Integrated Transcriptomic and Metabolomic Analysis Reveals Tissue-Specific Flavonoid Biosynthesis and MYB-Mediated Regulation of UGT71A1 in Panax quinquefolius
Abstract
1. Introduction
2. Results
2.1. Tissue-Specific Differential Accumulation of Flavonoid Metabolites in P. quinquefolius
2.2. Identification of Differentially Expressed Genes (DEGs) and Transcriptomic Analysis Among Three Tissues
2.3. Validation of DEGs in Flavonoid Biosynthesis of Panax quinquefolius by qRT-PCR
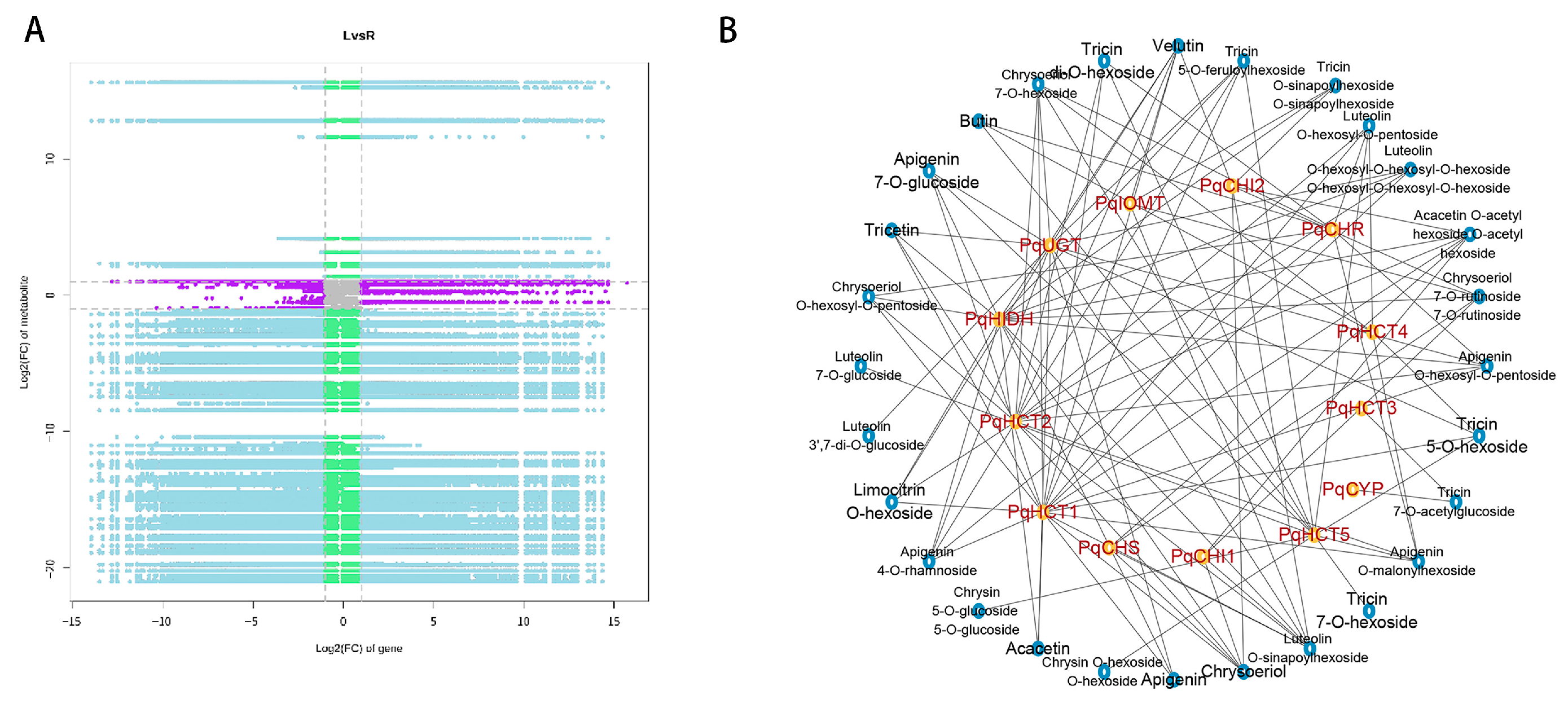
2.4. Correlation Analysis of Transcriptomic and Metabolomic Data
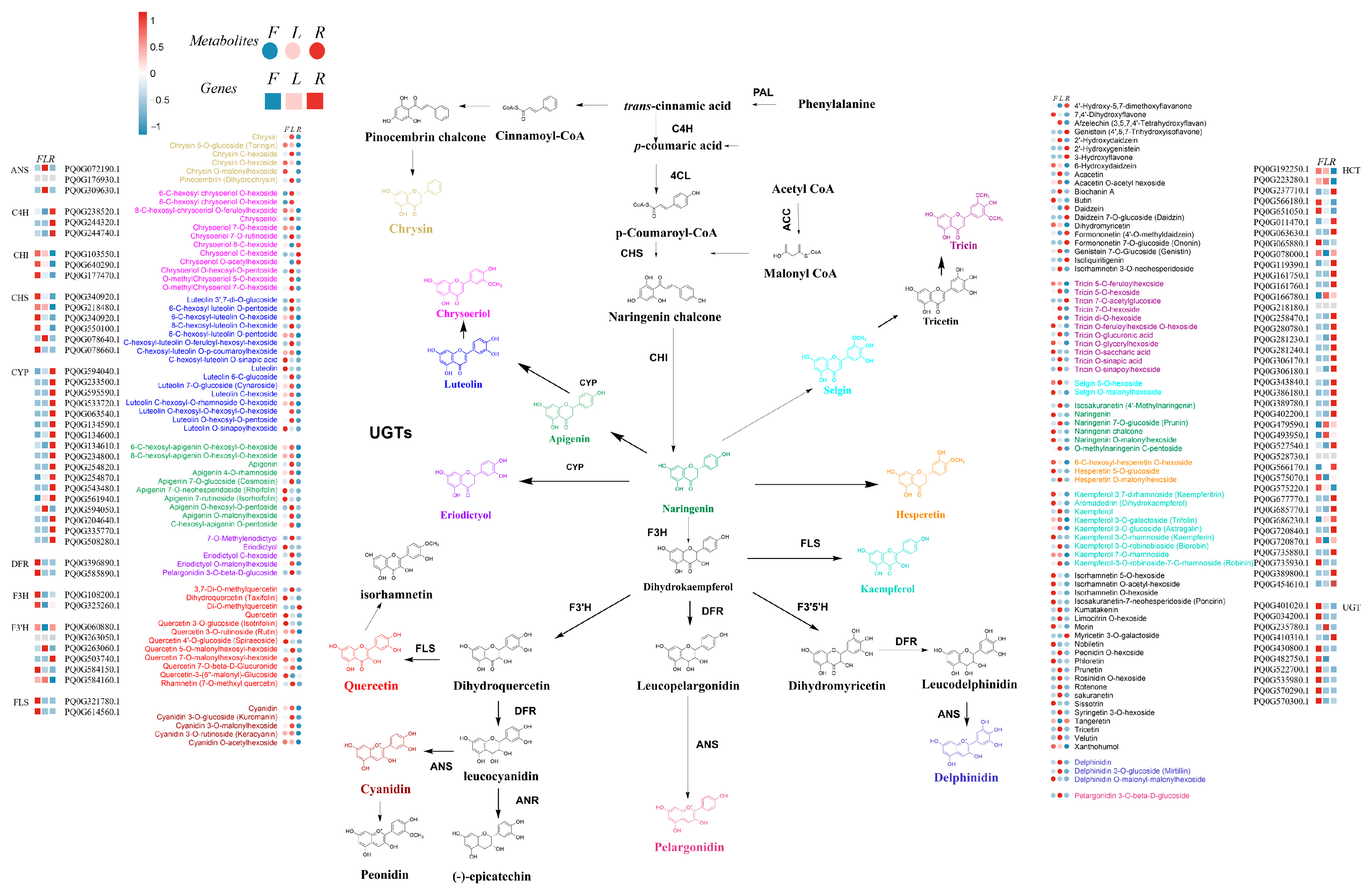
2.5. Changes in Genes and Metabolites in the Flavonoid Biosynthesis Regulatory Network
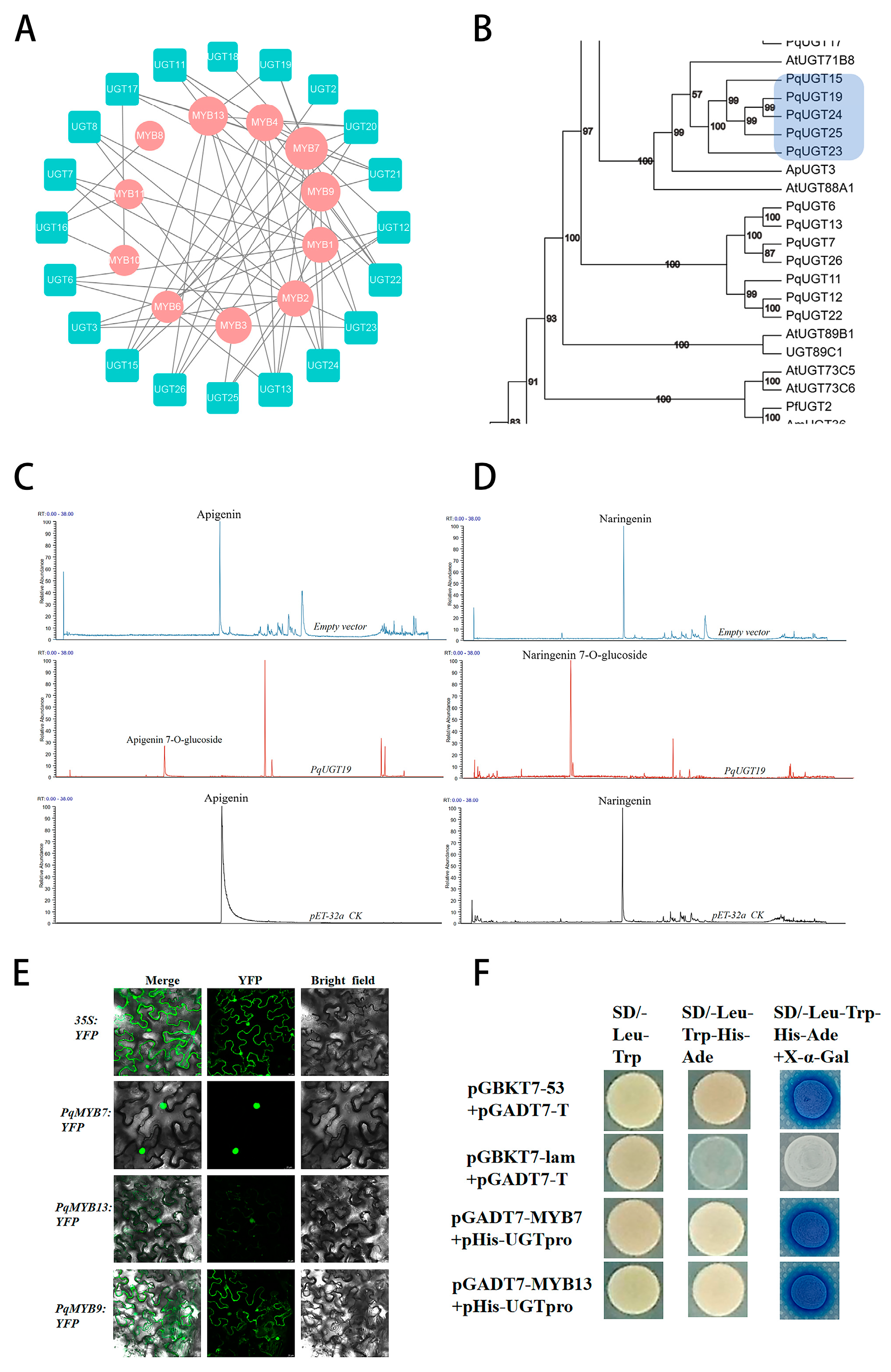
2.6. Functional Validation of Flavonoid-Related TFs and UGTs in P. quinquefolius
3. Discussion
3.1. Transcriptome Analysis and qRT-PCR Validation
3.2. Metabolite Profiling
3.3. Flavonoid Biosynthesis Pathway and Tissue-Specific Accumulation
3.4. Regulation of Flavonoid Biosynthesis by TFs
3.5. Glycosylation of Flavonoids by UGTs
3.6. Regulatory Network of UGTs and MYB Transcription Factors in Flavonoid Accumulation
4. Materials and Methods
4.1. Plant Materials and Sample Collection
4.2. Sample Preparation and Extraction
4.3. UPLC-MS/MS Determination of Flavonoid Compounds
4.4. Identification and Quantification of Flavonoid Metabolites
4.5. RNA Extraction and Transcriptome Sequencing
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.7. Correlation Analysis of Transcriptome and Metabolome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, D.H. Chemical Diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J. Ginseng Res. 2012, 36, 1. [Google Scholar] [CrossRef]
- Qu, C.; Li, B.; Lai, Y.; Li, H.; Windust, A.; Hofseth, L.J.; Nagarkatti, M.; Nagarkatti, P.; Wang, X.L.; Tang, D.; et al. Identifying panaxynol, a natural activator of nuclear factor erythroid-2 related factor 2 (Nrf2) from American ginseng as a suppressor of inflamed macrophage-induced cardiomyocyte hypertrophy. J. Ethnopharmacol. 2015, 168, 326–336. [Google Scholar] [CrossRef]
- Shao, Z.-H.; Xie, J.T.; Vanden Hoek, T.L.; Mehendale, S.; Aung, H.; Li, C.-Q.; Qin, Y.; Schumacker, P.T.; Becker, L.B.; Yuan, C.S. Antioxidant effects of American ginseng berry extract in cardiomyocytes exposed to acute oxidant stress. Biochim. Biophys. Acta 2004, 1670, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.K.; Yun, Y.S.; Han, I.W. Anticarcinogenic effect of long-term oral administration of red ginseng on newborn mice exposed to various chemical carcinogens. Cancer Detect. Prev. 1983, 6, 515–525. [Google Scholar] [PubMed]
