Single-Cell Analysis of Molecular Mechanisms in Rapid Antler Osteogenesis During Growth and Ossification Stages
Abstract
1. Introduction
2. Results
2.1. Antler Single-Cell RNA Sequencing
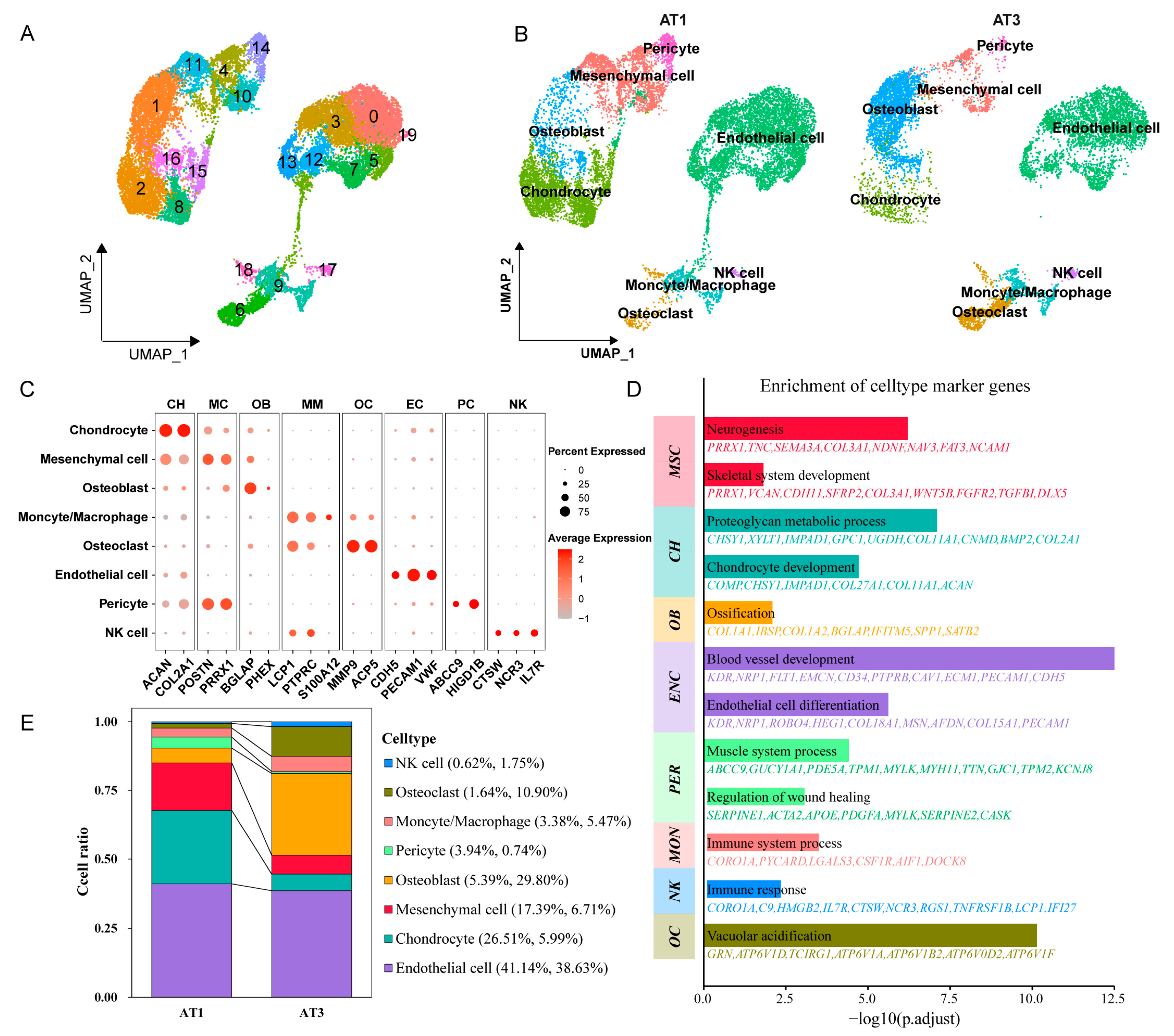
2.2. Cell Annotation
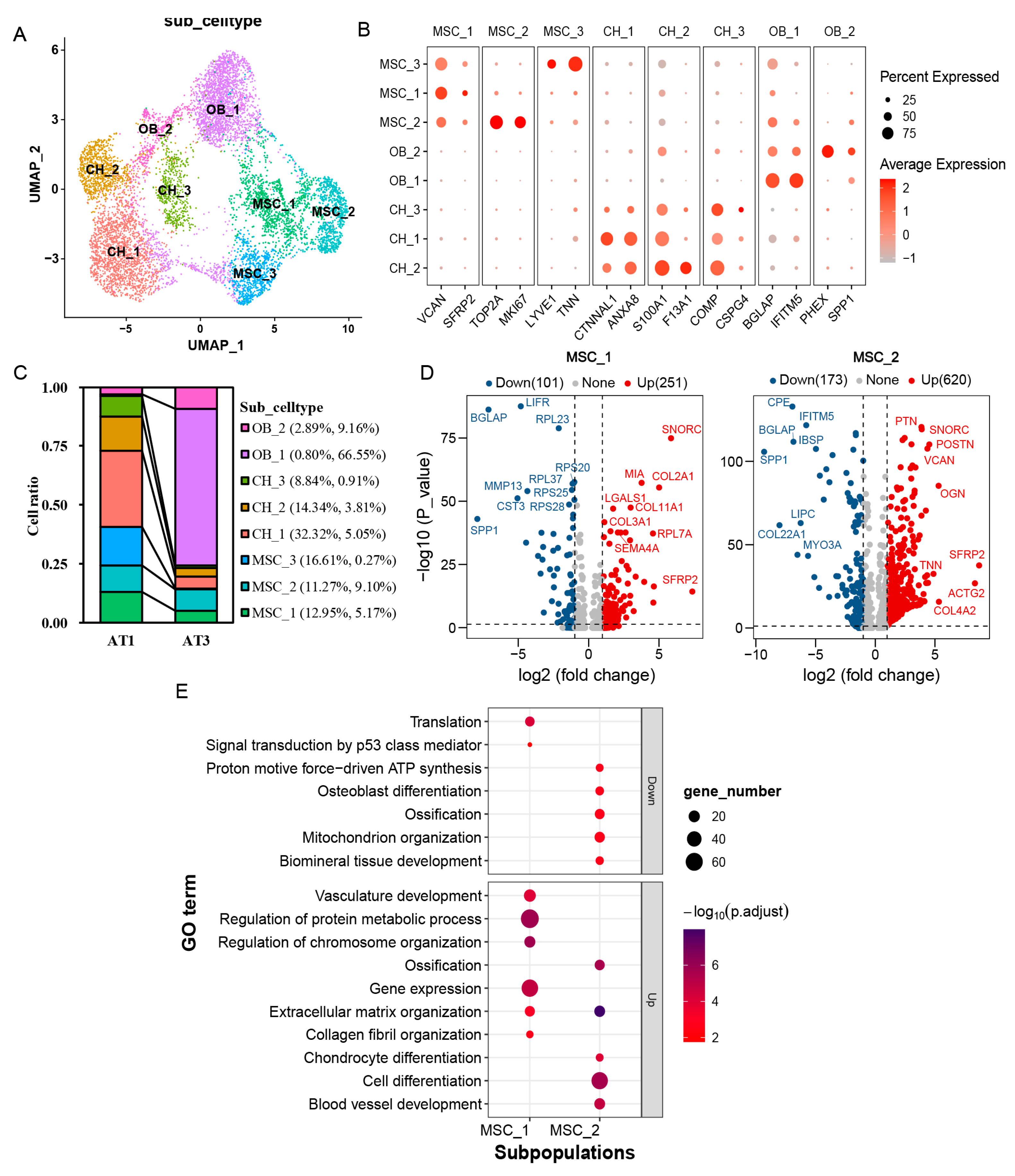
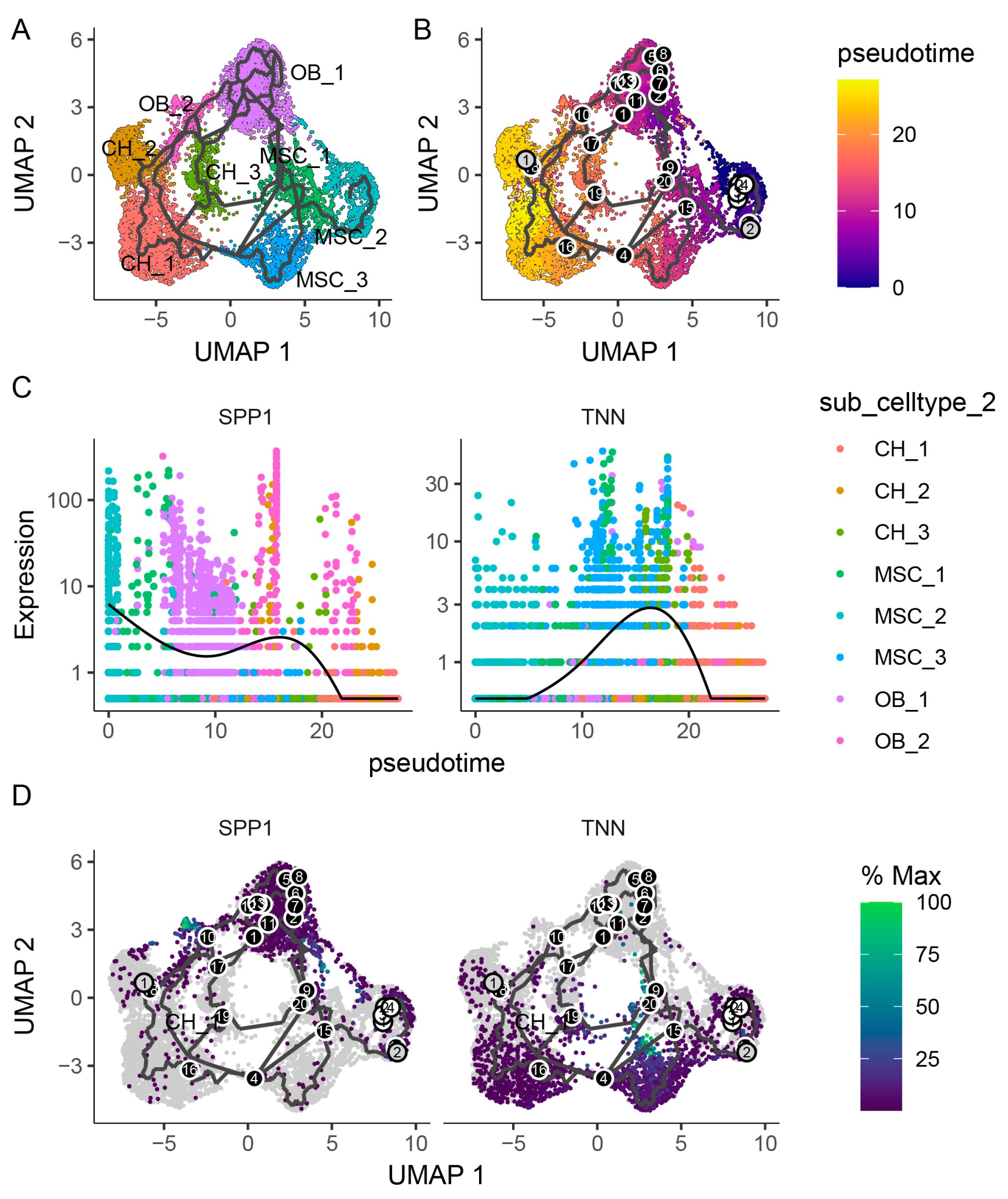
2.3. Subpopulation Analysis of Mesenchymal Cells, Chondrocytes, and Osteoblasts
2.4. Subpopulation Analysis of Endothelial Cells
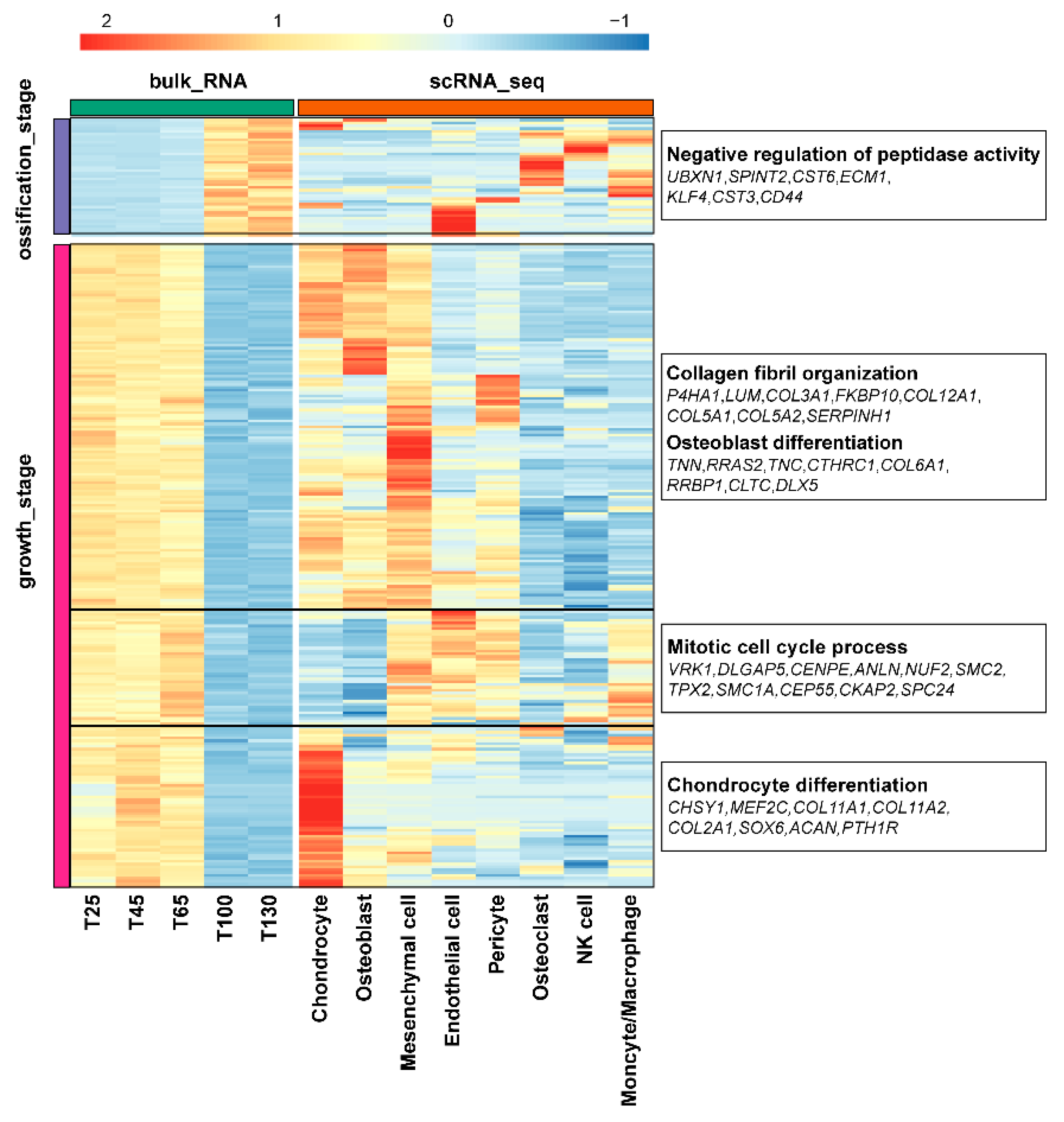
2.5. Combined Analysis of scRNA-seq and Bulk RNA-seq
3. Discussion
4. Materials and Methods
4.1. Sample Selection and Preparation
4.2. Cell Preparation
4.3. Single-Cell RNA Sequencing
4.4. Data Analysis
4.5. Cell Annotation and Differential Gene Expression Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Yang, Y.; Yu, B.; Gao, X.; Gao, X.; Nie, S.; Qin, T.; Hao, Y.; Guo, L.; Wu, H.; et al. A Novel Deer Antler-Inspired Bone Graft Triggers Rapid Bone Regeneration. Adv. Mater. 2024, 37, e2411571. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Zhang, G.; Ba, H.; Liu, H.; Wang, J.; Li, W.; Melino, G.; Shi, Y. Bone metabolism associated with annual antler regeneration: A deer insight into osteoporosis reversal. Biol. Direct 2024, 19, 123. [Google Scholar] [CrossRef]
- Wang, D.T.; Berg, D.; Ba, H.X.; Sun, H.M.; Wang, Z.; Li, C.Y. Deer antler stem cells are a novel type of cells that sustain full regeneration of a mammalian organ-deer antler. Cell Death Dis. 2019, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Ba, H.X.; Wang, D.T.; Wu, W.Y.; Sun, H.M.; Li, C.Y. Single-cell transcriptome provides novel insights into antler stem cells, a cell type capable of mammalian organ regeneration. Funct. Integr. Genom. 2019, 19, 555–564. [Google Scholar] [CrossRef]
