Modulation of Macrophages TLR4-Mediated Transcriptional Response by Lacticaseibacillus rhamnosus CRL1505 and Lactiplantibacillus plantarum CRL1506
Abstract
1. Introduction
2. Results
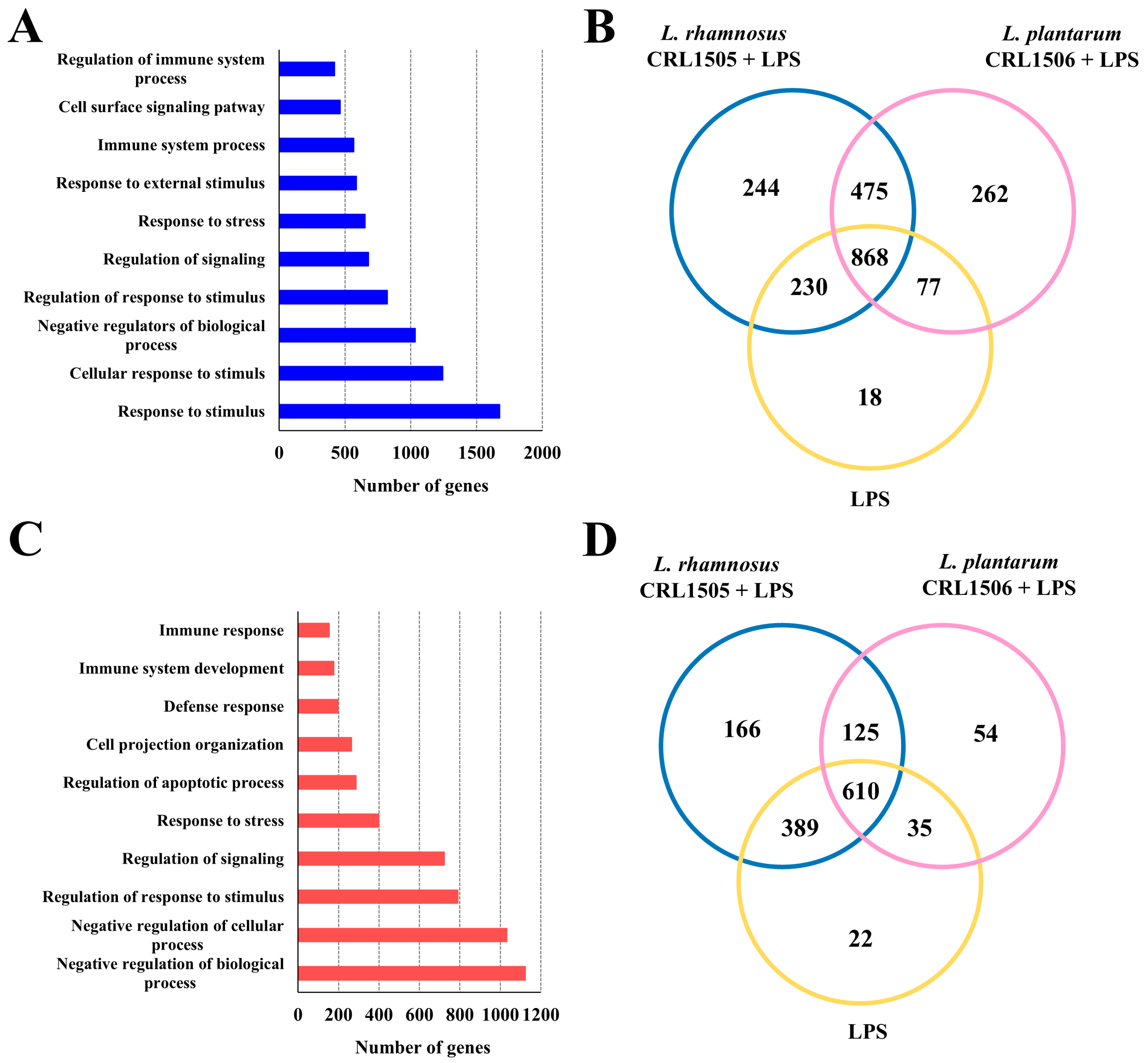
2.1. Effect of L. rhamnosus CRL1505 and L. plantarum CRL1506 on Macrophages TLR4-Mediated Transcriptomic Response
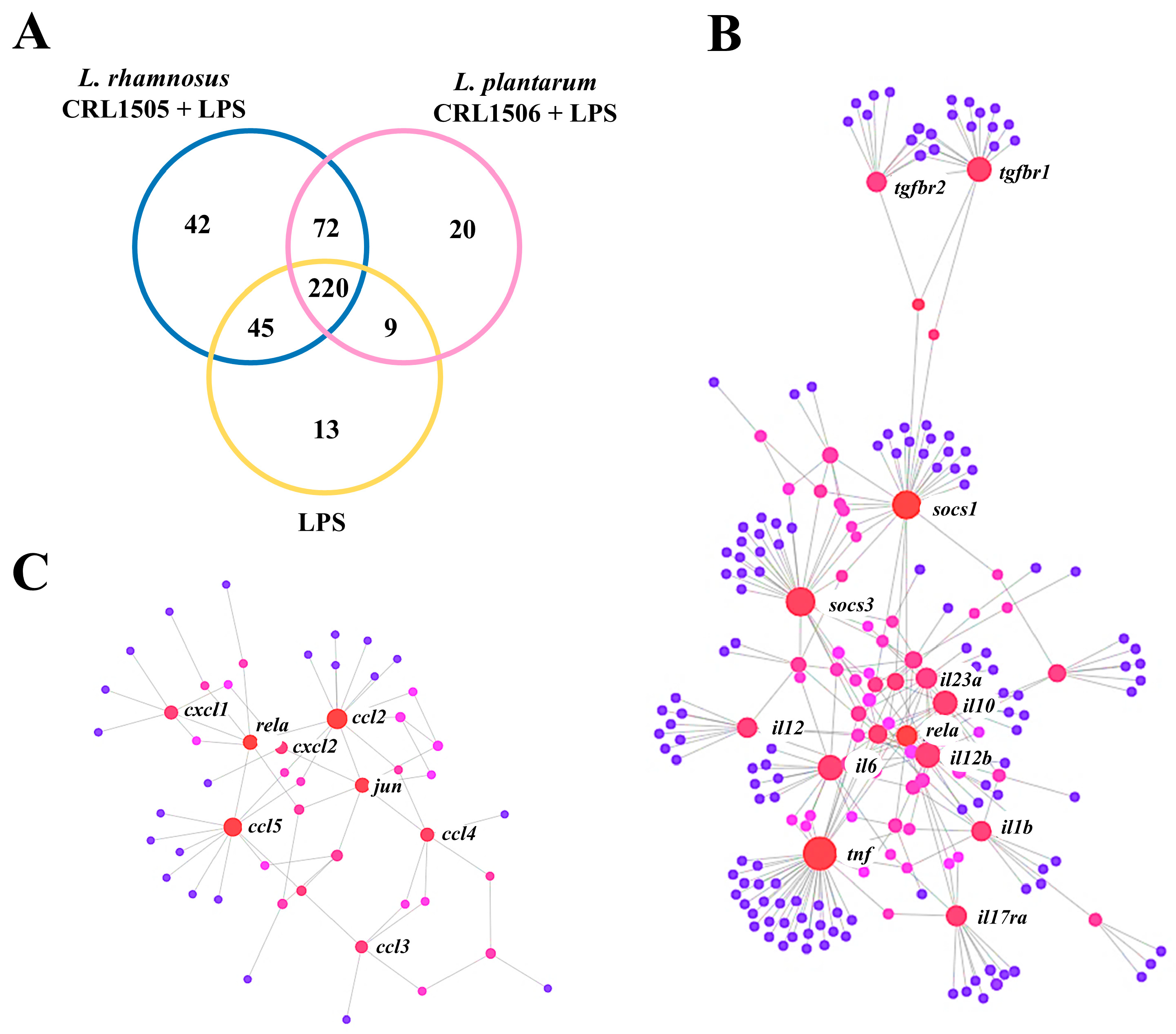
2.1.1. The CRL1505 and CRL1506 Strains Modulate Immune Factors in LPS-Stimulated RAW Macrophages
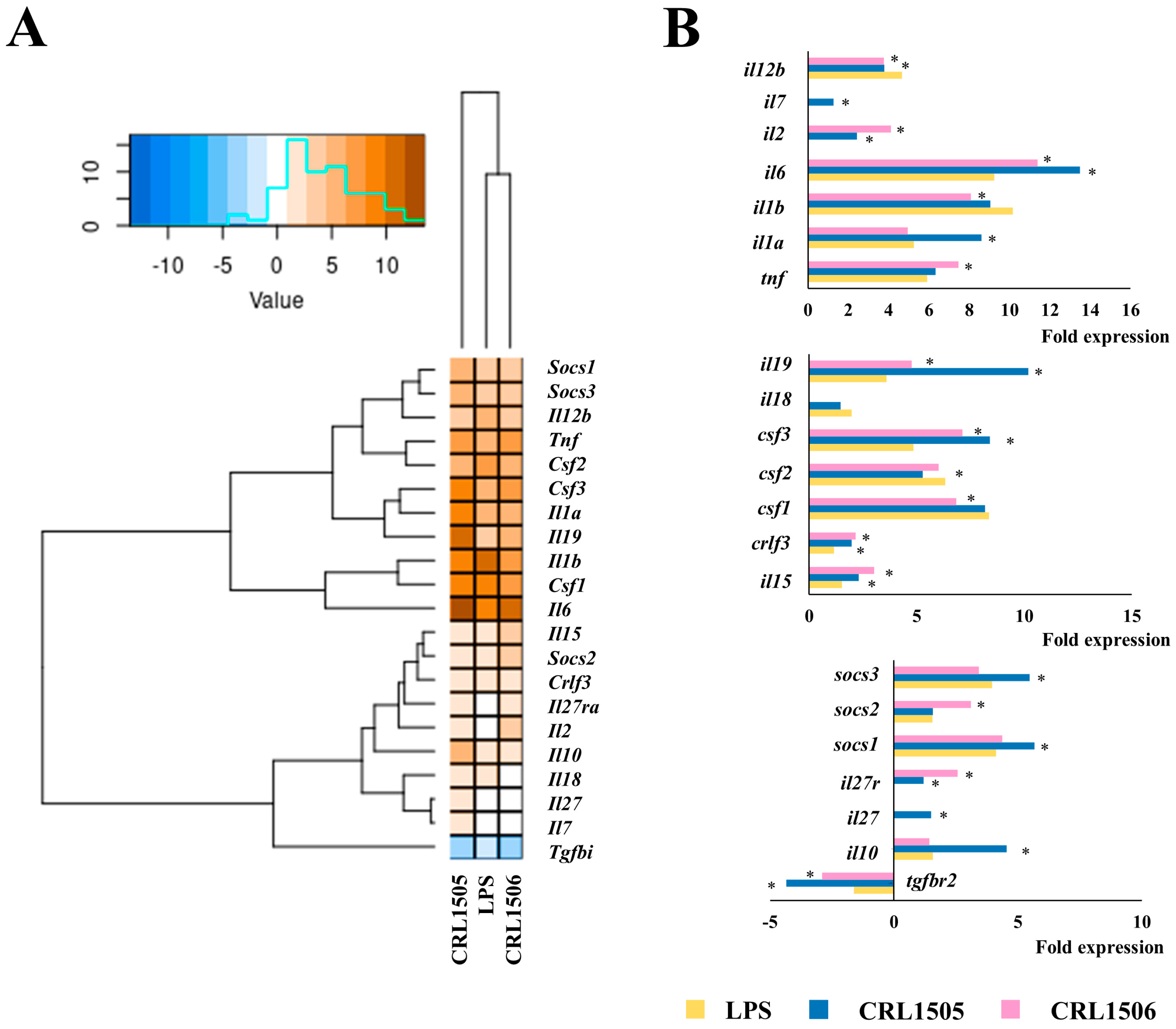
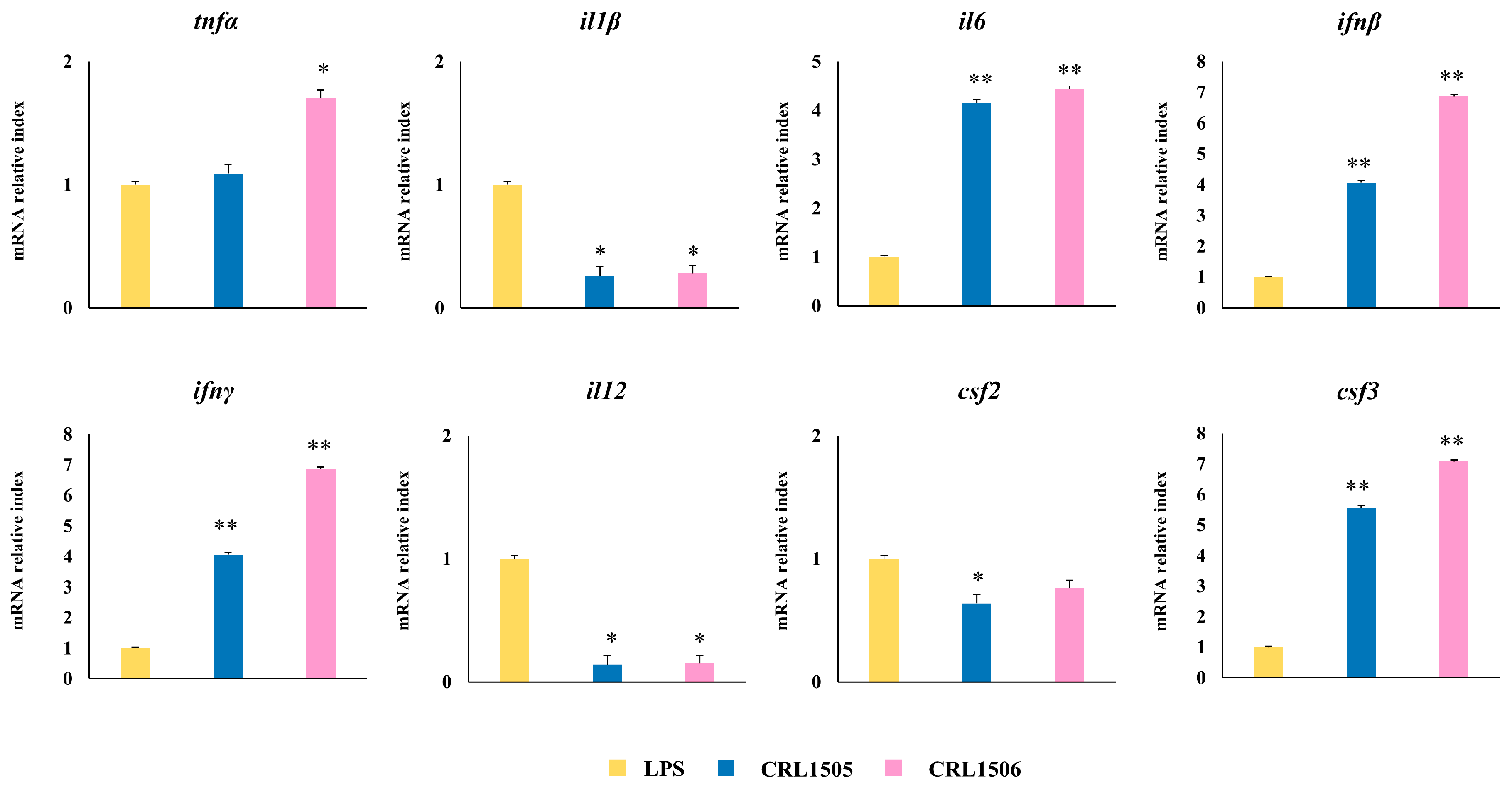
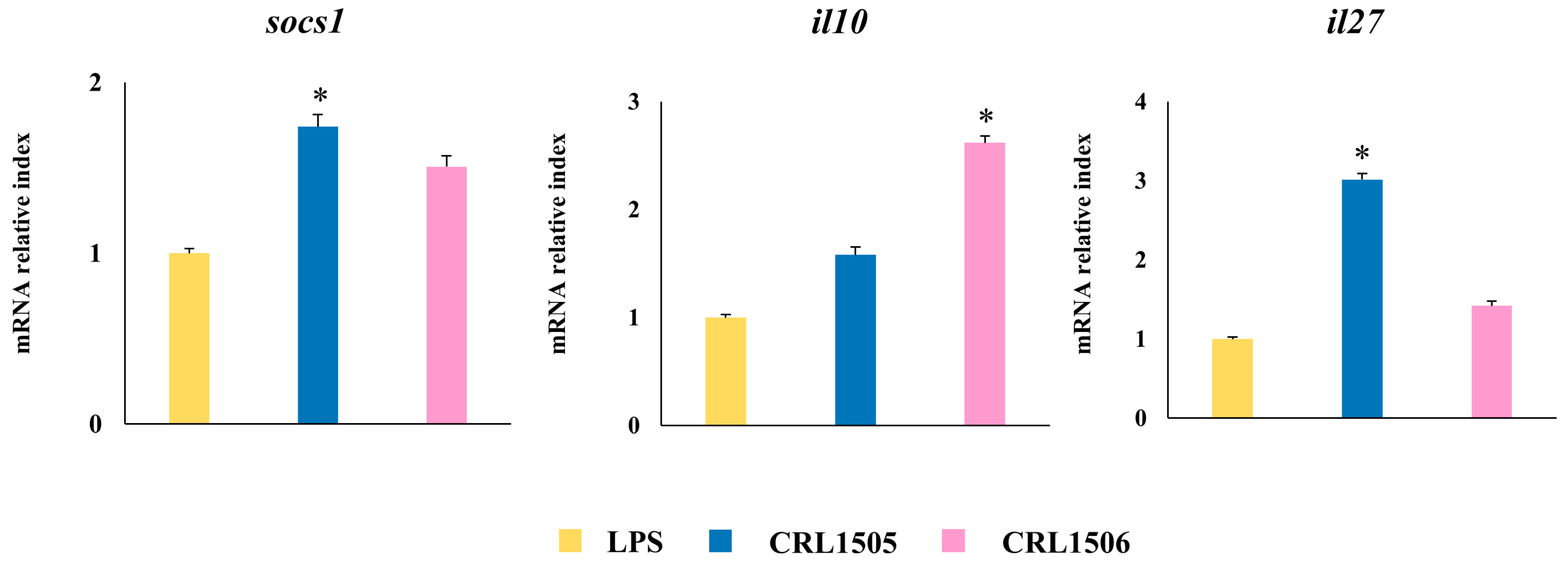
2.1.2. The CRL1505 and CRL1506 Strains Modulate Inflammatory and Regulatory Cytokines in LPS-Stimulated RAW Macrophages
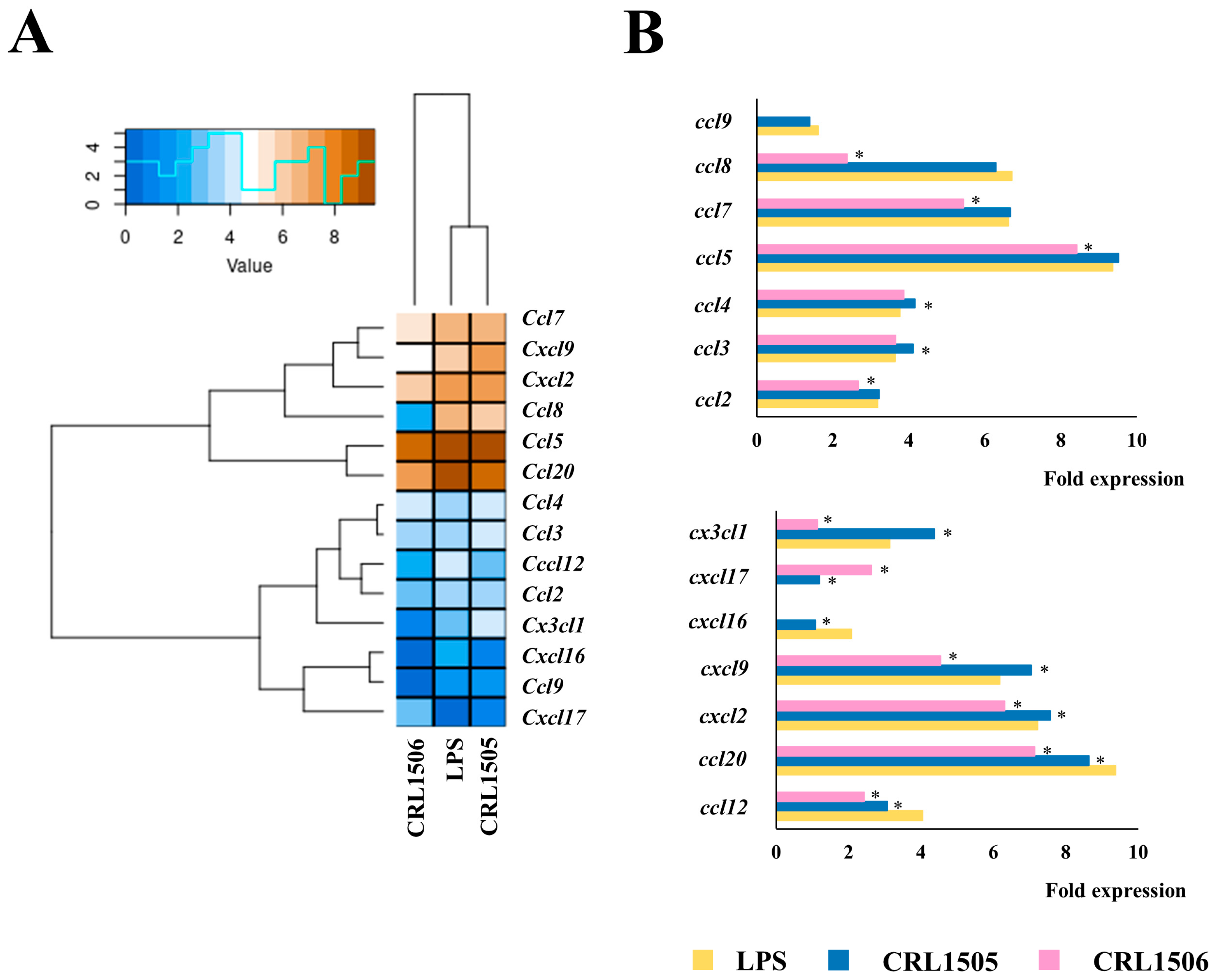
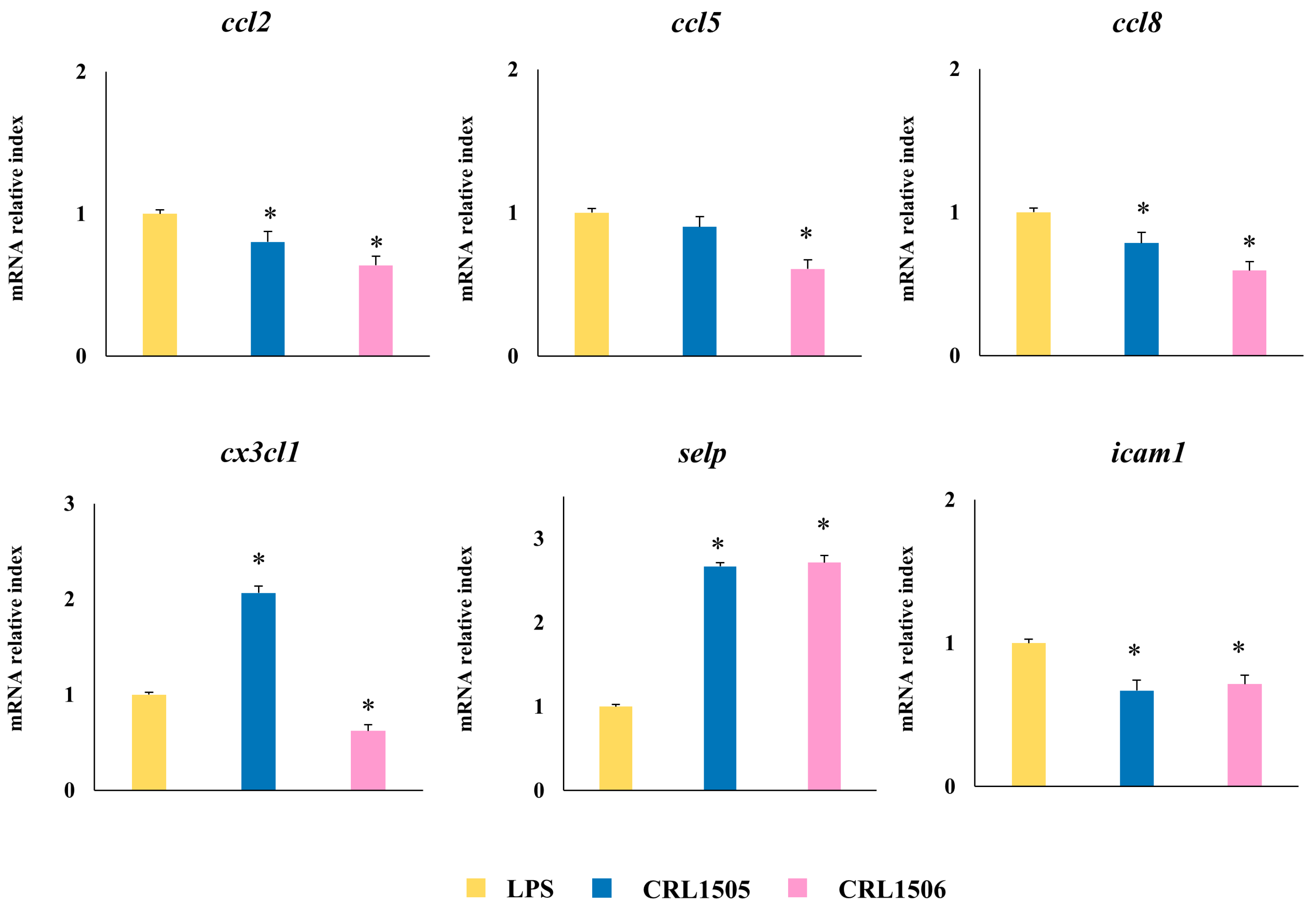
2.1.3. The CRL1505 and CRL1506 Strains Modulate Chemokines and Adhesion Molecules in LPS-Stimulated RAW Macrophages
2.1.4. Confirmation of Immune Factors Modulation by CRL1505 and CRL1506 Strains with qPCR
2.2. Effect of L. rhamnosus CRL1505 and L. plantarum CRL1506 on Peyer’s Patches APCs and Peritoneal Macrophages Cytokine Production in Response to LPS Stimulation
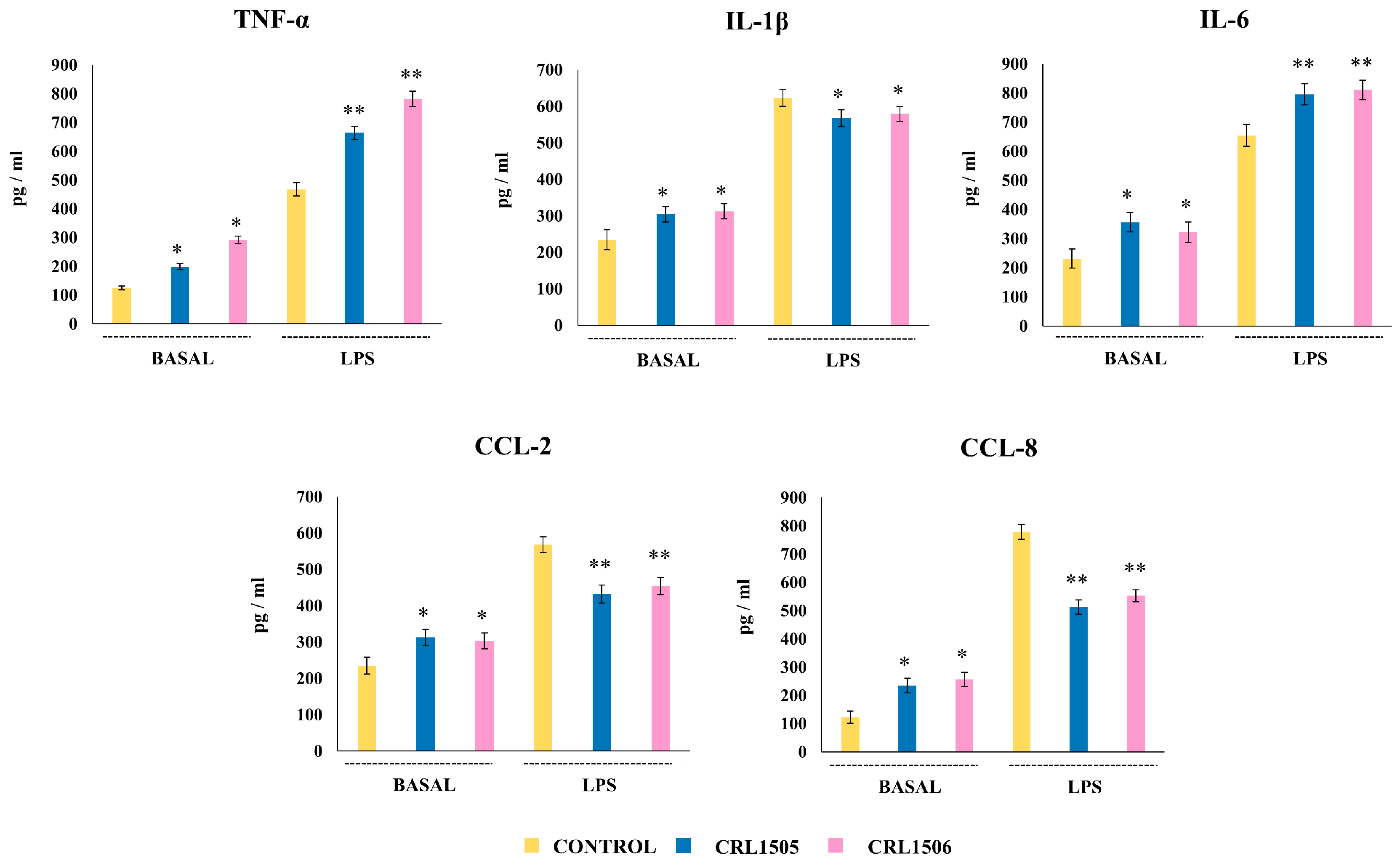
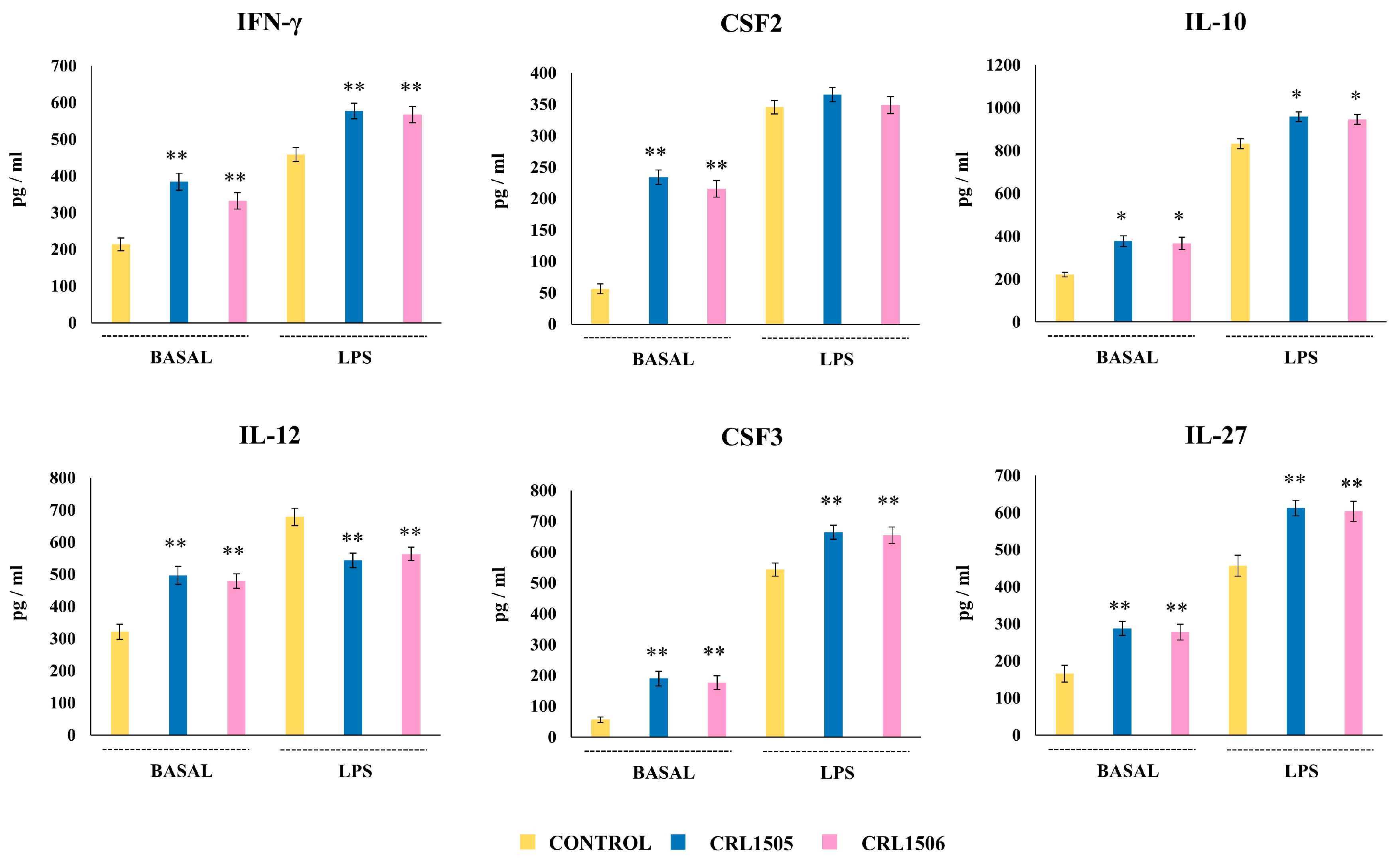
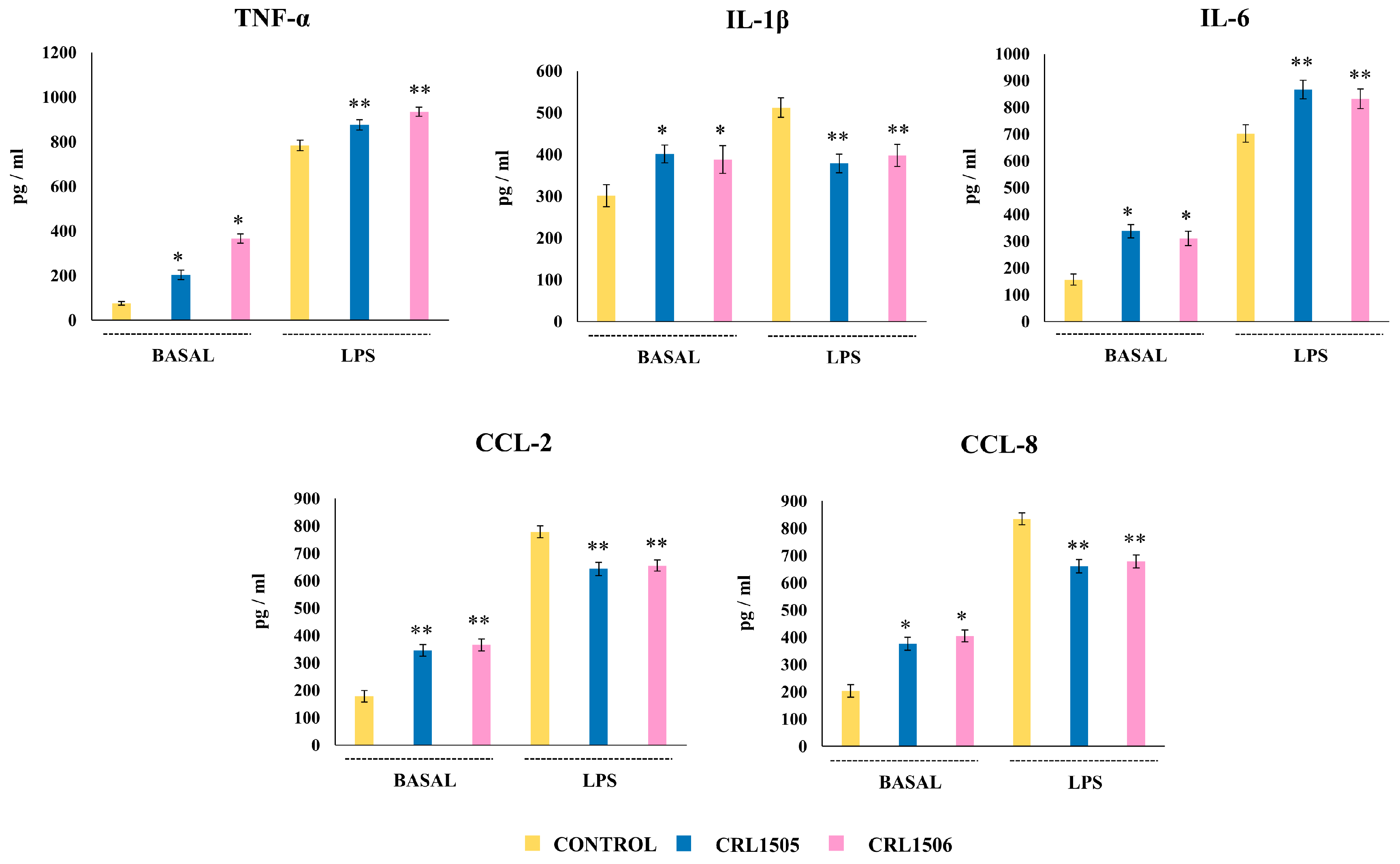
2.2.1. The CRL1505 and CRL1506 Strains Modulate Cytokine Production in LPS-Stimulated Peyer’s Patches Macrophages
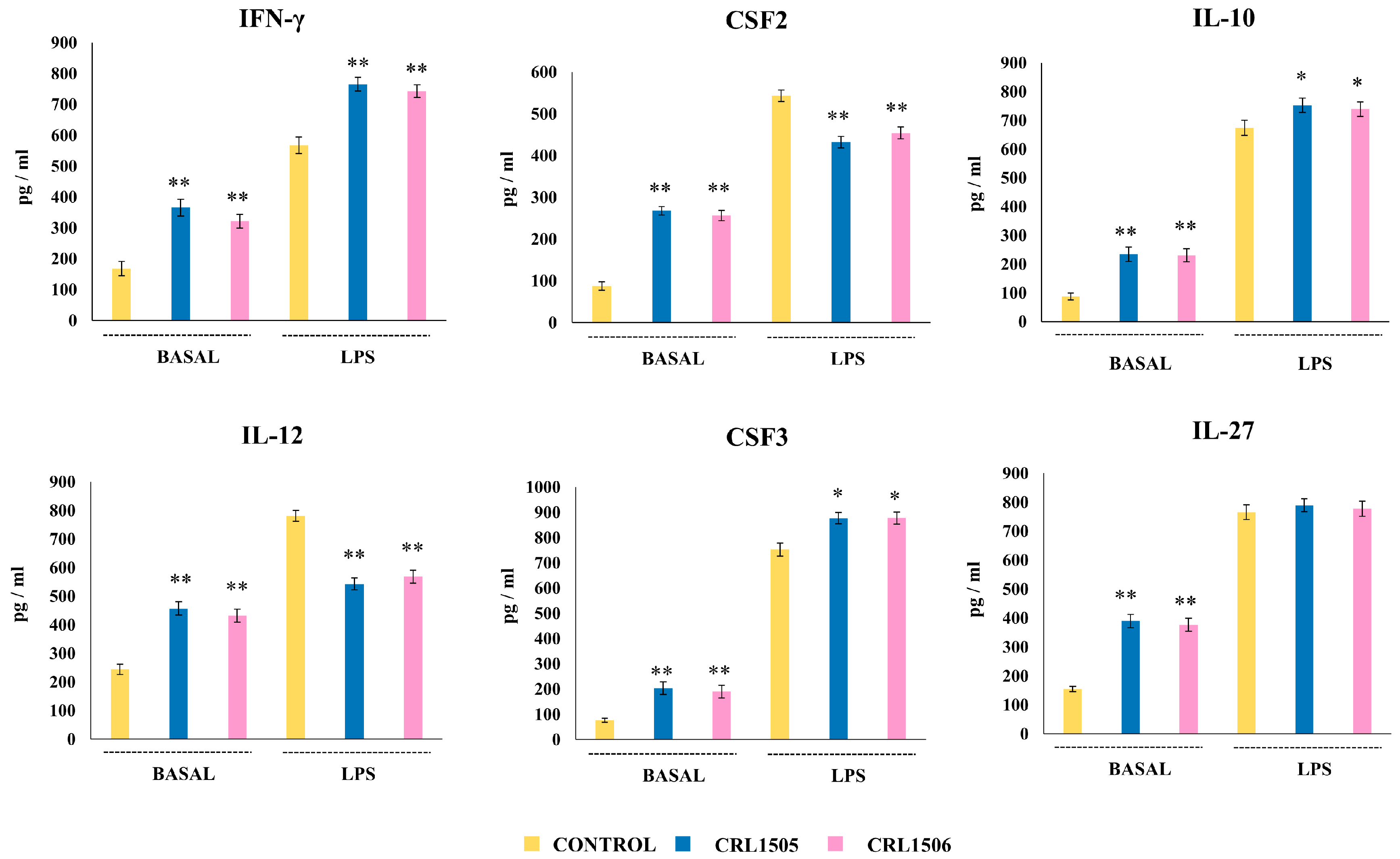
2.2.2. The CRL1505 and CRL1506 Strains Modulate Cytokine Production in LPS-Stimulated Peritoneal Macrophages
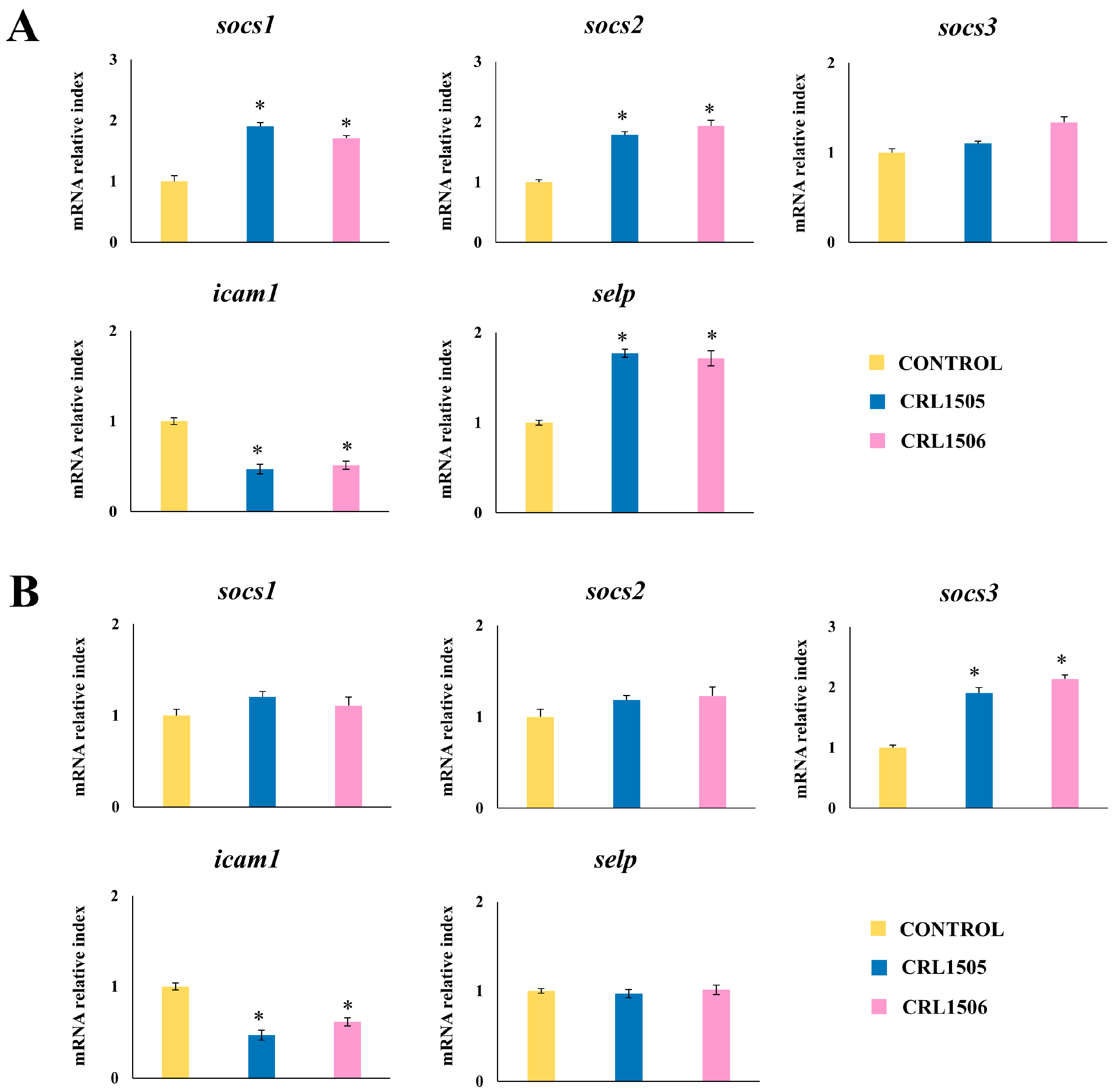
2.2.3. The CRL1505 and CRL1506 Strains Modulate Adhesion Molecules and Regulatory Factors Expression in LPS-Stimulated Peyer’s Patches and Peritoneal Macrophages
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Cell Cultures
4.3. Stimulation of Macrophages with Lactobacilli
4.4. Microarray
4.5. Quantitative Expression Analysis by qPCR
4.6. Animals, Feeding Procedures, and Immune Cell Isolation
4.7. Cytokine Protein Determinations
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, L.; Lei, X.; Wang, J.; Wang, L.; Zhong, Q.; Fang, X.; Li, P.; Du, B.; Wang, Y.; Liao, Z. Lipopolysaccharides Derived from Gram-Negative Bacterial Pool of Human Gut Microbiota Promote Inflammation and Obesity Development. Int. Rev. Immunol. 2022, 41, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Kessel, A.; Toubi, E.; Pavlotzky, E.; Mogilner, J.; Coran, A.G.; Lurie, M.; Karry, R.; Sukhotnik, I. Treatment with Glutamine Is Associated with Down-Regulation of Toll-like Receptor-4 and Myeloid Differentiation Factor 88 Expression and Decrease in Intestinal Mucosal Injury Caused by Lipopolysaccharide Endotoxaemia in a Rat. Clin. Exp. Immunol. 2008, 151, 341–347. [Google Scholar] [CrossRef]