- Dodd, G.F.; Williams, C.M.; Butler, L.T.; Spencer, J.P.E. Acute effects of flavonoid-rich blueberry on cognitive and vascular function in healthy older adults. Nutr. Healthy Aging 2019, 5, 119–132. [Google Scholar] [CrossRef]
- Chang, F.; Jia, F.; Lv, R.; Guan, M.; Jia, Q.; Sun, Y.; Li, Z. Effects of American Ginseng Cultivation on Bacterial Community Structure and Responses of Soil Nutrients in Different Ecological Niches. J. Microbiol. Biotechnol. 2022, 32, 419–429. [Google Scholar] [CrossRef]
- Qiang, B.; Miao, J.; Phillips, N.; Wei, K.; Gao, Y. Recent Advances in the Tissue Culture of American Ginseng (Panax quinquefolius). Chem. Biodivers. 2020, 17, e2000366. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Varela, C.L.; Costa, S.C.; Tavares-da-Silva, E.J. Phenolic Derivatives From Medicinal Herbs and Plant Extracts: Anticancer Effects and Synthetic Approaches to Modulate Biological Activity. Stud. Nat. Prod. Chem. 2018, 57, 115–156. [Google Scholar]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef]
- Wen, W.; Alseekh, S.; Fernie, A.R. Conservation and diversification of flavonoid metabolism in the plant kingdom. Curr. Opin. Plant Biol. 2020, 55, 100–108. [Google Scholar] [CrossRef]
- Yu, W.; Gong, F.; Xu, H. Molecular Mechanism of Exogenous ABA to Enhance UV-B Resistance in Rhododendron chrysanthum Pall. by Modulating Flavonoid Accumulation. Int. J. Mol. Sci. 2024, 25, 5248. [Google Scholar] [CrossRef]
- Kuhn, B.M.; Nodzyński, T.; Errafi, S.; Bucher, R.; Gupta, S.; Aryal, B.; Dobrev, P.; Bigler, L.; Geisler, M.; Zažímalová, E.; et al. Flavonol-induced changes in PIN2 polarity and auxin transport in the Arabidopsis thaliana rol1-2 mutant require phosphatase activity. Sci. Rep. 2017, 7, 41906. [Google Scholar] [CrossRef]
- Mughal, N.; Zhang, X.; Shoaib, N.; Deng, J.; Guo, J.; Zhang, J.; Yang, W.; Liu, J. Screening of soybean antifungal isoflavones based on targeted metabolomics analysis. Food Chem. 2025, 25, 102195. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Liu, C.; Sun, J.; Ding, M.; Ding, Y.; Xu, Y.; He, J.; Li, Q.; Jin, X. McWRKY43 Confers Cold Stress Tolerance in Michelia crassipes via Regulation of Flavonoid Biosynthesis. Int. J. Mol. Sci. 2024, 25, 9843. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.S.; Yamamoto, T.; Nozawa, A.; Tozawa, Y. Expression of Parsley Flavone Synthase I Establishes the Flavone Biosynthetic Pathway in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2008, 72, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Deng, B.; Tian, S.; Guo, M.; Liu, H.; Zhao, X. Metabolic and transcriptomic analyses reveal different metabolite biosynthesis profiles between leaf buds and mature leaves in Ziziphus jujuba mill. Food Chem. 2021, 347, 129005. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Q.; Le, L.; Zhou, T.; Yu, W.; Wang, G.; Fu, F.F.; Cao, F. Comparative histology, transcriptome, and metabolite profiling unravel the browning mechanisms of calli derived from ginkgo (Ginkgo biloba L.). J. For. Res. 2023, 34, 677–691. [Google Scholar] [CrossRef]
- Meng, X.; Li, G.; Gu, L.; Sun, Y.; Li, Z.; Liu, J.; Wu, X.; Dong, T.; Zhu, M. Comparative Metabolomic and Transcriptome Analysis Reveal Distinct Flavonoid Biosynthesis Regulation Between Petals of White and Purple Phalaenopsis amabilis. J. Plant Growth Regul. 2020, 39, 823–840. [Google Scholar] [CrossRef]
- Parkhomchuk, D.; Borodina, T.; Amstislavskiy, V.; Banaru, M.; Hallen, L.; Krobitsch, S.; Lehrach, H.; Soldatov, A. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res. 2009, 37, e123. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Liu, Q.; Zang, E.; Wu, L.; Hu, G.; Li, M. Transcriptome analysis of Saposhnikovia divaricata and mining of bolting and flowering genes. Chin. Herb. Med. 2023, 15, 574–587. [Google Scholar] [CrossRef]
- Covington, M.F.; Maloof, J.N.; Straume, M.; Kay, S.A.; Harmer, S.L. Global transcriptome analysis reveals circadian regulation of key pathways in plant growth and development. Genome Biol. 2008, 9, R130. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Kopka, J.; Dormann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bian, C.; Huang, Y.; Hou, W.; Xue, H.; Xing, Y.; Zheng, H.; Yang, Y.; Kang, T.; Xu, L.; et al. Targeted metabolomics analysis of three medicinal plants of the genus Pulsatilla. Med. Plant Biol. 2024, 3, e023. [Google Scholar] [CrossRef]
- Sumner, L.W.; Mendes, P.; Dixon, R.A. Plant metabolomics: Large-scale phytochemistry in the functional genomics era. Phytochemistry 2003, 62, 817–836. [Google Scholar] [CrossRef]
- Van Assche, R.; Broeckx, V.; Boonen, K.; Maes, E.; De Haes, W.; Schoofs, L.; Temmerman, L. Integrating -Omics: Systems Biology as Explored Through C-elegans Research. J. Mol. Biol. 2015, 427, 3441–3451. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fang, J.; Qi, X.; Lin, M.; Zhong, Y.; Sun, L.; Cui, W. Combined Analysis of the Fruit Metabolome and Transcriptome Reveals Candidate Genes Involved in Flavonoid Biosynthesis in Actinidia arguta. Int. J. Mol. Sci. 2018, 19, 1471. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, J.; Qi, K.; Li, Y.; Chen, Y. Combined analysis of transcriptomics and metabolomics revealed complex metabolic genes for diterpenoids biosynthesis in different organs of Anoectochilus roxburghii. Chin. Herb. Med. 2023, 15, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zheng, J.; Chen, X.; Shi, X.; Wang, H.; Fu, Q. Comprehensive Analysis of Transcriptome and Metabolome Reveals the Flavonoid Metabolic Pathway Is Associated with Fruit Peel Coloration of Melon. Molecules 2021, 26, 2830. [Google Scholar] [CrossRef]
- Lai, J.; Li, C.; Zhang, Y.; Wu, Z.; Li, W.; Zhang, Z.; Ye, W.; Guo, H.; Wang, C.; Long, T.; et al. Integrated Transcriptomic and Metabolomic Analyses Reveal the Molecular and Metabolic Basis of Flavonoids in Areca catechu L. J. Agric. Food Chem. 2023, 71, 4851–4862. [Google Scholar] [CrossRef]
- Yu, J.; Chen, W.; Wang, D.; Xu, L.; Wang, X. Cytotoxic constituents from the leaves and stems of Panax quinquefolius. Nat. Prod. Res. 2023, 37, 919–927. [Google Scholar] [CrossRef]
- Lim, E.K. Evolution of substrate recognition across a multigene family of glycosyltransferases in Arabidopsis. Glycobiology 2003, 3, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.E.; Wu, S.; Tian, L. PgUGT95B2 preferentially metabolizes flavones/flavonols and has evolved independently from flavone/flavonol UGTs identified in Arabidopsis thaliana. Phytochemistry 2019, 157, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, W.; Li, J.; Feng, X.; Li, C. Identification of a permissive glycosyltransferase UGT73AC11 for flavonoids glycosylation. Biochem. Eng. J. 2024, 209, 109372. [Google Scholar] [CrossRef]
- Chen, Y.; Zahavi, E.; Barak, P.; Umiel, N. Effects of Salinity Stresses on Tobacco: I. The Growth of Nicotiana tabacum Callus Cultures under Seawater, NaCl, and Mannitol Stresses. Z. Für Pflanzenphysiol. 1980, 98, 141–153. [Google Scholar] [CrossRef]
- Urrutia, M.; Blein-Nicolas, M.; Prigent, S.; Bernillon, S.; Deborde, C.; Balliau, T.; Maucourt, M.; Jacob, D.; Ballias, P.; Benard, C.; et al. Maize metabolome and proteome responses to controlled cold stress partly mimic early-sowing effects in the field and differ from those of Arabidopsis. Plant Cell Environ. 2021, 44, 1504–1521. [Google Scholar] [CrossRef]
- Niu, Y.; Muhammad, N.; Duan, Y.; Wang, Q.; Duan, Y.; Li, J.; Liu, M.; Liu, P.; Wang, L. Transcriptome and metabolome reveal the molecular mechanism of quality formation in different planting areas of Ziziphus jujuba Mill. ‘Jingudazao’ fruit. Ind. Crops Prod. 2024, 222, 119737. [Google Scholar] [CrossRef]
