiao, B.; Yang, H. Immature Dendritic Cell-Derived Exosomes: A Promise Subcellular Vaccine for Autoimmunity. Inflammation 2013, 36, 232–240. [Google Scholar] [CrossRef]
- Roos, M.A.; Gennero, L.; Denysenko, T.; Reguzzi, S.; Cavallo, G.; Pescarmona, G.P.; Ponzetto, A. Microparticles in Physiological and in Pathological Conditions. Cell Biochem. Funct. 2010, 28, 539–548. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Dai, Q. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Systemic Sclerosis: Role and Therapeutic Directions. Front. Cell Dev. Biol. 2024, 12, 1492821. [Google Scholar] [CrossRef]
- Rozier, P.; Maumus, M.; Maria, A.T.J.; Toupet, K.; Lai-Kee-Him, J.; Jorgensen, C.; Guilpain, P.; Noël, D. Mesenchymal Stromal Cells-Derived Extracellular Vesicles Alleviate Systemic Sclerosis via MiR-29a-3p. J. Autoimmun. 2021, 121, 102660. [Google Scholar] [CrossRef]
- Rozier, P.; Maumus, M.; Maria, A.T.J.; Toupet, K.; Jorgensen, C.; Guilpain, P.; Noël, D. Lung Fibrosis Is Improved by Extracellular Vesicles from IFNγ-Primed Mesenchymal Stromal Cells in Murine Systemic Sclerosis. Cells 2021, 10, 2727. [Google Scholar] [CrossRef]
- Rozier, P.; Maumus, M.; Bony, C.; Maria, A.T.J.; Sabatier, F.; Jorgensen, C.; Guilpain, P.; Noël, D. Extracellular Vesicles Are More Potent Than Adipose Mesenchymal Stromal Cells to Exert an Anti-Fibrotic Effect in an In Vitro Model of Systemic Sclerosis. Int. J. Mol. Sci. 2021, 22, 6837. [Google Scholar] [CrossRef] [PubMed]
- Distler, J.H.W.; Huber, L.C.; Gay, S.; Distler, O.; Pisetsky, D.S. Microparticles as Mediators of Cellular Cross-Talk in Inflammatory Disease. Autoimmunity 2006, 39, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.B.; Sander, T.; Kaul, S.; Wakim, B.T.; Halligan, B.; Twigger, S.; Pritchard, K.A.; Oldham, K.T.; Ou, J.-S. Comparative Proteomic Analysis of PAI-1 and TNF-Alpha-Derived Endothelial Microparticles. Proteomics 2008, 8, 2430–2446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Luo, H.; Skaug, B.; Tabib, T.; Li, Y.-N.; Tao, Y.; Matei, A.-E.; Lyons, M.A.; Schett, G.; Lafyatis, R.; et al. Fibroblast Subpopulations in Systemic Sclerosis: Functional Implications of Individual Subpopulations and Correlations with Clinical Features. J. Investig. Dermatol. 2024, 144, 1251-1261.e13. [Google Scholar] [CrossRef]
- Varga, J.A.; Trojanowska, M. Fibrosis in Systemic Sclerosis. Rheum. Dis. Clin. N. Am. 2008, 34, 115–143. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane Vesicles as Conveyors of Immune Responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Burnouf, T.; Chou, M.-L.; Goubran, H.; Cognasse, F.; Garraud, O.; Seghatchian, J. An Overview of the Role of Microparticles/Microvesicles in Blood Components: Are They Clinically Beneficial or Harmful? Transfus. Apher. Sci. 2015, 53, 137–145. [Google Scholar] [CrossRef]
- Perlish, J.S.; Bashey, R.I.; Stephens, R.E.; Fleischmajer, R. Connective Tissue Synthesis by Cultured Scleroderma Fibroblasts. I. In Vitro Collagen Synthesis by Normal and Scleroderma Dermal Fibroblasts. Arthritis Rheum. 1976, 19, 891–901. [Google Scholar] [CrossRef]
- Zurita-Salinas, C.S.; Krötzsch, E.; Díaz de León, L.; Alcocer-Varela, J. Collagen Turnover Is Diminished by Different Clones of Skin Fibroblasts from Early- but Not Late-Stage Systemic Sclerosis. Rheumatol. Int. 2004, 24, 283–290. [Google Scholar] [CrossRef]
- Corriveau, M.-P.; Boufaied, I.; Lessard, J.; Chabaud, S.; Senécal, J.-L.; Grodzicky, T.; Chartier, S.; Raymond, Y.; Moulin, V.J. The Fibrotic Phenotype of Systemic Sclerosis Fibroblasts Varies with Disease Duration and Severity of Skin Involvement: Reconstitution of Skin Fibrosis Development Using a Tissue Engineering Approach. J. Pathol. 2009, 217, 534–542. [Google Scholar] [CrossRef]