- Sui, Z.G.; Sun, H.M.; Weng, Y.J.; Zhang, X.D.; Sun, M.W.; Sun, R.; Zhao, B.F.; Liang, Z.; Zhang, Y.K.; Li, C.Y.; et al. Quantitative proteomics analysis of deer antlerogenic periosteal cells reveals potential bioactive factors in velvet antlers. J. Chromatogr. A 2020, 1609, 460496. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Qin, T.; Li, X.; Wang, Z.; Wang, Y.; Guan, Q.; Shi, W.; Xie, L.; Zhao, S.; Sun, H. Constructing the in vitro culture system of the sika deer (cervus nippon) antler periosteal cell to detect its function on antler regeneration. Front. Biosci. 2022, 27, 69. [Google Scholar] [CrossRef] [PubMed]
- Rolf, H.J.; Kierdorf, U.; Kierdorf, H.; Schulz, J.; Seymour, N.; Schliephake, H.; Napp, J.; Niebert, S.; Wolfel, H.; Wiese, K.G. Localization and characterization of STRO-1 cells in the deer pedicle and regenerating antler. PLoS ONE 2008, 3, e2064. [Google Scholar] [CrossRef]
- Song, H.; Park, K.H. Regulation and function of SOX9 during cartilage development and regeneration. Semin. Cancer Biol. 2020, 67, 12–23. [Google Scholar] [CrossRef]
- Jia, B.; Zhang, L.; Zhang, Y.; Ge, C.; Yang, F.; Du, R.; Ba, H. Integrated analysis of miRNA and mRNA transcriptomic reveals antler growth regulatory network. Mol. Genet. Genom. 2021, 296, 689–703. [Google Scholar] [CrossRef]
- Yao, B.J.; Zhang, M.; Liu, M.C.; Wang, Q.; Liu, M.X.; Zhao, Y. Sox9 Functions as a Master Regulator of Antler Growth by Controlling Multiple Cell Lineages. DNA Cell Biol. 2018, 37, 15–22. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef]