- Huang, X.; Rong, W.; Zhang, X.; Gao, Y.; Zhou, Y.; Su, J.; Luo, H.; Chu, G.; Wang, M. Transcriptome and metabolome analysis reveal the dynamic changes and biosynthesis pathways of alkaloids in Sophora alopecuroides L. under drought stress. Ind. Crops Prod. 2024, 212, 118365. [Google Scholar] [CrossRef]
- Peng, J.; Dong, B.Z.; Wang, D.; Zhao, Y.J.; Meng, H.W.; Zhou, H.Y. Analysis of differential metabolites of sunflower induced by virulent Verticillium dahlia V33 and hypovirulent Gibellulopsis nigrescens Vn-1. J. Phytopathol. 2022, 170, 349–358. [Google Scholar] [CrossRef]
- Wang, P.; Zhong, L.; Yang, H.; Hou, X.; Wu, C.; Zhang, R.; Yu, J.; Cheng, Y. Systematic transcriptomic and metabolomic analysis of walnut (Juglans regia L.) fruit to trace variations in antioxidant activity during ripening. Sci. Hortic. 2022, 295, 110849. [Google Scholar] [CrossRef]
- Chen, X.; Shi, X.; Ai, Q.; Han, J.; Wang, H.; Fu, Q. Transcriptomic and metabolomic analyses reveal that exogenous strigolactones alleviate the response of melon root to cadmium stress. Hortic. Plant J. 2022, 8, 637–649. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Huang, Z.; Lyu, L.; Li, W.; Wu, W. Integrative analysis of the metabolome and transcriptome provides insights into the mechanisms of flavonoid biosynthesis in blackberry. Food Res. Int. 2022, 153, 110948. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Li, Y.; Lu, Q.; Lu, H.; Li, J. Combined Analysis of the Metabolome and Transcriptome Identified Candidate Genes Involved in Phenolic Acid Biosynthesis in the Leaves of Cyclocarya paliurus. Int. J. Mol. Sci. 2020, 21, 1337. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Du, W.; Hao, Y.; Han, Y.; Li, H.; Liu, L.; Zhang, K.; Zhou, M.; Sun, Z. Elucidation of the Regulatory Network of Flavonoid Biosynthesis by Profiling the Metabolome and Transcriptome in Tartary Buckwheat. J. Agric. Food Chem. 2021, 69, 7218–7229. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Gong, W.; Yu, X.; Ji, K.; Chang, Y.; Wang, L.; Yuan, D. Integrative analysis of the metabolome and transcriptome provides novel insights into the mechanisms of flavonoid biosynthesis in Camellia lanceoleosa. Sci. Hortic. 2022, 304, 111357. [Google Scholar] [CrossRef]
- Nakamura, S.; Sugimoto, S.; Matsuda, H.; Yoshikawa, M. Medicinal flowers.: XVII.: New dammarane-type triterpene glycosides from flower buds of American ginseng, Panax quinquefolium L. Chem. Pharm. Bull. 2007, 55, 1342–1348. [Google Scholar] [CrossRef]