- Assassi, S.; Swindell, W.R.; Wu, M.; Tan, F.D.; Khanna, D.; Furst, D.E.; Tashkin, D.P.; Jahan-Tigh, R.R.; Mayes, M.D.; Gudjonsson, J.E.; et al. Dissecting the Heterogeneity of Skin Gene Expression Patterns in Systemic Sclerosis. Arthritis Rheumatol. 2015, 67, 3016–3026. [Google Scholar] [CrossRef] [PubMed]
- Skaug, B.; Lyons, M.A.; Swindell, W.R.; Salazar, G.A.; Wu, M.; Tran, T.M.; Charles, J.; Vershel, C.P.; Mayes, M.D.; Assassi, S. Large-Scale Analysis of Longitudinal Skin Gene Expression in Systemic Sclerosis Reveals Relationships of Immune Cell and Fibroblast Activity with Skin Thickness and a Trend towards Normalisation over Time. Ann. Rheum. Dis. 2022, 81, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Jinnin, M.; Yamane, K.; Honda, N.; Kajihara, I.; Makino, T.; Masuguchi, S.; Fukushima, S.; Okamoto, Y.; Hasegawa, M.; et al. Impaired IL-17 Signaling Pathway Contributes to the Increased Collagen Expression in Scleroderma Fibroblasts. J. Immunol. 2012, 188, 3573–3583. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A.; Carreira, P.E.; et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Arthritis Rheum 2013, 65, 2737–2747. [Google Scholar] [CrossRef]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A.; Rowell, N.; Wollheim, F. Scleroderma (Systemic Sclerosis): Classification, Subsets and Pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar]
- LeRoy, E.C.; Medsger, T.A. Criteria for the Classification of Early Systemic Sclerosis. J. Rheumatol. 2001, 28, 1573–1576. [Google Scholar]
- Argentino, G.; Barbieri, A.; Beri, R.; Bason, C.; Ruzzenente, A.; Olivieri, O.; Tinazzi, E.; Puccetti, A.; Vitali, C.; Del Papa, N.; et al. Profibrotic Effects of Endothelin-1 on Fibroblasts Are Mediated by Aldosterone In Vitro: Relevance to the Pathogenesis and Therapy of Systemic Sclerosis and Pulmonary Arterial Hypertension. Biomedicines 2022, 10, 2765. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Huber, L.C.; Hueber, A.J.; Reich, C.F.; Gay, S.; Distler, O.; Pisetsky, D.S. The Release of Microparticles by Apoptotic Cells and Their Effects on Macrophages. Apoptosis 2005, 10, 731–741. [Google Scholar] [CrossRef]
| Sex | Age | Disease Form | Disease Duration | |
|---|---|---|---|---|
| Healthy donors | 1 Male 1 Female | 32–45 | - | - |
| Patients | 1 Male 3 Female | 34–51 | 2 dcSSc 2 lcSSc | 3 < 5 years 1 > 5 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argentino, G.; Olivieri, B.; Morandi, M.; Bonisoli, G.; Beri, R.; Tinazzi, E.; Friso, S. Lymphomonocytic Extracellular Vesicles Influence Fibroblast Proliferation and Collagen Production in Systemic Sclerosis. Int. J. Mol. Sci. 2025, 26, 2699. https://doi.org/10.3390/ijms26062699
Argentino G, Olivieri B, Morandi M, Bonisoli G, Beri R, Tinazzi E, Friso S. Lymphomonocytic Extracellular Vesicles Influence Fibroblast Proliferation and Collagen Production in Systemic Sclerosis. International Journal of Molecular Sciences. 2025; 26(6):2699. https://doi.org/10.3390/ijms26062699
Chicago/Turabian StyleArgentino, Giuseppe, Bianca Olivieri, Matteo Morandi, Giulio Bonisoli, Ruggero Beri, Elisa Tinazzi, and Simonetta Friso. 2025. "Lymphomonocytic Extracellular Vesicles Influence Fibroblast Proliferation and Collagen Production in Systemic Sclerosis" International Journal of Molecular Sciences 26, no. 6: 2699. https://doi.org/10.3390/ijms26062699
APA StyleArgentino, G., Olivieri, B., Morandi, M., Bonisoli, G., Beri, R., Tinazzi, E., & Friso, S. (2025). Lymphomonocytic Extracellular Vesicles Influence Fibroblast Proliferation and Collagen Production in Systemic Sclerosis. International Journal of Molecular Sciences, 26(6), 2699. https://doi.org/10.3390/ijms26062699





