Antrodia cinnamomea Formula Suppresses Prostate Cancer Progression via Immune Modulation and PD-1/PD-L1 Pathway Inhibition
Abstract
1. Introduction
2. Results
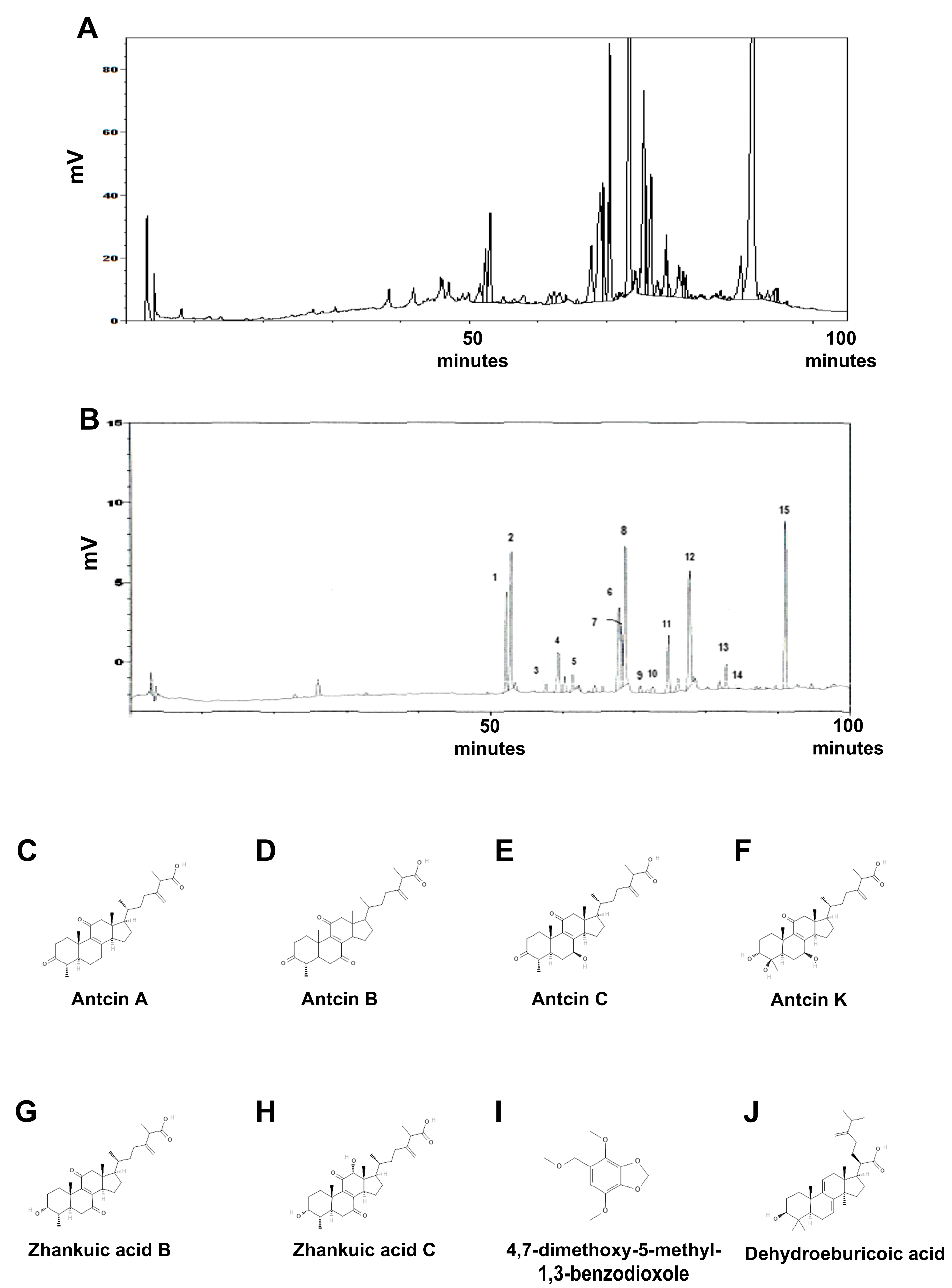
2.1. Identification of Reference Compounds in XZF by HPLC Analysis
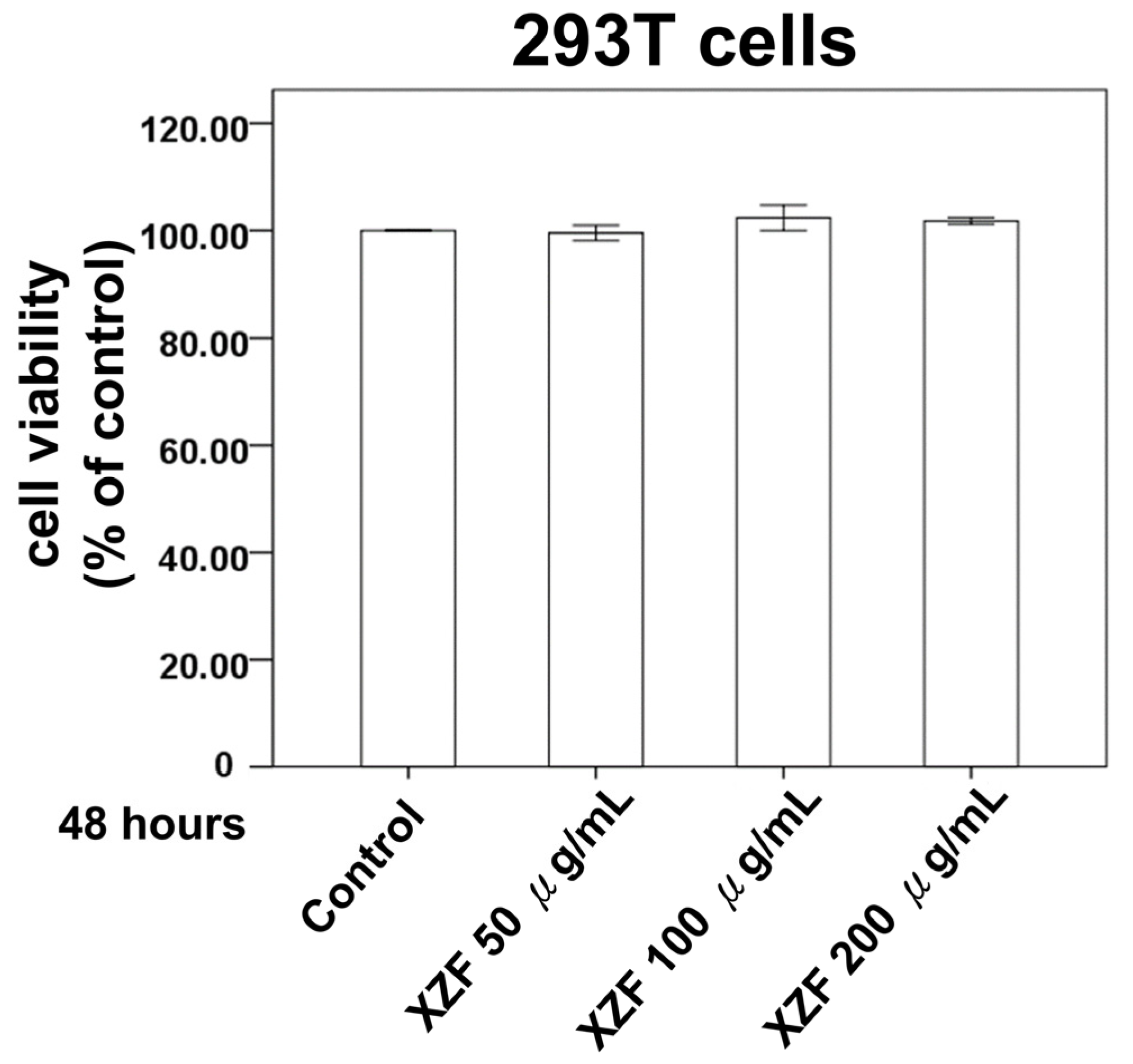
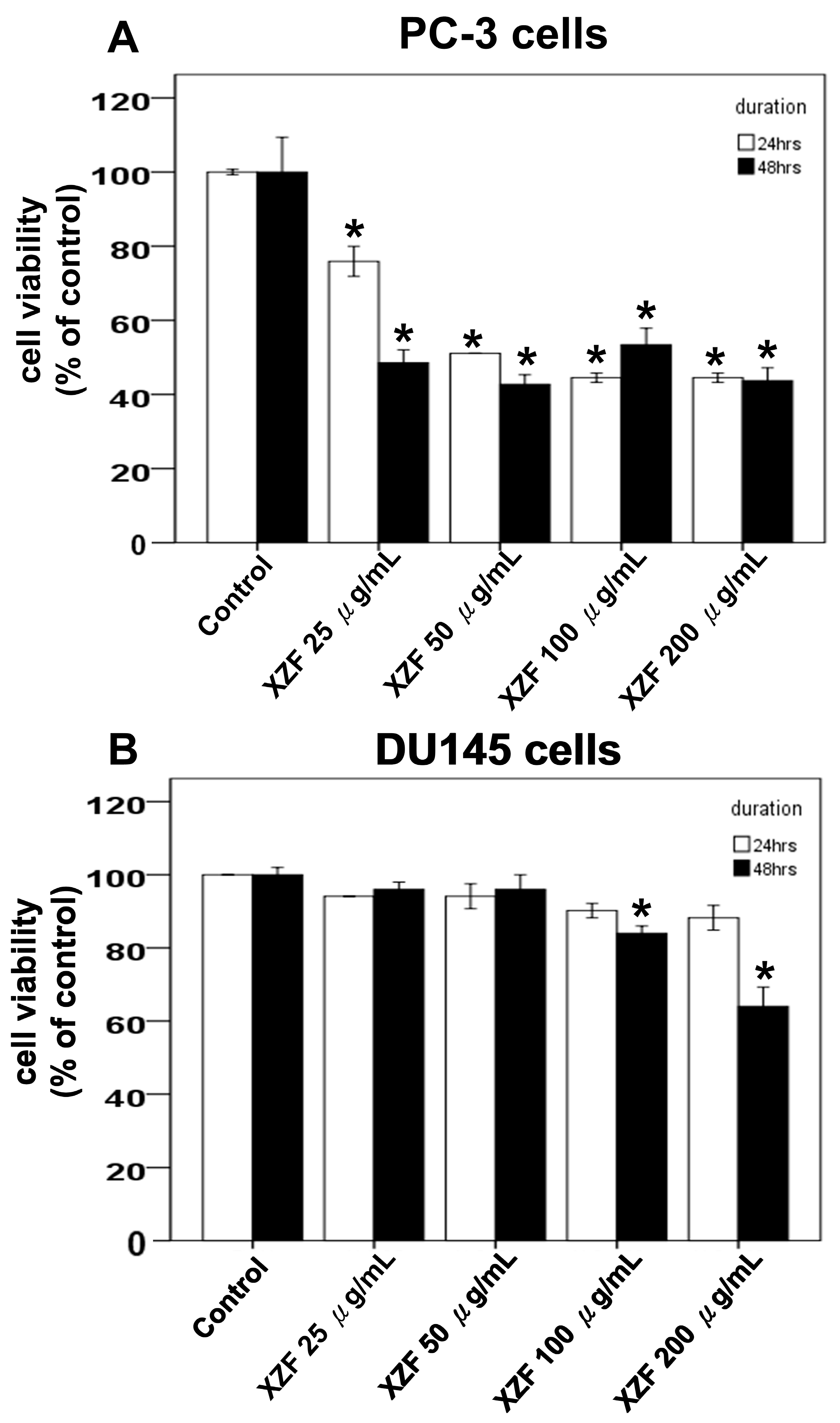
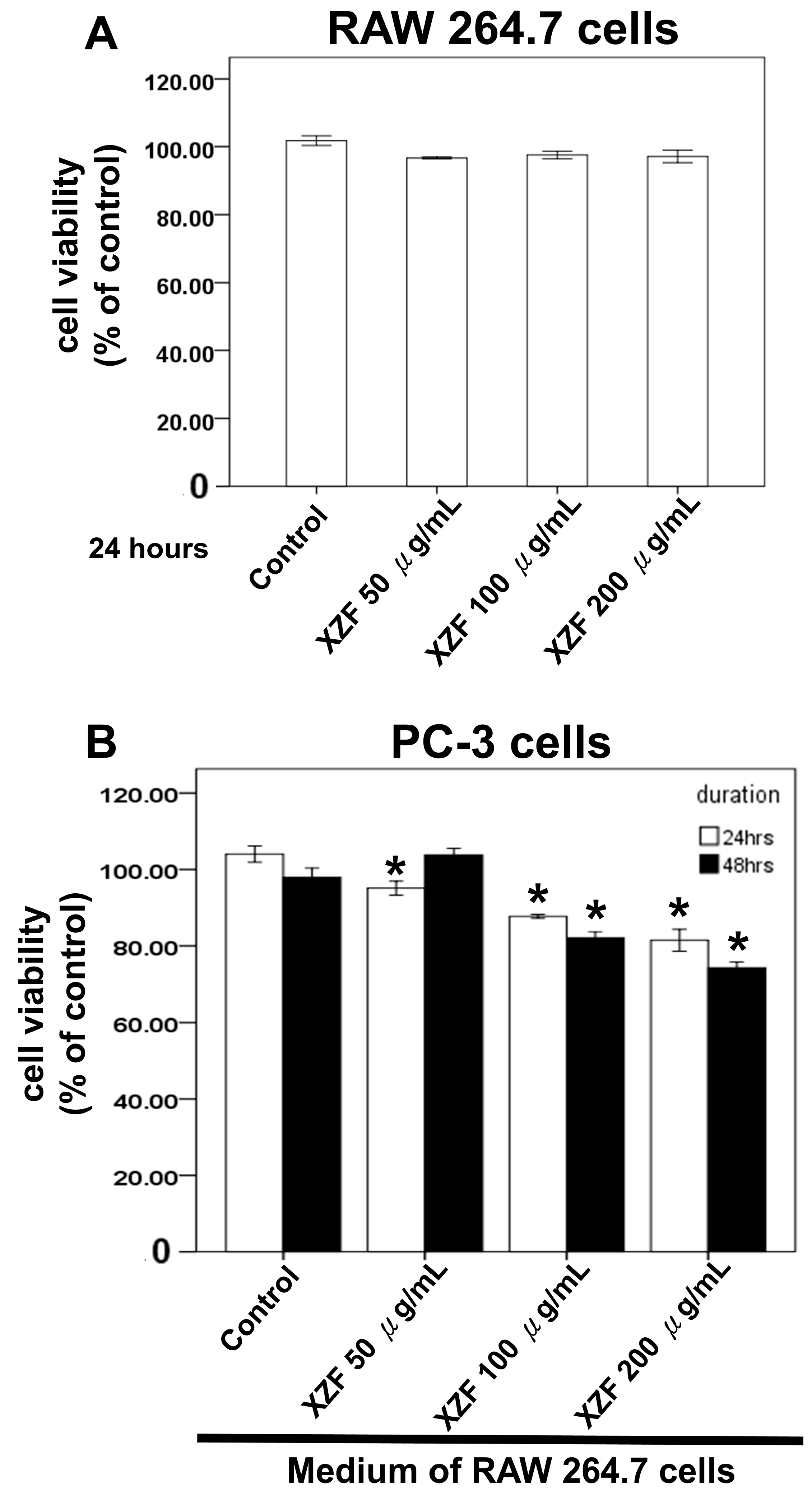
2.2. Effect of XZF on Cell Variability in the Non-Cancerous Cell Lines
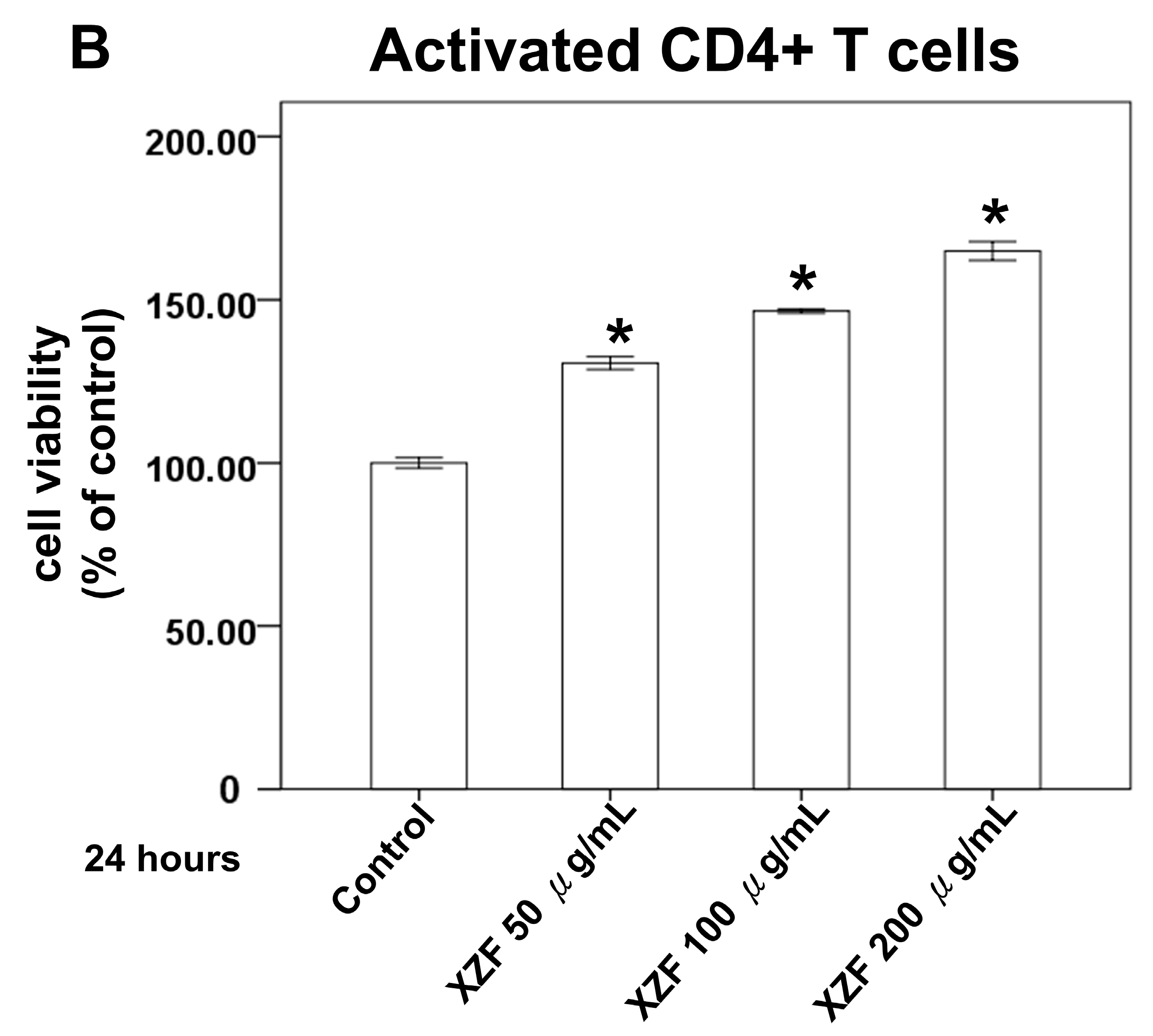
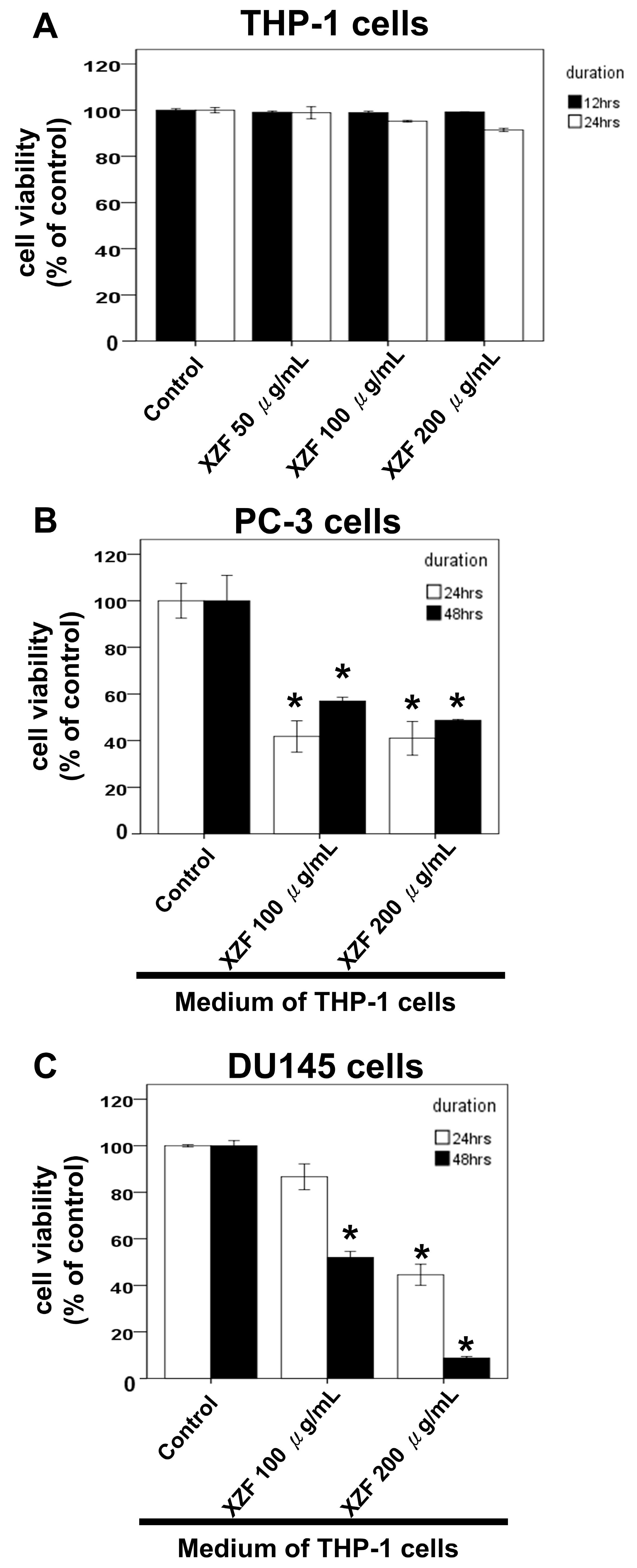
2.3. Effects of XZF on Cell Viability of T-Cells
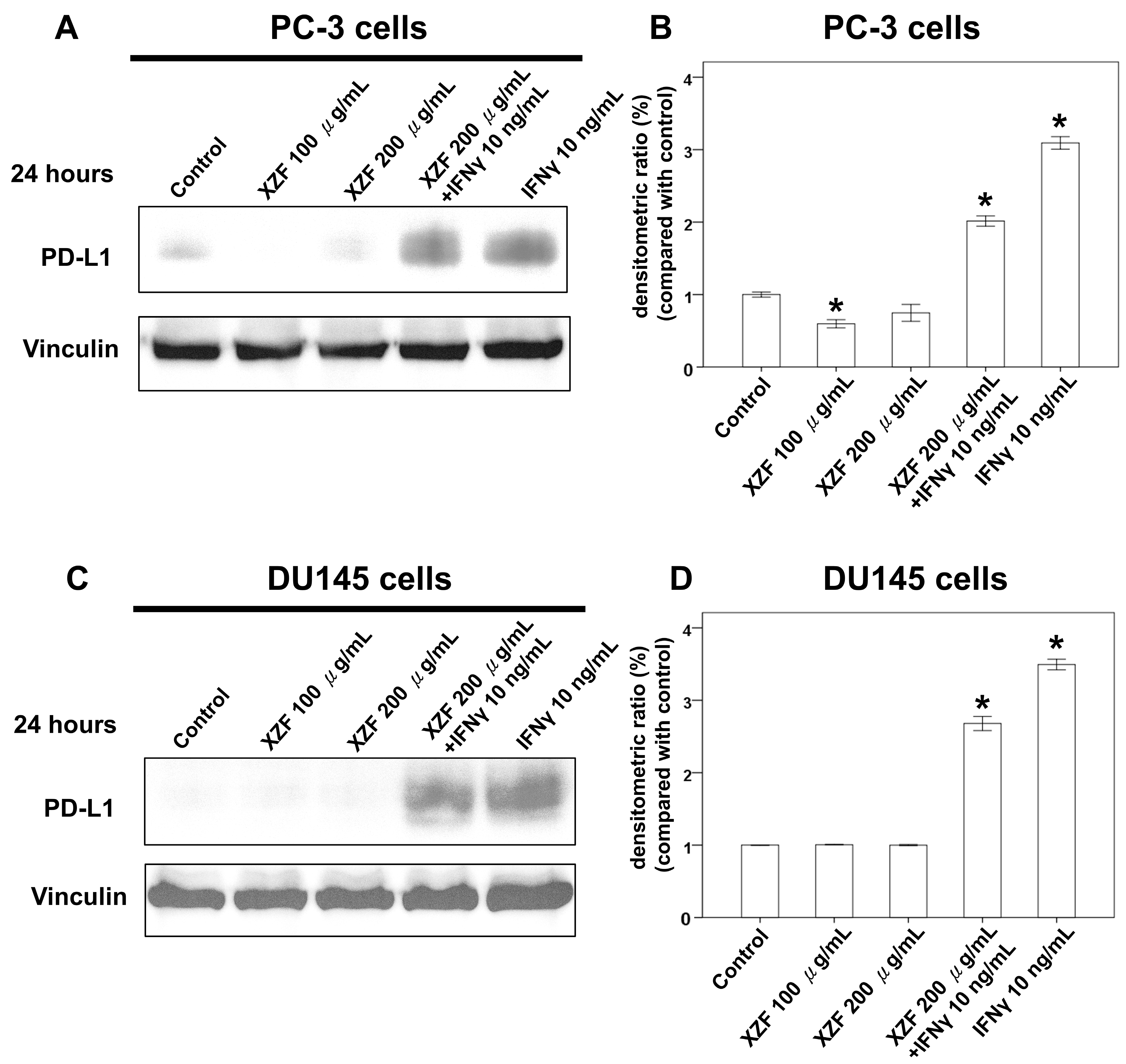
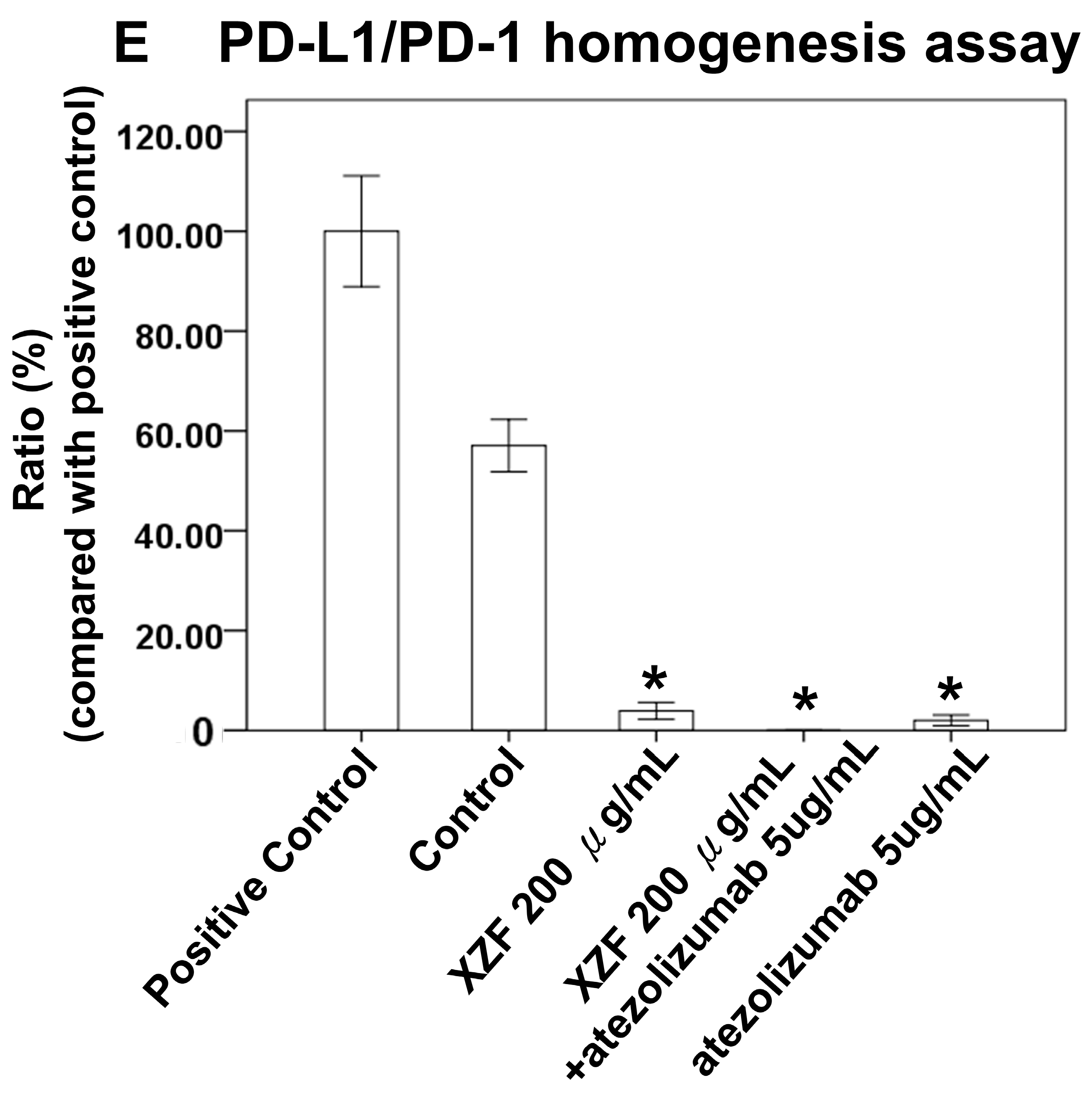
2.4. Effects of XZF on PD-L1/PD-1 Pathway
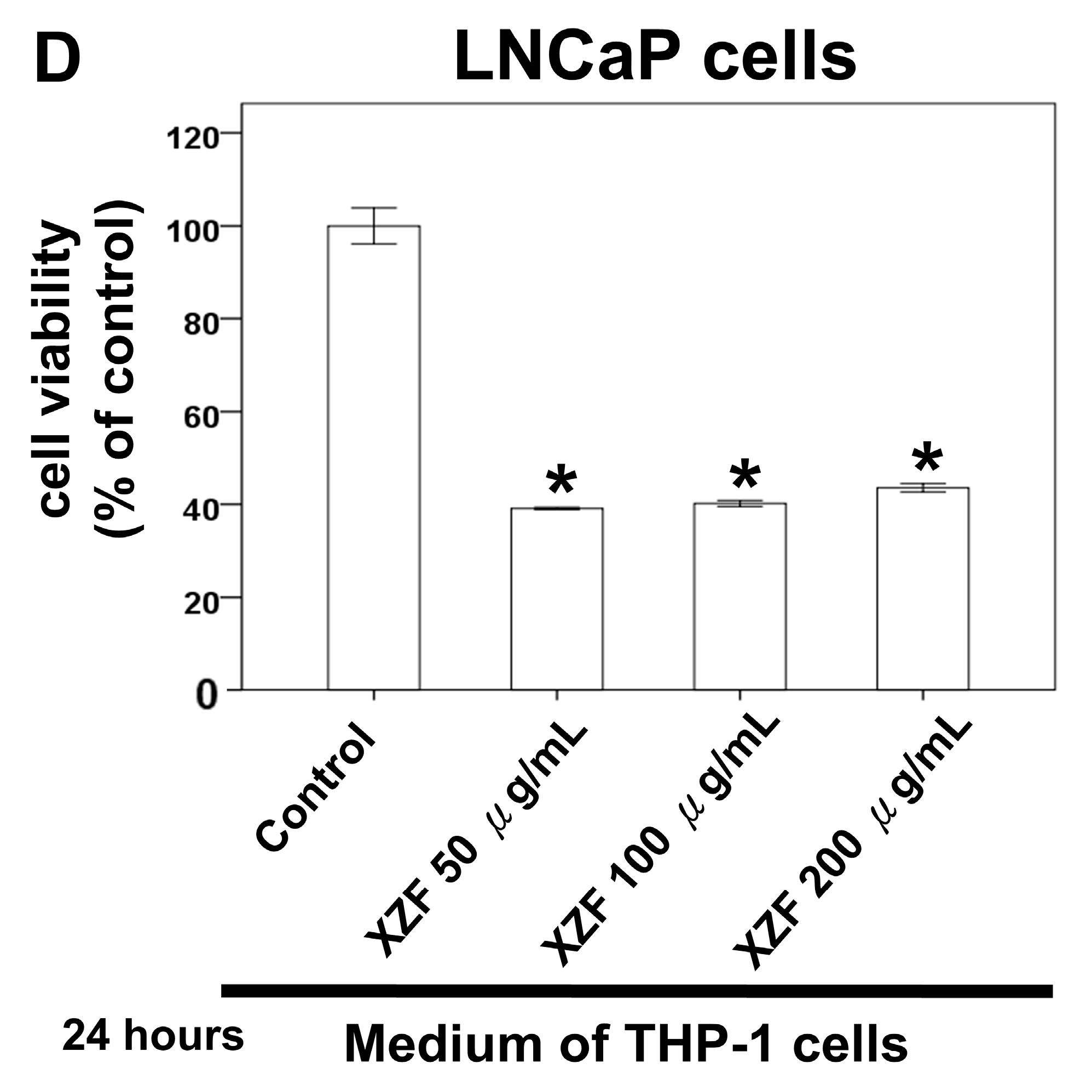
2.5. Effects of XZF on Cell Viability of Prostate Cancer Cells
2.6. The Effects of XZF Treatment on Prostate Cancer Cell Viability in Macrophage-Conditioned Medium
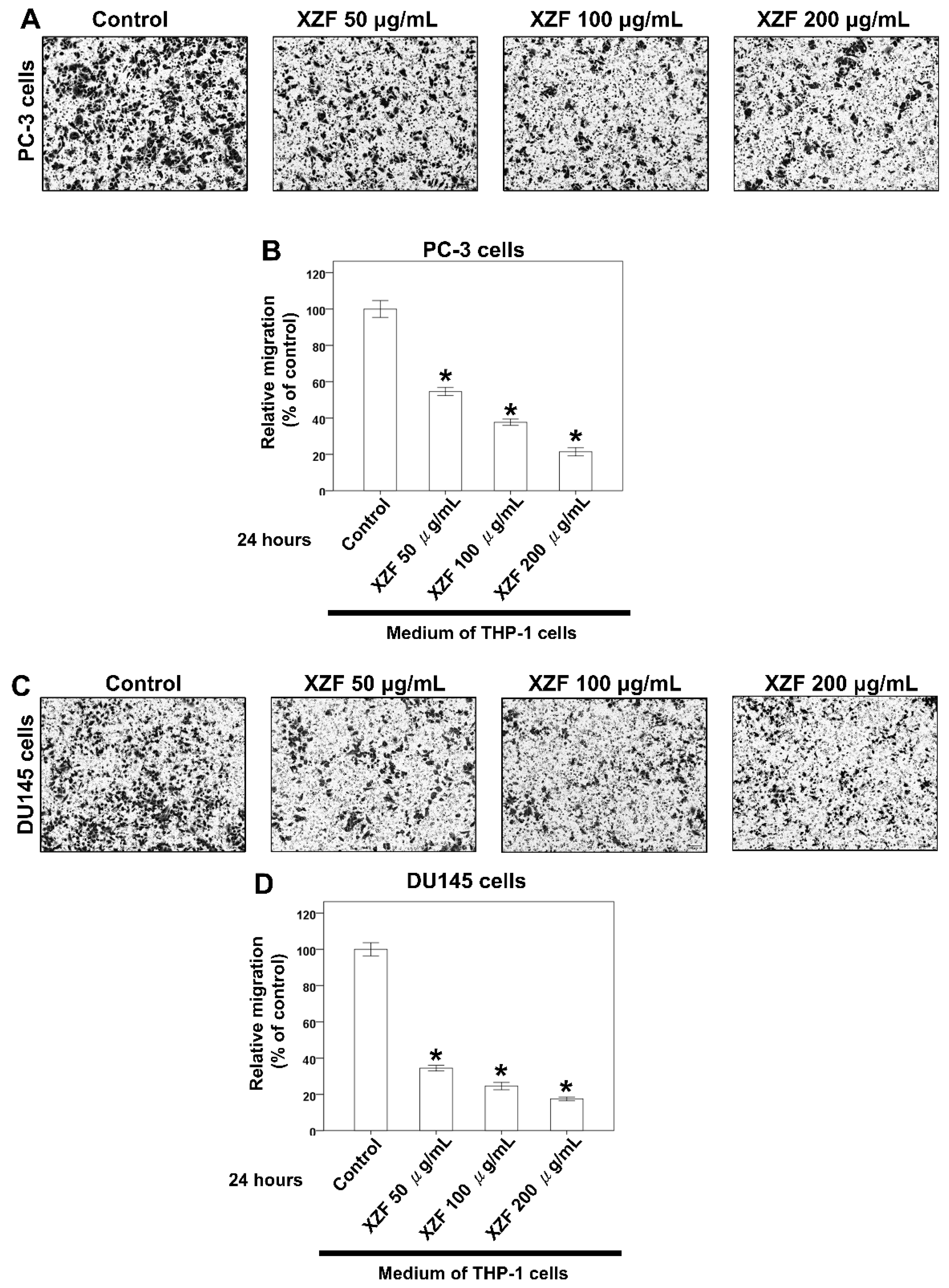
2.7. Effects of XZF on Migration of Prostate Cancer Cells with Macrophage-Conditioned Medium
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Quality Control of XZF
4.3. Cell Viability Assessment
4.4. Isolation of Splenic CD4+ and CD8+ T-Cells and T-Cell Activation Assays
4.5. Flow Cytometry Analysis of T-Cell Subpopulations
4.6. PD-1/PD-L1 Homogeneous Analysis
4.7. Western Blot Analysis
4.8. Protein Quantification Analysis
4.9. Migration Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Meng, L.; Yang, Y.; Mortazavi, A.; Zhang, J. Emerging Immunotherapy Approaches for Treating Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 14347. [Google Scholar] [CrossRef] [PubMed]
- Anti-PD-1-CTLA4 Combo Hits Prostate Cancer. Cancer Discov. 2019, 9, 569–570. [CrossRef] [PubMed]
- Wang, I.; Song, L.; Wang, B.Y.; Rezazadeh Kalebasty, A.; Uchio, E.; Zi, X. Prostate cancer immunotherapy: A review of recent advancements with novel treatment methods and efficacy. Am. J. Clin. Exp. Urol. 2022, 10, 210–233. [Google Scholar] [PubMed]
- Bilusic, M.; Madan, R.A.; Gulley, J.L. Immunotherapy of Prostate Cancer: Facts and Hopes. Clin. Cancer Res. 2017, 23, 6764–6770. [Google Scholar] [CrossRef]
- Kaur, H.B.; Guedes, L.B.; Lu, J.; Maldonado, L.; Reitz, L.; Barber, J.R.; De Marzo, A.M.; Tosoian, J.J.; Tomlins, S.A.; Schaeffer, E.M.; et al. Association of tumor-infiltrating T-cell density with molecular subtype, racial ancestry and clinical outcomes in prostate cancer. Mod. Pathol. 2018, 31, 1539–1552. [Google Scholar] [CrossRef]
- Krueger, T.E.; Thorek, D.L.J.; Meeker, A.K.; Isaacs, J.T.; Brennen, W.N. Tumor-infiltrating mesenchymal stem cells: Drivers of the immunosuppressive tumor microenvironment in prostate cancer? Prostate 2019, 79, 320–330. [Google Scholar] [CrossRef]
- de Bono, J.S.; Guo, C.; Gurel, B.; De Marzo, A.M.; Sfanos, K.S.; Mani, R.S.; Gil, J.; Drake, C.G.; Alimonti, A. Prostate carcinogenesis: Inflammatory storms. Nat. Rev. Cancer 2020, 20, 455–469. [Google Scholar] [CrossRef]
- Xu, P.; Wasielewski, L.J.; Yang, J.C.; Cai, D.; Evans, C.P.; Murphy, W.J.; Liu, C. The Immunotherapy and Immunosuppressive Signaling in Therapy-Resistant Prostate Cancer. Biomedicines 2022, 10, 1778. [Google Scholar] [CrossRef]
- Comito, G.; Giannoni, E.; Segura, C.P.; Barcellos-de-Souza, P.; Raspollini, M.R.; Baroni, G.; Lanciotti, M.; Serni, S.; Chiarugi, P. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene 2014, 33, 2423–2431. [Google Scholar] [CrossRef]