- Kim, M.S.; Jeon, S.J.; Youn, S.J.; Lee, H.; Park, Y.J.; Kim, D.O.; Kim, B.Y.; Kim, W.; Baik, M.Y. Enhancement of Minor Ginsenosides Contents and Antioxidant Capacity of American and Canadian Ginsengs (Panax quinquefolius) by Puffing. Antioxidants 2019, 8, 527. [Google Scholar] [CrossRef]
- Shou, W.U.; Xiao, G.U.O.; Peng, T.U.; Yong, J. Research progress on chemical constituents, biological activities, quality evaluation, and product development of Panax quinquefolium. China J. Chin. Mater. Medica 2022, 57, 1711–1725. [Google Scholar]
- Yan, N.; Gai, X.; Xue, L.; Du, Y.; Shi, J.; Liu, Y. Effects of NtSPS1 Overexpression on Solanesol Content, Plant Growth, Photosynthesis, and Metabolome of Nicotiana tabacum. Plants 2020, 9, 518. [Google Scholar] [CrossRef]
- Zou, H.; Jiang, H.; Li, L.; Huang, R. Integration of transcriptome and targeted metabolome profiling reveals hormone related genes involved in the growth of Bletilla striata. Sci. Rep. 2021, 11, 21950. [Google Scholar] [CrossRef]
- Ma, D.; Guo, Y.; Ali, I.; Lin, J.; Xu, Y.; Yang, M. Accumulation characteristics of plant flavonoids and effects of cultivation measures on their biosynthesis: A review. Plant Physiol. Biochem. 2024, 215, 108960. [Google Scholar] [CrossRef]
- Ramaroson, M.L.; Koutouan, C.; Helesbeux, J.J.; Le Clerc, V.; Hamama, L.; Geoffriau, E.; Briard, M. Role of Phenylpropanoids and Flavonoids in Plant Resistance to Pests and Diseases. Molecules 2022, 27, 8371. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Midega, C.A.O.; Hooper, A.; Pickett, J. Push-Pull: Chemical Ecology-Based Integrated Pest Management Technology. J. Chem. Ecol. 2016, 42, 689–697. [Google Scholar] [CrossRef]
- Ma, L.; Ma, S.; Chen, G.; Lu, X.; Zhang, C.; Wei, R.; Feng, X.; Xu, L.; Zhang, X.; Chai, Q.; et al. Transcriptomics and metabolomics revealed the synthesis and potential role of flavonoids in pea roots under continuous cropping obstacles. Physiol. Plant 2024, 176, e14185. [Google Scholar] [CrossRef]
- Albert, N.W.; Thrimawithana, A.H.; McGhie, T.K.; Clayton, W.A.; Deroles, S.C.; Schwinn, K.E.; Bowman, J.L.; Jordan, B.R.; Davies, K.M. Genetic analysis of the liverwort Marchantia polymorpha reveals that R2R3MYB activation of flavonoid production in response to abiotic stress is an ancient character in land plants. New Phytol. 2018, 218, 554–566. [Google Scholar] [CrossRef]
- Li, B.; Fan, R.; Guo, S.; Wang, P.; Zhu, X.; Fan, Y.; Chen, Y.; He, K.; Kumar, A.; Shi, J.; et al. The Arabidopsis MYB transcription factor, MYB111 modulates salt responses by regulating flavonoid biosynthesis. Environ. Exp. Bot. 2019, 166, 103807. [Google Scholar] [CrossRef]
