Evidence for Rab7b and Its Splice Isoforms Having Distinct Biological Functions from Rab7a
Abstract
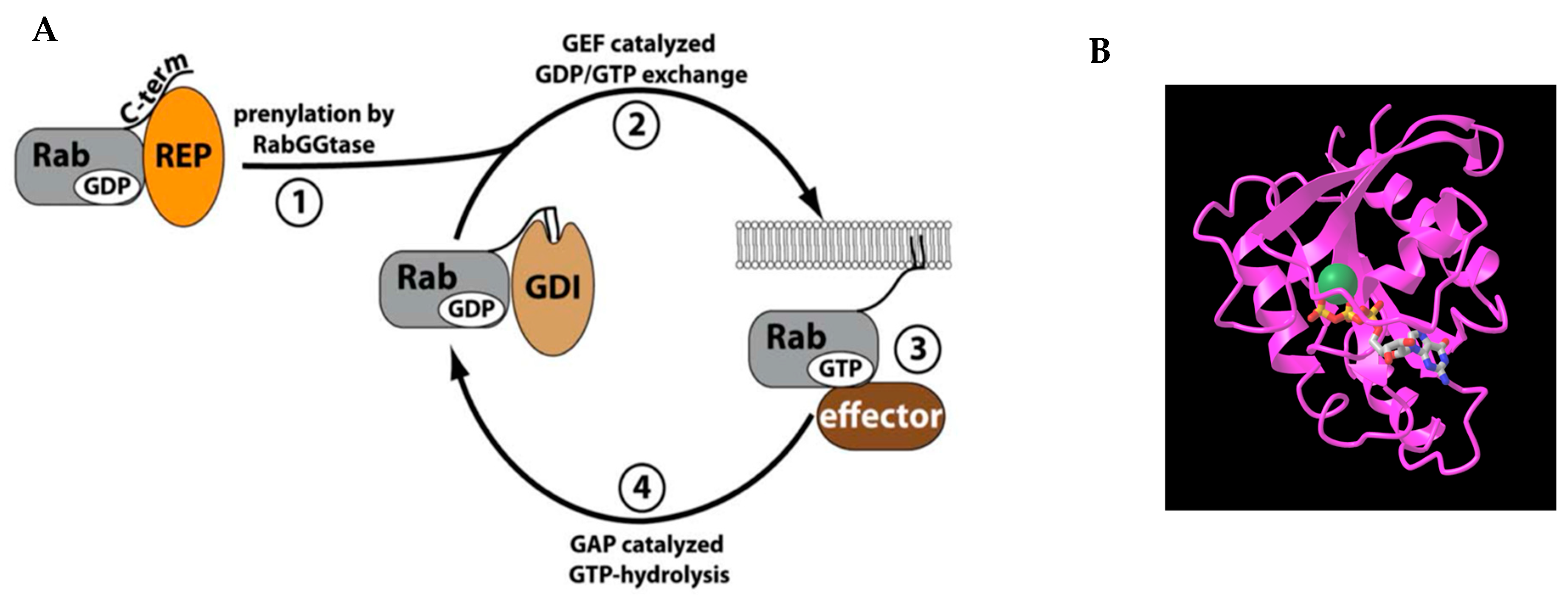
1. Introduction

2. Results
2.1. Clustal Alignment of Rab7a, Rab7b, and Its Splice Isoforms
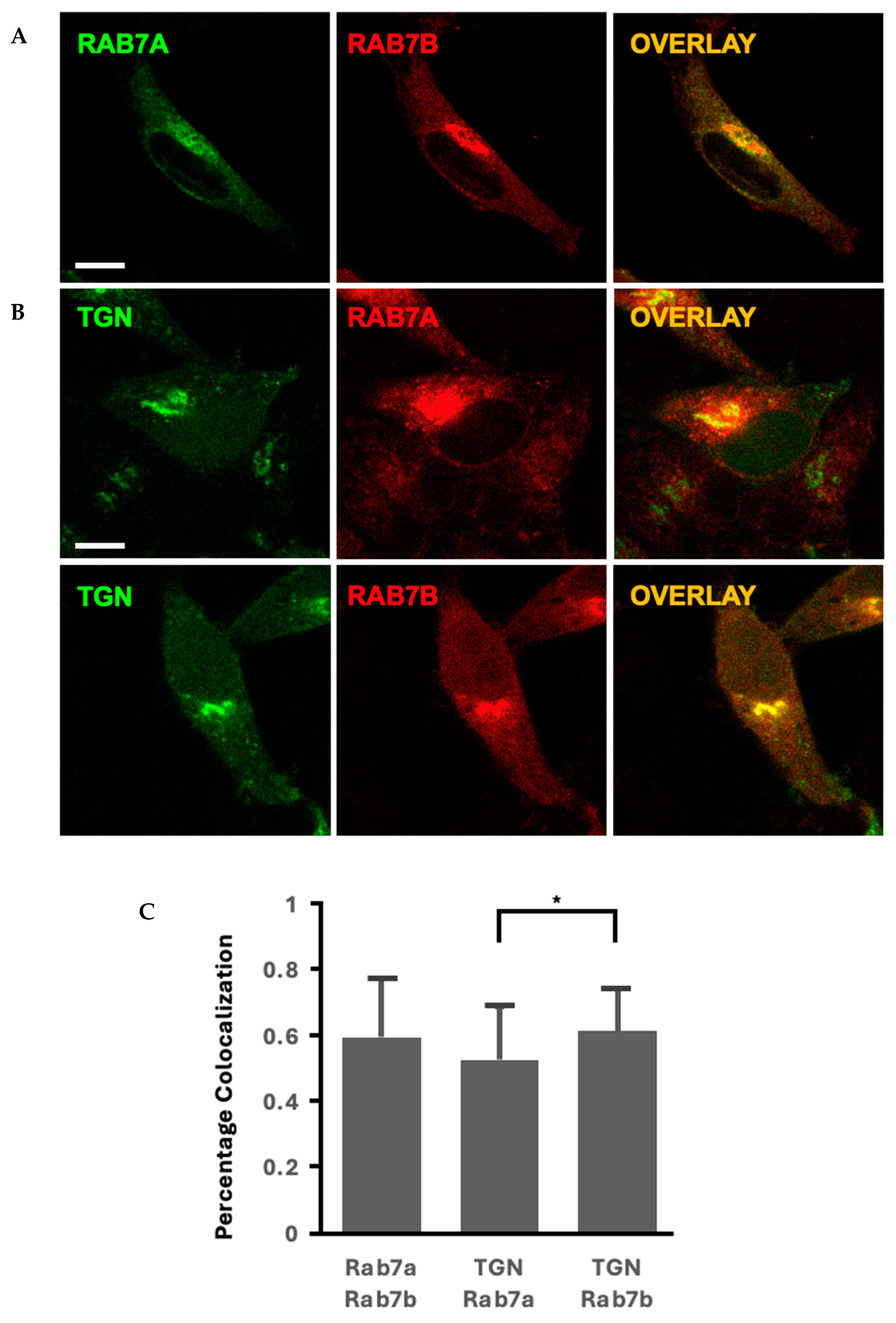
2.2. Rab7 Homologues Have Different Morphologies and Localisation
2.3. Subcellular Localization of Rab7a and Its Nucleotide Mutants in HeLa Cells
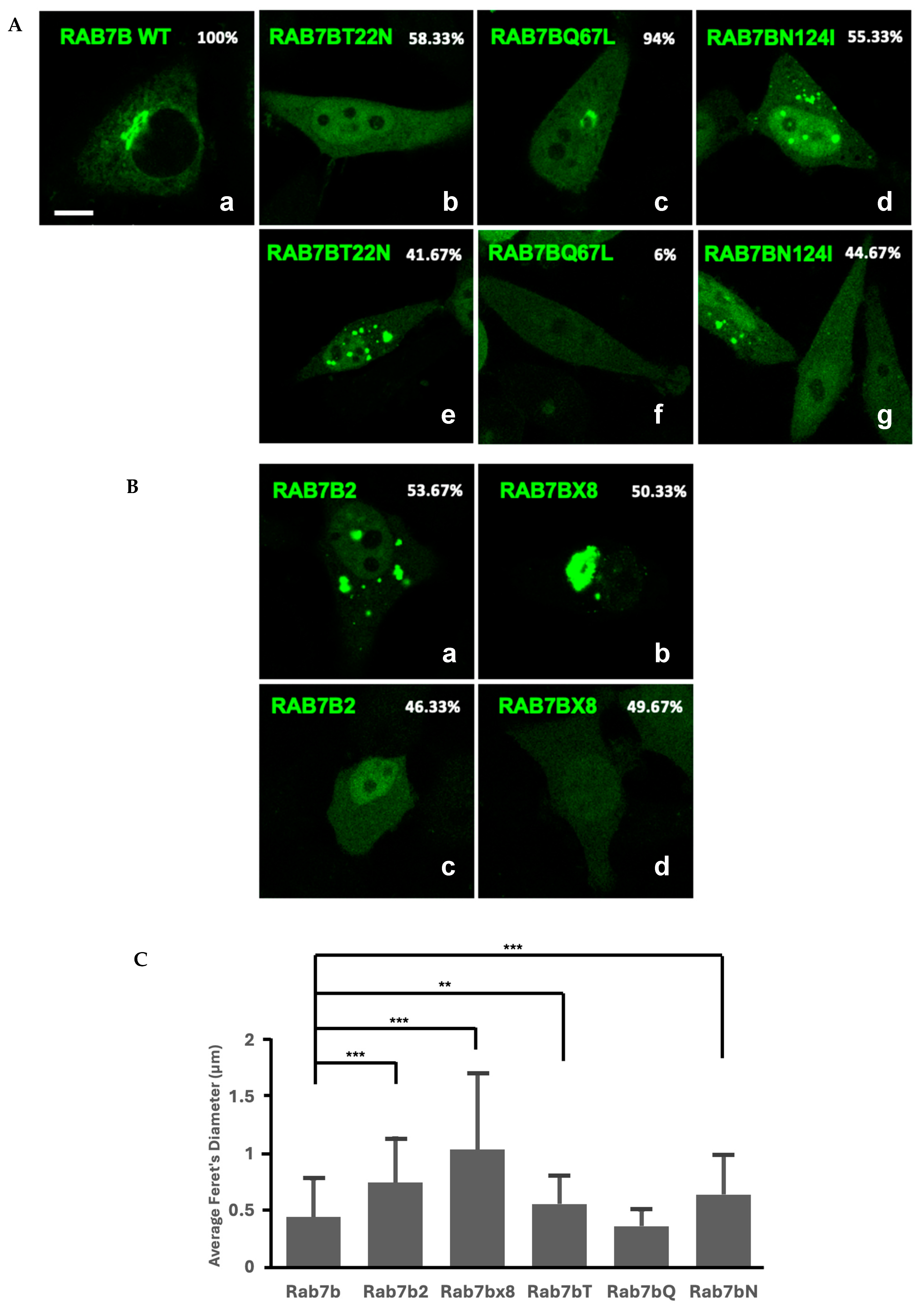
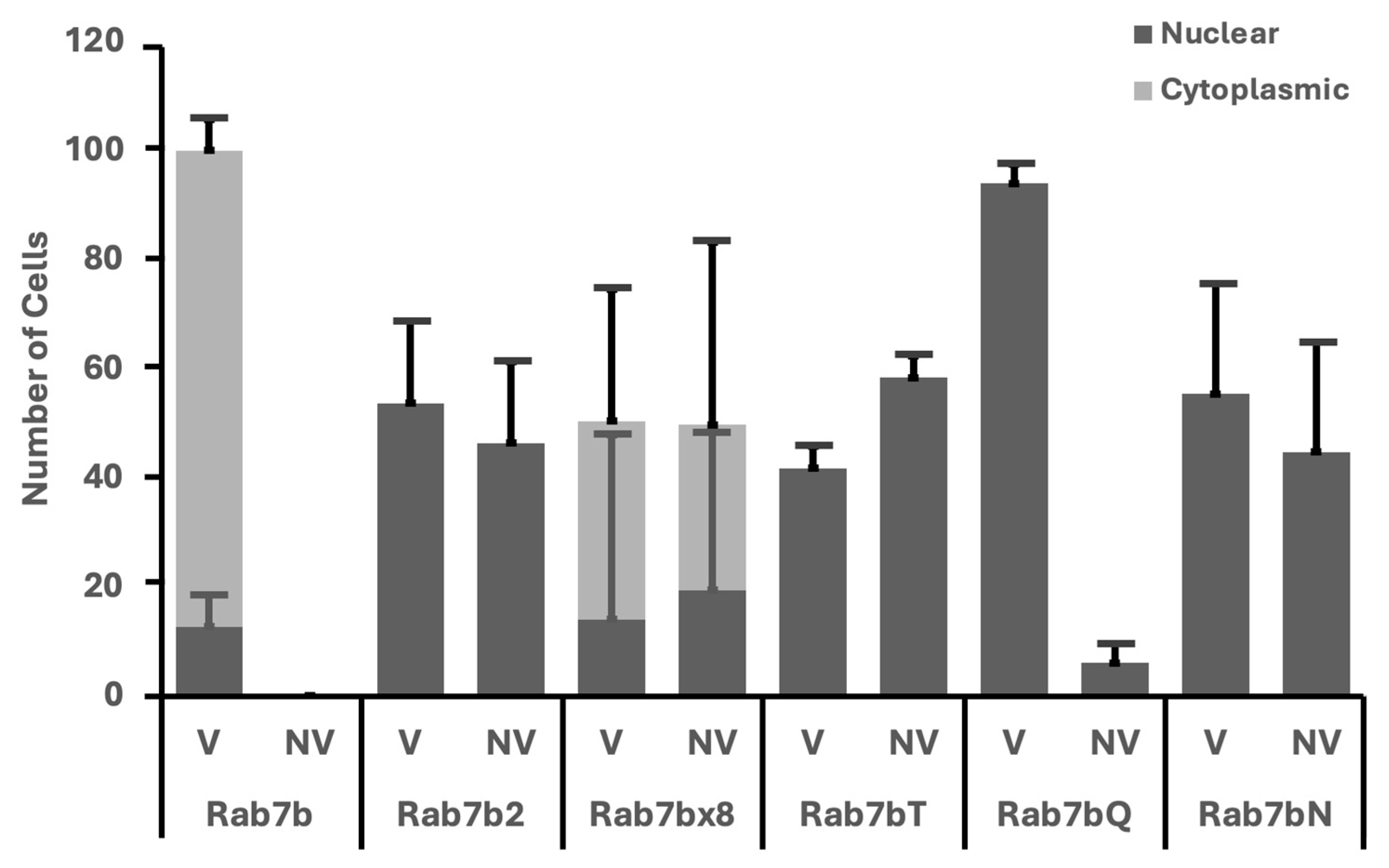
2.4. Subcellular Localization of Rab7b, Rab7b Nucleotide Mutants and Splice Isoforms in HeLa Cells