- Mattock, E.; Blocker, A.J. How Do the Virulence Factors of Shigella Work Together to Cause Disease? Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Santos, R.L.; Raffatellu, M.; Bevins, C.L.; Adams, L.G.; Tükel, Ç.; Tsolis, R.M.; Bäumler, A.J. Life in the Inflamed Intestine, Salmonella Style. Trends Microbiol. 2009, 17, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Huang, Z.; Jia, G.; Zhao, H.; Liu, G.; Chen, X. Naringin Mitigates LPS-Induced Intestinal Barrier Injury in Mice. Food Funct. 2023, 14, 1617–1626. [Google Scholar] [CrossRef]
- He, Y.; Wang, D.; Liu, K.; Deng, S.; Liu, Y. Sodium Humate Alleviates LPS-Induced Intestinal Barrier Injury by Improving Intestinal Immune Function and Regulating Gut Microbiota. Mol. Immunol. 2023, 161, 61–73. [Google Scholar] [CrossRef]
- Delfini, M.; Stakenborg, N.; Viola, M.F.; Boeckxstaens, G. Macrophages in the Gut: Masters in Multitasking. Immunity 2022, 55, 1530–1548. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage Biology in Development, Homeostasis and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Villena, J.; Kitazawa, H. Modulation of Intestinal TLR4-Inflammatory Signaling Pathways by Probiotic Microorganisms: Lessons Learned from Lactobacillus jensenii TL2937. Front. Immunol. 2014, 4, 512. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Lee, N.K.; Paik, H.D. Heat-Killed Latilactobacillus sakei CNSC001WB and Lactobacillus pentosus WB693 Have an Anti-Inflammatory Effect on LPS-Stimulated RAW 264.7 Cells. Probiotics Antimicrob. Proteins 2024, 16, 1875–1885. [Google Scholar] [CrossRef]
- Yoon, S.; Cho, H.; Nam, Y.; Park, M.; Lim, A.; Kim, J.H.; Park, J.; Kim, W. Multifunctional Probiotic and Functional Properties of Lactiplantibacillus plantarum LRCC5314, Isolated from Kimchi. J. Microbiol. Biotechnol. 2022, 32, 72–80. [Google Scholar] [CrossRef]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Kim, Y. Probiotic and Anti-Inflammatory Potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 Isolated from Infant Feces. PLoS ONE 2018, 13, e0192021. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Zheng, H.; Liu, M.; Hu, X.; Wang, T.; Zhang, X.; Jin, F.; Wang, L. Probiotic and Anti-Inflammatory Attributes of an Isolate Lactobacillus helveticus NS8 from Mongolian Fermented Koumiss. BMC Microbiol. 2015, 15, 196. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Albarracin, L.; Tsujikawa, Y.; Fukuyama, K.; Sakane, I.; Villena, J.; Kitazawa, H. Lactiplantibacillus plantarum LOC1 Isolated from Fresh Tea Leaves Modulates Macrophage Response to TLR4 Activation. Foods 2022, 11, 3257. [Google Scholar] [CrossRef]