- Simiyu, D.C.; Bayaraa, U.; Jang, J.H.; Lee, O.R. The R2R3-MYB transcription factor PgTT2 from Panax ginseng interacts with the WD40-repeat protein PgTTG1 during the regulation of proanthocyanidin biosynthesis and the response to salt stress. Plant Physiol. Biochem. 2024, 214, 108877. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, J.; Zheng, M.; Zhu, S.; Pei, T.; Cui, M.; Chang, L.; Xiao, H.; Yang, J.; Martin, C.; et al. SbMYB3 transcription factor promotes root-specific flavone biosynthesis in Scutellaria baicalensis. Hortic. Res. 2023, 10, uhac266. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, H.; Pan, J.; Zhang, S.; Guo, Y.; Xian, Y.; Sun, Z.; Zhang, Z. Transcriptomics and Metabolomics Changes Triggered by Inflorescence Removal in Panax notoginseng (Burk.). Front. Plant Sci. 2021, 12, 761821. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Fujimori, T.; An, S.; Iguchi, S.; Takenaka, Y.; Kajiura, H.; Yoshizawa, T.; Matsumura, H.; Kobayashi, M.; Ono, E.; et al. The apiosyltransferase celery UGT94AX1 catalyzes the biosynthesis of the flavone glycoside apiin. Plant Physiol. 2023, 193, 1758–1771. [Google Scholar] [CrossRef]
- Lu, C.; Wang, H.; Zheng, D.; Jia, S.; Xing, Q.; Wang, Z.; Li, Q.; Zhao, L. Cloning and Direct Evolution of a Novel 7-O-Glycosyltransferase from Cucurbita moschata and Its Application in the Efficient Biocatalytic Synthesis of Luteolin-7-O-glucoside. J. Agric. Food Chem. 2024, 72, 19093–19106. [Google Scholar] [CrossRef]
- Song, C.; Gu, L.; Liu, J.; Zhao, S.; Hong, X.; Schulenburg, K.; Schwab, W. Characterization of UGT71, a Major Glycosyltransferase Family for triterpenoids, flavonoids and phytohormones-biosynthetic in plants. ResearchGate. Plant Cell Physiol. 2015, 12, 2478–2493. [Google Scholar] [CrossRef] [PubMed]
- Okazawa, A.; Kusunose, T.; Ono, E.; Kim, H.J.; Satake, H.; Shimizu, B.; Mizutani, M.; Seki, H.; Muranaka, T. Glucosyltransferase activity of Arabidopsis UGT71C1 towards pinoresinol and lariciresinol. Plant Biotechnol. 2014, 31, 561–566. [Google Scholar] [CrossRef]
- Hou, M.; Nie, F.; Zhao, J.; Ju, Z.; Yang, L.; Wang, Q.; Zhao, S.; Wang, Z. New Glycosyltransferases in Panax notoginseng Perfect Main Ginsenosides Biosynthetic Pathways. J. Agric. Food Chem. 2023, 71, 963–973. [Google Scholar] [CrossRef]
