Methodological and Ethical Considerations in the Use of Chordate Embryos in Biomedical Research
Abstract
1. Introduction
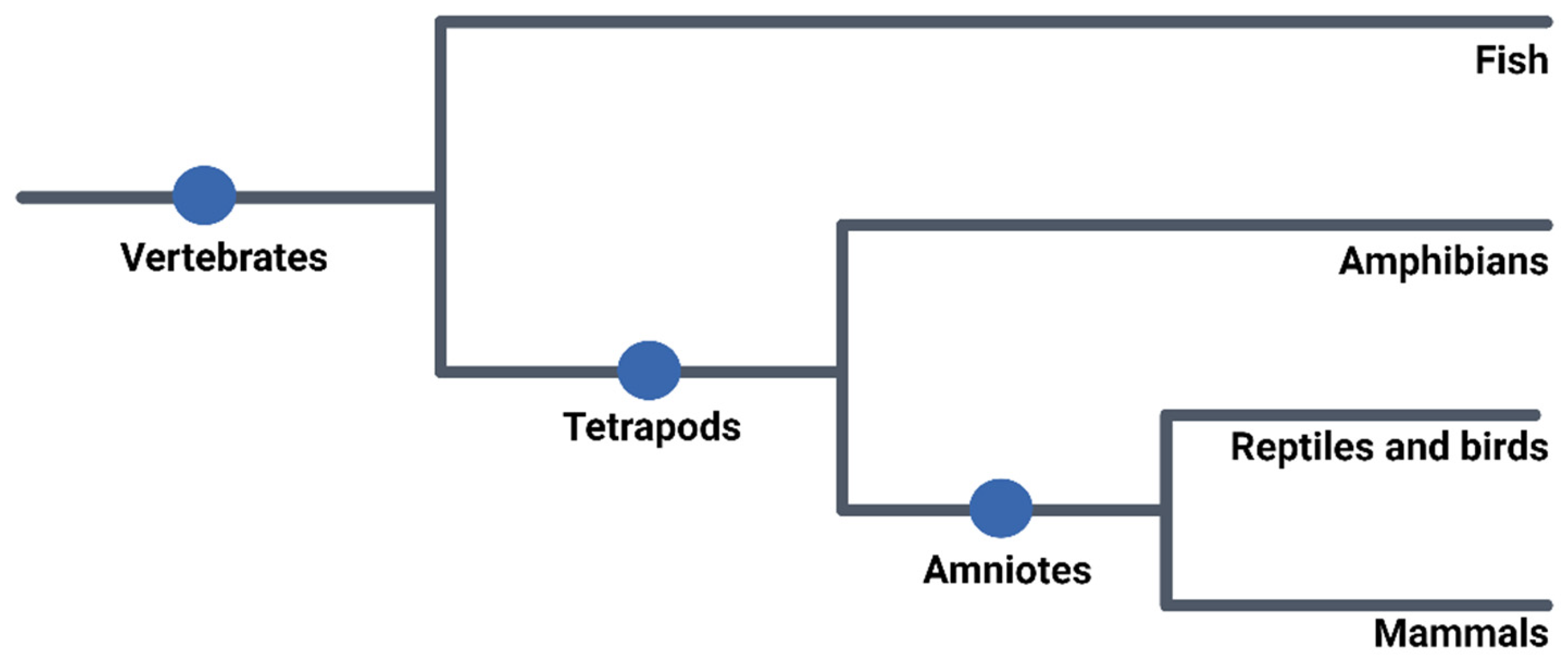
2. Use of Embryos in Research

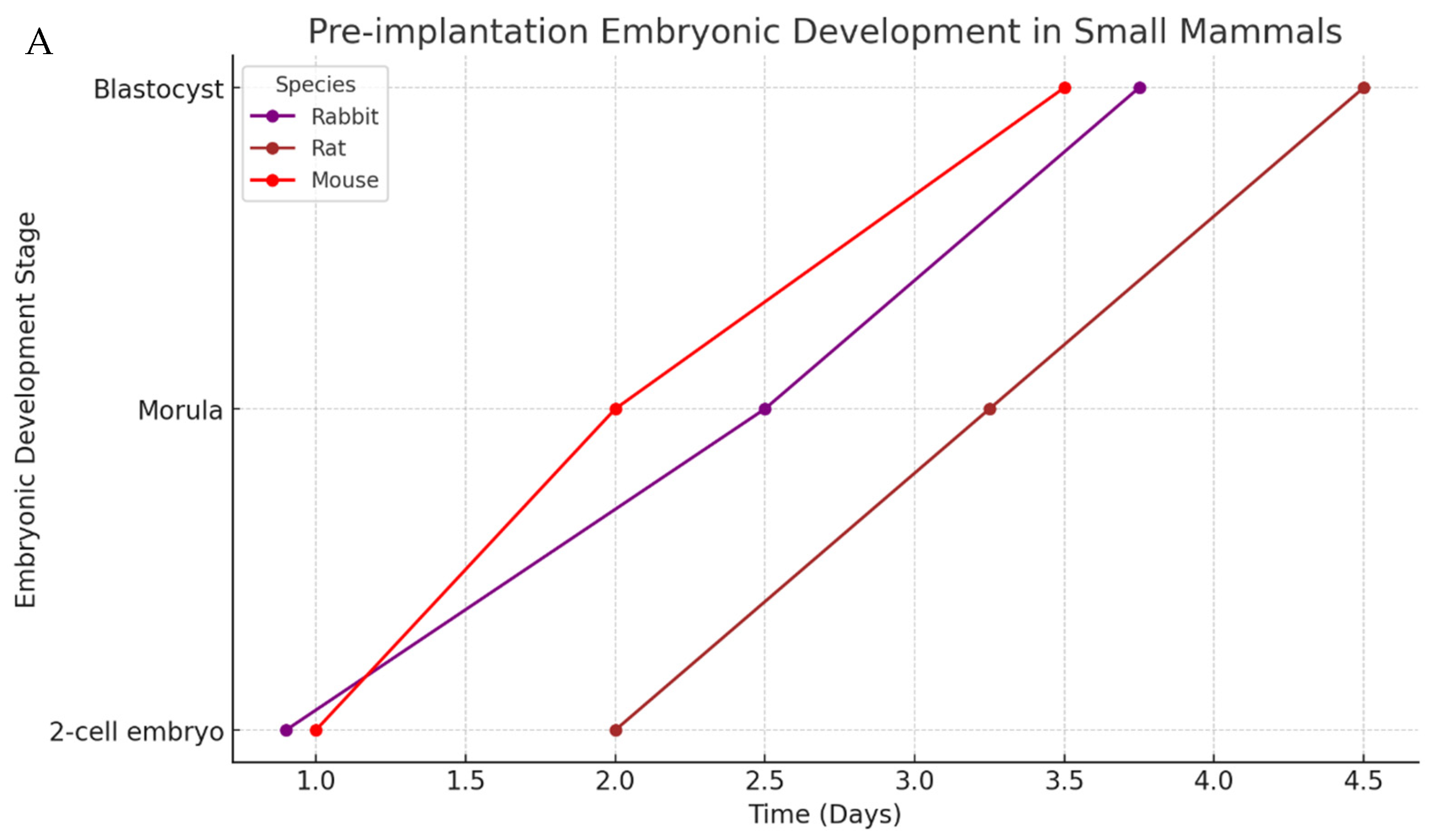
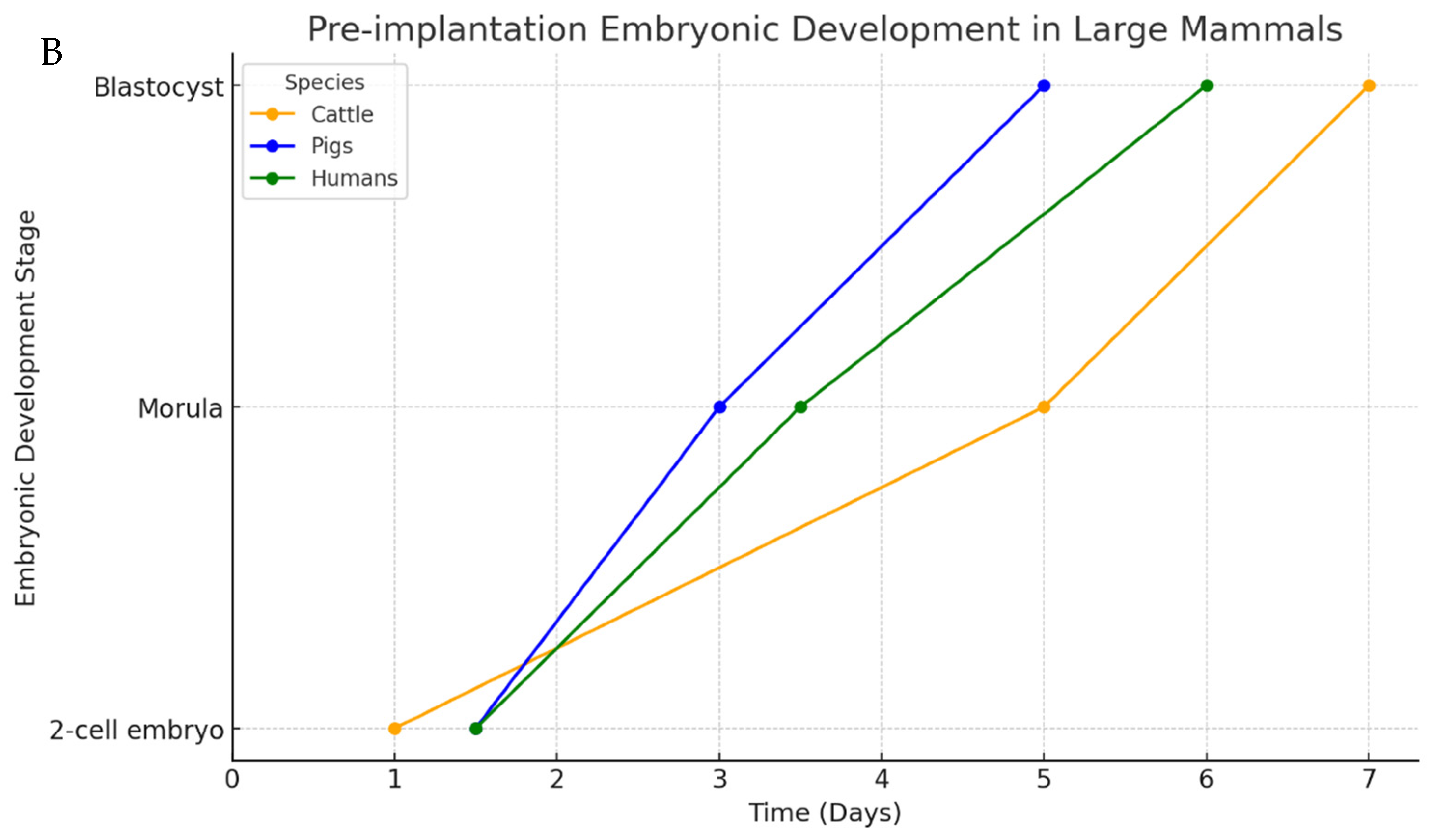
3. Mammalian Embryo

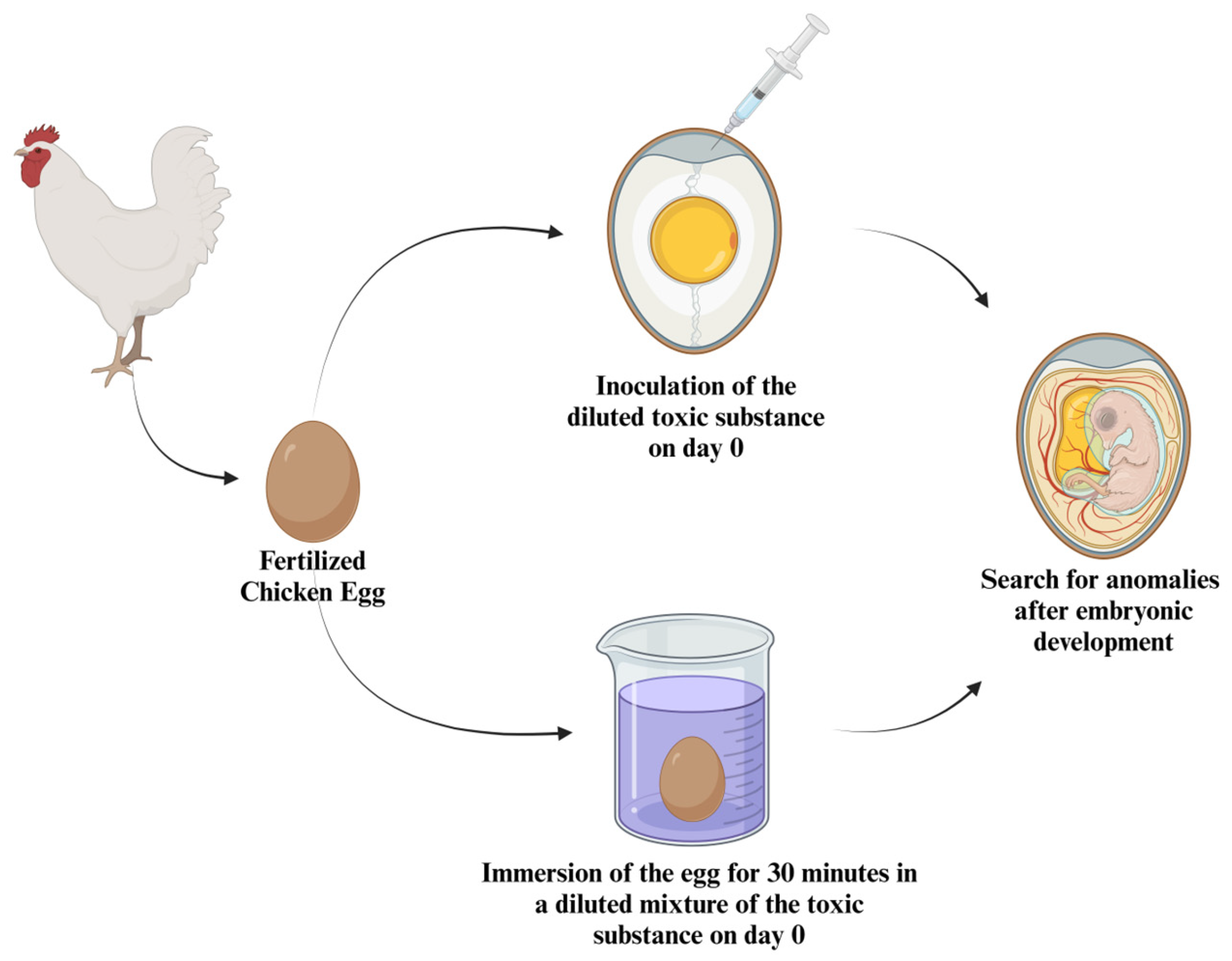
4. Avian Embryo
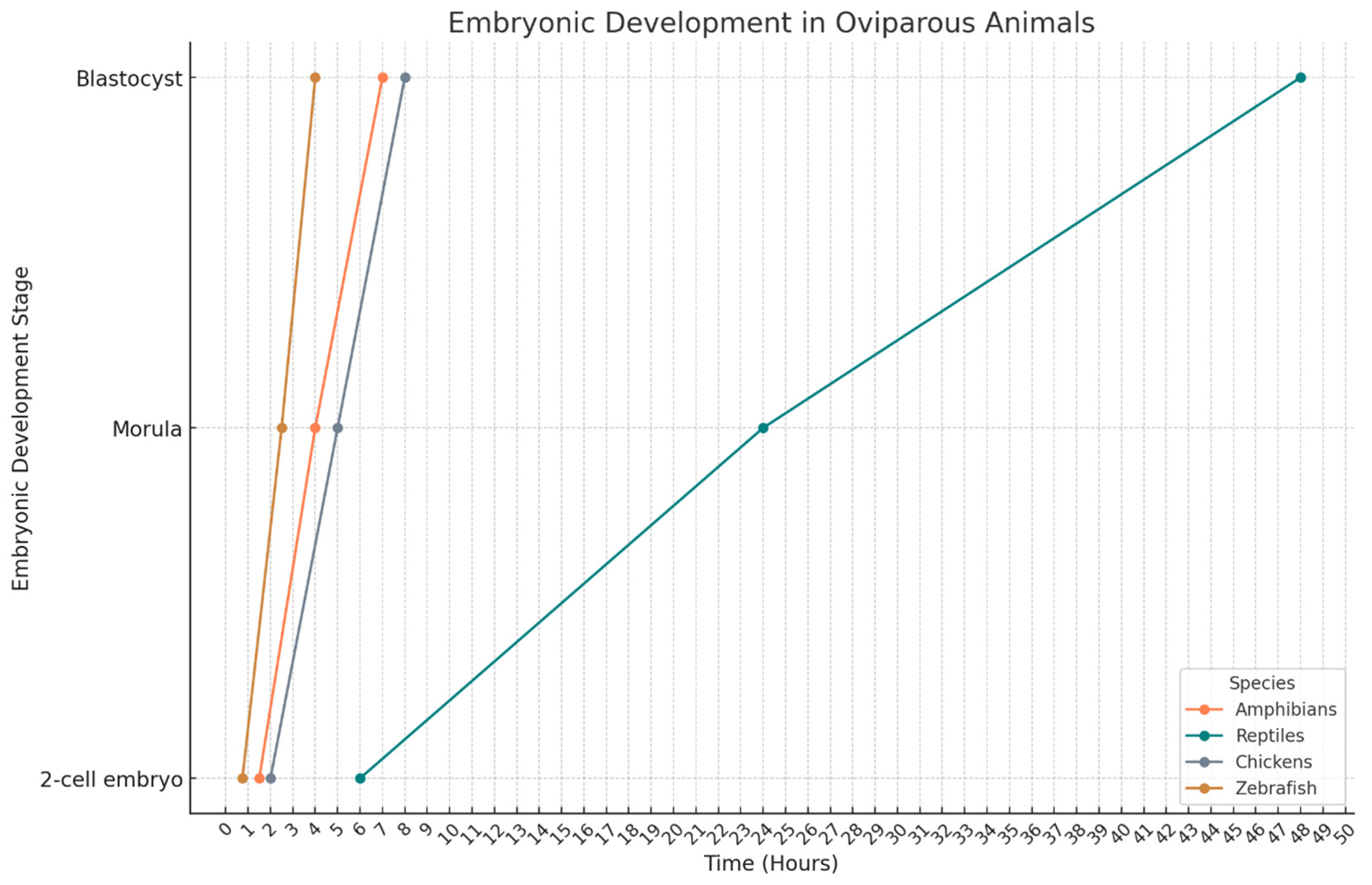
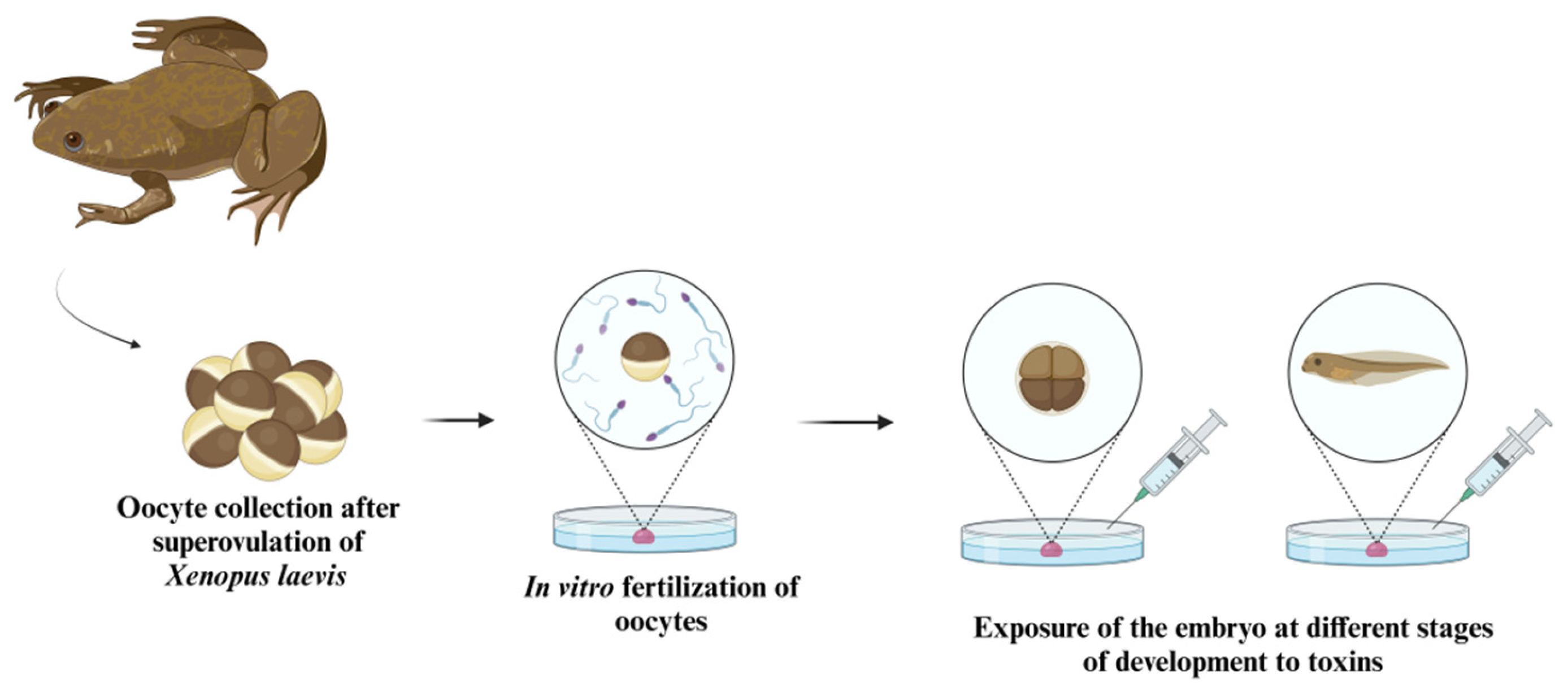
5. Amphibian Embryos
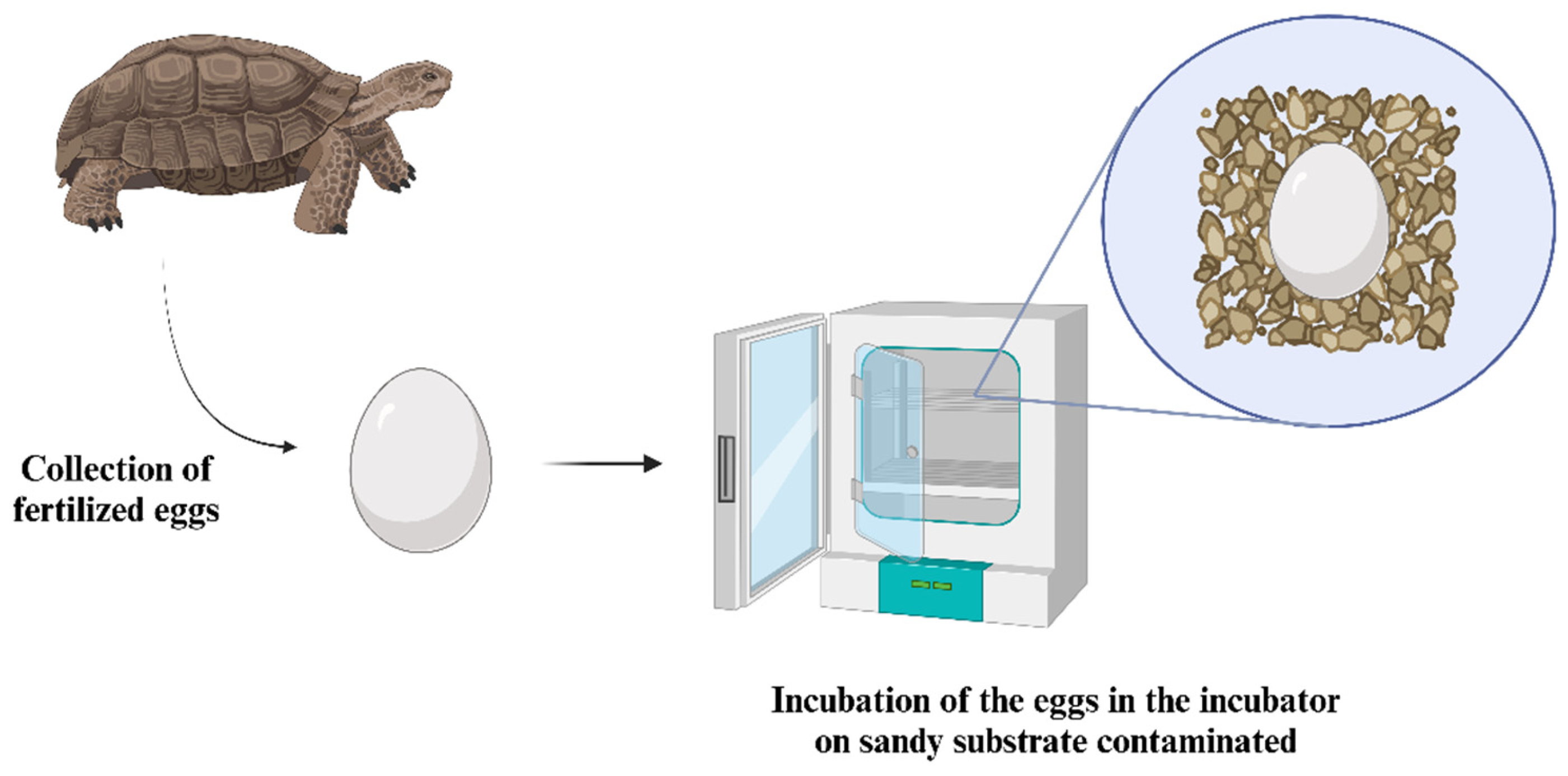
6. Reptile Embryos
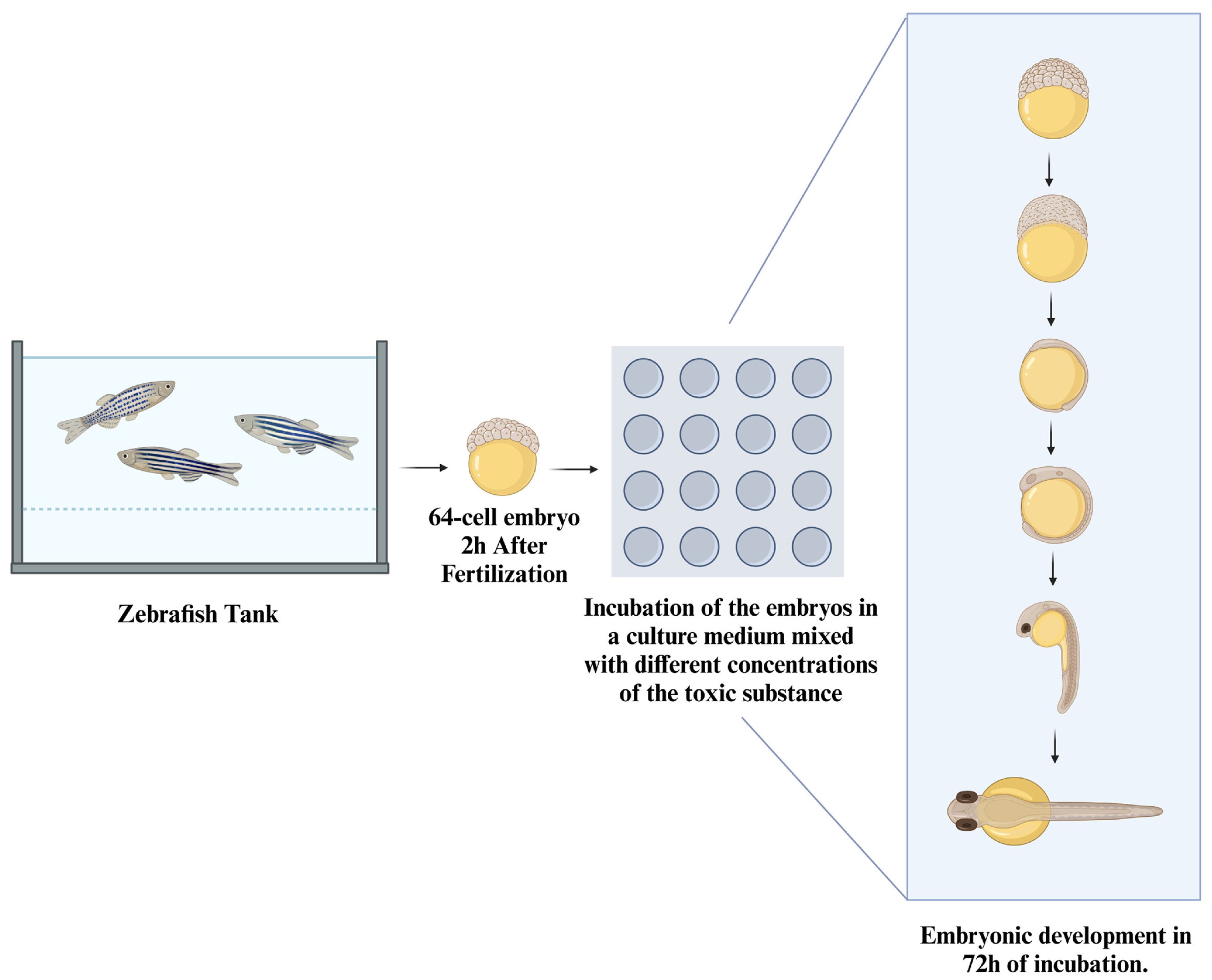
7. Fish Embryos
8. Human Embryo
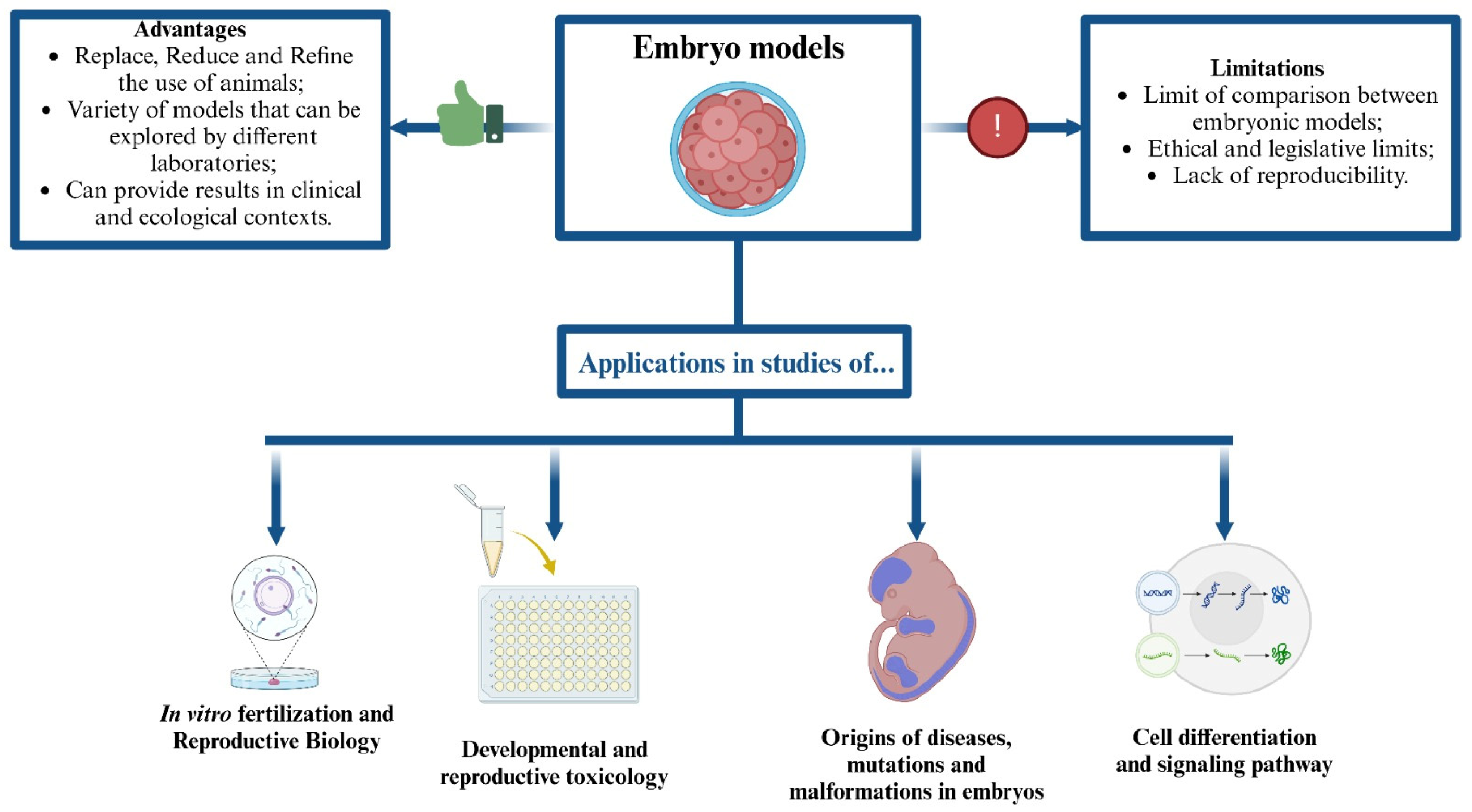
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Veissier, I.; Aubert, A.; Boissy, A. Animal welfare: A result of animal background and perception of its environment. Anim. Front. 2012, 2, 7–15. [Google Scholar] [CrossRef]
- Turner, P.V.; Barbee, R.W. Responsible Science and Research Animal Use. Ilar J. 2019, 60, 1–4. [Google Scholar] [CrossRef] [PubMed]
- McCausland, C. The Five Freedoms of Animal Welfare are Rights. J. Agric. Environ. Ethics 2014, 27, 649–662. [Google Scholar] [CrossRef]
- Kim, K.; Jo, W.; Lee, G.H. Why do we always care about the welfare of laboratory animals? Open Access Gov. 2023, 2023, 518–519. [Google Scholar] [CrossRef]
- Franco, N.H. Animal Experiments in Biomedical Research: A Historical Perspective. Animals 2013, 3, 238–273. [Google Scholar] [CrossRef]
- Kim, T.W.; Che, J.H.; Yun, J.W. Use of stem cells as alternative methods to animal experimentation in predictive toxicology. Regul. Toxicol. Pharmacol. 2019, 105, 15–29. [Google Scholar] [CrossRef]
- Freires, I.A.; Sardi, J.C.; de Castro, R.D.; Rosalen, P.L. Alternative Animal and Non-Animal Models for Drug Discovery and Development: Bonus or Burden? Pharm. Res. 2017, 34, 681–686. [Google Scholar] [CrossRef]
- Adler, S.; Basketter, D.; Creton, S.; Pelkonen, O.; van Benthem, J.; Zuang, V.; Andersen, K.E.; Angers-Loustau, A.; Aptula, A.; Bal-Price, A.; et al. Alternative (non-animal) methods for cosmetics testing: Current status and future prospects-2010. Arch. Toxicol. 2011, 85, 367–485. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Pedroso, A.R. Animal experimentation: A look into ethics, welfare and alternative methods. Rev. Assoc. Médica Bras. 2017, 63, 923–928. [Google Scholar] [CrossRef]
- Shin, H.K.; Huang, R.; Chen, M. In silico modeling-based new alternative methods to predict drug and herb-induced liver injury: A review. Food Chem. Toxicol. 2023, 179, 113948. [Google Scholar] [CrossRef]
- Wolf Pérez, A.M.; Lorenzen, N.; Vendruscolo, M.; Sormanni, P. Assessment of Therapeutic Antibody Developability by Combinations of In Vitro and In Silico Methods. Methods Mol. Biol. 2022, 2313, 57–113. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, J. In Silico Prediction of Target-Inhibitor Interaction. Adv. Exp. Med. Biol. 2016, 917, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Goyzueta-Mamani, L.D.; Barazorda-Ccahuana, H.L.; Mena-Ulecia, K.; Chávez-Fumagalli, M.A. Antiviral Activity of Metabolites from Peruvian Plants against SARS-CoV-2: An In Silico Approach. Molecules 2021, 26, 3882. [Google Scholar] [CrossRef] [PubMed]
- Khamlich, J.; Douiyeh, I.; Saih, A.; Moussamih, S.; Regragui, A.; Kettani, A.; Safi, A. Identification of small molecule glucokinase activators for the treatment of diabetes based on plants from the traditional Chinese medicine: In silico analysis. Microb. Pathog. 2024, 195, 106851. [Google Scholar] [CrossRef]
- Pamplona, J.H.; Zoehler, B.; Shigunov, P.; Barisón, M.J.; Severo, V.R.; Erich, N.M.; Ribeiro, A.L.; Horinouchi, C.; Suzukawa, A.A.; Robert, A.W.; et al. Alternative Methods as Tools for Obesity Research: In Vitro and In Silico Approaches. Life 2022, 13, 108. [Google Scholar] [CrossRef]
- Filaire, E.; Nachat-Kappes, R.; Laporte, C.; Harmand, M.F.; Simon, M.; Poinsot, C. Alternative in vitro models used in the main safety tests of cosmetic products and new challenges. Int. J. Cosmet. Sci. 2022, 44, 604–613. [Google Scholar] [CrossRef]
- Meigs, L.; Smirnova, L.; Rovida, C.; Leist, M.; Hartung, T. Animal testing and its alternatives—The most important omics is economics. Altex 2018, 35, 275–305. [Google Scholar] [CrossRef]