- Yoda, K.; He, F.; Kawase, M.; Miyazawa, K.; Hiramatsu, M. Oral Administration of Lactobacillus gasseri TMC0356 Stimulates Peritoneal Macrophages and Attenuates General Symptoms Caused by Enteropathogenic Escherichia coli Infection. J. Microbiol. Immunol. Infect. 2014, 47, 81–86. [Google Scholar] [CrossRef]
- Castillo, N.A.; de Moreno de Leblanc, A.; Galdeano, C.M.; Perdigón, G. Comparative Study of the Protective Capacity against Salmonella Infection between Probiotic and Nonprobiotic Lactobacilli. J. Appl. Microbiol. 2013, 114, 861–876. [Google Scholar] [CrossRef]
- Salva, S.; Villena, J.; Alvarez, S. Immunomodulatory Activity of Lactobacillus rhamnosus Strains Isolated from Goat Milk: Impact on Intestinal and Respiratory Infections. Int. J. Food Microbiol. 2010, 141, 82–89. [Google Scholar] [CrossRef]
- Baillo, A.; Villena, J.; Albarracín, L.; Tomokiyo, M.; Elean, M.; Fukuyama, K.; Quilodrán-Vega, S.; Fadda, S.; Kitazawa, H. Lactiplantibacillus plantarum Strains Modulate Intestinal Innate Immune Response and Increase Resistance to Enterotoxigenic Escherichia coli Infection. Microorganisms 2022, 11, 63. [Google Scholar] [CrossRef]
- Marranzino, G.; Villena, J.; Salva, S.; Alvarez, S. Stimulation of Macrophages by Immunobiotic Lactobacillus Strains: Influence beyond the Intestinal Tract. Microbiol. Immunol. 2012, 56, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Arroyo Portilla, C.; Fenouil, R.; Wagner, C.; Luciani, C.; Lagier, M.; Da Silva, C.; Hidalgo-Villeda, F.; Spinelli, L.; Fallet, M.; Tomas, J.; et al. Peyer’s Patch Phagocytes Acquire Specific Transcriptional Programs That Influence Their Maturation and Activation Profiles. Mucosal Immunol. 2023, 16, 527–547. [Google Scholar] [CrossRef]
- Bonnardel, J.; DaSilva, C.; Henri, S.; Tamoutounour, S.; Chasson, L.; Montañana-Sanchis, F.; Gorvel, J.P.; Lelouard, H. Innate and Adaptive Immune Functions of Peyer’s Patch Monocyte-Derived Cells. Cell Rep. 2015, 11, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Pamies, D.; Bal-Price, A.; Chesné, C.; Coecke, S.; Dinnyes, A.; Eskes, C.; Grillari, R.; Gstraunthaler, G.; Hartung, T.; Jennings, P.; et al. Advanced Good Cell Culture Practice for Human Primary, Stem Cell-Derived and Organoid Models as Well as Microphysiological Systems. ALTEX 2018, 35, 353–378. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Z.W.; Kozaki, T.; Ginhoux, F. Studying Tissue Macrophages in Vitro: Are IPSC-Derived Cells the Answer? Nat. Rev. Immunol. 2018, 18, 716–725. [Google Scholar] [CrossRef]