- Lo, C.H.; Lynch, C.C. Multifaceted Roles for Macrophages in Prostate Cancer Skeletal Metastasis. Front. Endocrinol. 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, M.; Masieri, L.; Raspollini, M.R.; Minervini, A.; Mari, A.; Comito, G.; Giannoni, E.; Carini, M.; Chiarugi, P.; Serni, S. The role of M1 and M2 macrophages in prostate cancer in relation to extracapsular tumor extension and biochemical recurrence after radical prostatectomy. Biomed Res. Int. 2014, 2014, 486798. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Isaacsson Velho, P.; Antonarakis, E.S. PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert Rev. Clin. Pharmacol. 2018, 11, 475–486. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Q.; Zhou, Y.; He, M.; Chen, J.; Gao, Y.; Wang, X. The Clinicopathologic and Prognostic Significance of Programmed Cell Death Ligand 1 (PD-L1) Expression in Patients With Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2018, 9, 1494. [Google Scholar] [CrossRef]
- Lu, X.; Horner, J.W.; Paul, E.; Shang, X.; Troncoso, P.; Deng, P.; Jiang, S.; Chang, Q.; Spring, D.J.; Sharma, P.; et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 2017, 543, 728–732. [Google Scholar] [CrossRef]
- Li, A.; Yi, M.; Qin, S.; Chu, Q.; Luo, S.; Wu, K. Prospects for combining immune checkpoint blockade with PARP inhibition. J. Hematol. Oncol. 2019, 12, 98. [Google Scholar] [CrossRef]
- Li, M.H.; Wu, H.C.; Yao, H.J.; Lin, C.C.; Wen, S.F.; Pan, I.H. Antrodia cinnamomea Extract Inhibits Th17 Cell Differentiation and Ameliorates Imiquimod-Induced Psoriasiform Skin Inflammation. Am. J. Chin. Med. 2015, 43, 1401–1417. [Google Scholar] [CrossRef]
- Lin, C.C.; Pan, I.H.; Li, Y.R.; Pan, Y.G.; Lin, M.K.; Lu, Y.H.; Wu, H.C.; Chu, C.L. The adjuvant effects of high-molecule-weight polysaccharides purified from Antrodia cinnamomea on dendritic cell function and DNA vaccines. PLoS ONE 2015, 10, e0116191. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Song, G.; Xie, S.; Jiang, W.; Chen, X.; Chu, M.; Hu, X.; Wang, Z.W. The roles of PD-1/PD-L1 in the prognosis and immunotherapy of prostate cancer. Mol. Ther. 2021, 29, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Palicelli, A.; Croci, S.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; Sanguedolce, F.; Ragazzi, M.; Zanelli, M.; Chaux, A.; et al. What Do We Have to Know about PD-L1 Expression in Prostate Cancer? A Systematic Literature Review. Part 3: PD-L1, Intracellular Signaling Pathways and Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 12330. [Google Scholar] [CrossRef]
- Dijkmans, R.; Billiau, A. Interferon gamma: A master key in the immune system. Curr. Opin. Immunol. 1988, 1, 269–274. [Google Scholar] [CrossRef]
- Zaidi, M.R.; Merlino, G. The two faces of interferon-gamma in cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Hamanishi, J.; Abiko, K.; Matsumura, N.; Baba, T.; Konishi, I. Dual Faces of IFNgamma in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity. Clin. Cancer Res. 2016, 22, 2329–2334. [Google Scholar] [CrossRef]
- Dallavalasa, S.; Beeraka, N.M.; Basavaraju, C.G.; Tulimilli, S.V.; Sadhu, S.P.; Rajesh, K.; Aliev, G.; Madhunapantula, S.V. The Role of Tumor Associated Macrophages (TAMs) in Cancer Progression, Chemoresistance, Angiogenesis and Metastasis—Current Status. Curr. Med. Chem. 2021, 28, 8203–8236. [Google Scholar] [CrossRef]