- McMullen, P.D.; Andersen, M.E.; Cholewa, B.; Clewell, H.J., 3rd; Dunnick, K.M.; Hartman, J.K.; Mansouri, K.; Minto, M.S.; Nicolas, C.I.; Phillips, M.B.; et al. Evaluating opportunities for advancing the use of alternative methods in risk assessment through the development of fit-for-purpose in vitro assays. Toxicol. In Vitro 2018, 48, 310–317. [Google Scholar] [CrossRef]
- Aboul-Soud, M.A.M.; Alzahrani, A.J.; Mahmoud, A. Induced Pluripotent Stem Cells (iPSCs)-Roles in Regenerative Therapies, Disease Modelling and Drug Screening. Cells 2021, 10, 2319. [Google Scholar] [CrossRef]
- Sakurai, M.; Ishitsuka, K.; Becker, H.J.; Yamazaki, S. Ex vivo expansion of human hematopoietic stem cells and clinical applications. Cancer Sci. 2024, 115, 698–705. [Google Scholar] [CrossRef]
- Castelo-Branco, D.; Amando, B.R.; Ocadaque, C.J.; Aguiar, L.; Paiva, D.D.Q.; Diógenes, E.M.; Guedes, G.M.M.; Costa, C.L.; Santos-Filho, A.S.P.; Andrade, A.R.C.; et al. Mini-review: From in vitro to ex vivo studies: An overview of alternative methods for the study of medical biofilms. Biofouling 2020, 36, 1129–1148. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.A.; Zhang, Y.; Rathnam, C.; Pongkulapa, T.; Lee, K.B. Bioengineering Approaches for the Advanced Organoid Research. Adv. Mater. 2021, 33, e2007949. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Mally, A.; Liedtke, D. Zebrafish Embryos and Larvae as Alternative Animal Models for Toxicity Testing. Int. J. Mol. Sci. 2021, 22, 13417. [Google Scholar] [CrossRef] [PubMed]
- Prior, H.; Casey, W.; Kimber, I.; Whelan, M.; Sewell, F. Reflections on the progress towards non-animal methods for acute toxicity testing of chemicals. Regul. Toxicol. Pharmacol. 2019, 102, 30–33. [Google Scholar] [CrossRef]
- Maluf, M.; Perin, P.M.; Foltran Januário, D.A.N.; Nascimento Saldiva, P.H. In vitro fertilization, embryo development, and cell lineage segregation after pre- and/or postnatal exposure of female mice to ambient fine particulate matter. Fertil. Steril. 2009, 92, 1725–1735. [Google Scholar] [CrossRef]
- Esfandiari, N.; Gubista, A. Mouse embryo assay for human in vitro fertilization quality control: A fresh look. J. Assist. Reprod. Genet. 2020, 37, 1123–1127. [Google Scholar] [CrossRef]
- Saitou, M.; Hayashi, K. Mammalian in vitro gametogenesis. Science 2021, 374, eaaz6830. [Google Scholar] [CrossRef]
- Oh, S.Y.; Na, S.B.; Kang, Y.K.; Do, J.T. In Vitro Embryogenesis and Gastrulation Using Stem Cells in Mice and Humans. Int. J. Mol. Sci. 2023, 24, 13655. [Google Scholar] [CrossRef]
- Xu, R.; Li, C.; Liu, X.; Gao, S. Insights into epigenetic patterns in mammalian early embryos. Protein Cell 2021, 12, 7–28. [Google Scholar] [CrossRef]
- Pamies, D.; Estevan, C.; Vilanova, E.; Sogorb, M.A. Chapter 11—Validated and Nonvalidated Mechanism-Based Methods for Testing Developmental Toxicity. In Reproductive and Developmental Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 193–209. [Google Scholar] [CrossRef]
- Green, M.L.; Lebron, J.A.; Tanis, K.Q.; Redfern, B.G.; Zhu, L.; Yu, Y.; Wang, E.; Kaczor, A.R.; Wysoczanski, E.; Chen, F.; et al. Use of Alternative Developmental Toxicity Assays to Assess Teratogenicity Potential of Pharmaceuticals. Appl. Vitr. Toxicol. 2017, 4, 44–53. [Google Scholar] [CrossRef]
- Alves-Pimenta, S.; Colaço, B.; Oliveira, P.A.; Venâncio, C. Biological Concerns on the Selection of Animal Models for Teratogenic Testing. In Teratogenicity Testing: Methods and Protocols; Félix, L., Ed.; Springer: New York, NY, USA, 2018; pp. 61–93. [Google Scholar] [CrossRef]
- Webster, W.S.; Brown-Woodman, P.D.; Ritchie, H.E. A review of the contribution of whole embryo culture to the determination of hazard and risk in teratogenicity testing. Int. J. Dev. Biol. 1997, 41, 329–335. [Google Scholar] [PubMed]
- Sogorb, M.A.; Pamies, D.; de Lapuente, J.; Estevan, C.; Estévez, J.; Vilanova, E. An integrated approach for detecting embryotoxicity and developmental toxicity of environmental contaminants using in vitro alternative methods. Toxicol. Lett. 2014, 230, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Umanzor-Alvarez, J.; Wade, E.C.; Gifford, A.; Nontapot, K.; Cruz-Reese, A.; Gotoh, T.; Sible, J.C.; Khodaparast, G.A. Near-infrared laser delivery of nanoparticles to developing embryos: A study of efficacy and viability. Biotechnol. J. 2011, 6, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, N.; Bintig, W.; Perret, P.L.; Rees, J.; Viperino, A.; Eickholt, B.; Kuebler, W.M.; Höpfner, M.; Nitzsche, B.; Grune, J. In-ovo echocardiography for application in cardiovascular research. Basic. Res. Cardiol. 2023, 118, 19. [Google Scholar] [CrossRef]