- Hung, Y.L.; Wang, S.C.; Suzuki, K.; Fang, S.H.; Chen, C.S.; Cheng, W.C.; Su, C.C.; Yeh, H.C.; Tu, H.P.; Liu, P.L.; et al. Bavachin Attenuates LPS-Induced Inflammatory Response and Inhibits the Activation of NLRP3 Inflammasome in Macrophages. Phytomedicine 2019, 59, 152785. [Google Scholar] [CrossRef]
- He, G.; Zhang, X.; Chen, Y.; Chen, J.; Li, L.; Xie, Y. Isoalantolactone Inhibits LPS-Induced Inflammation via NF-ΚB Inactivation in Peritoneal Macrophages and Improves Survival in Sepsis. Biomed. Pharmacother. 2017, 90, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Rusanov, A.L.; Kozhin, P.M.; Tikhonova, O.V.; Zgoda, V.G.; Loginov, D.S.; Chlastáková, A.; Selinger, M.; Sterba, J.; Grubhoffer, L.; Luzgina, N.G. Proteome Profiling of PMJ2-R and Primary Peritoneal Macrophages. Int. J. Mol. Sci. 2021, 22, 6323. [Google Scholar] [CrossRef]
- Ning, S.; Zhang, Z.; Zhou, C.; Wang, B.; Liu, Z.; Feng, B. Cross-Talk between Macrophages and Gut Microbiota in Inflammatory Bowel Disease: A Dynamic Interplay Influencing Pathogenesis and Therapy. Front. Med. 2024, 11, 1457218. [Google Scholar] [CrossRef]
- Wagner, C.; Bonnardel, J.; Da Silva, C.; Martens, L.; Gorvel, J.P.; Lelouard, H. Some News from the Unknown Soldier, the Peyer’s Patch Macrophage. Cell. Immunol. 2018, 330, 159–167. [Google Scholar] [CrossRef]
- Ardavín, C.; Alvarez-Ladrón, N.; Ferriz, M.; Gutiérrez-González, A.; Vega-Pérez, A. Mouse Tissue-Resident Peritoneal Macrophages in Homeostasis, Repair, Infection, and Tumor Metastasis. Adv. Sci. 2023, 10, 2206617. [Google Scholar] [CrossRef] [PubMed]
- Gautiar, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-Expression Profiles and Transcriptional Regulatory Pathways That Underlie the Identity and Diversity of Mouse Tissue Macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef]
- Roberts, A.W.; Lee, B.L.; Deguine, J.; John, S.; Shlomchik, M.J.; Barton, G.M. Tissue-Resident Macrophages Are Locally Programmed for Silent Clearance of Apoptotic Cells. Immunity 2017, 47, 913–927.e6. [Google Scholar] [CrossRef] [PubMed]
- Vega-Pérez, A.; Villarrubia, L.H.; Godio, C.; Gutiérrez-González, A.; Feo-Lucas, L.; Ferriz, M.; Martínez-Puente, N.; Alcaín, J.; Mora, A.; Sabio, G.; et al. Resident Macrophage-Dependent Immune Cell Scaffolds Drive Anti-Bacterial Defense in the Peritoneal Cavity. Immunity 2021, 54, 2578–2594.e5. [Google Scholar] [CrossRef]