- Messex, J.K.; Liou, G.Y. Impact of Immune Cells in the Tumor Microenvironment of Prostate Cancer Metastasis. Life 2023, 13, 333. [Google Scholar] [CrossRef]
- Kumari, N.; Choi, S.H. Tumor-associated macrophages in cancer: Recent advancements in cancer nanoimmunotherapies. J. Exp. Clin. Cancer Res. 2022, 41, 68. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, D.; Liu, Y.; Li, C.; Zhao, X.; Li, Y.; Li, W. Ganoderma lucidum polysaccharide inhibits prostate cancer cell migration via the protein arginine methyltransferase 6 signaling pathway. Mol. Med. Rep. 2018, 17, 147–157. [Google Scholar] [CrossRef]
- Jiang, J.; Slivova, V.; Valachovicova, T.; Harvey, K.; Sliva, D. Ganoderma lucidum inhibits proliferation and induces apoptosis in human prostate cancer cells PC-3. Int. J. Oncol. 2004, 24, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xie, Z.P.; Huang, Z.S.; Li, H.; Wei, A.Y.; Di, J.M.; Xiao, H.J.; Zhang, Z.G.; Cai, L.H.; Tao, X.; et al. Total triterpenoids from Ganoderma Lucidum suppresses prostate cancer cell growth by inducing growth arrest and apoptosis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015, 35, 736–741. [Google Scholar] [CrossRef]
- Rahimnia, R.; Akbari, M.R.; Yasseri, A.F.; Taheri, D.; Mirzaei, A.; Ghajar, H.A.; Farashah, P.D.; Baghdadabad, L.Z.; Aghamir, S.M.K. The effect of Ganoderma lucidum polysaccharide extract on sensitizing prostate cancer cells to flutamide and docetaxel: An in vitro study. Sci. Rep. 2023, 13, 18940. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, B.; Zhou, L.; Wang, X.; Veeraraghavan, V.P.; Mohan, S.K.; Xin, F. Ganoderma lucidum put forth anti-tumor activity against PC-3 prostate cancer cells via inhibition of Jak-1/STAT-3 activity. Saudi J. Biol. Sci. 2020, 27, 2632–2637. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Jin, X.; Xie, M.; Liu, J.; Gontcharov, A.A.; Wang, H.; Lv, R.; Liu, D.; Wang, Q.; Li, Y. Characterization of a polysaccharide from Sanghuangporus vaninii and its antitumor regulation via activation of the p53 signaling pathway in breast cancer MCF-7 cells. Int. J. Biol. Macromol. 2020, 163, 865–877. [Google Scholar] [CrossRef]
- Guo, S.; Duan, W.; Wang, Y.; Chen, L.; Yang, C.; Gu, X.; Xue, Q.; Li, R.; Zhang, Z. Component Analysis and Anti-Colorectal Cancer Mechanism via AKT/mTOR Signalling Pathway of Sanghuangporus vaninii Extracts. Molecules 2022, 27, 1153. [Google Scholar] [CrossRef]
- Youn, M.J.; Kim, J.K.; Park, S.Y.; Kim, Y.; Park, C.; Kim, E.S.; Park, K.I.; So, H.S.; Park, R. Potential anticancer properties of the water extract of Inonotus [corrected] obliquus by induction of apoptosis in melanoma B16-F10 cells. J. Ethnopharmacol. 2009, 121, 221–228. [Google Scholar] [CrossRef]
- Chung, M.J.; Chung, C.K.; Jeong, Y.; Ham, S.S. Anticancer activity of subfractions containing pure compounds of Chaga mushroom (Inonotus obliquus) extract in human cancer cells and in Balbc/c mice bearing Sarcoma-180 cells. Nutr. Res. Pract. 2010, 4, 177–182. [Google Scholar] [CrossRef]
- Zaidman, B.Z.; Wasser, S.P.; Nevo, E.; Mahajna, J. Androgen receptor-dependent and -independent mechanisms mediate Ganoderma lucidum activities in LNCaP prostate cancer cells. Int. J. Oncol. 2007, 31, 959–967. [Google Scholar] [CrossRef]
- Stanley, G.; Harvey, K.; Slivova, V.; Jiang, J.; Sliva, D. Ganoderma lucidum suppresses angiogenesis through the inhibition of secretion of VEGF and TGF-beta1 from prostate cancer cells. Biochem. Biophys. Res. Commun. 2005, 330, 46–52. [Google Scholar] [CrossRef]