- Kurth, T.; Weiche, S.; Vorkel, D.; Kretschmar, S.; Menge, A. Histology of plastic embedded amphibian embryos and larvae. Genesis 2012, 50, 235–250. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, Y.Y.; Han, J.Y.; Kim, S.W.; Kim, H.; Ku, S.-Y. Advancements in Human Embryonic Stem Cell Research: Clinical Applications and Ethical Issues. Tissue Eng. Regen. Med. 2024, 21, 379–394. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Palasca, O.; Yarani, R.; Litman, T.; Anthon, C.; Groenen, M.A.M.; Stadler, P.F.; Pociot, F.; Jensen, L.J.; Gorodkin, J. Human pathways in animal models: Possibilities and limitations. Nucleic Acids Res. 2021, 49, 1859–1871. [Google Scholar] [CrossRef]
- Scialdone, A.; Rivron, N. In preprints: Improving and interrogating embryo models. Development 2022, 149, dev201404. [Google Scholar] [CrossRef]
- Ajduk, A.; Zernicka-Goetz, M. Advances in embryo selection methods. F1000 Biol. Rep. 2012, 4, 11. [Google Scholar] [CrossRef]
- Dimitriadis, I.; Zaninovic, N.; Badiola, A.C.; Bormann, C.L. Artificial intelligence in the embryology laboratory: A review. Reprod. BioMed. Online 2022, 44, 435–448. [Google Scholar] [CrossRef]
- Gupta, A.; Lutolf, M.P.; Hughes, A.J.; Sonnen, K.F. Bioengineering in vitro models of embryonic development. Stem Cell Rep. 2021, 16, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Van Soom, A.; Wydooghe, E.; Heras, S.; Vandaele, L. Alternative models for the study of embryo-maternal cross-talk and signaling molecules from fertilisation to implantation. Reprod. Fertil. Dev. 2011, 23, iii–v. [Google Scholar] [CrossRef] [PubMed]
- Posfai, E.; Lanner, F.; Mulas, C.; Leitch, H.G. All models are wrong, but some are useful: Establishing standards for stem cell-based embryo models. Stem Cell Rep. 2021, 16, 1117–1141. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Zhang, X.; Zhang, J.; Wu, C. Use of Chicken Embryo Model in Toxicity Studies of Endocrine-Disrupting Chemicals and Nanoparticles. Chem. Res. Toxicol. 2022, 35, 550–568. [Google Scholar] [CrossRef]
- Johnston, H.J.; Verdon, R.; Gillies, S.; Brown, D.M.; Fernandes, T.F.; Henry, T.B.; Rossi, A.G.; Tran, L.; Tucker, C.; Tyler, C.R.; et al. Adoption of in vitro systems and zebrafish embryos as alternative models for reducing rodent use in assessments of immunological and oxidative stress responses to nanomaterials. Crit. Rev. Toxicol. 2018, 48, 252–271. [Google Scholar] [CrossRef]
- Driessen, M.; Kienhuis, A.S.; Pennings, J.L.; Pronk, T.E.; van de Brandhof, E.J.; Roodbergen, M.; Spaink, H.P.; van de Water, B.; van der Ven, L.T. Exploring the zebrafish embryo as an alternative model for the evaluation of liver toxicity by histopathology and expression profiling. Arch. Toxicol. 2013, 87, 807–823. [Google Scholar] [CrossRef]
- Perkins, E.J.; Ankley, G.T.; Crofton, K.M.; Garcia-Reyero, N.; LaLone, C.A.; Johnson, M.S.; Tietge, J.E.; Villeneuve, D.L. Current perspectives on the use of alternative species in human health and ecological hazard assessments. Environ. Health Perspect. 2013, 121, 1002–1010. [Google Scholar] [CrossRef]
- Chu, P.Y.; Koh, A.P.; Antony, J.; Huang, R.Y. Applications of the Chick Chorioallantoic Membrane as an Alternative Model for Cancer Studies. Cells Tissues Organs 2022, 211, 222–237. [Google Scholar] [CrossRef]
- Deeming, D. Reptilian Incubation: Environment, Evolution and Behaviour; Nottingham University Press: Nottingham, UK, 2004. [Google Scholar]
- Crossley, D.A.; Crossley, J.L.; Conner, J.L.; Smith, B.; Elsey, R.; Nelson, D.; Wang, T. Short communication: Characterizing arterial and venous blood gases over the gas exchange surface, the chorioallantoic membrane, of embryonic American alligators (Alligator mississippiensis) at two points of development. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2024, 290, 111575. [Google Scholar] [CrossRef]
- Zhao, B.; Li, T.; Shine, R.; Du, W.-G. Turtle embryos move to optimal thermal environments within the egg. Biol. Lett. 2013, 9, 20130337. [Google Scholar] [CrossRef]
- Li, T.; Zhao, B.; Zhou, Y.-K.; Hu, R.; Du, W.-G.; Natural History Editor: Mark, A.M. Thermoregulatory Behavior Is Widespread in the Embryos of Reptiles and Birds. Am. Nat. 2014, 183, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Du, W.G.; Zhao, B.; Chen, Y.; Shine, R. Behavioral thermoregulation by turtle embryos. Proc. Natl. Acad. Sci. USA 2011, 108, 9513–9515. [Google Scholar] [CrossRef]