- Yang, L.; Gong, T.; Shen, H.; Pei, J.; Zhang, L.; Zhang, Q.; Huang, Y.; Hu, Z.; Pan, Z.; Yang, P.; et al. Precision N-Glycoproteomic Profiling of Murine Peritoneal Macrophages After Different Stimulations. Front. Immunol. 2021, 12, 722293. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Jenkins, S.J. The Biology of Serous Cavity Macrophages. Cell Immunol. 2018, 330, 126–135. [Google Scholar] [CrossRef]
- Gordon, M.A.; Jack, D.L.; Dockrell, D.H.; Lee, M.E.; Read, R.C. Gamma Interferon Enhances Internalization and Early Nonoxidative Killing of Salmonella Enterica Serovar typhimurium by Human Macrophages and Modifies Cytokine Responses. Infect. Immun. 2005, 73, 3445–3452. [Google Scholar] [CrossRef]
- Ho, N.K.; Ossa, J.C.; Silphaduang, U.; Johnson, R.; Johnson-Henry, K.C.; Sherman, P.M. Enterohemorrhagic Escherichia coli O157:H7 Shiga Toxins Inhibit Gamma Interferon-Mediated Cellular Activation. Infect. Immun. 2012, 80, 2307–2315. [Google Scholar] [CrossRef]
- Pham, T.H.M.; Brewer, S.M.; Thurston, T.; Massis, L.M.; Honeycutt, J.; Lugo, K.; Jacobson, A.R.; Vilches-Moure, J.G.; Hamblin, M.; Helaine, S.; et al. Salmonella-Driven Polarization of Granuloma Macrophages Antagonizes TNF-Mediated Pathogen Restriction during Persistent Infection. Cell Host Microbe 2020, 27, 54–67.e5. [Google Scholar] [CrossRef]
- Porbahaie, M.; van den Belt, M.; Ulfman, L.; Ruijschop, R.M.A.J.; Lucas-Van de Bos, E.; Hartog, A.; Lenz, S.; van Alen-Boerrigter, I.J.; Teodorowicz, M.; Savelkoul, H.F.J.; et al. Low Doses of Diarrhoeagenic E. coli Induce Enhanced Monocyte and MDC Responses and Prevent Development of Symptoms after Homologous Rechallenge. PLoS ONE 2023, 18, e0279626. [Google Scholar] [CrossRef]
- Ingersoll, M.A.; Kline, K.A.; Nielsen, H.V.; Hultgren, S.J. G-CSF Induction Early in Uropathogenic Escherichia coli Infection of the Urinary Tract Modulates Host Immunity. Cell. Microbiol. 2008, 10, 2568–2578. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zadeh, M.; Sharma, C.; Lin, Y.D.; Soshnev, A.A.; Mohamadzadeh, M. Controlling Functional Homeostasis of Ileal Resident Macrophages by Vitamin B12 during Steady State and Salmonella Infection in Mice. Mucosal Immunol. 2024, 17, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Coon, C.; Beagley, K.W.; Bao, S. The Role of Granulocyte Macrophage-Colony Stimulating Factor in Gastrointestinal Immunity to Salmonellosis. Scand. J. Immunol. 2009, 70, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Gong, Z.; Zhang, S.; Cao, J.; Mao, W.; Yao, Y.; Zhao, J.; Li, Q.; Liu, K.; Liu, B.; et al. Besides TLR2 and TLR4, NLRP3 Is Also Involved in Regulating Escherichia coli Infection-Induced Inflammatory Responses in Mice. Int. Immunopharmacol. 2023, 121, 110556. [Google Scholar] [CrossRef]