- Huang, T.F.; Wang, S.W.; Lai, Y.W.; Liu, S.C.; Chen, Y.J.; Hsueh, T.Y.; Lin, C.C.; Lin, C.H.; Chung, C.H. 4-Acetylantroquinonol B Suppresses Prostate Cancer Growth and Angiogenesis via a VEGF/PI3K/ERK/mTOR-Dependent Signaling Pathway in Subcutaneous Xenograft and In Vivo Angiogenesis Models. Int. J. Mol. Sci. 2022, 23, 1446. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Tzeng, D.T.W.; Huang, Y.P.; Lin, C.J.; Lo, U.G.; Wu, C.L.; Lin, H.; Hsieh, J.T.; Tang, C.H.; Lai, C.H. Antrocin Sensitizes Prostate Cancer Cells to Radiotherapy Through Inhibiting PI3K/AKT and MAPK Signaling Pathways. Cancers 2018, 11, 34. [Google Scholar] [CrossRef]
- Rios, J.L.; Andujar, I.; Recio, M.C.; Giner, R.M. Lanostanoids from fungi: A group of potential anticancer compounds. J. Nat. Prod. 2012, 75, 2016–2044. [Google Scholar] [CrossRef]
- Jayakumar, S.; Kunwar, A.; Sandur, S.K.; Pandey, B.N.; Chaubey, R.C. Differential response of DU145 and PC3 prostate cancer cells to ionizing radiation: Role of reactive oxygen species, GSH and Nrf2 in radiosensitivity. Biochim. Biophys. Acta 2014, 1840, 485–494. [Google Scholar] [CrossRef]
- Dulinska-Litewka, J.; Dykas, K.; Boznanski, S.; Halubiec, P.; Kaczor-Kaminska, M.; Zagajewski, J.; Bohn, T.; Wator, G. The Influence of beta-Carotene and Its Liposomal Form on the Expression of EMT Markers and Androgen-Dependent Pathways in Different Prostate Cell Lines. Antioxidants 2024, 13, 902. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, J.Y.; Lei, Z.M.; Wan, L.J.; Zhu, X.W.; Ye, F.; Tong, Y.Y. Anti-proliferative effects of paeonol on human prostate cancer cell lines DU145 and PC-3. J. Physiol. Biochem. 2017, 73, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, X.; Wang, L.; Zhang, D.; Luo, Q.; Wang, B. Overexpressing miR-335 inhibits DU145 cell proliferation by targeting early growth response 3 in prostate cancer. Int. J. Oncol. 2019, 54, 1981–1994. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Y.; Liu, W.; Li, Z.; Wei, Z.; Jiang, R. Microtubule-associated protein tau is associated with the resistance to docetaxel in prostate cancer cell lines. Res. Rep. Urol. 2017, 9, 71–77. [Google Scholar] [CrossRef]
- Molter, C.W.; Muszynski, E.F.; Tao, Y.; Trivedi, T.; Clouvel, A.; Ehrlicher, A.J. Prostate cancer cells of increasing metastatic potential exhibit diverse contractile forces, cell stiffness, and motility in a microenvironment stiffness-dependent manner. Front. Cell Dev. Biol. 2022, 10, 932510. [Google Scholar] [CrossRef]
- Ahmed, K.; Omarova, Z.; Sheikh, A.; Abuova, G.; Ghias, K.; Abidi, S.H. Comparison of baseline global gene expression profiles of prostate cancer cell lines LNCaP and DU145. BMC Res. Notes 2024, 17, 398. [Google Scholar] [CrossRef]
- Guan, X.; Polesso, F.; Wang, C.; Sehrawat, A.; Hawkins, R.M.; Murray, S.E.; Thomas, G.V.; Caruso, B.; Thompson, R.F.; Wood, M.A.; et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 2022, 606, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.H.; Withers, H.G.; Garbens, A.; Love, H.D.; Magnoni, L.; Hayward, S.W.; Moyes, C.D. Hypoxia and the metabolic phenotype of prostate cancer cells. Biochim. Biophys. Acta 2009, 1787, 1433–1443. [Google Scholar] [CrossRef]