- Mozdziak, P.E.; Petitte, J.N. Transgenic Snakes and Methods of Making; Google Patents: Mountain View, CA, USA, 2010. [Google Scholar]
- Itoh, K.; Reis, A.H.; Hayhurst, A.; Sokol, S.Y. Isolation of nanobodies against Xenopus embryonic antigens using immune and non-immune phage display libraries. PLoS ONE 2019, 14, e0216083. [Google Scholar] [CrossRef] [PubMed]
- El Mir, J.; Nasrallah, A.; Thézé, N.; Cario, M.; Fayyad-Kazan, H.; Thiébaud, P.; Rezvani, H.R. Xenopus as a model system for studying pigmentation and pigmentary disorders. Pigment. Cell Melanoma Res. 2024, 38, e13178. [Google Scholar] [CrossRef]
- Menegola, E.; Broccia, M.; Di Renzo, F.; Prati, M.; Giavini, E. In vitro teratogenic potential of two antifungal triazoles: Triadimefon and triadimenol. Vitr. Cell. Dev. Biology. Anim. 2000, 36, 88–95. [Google Scholar]
- Gilbert, S.F.; Barresi, M.J.F. Biologia do Desenvolvimento; Artmed: Bucuresti, Romania, 2019. [Google Scholar]
- Mahajan, T.; Ganguly, S.; Pagrut, N. Embryogenesis: A comprehensive review. J. Entomol. Zool. Stud. 2018, 6, 1151–1153. [Google Scholar] [CrossRef]
- Santos, R.R.; Schoevers, E.J.; Roelen, B.A. Usefulness of bovine and porcine IVM/IVF models for reproductive toxicology. Reprod. Biol. Endocrinol. 2014, 12, 117. [Google Scholar] [CrossRef]
- Ferré, L.B.; Kjelland, M.E.; Strøbech, L.B.; Hyttel, P.; Mermillod, P.; Ross, P.J. Review: Recent advances in bovine in vitro embryo production: Reproductive biotechnology history and methods. Animal 2020, 14, 991–1004. [Google Scholar] [CrossRef]
- Idrees, M.; Kumar, V.; Khan, A.M.; Joo, M.D.; Uddin, Z.; Lee, K.W.; Kong, I.K. Hesperetin activated SIRT1 neutralizes cadmium effects on the early bovine embryo development. Theriogenology 2022, 189, 209–221. [Google Scholar] [CrossRef]
- Naspinska, R.; Moreira da Silva, M.H.; Moreira da Silva, F. Current Advances in Bovine In Vitro Maturation and Embryo Production Using Different Antioxidants: A Review. J. Dev. Biol. 2023, 11, 36. [Google Scholar] [CrossRef]
- Saleh, A.C.; Sabry, R.; Mastromonaco, G.F.; Favetta, L.A. BPA and BPS affect the expression of anti-Mullerian hormone (AMH) and its receptor during bovine oocyte maturation and early embryo development. Reprod. Biol. Endocrinol. 2021, 19, 119. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, V.B.; Marikawa, Y. Trophectoderm formation: Regulation of morphogenesis and gene expressions by RHO, ROCK, cell polarity, and HIPPO signaling. Reproduction 2022, 164, R75–R86. [Google Scholar] [CrossRef] [PubMed]
- Caine, E.A.; Jagger, B.W.; Diamond, M.S. Animal Models of Zika Virus Infection during Pregnancy. Viruses 2018, 10, 598. [Google Scholar] [CrossRef]
- Cambra, J.M.; Martinez, C.A.; Rodriguez-Martinez, H.; Martinez, E.A.; Cuello, C.; Gil, M.A. N-(2-mercaptopropionyl)-glycine enhances in vitro pig embryo production and reduces oxidative stress. Sci. Rep. 2020, 10, 18632. [Google Scholar] [CrossRef]
- Chiminelli, I.; Spicer, L.J.; Maylem, E.R.S.; Caloni, F. Emerging mycotoxins and reproductive effects in animals: A short review. J. Appl. Toxicol. 2022, 42, 1901–1909. [Google Scholar] [CrossRef]
- Lucas, C.G.; Redel, B.K.; Chen, P.R.; Spate, L.D.; Prather, R.S.; Wells, K.D. Effects of RAD51-stimulatory compound 1 (RS-1) and its vehicle, DMSO, on pig embryo culture. Reprod. Toxicol. 2021, 105, 44–52. [Google Scholar] [CrossRef]
- Garcia-Dominguez, X.; Juarez, J.D.; Vicente, J.S.; Marco-Jiménez, F. Impact of embryo technologies on secondary sex ratio in rabbit. Cryobiology 2020, 97, 60–65. [Google Scholar] [CrossRef]
- Honda, A.; Ogura, A. Rabbit models for biomedical research revisited via genome editing approaches. J. Reprod. Dev. 2017, 63, 435–438. [Google Scholar] [CrossRef]
- Catlin, N.; Waidyanatha, S.; Mylchreest, E.; Miller-Pinsler, L.; Cunny, H.; Foster, P.; Sutherland, V.; McIntyre, B. Embryo-fetal development studies with the dietary supplement vinpocetine in the rat and rabbit. Birth Defects Res. 2018, 110, 883–896. [Google Scholar] [CrossRef]
- Barlow, S.M.; Terry, C.; Gehen, S.; Corvaro, M. Developmental toxicity studies on triclopyr acid, triclopyr butoxyethyl ester and triclopyr triethylamine salt in the rabbit. Food Chem. Toxicol. 2022, 161, 112845. [Google Scholar] [CrossRef]