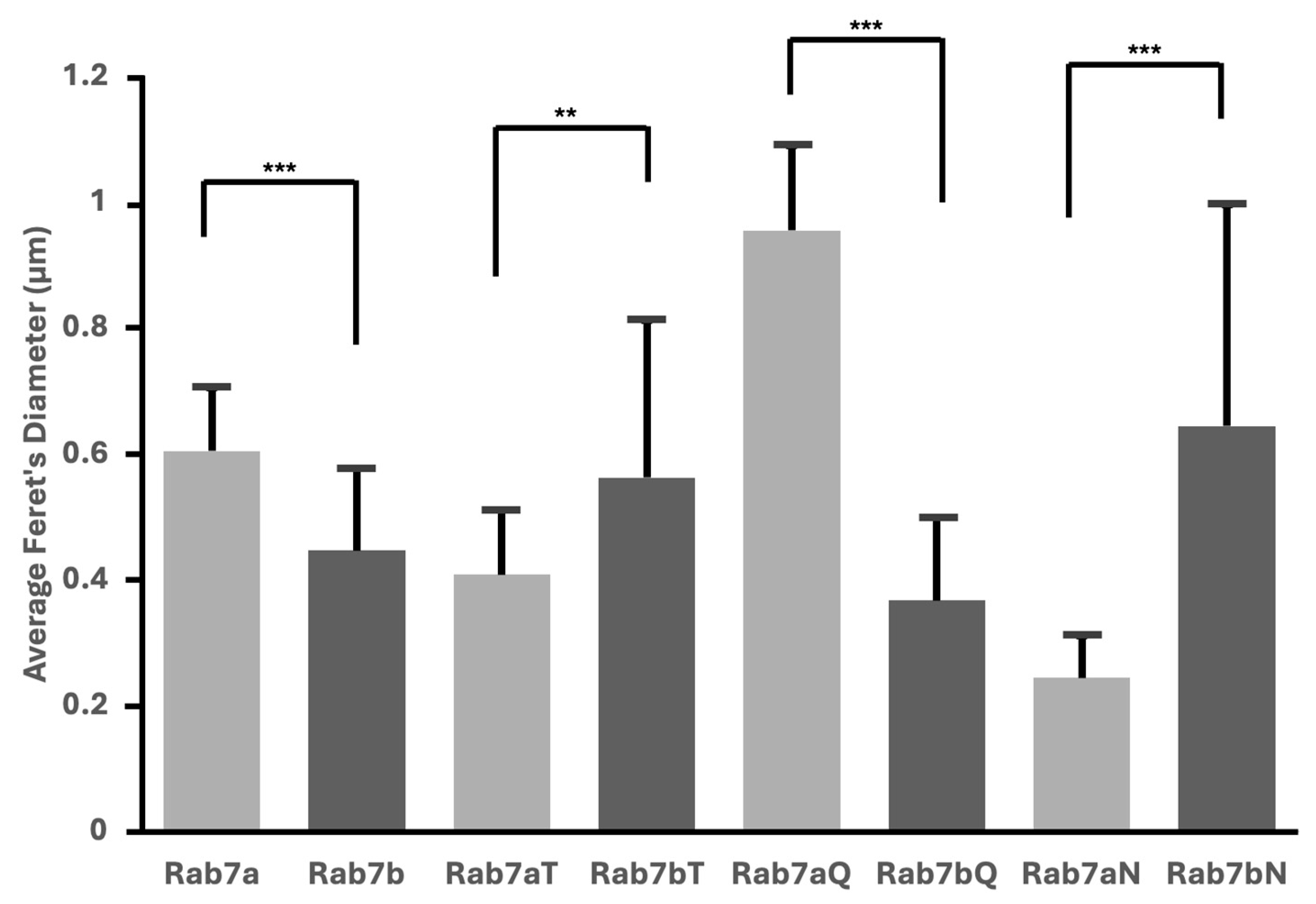
2.5. Nucleotide Mutants of Rab7a and Rab7b Cause Variations in Vesicular Size
2.6. Rab7a and Rab7b Nucleotide Mutants and Splice Isoforms Have Nuclear Vesicles
3. Discussion
3.1. Evidence That Rab7a and Rab7b Have Different Functions Based on Localization
3.2. Only Rab7b Is Able to Make Splice Isoforms
3.3. Presence of Vesicles and Nuclear Phenotypes in Rab7a and Rab7b Nucleotide Mutants
4. Materials and Methods
4.1. Plasmids
4.2. HeLa Cell Transfection
4.3. Confocal Microscopy
4.4. Quantitative and Statistical Analysis