- Su, C.Y.; Huang, G.C.; Chang, Y.C.; Chen, Y.J.; Fang, H.W. Analyzing the Expression of Biomarkers in Prostate Cancer Cell Lines. In Vivo 2021, 35, 1545–1548. [Google Scholar] [CrossRef]
- Chen, Y.C.; Liu, Y.C.; El-Shazly, M.; Wu, T.Y.; Chang, J.G.; Wu, Y.C. Antrodia cinnamomea, a Treasured Medicinal Mushroom, Induces Growth Arrest in Breast Cancer Cells, T47D Cells: New Mechanisms Emerge. Int. J. Mol. Sci. 2019, 20, 833. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Liu, Y.K.; Lan, K.L.; Lee, Y.W.; Tsai, T.H.; Chen, Y.J. Medicinal Fungus Antrodia cinnamomea Inhibits Growth and Cancer Stem Cell Characteristics of Hepatocellular Carcinoma. Evid. Based Complement. Altern. Med. 2013, 2013, 569737. [Google Scholar] [CrossRef][Green Version]
- Chung, C.H.; Yeh, S.C.; Chen, C.J.; Lee, K.T. Coenzyme Q0 from Antrodia cinnamomea in Submerged Cultures Induces Reactive Oxygen Species-Mediated Apoptosis in A549 Human Lung Cancer Cells. Evid. Based Complement. Altern. Med. 2014, 2014, 246748. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Ma, J.; Han, C.; Jin, Y.; Zhao, G.; He, X. Extraction and antioxidant activity of total triterpenoids in the mycelium of a medicinal fungus, Sanghuangporus sanghuang. Sci. Rep. 2019, 9, 7418. [Google Scholar] [CrossRef]
- Wang, W.; Song, J.; Lu, N.; Yan, J.; Chen, G. Sanghuangporus sanghuang extract inhibits the proliferation and invasion of lung cancer cells in vitro and in vivo. Nutr. Res. Pract. 2023, 17, 1070–1083. [Google Scholar] [CrossRef]
- Martinez-Montemayor, M.M.; Acevedo, R.R.; Otero-Franqui, E.; Cubano, L.A.; Dharmawardhane, S.F. Ganoderma lucidum (Reishi) inhibits cancer cell growth and expression of key molecules in inflammatory breast cancer. Nutr. Cancer 2011, 63, 1085–1094. [Google Scholar] [CrossRef]
- Song, M.; Li, Z.H.; Gu, H.S.; Tang, R.Y.; Zhang, R.; Zhu, Y.L.; Liu, J.L.; Zhang, J.J.; Wang, L.Y. Ganoderma lucidum Spore Polysaccharide Inhibits the Growth of Hepatocellular Carcinoma Cells by Altering Macrophage Polarity and Induction of Apoptosis. J. Immunol. Res. 2021, 2021, 6696606. [Google Scholar] [CrossRef]
- Lin, W.; Gu, L.; Zhu, L.Y.; Zhou, S.; Lian, D.; Xu, Y.; Zheng, L.; Liu, X.; Li, L. Extract of Ganoderma sinensis spores induces cell cycle arrest of hepatoma cell via endoplasmic reticulum stress. Pharm. Biol. 2021, 59, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chang, Y.; Liu, Y.; Zhang, M.; Luo, H.; Hao, C.; Zeng, P.; Sun, Y.; Wang, H.; Zhang, L. Overview of Ganoderma sinense polysaccharide-an adjunctive drug used during concurrent Chemo/Radiation therapy for cancer treatment in China. Biomed. Pharmacother. 2017, 96, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Hwang, H.S.; Yun, J.W. Antitumor activity of water extract of a mushroom, Inonotus obliquus, against HT-29 human colon cancer cells. Phytother. Res. 2009, 23, 1784–1789. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, Y.; Xue, Z.; Li, N.; Liu, J.; Chen, H. Recent Developments in Inonotus obliquus (Chaga mushroom) Polysaccharides: Isolation, Structural Characteristics, Biological Activities and Application. Polymers 2021, 13, 1441. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wan, P.; Qiao, J.; Liu, Y.; Peng, Q.; Zhang, Z.; Shu, X.; Xia, Y.; Sun, B. Current and further outlook on the protective potential of Antrodia camphorata against neurological disorders. Front. Pharmacol. 2024, 15, 1372110. [Google Scholar] [CrossRef]