- Theunissen, P.T.; Beken, S.; Beyer, B.K.; Breslin, W.J.; Cappon, G.D.; Chen, C.L.; Chmielewski, G.; De Schaepdrijver, L.; Enright, B.; Foreman, J.E.; et al. Comparison of rat and rabbit embryo-fetal developmental toxicity data for 379 pharmaceuticals: On the nature and severity of developmental effects. Crit. Rev. Toxicol. 2016, 46, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Tesarik, J. Control of Maternal-to-Zygotic Transition in Human Embryos and Other Animal Species (Especially Mouse): Similarities and Differences. Int. J. Mol. Sci. 2022, 23, 8562. [Google Scholar] [CrossRef]
- Greenlee, A.R.; Ellis, T.M.; Berg, R.L. Low-dose agrochemicals and lawn-care pesticides induce developmental toxicity in murine preimplantation embryos. Environ. Health Perspect. 2004, 112, 703–709. [Google Scholar] [CrossRef]
- Dias, P.F.; MÜLler, Y.M.R. Características do desenvolvimento embrionário de Gallus gallus domesticus, em temperaturas e períodos diferentes de incubação. Braz. J. Vet. Res. Anim. Sci. 1998, 35, 233–235. [Google Scholar] [CrossRef]
- Bednarczyk, M.; Dunislawska, A.; Stadnicka, K.; Grochowska, E. Chicken embryo as a model in epigenetic research. Poult. Sci. 2021, 100, 101164. [Google Scholar] [CrossRef]
- Wittig, J.G.; Münsterberg, A. The Chicken as a Model Organism to Study Heart Development. Cold Spring Harb. Perspect. Biol. 2020, 12, a037218. [Google Scholar] [CrossRef]
- Flentke, G.R.; Smith, S.M. The avian embryo as a model for fetal alcohol spectrum disorder. Biochem. Cell Biol. 2018, 96, 98–106. [Google Scholar] [CrossRef]
- Garcia, P.; Wang, Y.; Viallet, J.; Macek Jilkova, Z. The Chicken Embryo Model: A Novel and Relevant Model for Immune-Based Studies. Front. Immunol. 2021, 12, 791081. [Google Scholar] [CrossRef]
- Kue, C.S.; Tan, K.Y.; Lam, M.L.; Lee, H.B. Chick embryo chorioallantoic membrane (CAM): An alternative predictive model in acute toxicological studies for anti-cancer drugs. Exp. Anim. 2015, 64, 129–138. [Google Scholar] [CrossRef]
- Jota Baptista, C.V.; Faustino-Rocha, A.I.; Oliveira, P.A. Animal Models in Pharmacology: A Brief History Awarding the Nobel Prizes for Physiology or Medicine. Pharmacology 2021, 106, 356–368. [Google Scholar] [CrossRef]
- Hruba, H.; Abdelsalam, E.E.E.; Anisimov, N.; Bandouchova, H.; Havelkova, B.; Heger, T.; Kanova, M.; Kovacova, V.; Nemcova, M.; Piacek, V.; et al. Reproductive toxicity of fluoroquinolones in birds. BMC Vet. Res. 2019, 15, 209. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Sharifi, I.; Tavakkoli, H.; Derakhshanfar, A.; Keyhani, A.R.; Salari, Z.; Mosallanejad, S.S.; Bamorovat, M. Embryonic toxico-pathological effects of meglumine antimoniate using a chick embryo model. PLoS ONE 2018, 13, e0196424. [Google Scholar] [CrossRef]
- Kurantowicz, N.; Sawosz, E.; Halik, G.; Strojny, B.; Hotowy, A.; Grodzik, M.; Piast, R.; Pasanphan, W.; Chwalibog, A. Toxicity studies of six types of carbon nanoparticles in a chicken-embryo model. Int. J. Nanomed. 2017, 12, 2887–2898. [Google Scholar] [CrossRef] [PubMed]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, S.; Mundim, A.V.; Silva, R.R.; Queiroz, J.S.; Rios, M.P.; Notário, F.O.; Medeiros Ronchi, A.A.; Beletti, M.E.; Franco, R.R.; Espindola, F.S.; et al. Physiological Changes in Chicken Embryos Inoculated with Drugs and Viruses Highlight the Need for More Standardization of this Animal Model. Animals 2022, 12, 1156. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem. Cell Biol. 2008, 130, 1119–1130. [Google Scholar] [CrossRef]
- Guy, J.S. Isolation and propagation of coronaviruses in embryonated eggs. Methods Mol. Biol. 2008, 454, 109–117. [Google Scholar] [CrossRef]
- Miebach, L.; Berner, J.; Bekeschus, S. In ovo model in cancer research and tumor immunology. Front. Immunol. 2022, 13, 1006064. [Google Scholar] [CrossRef]
- Lehel, J.; Szemerédy, G.; Szabó, R.; Major, L.; Grúz, A.; Budai, P. Reproductive toxicological changes in avian embryos due to a pesticide and an environmental contaminant. Acta Vet. Hung. 2021, 69, 363–371. [Google Scholar] [CrossRef]
- Maia, L.A.; Velloso, I.; Abreu, J.G. Advances in the use of Xenopus for successful drug screening. Expert. Opin. Drug Discov. 2017, 12, 1153–1159. [Google Scholar] [CrossRef]
- Bastos, V. Desenvolvimento e estabelecimento de linha celular de blástula de Xenopus laevis. Rev. Captar Ciência E Ambiente Para Todos 2020, 9, 68–80. [Google Scholar]