- Feng, X.; Bai, S.; Zhou, L.; Song, Y.; Jia, S.; Guo, Q.; Zhang, C. Integrated Analysis of Transcriptome and Metabolome Provides Insights into Flavonoid Biosynthesis of Blueberry Leaves in Response to Drought Stress. Int. J. Mol. Sci. 2024, 25, 11135. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Yan, Y.; Guo, C.; Chen, J.; Wang, Y.; Wang, Y.; Zhang, J.; Gao, C.; Lian, J.; Piao, X.; et al. Identification and Expression Analysis of R2R3-MYB Transcription Factors Associated with Flavonoid Biosynthesis in Panax quinquefolius. Int. J. Mol. Sci. 2024, 25, 3709. [Google Scholar] [CrossRef]
- Di, P.; Yan, Y.; Wang, P.; Yan, M.; Wang, Y.-P.; Huang, L.Q. Integrative SMRT sequencing and ginsenoside profiling analysis provide insights into the biosynthesis of ginsenoside in Panax quinquefolium. Chin. J. Nat. Med. 2022, 20, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Wang, X.F.; Lu, T.; Li, M.R.; Jiang, P.; Zhao, J.; Liu, S.T.; Fu, X.Q.; Wendel, J.F.; Van de Peer, Y.; et al. Reshuffling of the ancestral core-eudicot genome shaped chromatin topology and epigenetic modification in Panax. Nat. Commun. 2022, 13, 1902. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie, and Ballgown. Nat. Protoc. 2016, 11, 1650. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, J.; Wang, P.; Li, Y.; Wang, Y.; Yan, Y.; Chi, J.; Chen, J.; Lian, J.; Piao, X.; et al. Integrated Transcriptomic and Metabolomic Analysis Reveals Tissue-Specific Flavonoid Biosynthesis and MYB-Mediated Regulation of UGT71A1 in Panax quinquefolius. Int. J. Mol. Sci. 2025, 26, 2669. https://doi.org/10.3390/ijms26062669
Wang Y, Zhang J, Wang P, Li Y, Wang Y, Yan Y, Chi J, Chen J, Lian J, Piao X, et al. Integrated Transcriptomic and Metabolomic Analysis Reveals Tissue-Specific Flavonoid Biosynthesis and MYB-Mediated Regulation of UGT71A1 in Panax quinquefolius. International Journal of Molecular Sciences. 2025; 26(6):2669. https://doi.org/10.3390/ijms26062669
Chicago/Turabian StyleWang, Yumeng, Jiaxin Zhang, Ping Wang, Yongkang Li, Yihan Wang, Yan Yan, Junwen Chi, Jiankang Chen, Junmei Lian, Xiangmin Piao, and et al. 2025. "Integrated Transcriptomic and Metabolomic Analysis Reveals Tissue-Specific Flavonoid Biosynthesis and MYB-Mediated Regulation of UGT71A1 in Panax quinquefolius" International Journal of Molecular Sciences 26, no. 6: 2669. https://doi.org/10.3390/ijms26062669
APA StyleWang, Y., Zhang, J., Wang, P., Li, Y., Wang, Y., Yan, Y., Chi, J., Chen, J., Lian, J., Piao, X., Lei, X., Xiao, Y., Murray, J., Deyholos, M. K., Wang, Y., Di, P., & Zhang, J. (2025). Integrated Transcriptomic and Metabolomic Analysis Reveals Tissue-Specific Flavonoid Biosynthesis and MYB-Mediated Regulation of UGT71A1 in Panax quinquefolius. International Journal of Molecular Sciences, 26(6), 2669. https://doi.org/10.3390/ijms26062669