- Teng, Y.; Liang, H.; Zhang, Z.; He, Y.; Pan, Y.; Yuan, S.; Wu, X.; Zhao, Q.; Yang, H.; Zhou, P. Biodistribution and immunomodulatory activities of a proteoglycan isolated from Ganoderma lucidum. J. Funct. Foods 2020, 74, 104193. [Google Scholar] [CrossRef]
- Khoroshutin, P.; Reva, G.; Yamamoto, T.; Reva, I. Pharmacokinetics and pharmacodynamics of Chaga birch mushroom components (Inonotus obliquus). Arch. Euromedica 2021, 11, 31–38. [Google Scholar] [CrossRef]
- Ling, T.; Arroyo-Cruz, L.V.; Smither, W.R.; Seighman, E.K.; Martinez-Montemayor, M.M.; Rivas, F. Early Preclinical Studies of Ergosterol Peroxide and Biological Evaluation of Its Derivatives. ACS Omega 2024, 9, 37117–37127. [Google Scholar] [CrossRef]
- Zhang, F.F.; Liu, R.M. Pharmacokinetics of ganoderic acids. Zhongguo Zhong Yao Za Zhi 2019, 44, 905–911. [Google Scholar] [CrossRef]
- Oh, K.K.; Adnan, M.; Cho, D.H. A network pharmacology analysis on drug-like compounds from Ganoderma lucidum for alleviation of atherosclerosis. J. Food Biochem. 2021, 45, e13906. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Q.; Lv, B.; Li, N.; Bian, X.; Chen, L.; Kong, M.; Shen, Y.; Zheng, W.; Zhang, J.; et al. Ganoderma lucidum Polysaccharide Supplementation Significantly Activates T-Cell-Mediated Antitumor Immunity and Enhances Anti-PD-1 Immunotherapy Efficacy in Colorectal Cancer. J. Agric. Food Chem. 2024, 72, 12072–12082. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.C.; Huang, C.C.; Chiang, P.F.; Lin, C.N.; Li, L.L.; Lee, T.W.; Lin, B.; Chen, I.C.; Chang, K.W.; Fan, C.K.; et al. Radioprotective effects of Antrodia cinnamomea are enhanced on immune cells and inhibited on cancer cells. Int. J. Radiat. Biol. 2014, 90, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.Y.; Pan, M.H.; Lai, C.S.; Lin, T.T.; Chen, C.T.; Chung, T.S.; Chen, C.L.; Lin, C.H.; Chuang, W.C.; Lee, M.C.; et al. CCM111, the water extract of Antrodia cinnamomea, regulates immune-related activity through STAT3 and NF-kappaB pathways. Sci. Rep. 2017, 7, 4862. [Google Scholar] [CrossRef]
- Yue, G.G.; Chan, B.C.; Han, X.Q.; Cheng, L.; Wong, E.C.; Leung, P.C.; Fung, K.P.; Ng, M.C.; Fan, K.; Sze, D.M.; et al. Immunomodulatory activities of Ganoderma sinense polysaccharides in human immune cells. Nutr. Cancer 2013, 65, 765–774. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Q.; He, Y.M. The effect of Ganoderma lucidum extract on immunological function and identify its anti-tumor immunostimulatory activity based on the biological network. Sci. Rep. 2018, 8, 12680. [Google Scholar] [CrossRef]
- Song, F.Q.; Liu, Y.; Kong, X.S.; Chang, W.; Song, G. Progress on understanding the anticancer mechanisms of medicinal mushroom: Inonotus obliquus. Asian Pac. J. Cancer Prev. 2013, 14, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, W.; Di, T.; Meng, J.; Qi, Y.; Li, G.; Zhang, Y.; Su, H.; Yan, W. Water extract of sporoderm-broken spores of Ganoderma lucidum enhanced pd-l1 antibody efficiency through downregulation and relieved complications of pd-l1 monoclonal antibody. Biomed. Pharmacother. 2020, 131, 110541. [Google Scholar] [CrossRef]
- Lin, W.C.; Deng, J.S.; Huang, S.S.; Wu, S.H.; Chen, C.C.; Lin, W.R.; Lin, H.Y.; Huang, G.J. Anti-Inflammatory Activity of Sanghuangporus sanghuang Mycelium. Int. J. Mol. Sci. 2017, 18, 347. [Google Scholar] [CrossRef]
- Wang, S.Y.; Hsu, M.L.; Hsu, H.C.; Tzeng, C.H.; Lee, S.S.; Shiao, M.S.; Ho, C.K. The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int. J. Cancer 1997, 70, 699–705. [Google Scholar] [CrossRef]