- Elinson, R.P.; Rowning, B. A transient array of parallel microtubules in frog eggs: Potential tracks for a cytoplasmic rotation that specifies the dorso-ventral axis. Dev. Biol. 1988, 128, 185–197. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, E.M.; Gurdon, J.B. A Brief History of Xenopus in Biology. Cold Spring Harb. Protoc. 2021, 2021, pdb-top107615. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Etkin, L. Patterning and lineage specification in the amphibian embryo. Curr. Top. Dev. Biol. 2001, 51, 1–67. [Google Scholar] [CrossRef]
- Keller, R. Early embryonic development of Xenopus laevis. Methods Cell Biol. 1991, 36, 61–113. [Google Scholar] [CrossRef]
- Sanz-Ezquerro, J.J.; Münsterberg, A.E.; Stricker, S. Editorial: Signaling Pathways in Embryonic Development. Front. Cell Dev. Biol. 2017, 5, 76. [Google Scholar] [CrossRef]
- Koser, D.E.; Thompson, A.J.; Foster, S.K.; Dwivedy, A.; Pillai, E.K.; Sheridan, G.K.; Svoboda, H.; Viana, M.; Costa, L.D.; Guck, J.; et al. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 2016, 19, 1592–1598. [Google Scholar] [CrossRef]
- Amado, N.G.; Fonseca, B.F.; Cerqueira, D.M.; Reis, A.H.; Simas, A.B.; Kuster, R.M.; Mendes, F.A.; Abreu, J.G. Effects of natural compounds on Xenopus embryogenesis: A potential read out for functional drug discovery targeting Wnt/β-catenin signaling. Curr. Top. Med. Chem. 2012, 12, 2103–2113. [Google Scholar] [CrossRef]
- Gao, J.; Shen, W. Xenopus in revealing developmental toxicity and modeling human diseases. Environ. Pollut. 2021, 268, 115809. [Google Scholar] [CrossRef]
- Fort, D.J.; Dawson, D.A.; Bantle, J.A. Development of a metabolic activation system for the frog embryo teratogenesis assay: Xenopus (FETAX). Teratog.Carcinog. Mutagen. 1988, 8, 251–263. [Google Scholar] [CrossRef]
- Gaudet-Hull, A.M.; Rayburn, J.R.; Bantle, J.A.; Burton, D.T.; Turley, S.D.; Dawson, D.A.; Dumont, J.N.; Finch, R.A.; Maurice, M.A.; Fort, D.J.; et al. Fetax interlaboratory validation study: Phase II testing. Environ. Toxicol. Chem. 1994, 13, 1629–1637. [Google Scholar] [CrossRef]
- Truter, J.C.; Myburgh, J. In ovo manipulation of Nile crocodile embryos: Egg windowing and potential dental research applications. Int. J. Dev. Biol. 2021, 65, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, J.I.; Richman, J.M. Resilience of the replacing dentition in adult reptiles. Dev. Biol. 2024, 516, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Olmo, E. Reptile Evolution and Genetics: An Overview. Animals 2023, 13, 1924. [Google Scholar] [CrossRef]
- Tollis, M.; Hutchins, E.D.; Kusumi, K. Reptile genomes open the frontier for comparative analysis of amniote development and regeneration. Int. J. Dev. Biol. 2014, 58, 863–871. [Google Scholar] [CrossRef]
- Rasys, A.M.; Park, S.; Ball, R.E.; Alcala, A.J.; Lauderdale, J.D.; Menke, D.B. CRISPR-Cas9 Gene Editing in Lizards through Microinjection of Unfertilized Oocytes. Cell Rep. 2019, 28, 2288–2292.e2283. [Google Scholar] [CrossRef]
- Lozito, T.P.; Londono, R.; Sun, A.X.; Hudnall, M.L. Introducing dorsoventral patterning in adult regenerating lizard tails with gene-edited embryonic neural stem cells. Nat. Commun. 2021, 12, 6010. [Google Scholar] [CrossRef]
- Hyder, F.; Rothman, D.L.; Bennett, M.R. Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc. Natl. Acad. Sci. USA 2013, 110, 3549–3554. [Google Scholar] [CrossRef]
- Londono, R.; Wenzhong, W.; Wang, B.; Tuan, R.S.; Lozito, T.P. Cartilage and Muscle Cell Fate and Origins during Lizard Tail Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 70. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Jiang, S.; Zhou, W.; Liu, Y.; Wang, Y.; Gu, Q.; Gu, Y.; Dong, Y.; Liu, M.; et al. Gecko CD59 is implicated in proximodistal identity during tail regeneration. PLoS ONE 2011, 6, e17878. [Google Scholar] [CrossRef]
- de Solla, S.R.; Palonen, K.E.; Martin, P.A. Toxicity of pesticides associated with potato production, including soil fumigants, to snapping turtle eggs (Chelydra Serpentina). Environ. Toxicol. Chem. 2013, 33, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Canzanella, S.; Iaccarino, D.; Pepe, A.; Di Nocera, F.; Bruno, T.; Marigliano, L.; Sansone, D.; Hochscheid, S.; Gallo, P.; et al. Trace Elements and Persistent Organic Pollutants in Unhatched Loggerhead Turtle Eggs from an Emerging Nesting Site along the Southwestern Coasts of Italy, Western Mediterranean Sea. Animals 2023, 13, 1075. [Google Scholar] [CrossRef] [PubMed]