- Komori, T. Whole Aspect of Runx2 Functions in Skeletal Development. Int. J. Mol. Sci. 2022, 23, 5776. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Wang, N.; Li, Z.; Heller, R.; Liu, R.; Zhao, Y.; Han, J.; Pan, X.; Zheng, Z.; et al. Genetic basis of ruminant headgear and rapid antler regeneration. Science 2019, 364, 1153. [Google Scholar] [CrossRef]
- Liu, Z.P.; Li, W.; Geng, L.L.; Sun, L.; Wang, Q.R.; Yu, Y.; Yan, P.Z.; Liang, C.Q.; Ren, J.; Song, M.S.; et al. Cross-species metabolomic analysis identifies uridine as a potent regeneration promoting factor. Cell Discov. 2022, 8, 6. [Google Scholar] [CrossRef]
- Qin, T.; Zhang, G.K.; Zheng, Y.; Li, S.Y.; Yuan, Y.; Li, Q.J.; Hu, M.L.; Si, H.Z.; Wei, G.N.; Gao, X.L.; et al. A population of stem cells with strong regenerative potential discovered in deer antlers. Science 2023, 379, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Dong, Y.M.; Xing, X.M. Comprehensive transcriptome analysis of sika deer antler using PacBio and Illumina sequencing. Sci. Rep. 2022, 12, 16161. [Google Scholar] [CrossRef]
- Zhang, R.R.; Li, Y.; Xing, X.M. Comparative antler proteome of sika deer from different developmental stages. Sci. Rep. 2021, 11, 10484. [Google Scholar] [CrossRef]
- De Zoysa, P.; Toubat, O.; Harvey, D.C.; Yi, C.; Liu, J.; Cavallero, S.; Hong, Y.K.; Sucov, H.M.; Kumar, S.R. Delta-like ligand-4 regulates Notch-mediated maturation of second heart field progenitor-derived pharyngeal arterial endothelial cells. J. Cell. Mol. Med. 2022, 26, 5181–5194. [Google Scholar] [CrossRef]
- Kenswil, K.J.G.; Pisterzi, P.; Sanchez-Duffhues, G.; van Dijk, C.; Lolli, A.; Knuth, C.; Vanchin, B.; Jaramillo, A.C.; Hoogenboezem, R.M.; Sanders, M.A.; et al. Endothelium-derived stromal cells contribute to hematopoietic bone marrow niche formation. Cell Stem Cell 2021, 28, 653–670.e11. [Google Scholar] [CrossRef]