- Han, X.Q.; Yue, G.L.; Yue, R.Q.; Dong, C.X.; Chan, C.L.; Ko, C.H.; Cheung, W.S.; Luo, K.W.; Dai, H.; Wong, C.K.; et al. Structure elucidation and immunomodulatory activity of a beta glucan from the fruiting bodies of Ganoderma sinense. PLoS ONE 2014, 9, e100380. [Google Scholar] [CrossRef]
- Ern, P.T.Y.; Quan, T.Y.; Yee, F.S.; Yin, A.C.Y. Therapeutic properties of Inonotus obliquus (Chaga mushroom): A review. Mycology 2024, 15, 144–161. [Google Scholar] [CrossRef]
- Lin, Z.H.; Lu, M.K.; Lo, H.C.; Chang, C.C.; Tseng, A.J.; Chao, C.H.; Lin, T.Y. ZnF3, a sulfated polysaccharide from Antrodia cinnamomea, inhibits lung cancer cells via induction of apoptosis and activation of M1-like macrophage-induced cell death. Int. J. Biol. Macromol. 2023, 238, 124144. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Wang, Y.; Hao, C.; Cheng, Y.; Guo, X.; He, Y.; Shi, Y.; Wang, S.; Li, Y.; Shi, W. Sanghuangporus sanghuang extract extended the lifespan and healthspan of Caenorhabditis elegans via DAF-16/SIR-2.1. Front. Pharmacol. 2023, 14, 1136897. [Google Scholar] [CrossRef]
- Li, G.L.; Tang, J.F.; Tan, W.L.; Zhang, T.; Zeng, D.; Zhao, S.; Ran, J.H.; Li, J.; Wang, Y.P.; Chen, D.L. The anti-hepatocellular carcinoma effects of polysaccharides from Ganoderma lucidum by regulating macrophage polarization via the MAPK/NF-kappaB signaling pathway. Food Funct. 2023, 14, 3155–3168. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.S.; Zhang, C.; Dong, H.L.; Li, K.K.; Han, Q.B.; Wan, Y.; Chen, R.; Yang, F.; Li, H.L.; Ko, C.H.; et al. GSP-2, a polysaccharide extracted from Ganoderma sinense, is a novel toll-like receptor 4 agonist. PLoS ONE 2019, 14, e0221636. [Google Scholar] [CrossRef] [PubMed]
- Wold, C.W.; Christopoulos, P.F.; Arias, M.A.; Dzovor, D.E.; Oynebraten, I.; Corthay, A.; Inngjerdingen, K.T. Fungal polysaccharides from Inonotus obliquus are agonists for Toll-like receptors and induce macrophage anti-cancer activity. Commun. Biol. 2024, 7, 222. [Google Scholar] [CrossRef]
- Huang, Y.J.; Yadav, V.K.; Srivastava, P.; Wu, A.T.; Huynh, T.T.; Wei, P.L.; Huang, C.F.; Huang, T.H. Antrodia cinnamomea Enhances Chemo-Sensitivity of 5-FU and Suppresses Colon Tumorigenesis and Cancer Stemness via Up-Regulation of Tumor Suppressor miR-142-3p. Biomolecules 2019, 9, 306. [Google Scholar] [CrossRef]
- Chen, J.H.; Wu, A.T.H.; Tzeng, D.T.W.; Huang, C.C.; Tzeng, Y.M.; Chao, T.Y. Antrocin, a bioactive component from Antrodia cinnamomea, suppresses breast carcinogenesis and stemness via downregulation of beta-catenin/Notch1/Akt signaling. Phytomedicine 2019, 52, 70–78. [Google Scholar] [CrossRef]
- Jiao, C.; Qiu, J.; Gong, C.; Li, X.; Liang, H.; He, C.; Cen, S.; Xie, Y. Ganoderma lucidum extract reverses multidrug resistance in breast cancer cells through inhibiting ATPase activity of the P-glycoprotein via MAPK/ERK signaling pathway. Exp. Cell Res. 2025, 444, 114355. [Google Scholar] [CrossRef]
- Zhong, C.; Li, Y.; Li, W.; Lian, S.; Li, Y.; Wu, C.; Zhang, K.; Zhou, G.; Wang, W.; Xu, H.; et al. Ganoderma lucidum extract promotes tumor cell pyroptosis and inhibits metastasis in breast cancer. Food Chem. Toxicol. 2023, 174, 113654. [Google Scholar] [CrossRef]