- Mauch, T.J.; Schoenwolf, G.C. Developmental Biology. Sixth Edition. By Scott F. Gilbert. Am. J. Med. Genet. 2001, 99, 170–171. [Google Scholar] [CrossRef]
- Dey, A.; Flajšhans, M.; Pšenička, M.; Gazo, I. DNA repair genes play a variety of roles in the development of fish embryos. Front. Cell Dev. Biol. 2023, 11, 1119229. [Google Scholar] [CrossRef]
- Babin, P.; Cerdà, J.; Lubzens, E. The Fish Oocyte: From Basic Studies to Biotechnological Applications; Springer Science & Business Media: New York, NY, USA, 2007; pp. 1–508. [Google Scholar] [CrossRef]
- Polačik, M.; Vrtílek, M.; Reichard, M.; Žák, J.; Blažek, R.; Podrabsky, J. Embryo ecology: Developmental synchrony and asynchrony in the embryonic development of wild annual fish populations. Ecol. Evol. 2021, 11, 4945–4956. [Google Scholar] [CrossRef]
- Kirchmaier, S.; Naruse, K.; Wittbrodt, J.; Loosli, F. The Genomic and Genetic Toolbox of the Teleost Medaka (Oryzias latipes). Genetics 2015, 199, 905–918. [Google Scholar] [CrossRef]
- Feitosa, N.M.; Calderon, E.N.; da Silva, R.N.; de Melo, S.L.R.; Souza-Menezes, J.; Nunes-da-Fonseca, R.; Reynier, M.V. Brazilian silverside, Atherinella brasiliensis (Quoy & Gaimard,1825) embryos as a test-species for marine fish ecotoxicological tests. PeerJ 2021, 9, e11214. [Google Scholar] [CrossRef]
- Rafaella, S.B.; Aryelle, C.; Davi, F.; Thiago Lopes, R. Transgenic zebrafish (Danio rerio) as an emerging model system in ecotoxicology and toxicology: Historical review, recent advances, and trends. Sci. Total Environ. 2022, 848, 157665. [Google Scholar] [CrossRef]
- Blechinger, S.; Warren, J.; Kuwada, J.; Krone, P. Developmental Toxicology of Cadmium in Living Embryos of a Stable Transgenic Zebrafish Line. Environ. Health Perspect. 2002, 110, 1041–1046. [Google Scholar] [CrossRef]
- Roosen-Runge, E. Observations of the early development of the zebra fish, Brachydanio rerio. Anat. Rec. 1937, 70, 103. [Google Scholar]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Bondue, T.; Berlingerio, S.P.; van den Heuvel, L.; Levtchenko, E. The Zebrafish Embryo as a Model Organism for Testing mRNA-Based Therapeutics. Int. J. Mol. Sci. 2023, 24, 11224. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Ramdas Nair, A.; Delaney, P.; Koomson, A.A.; Ranjan, S.; Sadler, K.C. Systematic Evaluation of the Effects of Toxicant Exposure on Survival in Zebrafish Embryos and Larvae. Curr. Protoc. 2021, 1, e231. [Google Scholar] [CrossRef]
- Arteaga, C.; Boix, N.; Teixido, E.; Marizande, F.; Cadena, S.; Bustillos, A. The Zebrafish Embryo as a Model to Test Protective Effects of Food Antioxidant Compounds. Molecules 2021, 26, 5786. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, X.; Qin, Y.; Hou, Y.; Hou, J.; Wu, Q.; Xu, W. Zebrafish as model organisms for toxicological evaluations in the field of food science. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3481–3505. [Google Scholar] [CrossRef]
- Li, K.; Wu, J.Q.; Jiang, L.L.; Shen, L.Z.; Li, J.Y.; He, Z.H.; Wei, P.; Lv, Z.; He, M.F. Developmental toxicity of 2,4-dichlorophenoxyacetic acid in zebrafish embryos. Chemosphere 2017, 171, 40–48. [Google Scholar] [CrossRef]
- Niakan, K.K.; Han, J.; Pedersen, R.A.; Simon, C.; Pera, R.A. Human pre-implantation embryo development. Development 2012, 139, 829–841. [Google Scholar] [CrossRef]
- Ghimire, S.; Mantziou, V.; Moris, N.; Martinez Arias, A. Human gastrulation: The embryo and its models. Dev. Biol. 2021, 474, 100–108. [Google Scholar] [CrossRef]
- Rossant, J. Why study human embryo development? Dev. Biol. 2024, 509, 43–50. [Google Scholar] [CrossRef]
- Nazari, E.M. Embriologia Humana; BIOLOGIA/EAD/UFSC: Florianópolis, Brazil, 2011; p. 170. [Google Scholar]
- Rivron, N.C.; Martinez Arias, A.; Pera, M.F.; Moris, N.; M’Hamdi, H.I. An ethical framework for human embryology with embryo models. Cell 2023, 186, 3548–3557. [Google Scholar] [CrossRef] [PubMed]
- Savulescu, J.; Labude, M.; Barcellona, C.; Huang, Z.; Leverentz, M.K.; Xafis, V.; Lysaght, T. Two kinds of embryo research: Four case examples. J. Med. Ethics 2022, 48, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Appleby, J.B.; Bredenoord, A.L. Should the 14-day rule for embryo research become the 28-day rule? EMBO Mol. Med. 2018, 10, e9437. [Google Scholar] [CrossRef] [PubMed]