- Tchernychev, B.; Furie, B.; Furie, B.C. Peritoneal Macrophages Express Both P-Selectin and PSGL-1. J. Cell Biol. 2003, 163, 1145–1155. [Google Scholar] [CrossRef]
- Winterberg, T.; Vieten, G.; Feldmann, L.; Yu, Y.; Hansen, G.; Hennig, C.; Ure, B.M.; Kuebler, J.F. Neonatal Murine Macrophages Show Enhanced Chemotactic Capacity upon Toll-like Receptor Stimulation. Pediatr. Surg. Int. 2014, 30, 159–164. [Google Scholar] [CrossRef]
- Kum, W.W.S.; Lo, B.C.; Deng, W.; Ziltener, H.J.; Finlay, B.B. Impaired Innate Immune Response and Enhanced Pathology during Citrobacter rodentium Infection in Mice Lacking Functional P-Selectin. Cell. Microbiol. 2010, 12, 1250–1271. [Google Scholar] [CrossRef]
- Kum, W.W.S.; Lee, S.; Grassl, G.A.; Bidshahri, R.; Hsu, K.; Ziltener, H.J.; Finlay, B.B. Lack of Functional P-Selectin Ligand Exacerbates Salmonella Serovar typhimurium Infection. J. Immunol. 2009, 182, 6550–6561. [Google Scholar] [CrossRef]
- Waters, S.; Luther, S.; Joerger, T.; Richards, G.P.; Boyd, E.F.; Parent, M.A. Murine Macrophage Inflammatory Cytokine Production and Immune Activation in Response to Vibrio parahaemolyticus Infection. Microbiol. Immunol. 2013, 57, 323–328. [Google Scholar] [CrossRef]
- Qin, K.; Fu, K.; Liu, J.; Wu, C.; Wang, Y.; Zhou, L. Vibrio vulnificus Cytolysin Induces Inflammatory Responses in RAW264.7 Macrophages through Calcium Signaling and Causes Inflammation in Vivo. Microb. Pathog. 2019, 137, 103789. [Google Scholar] [CrossRef]
- Yu, G.; Wang, J.; Zhang, W.; Yang, Q.; Liu, G.; Wang, L.; Bello, B.K.; Zhang, X.; Zhang, T.; Fan, H.; et al. NLRP3 Inflammasome Signal Pathway Involves in Vibrio harveyi-Induced Inflammatory Response in Murine Peritoneal Macrophages in Vitro. Acta Biochim. Biophys. Sin. 2021, 53, 1590–1601. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Shao, L.; Liu, R.; Msuthwana, P.; Hu, J.; Wang, C. Lactobacillus rhamnosus GG Defense against Salmonella Enterica Serovar typhimurium Infection through Modulation of M1 Macrophage Polarization. Microb. Pathog. 2021, 156, 104939. [Google Scholar] [CrossRef] [PubMed]
- Wormald, S.; Hilton, D.J. Inhibitors of Cytokine Signal Transduction. J. Biol. Chem. 2004, 279, 821–824. [Google Scholar] [CrossRef]
- De Oca, M.M.; De Labastida Rivera, F.; Winterford, C.; Frame, T.C.M.; Ng, S.S.; Amante, F.H.; Edwards, C.L.; Bukali, L.; Wang, Y.; Uzonna, J.E.; et al. IL-27 Signalling Regulates Glycolysis in Th1 Cells to Limit Immunopathology during Infection. PLoS Pathog. 2020, 16, e1008994. [Google Scholar] [CrossRef]