- Xiao, S.M.; Kung, A.W.; Gao, Y.; Lau, K.S.; Ma, A.; Zhang, Z.L.; Liu, J.M.; Xia, W.; He, J.W.; Zhao, L.; et al. Post-genome wide association studies and functional analyses identify association of MPP7 gene variants with site-specific bone mineral density. Hum. Mol. Genet. 2012, 21, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Huynh, N.C.N.; Okamoto, K.; Muro, R.; Terashima, A.; Kurikawa, Y.; Komatsu, N.; Pluemsakunthai, W.; Nitta, T.; Abe, T.; et al. Stepwise cell fate decision pathways during osteoclastogenesis at single-cell resolution. Nat. Metab. 2020, 2, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- de Castro, L.F.; Sworder, B.J.; Mui, B.; Futrega, K.; Berendsen, A.; Phillips, M.D.; Burbach, N.J.; Cherman, N.; Kuznetsov, S.; Gabet, Y.; et al. Secreted frizzled related-protein 2 (Sfrp2) deficiency decreases adult skeletal stem cell function in mice. Bone Res. 2021, 9, 49. [Google Scholar] [CrossRef]
- Yin, B.; Shen, F.; Ma, Q.; Liu, Y.; Han, X.; Cai, X.; Shi, Y.; Ye, L. Identification of Postn+ periosteal progenitor cells with bone regenerative potential. JCI Insight 2024, 9, e182524. [Google Scholar] [CrossRef] [PubMed]
- Bouderlique, T.; Henault, E.; Lebouvier, A.; Frescaline, G.; Bierling, P.; Rouard, H.; Courty, J.; Albanese, P.; Chevallier, N. Pleiotrophin commits human bone marrow mesenchymal stromal cells towards hypertrophy during chondrogenesis. PLoS ONE 2014, 9, e88287. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Zheng, X.; Zhu, J.; Jin, L.; Gao, R. Pleiotrophin inhibited chondrogenic differentiation potential of dental pulp stem cells. Oral Dis. 2024, 30, 1439–1450. [Google Scholar] [CrossRef]