- Abugomaa, A.; Elbadawy, M.; Ishihara, Y.; Yamamoto, H.; Kaneda, M.; Yamawaki, H.; Shinohara, Y.; Usui, T.; Sasaki, K. Anti-cancer activity of Chaga mushroom (Inonotus obliquus) against dog bladder cancer organoids. Front. Pharmacol. 2023, 14, 1159516. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, R.; Jiao, Z.; Chen, W.; Peng, D.; Wang, L.; Yu, N.; Peng, C.; Cai, B.; Song, H.; et al. Current Advancements in Antitumor Properties and Mechanisms of Medicinal Components in Edible Mushrooms. Nutrients 2022, 14, 2622. [Google Scholar] [CrossRef]
- Duru, K.C.; Kovaleva, E.G.; Danilova, I.G.; van der Bijl, P. The pharmacological potential and possible molecular mechanisms of action of Inonotus obliquus from preclinical studies. Phytother. Res. 2019, 33, 1966–1980. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.K.; Shih, P.H.; Lee, W.H.; Bamodu, O.A.; Wu, A.T.H.; Huang, C.C.; Tzeng, Y.M.; Hsiao, M.; Yeh, C.T.; Lin, C.M. Antrodia cinnamomea sensitizes radio-/chemo-therapy of cancer stem-like cells by modulating microRNA expression. J. Ethnopharmacol. 2017, 207, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-H.; Shiou, W.-Y.; Wang, K.-C.; Huang, S.-Y.; Shie, Y.-T.; Tsai, C.-M.; Shie, J.-F.; Chen, K.-D. Chemotaxonomy of triterpenoid pattern of HPLC of Ganoderma lucidum and Ganoderma tsugae. J. Chin. Chem. Soc. 1999, 46, 5. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lin, Y.S.; Yang, Y.H.; Shu, L.H.; Cheng, Y.C.; Liu, H.T. GB-2 inhibits ACE2 and TMPRSS2 expression: In vivo and in vitro studies. Biomed. Pharmacother. 2020, 132, 110816. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Yang, Y.H.; Lin, Y.S.; Shu, L.H.; Liu, H.T.; Lu, C.K.; Wu, Y.H.; Wu, Y.H. The Effect and Mechanism of Astragalus Polysaccharides on T Cells and Macrophages in Inhibiting Prostate Cancer. Biomed. J. 2024, 48, 100741. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lin, Y.S.; Yang, Y.H.; Shu, L.H.; Cheng, Y.C.; Liu, H.T. Potential Simultaneous Inhibitors of Angiotensin-Converting Enzyme 2 and Transmembrane Protease, Serine 2. Front. Pharmacol. 2020, 11, 584158. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-Y.; Lu, C.-K.; Shu, L.-H.; Liu, H.-T.; Wu, Y.-H.; Lin, Y.-S.; Yang, Y.-H.; Shih, W.-T.; Lee, I.-Y.; Wu, Y.-H.; et al. Antrodia cinnamomea Formula Suppresses Prostate Cancer Progression via Immune Modulation and PD-1/PD-L1 Pathway Inhibition. Int. J. Mol. Sci. 2025, 26, 2684. https://doi.org/10.3390/ijms26062684
Tsai M-Y, Lu C-K, Shu L-H, Liu H-T, Wu Y-H, Lin Y-S, Yang Y-H, Shih W-T, Lee I-Y, Wu Y-H, et al. Antrodia cinnamomea Formula Suppresses Prostate Cancer Progression via Immune Modulation and PD-1/PD-L1 Pathway Inhibition. International Journal of Molecular Sciences. 2025; 26(6):2684. https://doi.org/10.3390/ijms26062684
Chicago/Turabian StyleTsai, Ming-Yen, Chung-Kuang Lu, Li-Hsin Shu, Hung-Te Liu, Yu-Huei Wu, Yu-Shih Lin, Yao-Hsu Yang, Wei-Tai Shih, I-Yun Lee, Yu-Heng Wu, and et al. 2025. "Antrodia cinnamomea Formula Suppresses Prostate Cancer Progression via Immune Modulation and PD-1/PD-L1 Pathway Inhibition" International Journal of Molecular Sciences 26, no. 6: 2684. https://doi.org/10.3390/ijms26062684
APA StyleTsai, M.-Y., Lu, C.-K., Shu, L.-H., Liu, H.-T., Wu, Y.-H., Lin, Y.-S., Yang, Y.-H., Shih, W.-T., Lee, I.-Y., Wu, Y.-H., & Wu, C.-Y. (2025). Antrodia cinnamomea Formula Suppresses Prostate Cancer Progression via Immune Modulation and PD-1/PD-L1 Pathway Inhibition. International Journal of Molecular Sciences, 26(6), 2684. https://doi.org/10.3390/ijms26062684