- Sobah, M.L.; Liongue, C.; Ward, A.C. SOCS Proteins in Immunity, Inflammatory Diseases, and Immune-Related Cancer. Front. Med. 2021, 8, 727987. [Google Scholar] [CrossRef]
- Fathima, A.; Jamma, T. UDCA Ameliorates Inflammation Driven EMT by Inducing TGR5 Dependent SOCS1 Expression in Mouse Macrophages. Sci. Rep. 2024, 14, 24285. [Google Scholar] [CrossRef]
- Ipseiz, N.; Pickering, R.J.; Rosas, M.; Tyrrell, V.J.; Davies, L.C.; Orr, S.J.; Czubala, M.A.; Fathalla, D.; Robertson, A.A.; Bryant, C.E.; et al. Tissue-Resident Macrophages Actively Suppress IL-1beta Release via a Reactive Prostanoid/IL-10 Pathway. EMBO J. 2020, 39, e103454. [Google Scholar] [CrossRef]
- Brubaker, J.; Zhang, X.; Bourgeois, A.L.; Harro, C.; Sack, D.A.; Chakraborty, S. Intestinal and Systemic Inflammation Induced by Symptomatic and Asymptomatic Enterotoxigenic E. coli Infection and Impact on Intestinal Colonization and ETEC Specific Immune Responses in an Experimental Human Challenge Model. Gut Microbes 2021, 13, 1891852. [Google Scholar] [CrossRef]
- Andrews, C.; McLean, M.H.; Hixon, J.A.; Pontejo, S.M.; Starr, T.; Malo, C.; Cam, M.; Ridnour, L.; Hickman, H.; Steele-Mortimer, O.; et al. IL-27 Induces an IFN-like Signature in Murine Macrophages Which in Turn Modulate Colonic Epithelium. Front. Immunol. 2023, 14, 1021824. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Gordon, R.A.; Ivashkiv, L.B. Suppression of TNF-α and IL-1 Signaling Identifies a Mechanism of Homeostatic Regulation of Macrophages by IL-27. J. Immunol. 2010, 185, 7047–7056. [Google Scholar] [CrossRef]
- Clua, P.; Tomokiyo, M.; Raya Tonetti, F.; Islam, M.A.; García Castillo, V.; Marcial, G.; Salva, S.; Alvarez, S.; Takahashi, H.; Kurata, S.; et al. The Role of Alveolar Macrophages in the Improved Protection against Respiratory Syncytial Virus and Pneumococcal Superinfection Induced by the Peptidoglycan of Lactobacillus rhamnosus CRL1505. Cells 2020, 9, 1653. [Google Scholar] [CrossRef]
- Mizuno, H.; Arce, L.; Tomotsune, K.; Albarracin, L.; Funabashi, R.; Vera, D.; Islam, M.A.; Vizoso-Pinto, M.G.; Takahashi, H.; Sasaki, Y.; et al. Lipoteichoic Acid Is Involved in the Ability of the Immunobiotic Strain Lactobacillus plantarum CRL1506 to Modulate the Intestinal Antiviral Innate Immunity Triggered by TLR3 Activation. Front. Immunol. 2020, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castillo, V.; Tomokiyo, M.; Raya Tonetti, F.; Islam, M.A.; Takahashi, H.; Kitazawa, H.; Villena, J. Alveolar Macrophages Are Key Players in the Modulation of the Respiratory Antiviral Immunity Induced by Orally Administered Lacticaseibacillus rhamnosus CRL1505. Front. Immunol. 2020, 11, 568636. [Google Scholar] [CrossRef]