- Kimura, H.; Akiyama, H.; Nakamura, T.; de Crombrugghe, B. Tenascin-W inhibits proliferation and differentiation of preosteoblasts during endochondral bone formation. Biochem. Biophys. Res. Commun. 2007, 356, 935–941. [Google Scholar] [CrossRef]
- Komori, T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010, 339, 189–195. [Google Scholar] [CrossRef]
- Heinonen, J.; Taipaleenmäki, H.; Roering, P.; Takatalo, M.; Harkness, L.; Sandholm, J.; Uusitalo-Järvinen, H.; Kassem, M.; Kiviranta, I.; Laitala-Leinonen, T.; et al. Snorc is a novel cartilage specific small membrane proteoglycan expressed in differentiating and articular chondrocytes. Osteoarthr. Cartil. 2011, 19, 1026–1035. [Google Scholar] [CrossRef]
- Heinonen, J.; Zhang, F.P.; Surmann-Schmitt, C.; Honkala, S.; Stock, M.; Poutanen, M.; Säämänen, A.M. Defects in chondrocyte maturation and secondary ossification in mouse knee joint epiphyses due to Snorc deficiency. Osteoarthr. Cartil. 2017, 25, 1132–1142. [Google Scholar] [CrossRef]
- Bosserhoff, A.K.; Buettner, R. Establishing the protein MIA (melanoma inhibitory activity) as a marker for chondrocyte differentiation. Biomaterials 2003, 24, 3229–3234. [Google Scholar] [CrossRef] [PubMed]
- Tscheudschilsuren, G.; Bosserhoff, A.K.; Schlegel, J.; Vollmer, D.; Anton, A.; Alt, V.; Schnettler, R.; Brandt, J.; Proetzel, G. Regulation of mesenchymal stem cell and chondrocyte differentiation by MIA. Exp. Cell Res. 2006, 312, 63–72. [Google Scholar] [CrossRef]
- Meng, X.; Tackmann, N.R.; Liu, S.J.; Yang, J.; Dong, J.H.; Wu, C.Y.; Cox, A.D.; Zhang, Y.P. RPL23 Links Oncogenic RAS Signaling to p53-Mediated Tumor Suppression. Cancer Res. 2016, 76, 5030–5039. [Google Scholar] [CrossRef]