- Cockerell, A.; Wright, L.; Dattani, A.; Guo, G.; Smith, A.; Tsaneva-Atanasova, K.; Richards, D.M. Biophysical models of early mammalian embryogenesis. Stem Cell Rep. 2023, 18, 26–46. [Google Scholar] [CrossRef]
- Gerri, C.; McCarthy, A.; Alanis-Lobato, G.; Demtschenko, A.; Bruneau, A.; Loubersac, S.; Fogarty, N.M.E.; Hampshire, D.; Elder, K.; Snell, P.; et al. Initiation of a conserved trophectoderm program in human, cow and mouse embryos. Nature 2020, 587, 443–447. [Google Scholar] [CrossRef]
- Nicolas, P.; Etoc, F.; Brivanlou, A.H. The ethics of human-embryoids model: A call for consistency. J. Mol. Med. 2021, 99, 569–579. [Google Scholar] [CrossRef]
- Weatherbee, B.A.T.; Gantner, C.W.; Iwamoto-Stohl, L.K.; Daza, R.M.; Hamazaki, N.; Shendure, J.; Zernicka-Goetz, M. Pluripotent stem cell-derived model of the post-implantation human embryo. Nature 2023, 622, 584–593. [Google Scholar] [CrossRef]
- Oldak, B.; Wildschutz, E.; Bondarenko, V.; Comar, M.-Y.; Zhao, C.; Aguilera-Castrejon, A.; Tarazi, S.; Viukov, S.; Pham, T.X.A.; Ashouokhi, S.; et al. Complete human day 14 post-implantation embryo models from naive ES cells. Nature 2023, 622, 562–573. [Google Scholar] [CrossRef]
- Bao, M.; Cornwall-Scoones, J.; Zernicka-Goetz, M. Stem-cell-based human and mouse embryo models. Curr. Opin. Genet. Dev. 2022, 76, 101970. [Google Scholar] [CrossRef]
| Embryo Model | Key Characteristics | Main Applications | Main Advantages | Main Disadvantages |
|---|---|---|---|---|
| Mammals | Development inside the mother; like humans | Reproductive health, toxicity studies, genetic selection, teratogenesis | High genetic similarity to humans; well-characterized | Require strict protocols; higher investment; results inferred without maternal interaction |
| Birds | External development; has a structure similar to mammal placenta | Cancer research, immune response studies, vaccine studies | Rapid development; easy manipulation | Many tests not standardized |
| Amphibians | Shares processes and genes with other vertebrates. Amniotic egg; Polarized development | Cell signaling, genetic mapping, toxicity testing, teratogenesis testing | Easy maintenance; produces many embryos | Take time to reach reproductive age |
| Reptiles | Differentiation and growth vary with environment | Environmental variation studies; toxicity testing | Respond to environmental changes | Less commonly used |
| Fish | Transparent embryos allow for easy observation | Toxicity testing; safety evaluation | Produce many embryos; low maintenance cost | Greater evolutionary distance from humans |
| Humans | Use is restricted or limited by the 14-day rule | Embryonic development and disease studies | Unique data on human development | Ethical and legal restrictions in many countries |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campitelli, L.M.M.; Lopes, K.P.; de Lima, I.L.; Ferreira, F.B.; Isidoro, N.D.; Ferreira, G.M.; Ponce, M.C.F.; Ferreira, M.C.d.O.; Mendes, L.S.; Marcelino, P.H.R.; et al. Methodological and Ethical Considerations in the Use of Chordate Embryos in Biomedical Research. Int. J. Mol. Sci. 2025, 26, 2624. https://doi.org/10.3390/ijms26062624
Campitelli LMM, Lopes KP, de Lima IL, Ferreira FB, Isidoro ND, Ferreira GM, Ponce MCF, Ferreira MCdO, Mendes LS, Marcelino PHR, et al. Methodological and Ethical Considerations in the Use of Chordate Embryos in Biomedical Research. International Journal of Molecular Sciences. 2025; 26(6):2624. https://doi.org/10.3390/ijms26062624
Chicago/Turabian StyleCampitelli, Laura Maria Mendes, Karina Pereira Lopes, Isabela Lemos de Lima, Flávia Batista Ferreira, Nayara Delfim Isidoro, Giovana Magalhães Ferreira, Maria Clara Fioravanti Ponce, Milene Caroline de Oliveira Ferreira, Ludmilla Silva Mendes, Pedro Henrique Ribeiro Marcelino, and et al. 2025. "Methodological and Ethical Considerations in the Use of Chordate Embryos in Biomedical Research" International Journal of Molecular Sciences 26, no. 6: 2624. https://doi.org/10.3390/ijms26062624
APA StyleCampitelli, L. M. M., Lopes, K. P., de Lima, I. L., Ferreira, F. B., Isidoro, N. D., Ferreira, G. M., Ponce, M. C. F., Ferreira, M. C. d. O., Mendes, L. S., Marcelino, P. H. R., Neves, M. M., Klein, S. G., Fonseca, B. B., Polveiro, R. C., & da Silva, M. V. (2025). Methodological and Ethical Considerations in the Use of Chordate Embryos in Biomedical Research. International Journal of Molecular Sciences, 26(6), 2624. https://doi.org/10.3390/ijms26062624