- Da Silva, C.; Wagner, C.; Bonnardel, J.; Gorvel, J.P.; Lelouard, H. The Peyer’s Patch Mononuclear Phagocyte System at Steady State and during Infection. Front. Immunol. 2017, 8, 1254. [Google Scholar] [CrossRef] [PubMed]
- Lelouard, H.; Henri, S.; De Bovis, B.; Mugnier, B.; Chollat-Namy, A.; Malissen, B.; Méresse, S.; Gorvel, J.P. Pathogenic Bacteria and Dead Cells Are Internalized by a Unique Subset of Peyer’s Patch Dendritic Cells That Express Lysozyme. Gastroenterology 2010, 138, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, E.; Liu, F.; Zhang, Y.; Zhong, M.; Li, Y.; Zhou, D.; Chen, Y.; Cao, Y.; Xiao, Y.; et al. Flagellins of Salmonella typhi and Nonpathogenic Escherichia coli Are Differentially Recognized through the NLRC4 Pathway in Macrophages. J. Innate Immun. 2014, 6, 47–57. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, M.; Baillo, A.; Albarracin, L.; Elean, M.; Serda, R.; Suda, Y.; Namai, F.; Nishiyama, K.; Kitazawa, H.; Villena, J. Modulation of Macrophages TLR4-Mediated Transcriptional Response by Lacticaseibacillus rhamnosus CRL1505 and Lactiplantibacillus plantarum CRL1506. Int. J. Mol. Sci. 2025, 26, 2688. https://doi.org/10.3390/ijms26062688
Suzuki M, Baillo A, Albarracin L, Elean M, Serda R, Suda Y, Namai F, Nishiyama K, Kitazawa H, Villena J. Modulation of Macrophages TLR4-Mediated Transcriptional Response by Lacticaseibacillus rhamnosus CRL1505 and Lactiplantibacillus plantarum CRL1506. International Journal of Molecular Sciences. 2025; 26(6):2688. https://doi.org/10.3390/ijms26062688
Chicago/Turabian StyleSuzuki, Masahiko, Ayelen Baillo, Leonardo Albarracin, Mariano Elean, Rodrigo Serda, Yoshihito Suda, Fu Namai, Keita Nishiyama, Haruki Kitazawa, and Julio Villena. 2025. "Modulation of Macrophages TLR4-Mediated Transcriptional Response by Lacticaseibacillus rhamnosus CRL1505 and Lactiplantibacillus plantarum CRL1506" International Journal of Molecular Sciences 26, no. 6: 2688. https://doi.org/10.3390/ijms26062688
APA StyleSuzuki, M., Baillo, A., Albarracin, L., Elean, M., Serda, R., Suda, Y., Namai, F., Nishiyama, K., Kitazawa, H., & Villena, J. (2025). Modulation of Macrophages TLR4-Mediated Transcriptional Response by Lacticaseibacillus rhamnosus CRL1505 and Lactiplantibacillus plantarum CRL1506. International Journal of Molecular Sciences, 26(6), 2688. https://doi.org/10.3390/ijms26062688