- Daftuar, L.; Zhu, Y.; Jacq, X.; Prives, C. Ribosomal Proteins RPL37, RPS15 and RPS20 Regulate the Mdm2-p53-MdmX Network. PLoS ONE 2013, 8, e68667. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Ha, H.I.; Kim, N.; Yi, O.; Lee, S.H.; Choi, Y. Adrm1 interacts with Atp6v0d2 and regulates osteoclast differentiation. Biochem. Biophys. Res. Commun. 2009, 390, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, H.; Egami, K.; Wada, N.; Tou, K.; Horiuchi, M.; Matsusaki, H.; Miura, R.; Yoshie, O.; Kukita, T. Identification of genes differentially expressed in osteoclast-like cells. J. Interf. Cytokine Res. 2005, 25, 227–231. [Google Scholar] [CrossRef]
- Vives, V.; Laurin, M.; Cres, G.; Larrousse, P.; Morichaud, Z.; Noel, D.; Côté, J.F.; Blangy, A. The Rac1 Exchange Factor Dock5 Is Essential for Bone Resorption by Osteoclasts. J. Bone Miner. Res. 2011, 26, 1099–1110. [Google Scholar] [CrossRef]
- Guimbal, S.; Morel, A.; Guérit, D.; Chardon, M.; Blangy, A.; Vives, V. Dock5 is a new regulator of microtubule dynamic instability in osteoclasts. Biol. Cell 2019, 111, 271–283. [Google Scholar] [CrossRef]
- Lombardi, M.S.; Gilliéron, C.; Berkelaar, M.; Gabay, C. Salt-inducible kinases (SIK) inhibition reduces RANKL-induced osteoclastogenesis. PLoS ONE 2017, 12, e0185426. [Google Scholar] [CrossRef]
- Tomlinson, R.E.; McKenzie, J.A.; Schmieder, A.H.; Wohl, G.R.; Lanza, G.M.; Silva, M.J. Angiogenesis is required for stress fracture healing in rats. Bone 2013, 52, 212–219. [Google Scholar] [CrossRef]
- Zhang, W.; Chu, W.H.; Liu, Q.X.; Coates, D.; Shang, Y.D.; Li, C.Y. Deer thymosin beta 10 functions as a novel factor for angiogenesis and chondrogenesis during antler growth and regeneration. Stem Cell Res. Ther. 2018, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, C.D.; Allori, A.C.; Reformat, D.D.; Sailon, A.M.; Allen, R.J.; Davidson, E.H.; Alikhani, M.; Bromage, T.G.; Ricci, J.L.; Warren, S.M. Characterization of Adipose-Derived Mesenchymal Stem Cell Combinations for Vascularized Bone Engineering. Tissue Eng. Part A 2013, 19, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Ai, C.; Wang, T.; Li, Y.; Liu, H.; Hu, P.; Wang, G.; Liu, H.; Wang, H.; Zhang, R.; et al. The First High-quality Reference Genome of Sika Deer Provides Insights into High-tannin Adaptation. Genom. Proteom. Bioinform. 2023, 21, 203–215. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Xing, X. Single-Cell Analysis of Molecular Mechanisms in Rapid Antler Osteogenesis During Growth and Ossification Stages. Int. J. Mol. Sci. 2025, 26, 2642. https://doi.org/10.3390/ijms26062642
Zhang R, Xing X. Single-Cell Analysis of Molecular Mechanisms in Rapid Antler Osteogenesis During Growth and Ossification Stages. International Journal of Molecular Sciences. 2025; 26(6):2642. https://doi.org/10.3390/ijms26062642
Chicago/Turabian StyleZhang, Ranran, and Xiumei Xing. 2025. "Single-Cell Analysis of Molecular Mechanisms in Rapid Antler Osteogenesis During Growth and Ossification Stages" International Journal of Molecular Sciences 26, no. 6: 2642. https://doi.org/10.3390/ijms26062642
APA StyleZhang, R., & Xing, X. (2025). Single-Cell Analysis of Molecular Mechanisms in Rapid Antler Osteogenesis During Growth and Ossification Stages. International Journal of Molecular Sciences, 26(6), 2642. https://doi.org/10.3390/ijms26062642