4.5. Clustal Alignment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsumoto, N.; Sekiya, M.; Tohyama, K.; Ishiyama-Matsuura, E.; Sun-Wada, G.-H.; Wada, Y.; Futai, M.; Nakanishi-Matsui, M. Essential Role of the a3 Isoform of V-ATPase in Secretory Lysosome Trafficking via Rab7 Recruitment. Sci. Rep. 2018, 8, 6701. [Google Scholar] [CrossRef] [PubMed]
- Bucci, C.; Bakke, O.; Progida, C. Rab7b and receptors trafficking. Commun. Integr. Biol. 2010, 3, 401–404. [Google Scholar] [CrossRef]
- Li, G.; Marlin, M.C. Rab Family of GTPases; Methods in Molecular Biology; Springer Nature: Clifton, NJ, USA, 2015; Volume 1298, pp. 1–15. [Google Scholar]
- Oesterlin, L.K.; Pylypenko, O.; Goud, B. Effectors of Rab GTPases: Rab Binding Specificity and Their Role in Coordination of Rab Function and Localization. In Ras Superfamily Small G Proteins: Biology and Mechanisms 2 [Internet]; Wittinghofer, A., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 39–66. Available online: https://link.springer.com/10.1007/978-3-319-07761-1_3 (accessed on 30 January 2025).
- Pylypenko, O.; Hammich, H.; Yu, I.-M.; Houdusse, A. Rab GTPases and their interacting protein partners: Structural insights into Rab functional diversity. Small GTPases 2018, 9, 22–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Youkharibache, P.; Marchler-Bauer, A.; Lanczycki, C.; Zhang, D.; Lu, S.; Madej, T.; Marchler, G.H.; Cheng, T.; Chong, L.C.; et al. iCn3D: From Web-Based 3D Viewer to Structural Analysis Tool in Batch Mode. Front. Mol. Biosci. 2022, 9, 831740. [Google Scholar] [CrossRef]
- Parray, Z.A. A review on evolution, structural characteristics, interactions, and regulation of the membrane transport protein: The family of Rab proteins. Int. J. Biol. Macromol. 2025, 296, 139828. [Google Scholar] [CrossRef]
- Waschbüsch, D.; Khan, A.R. Phosphorylation of Rab GTPASES in the regulation of membrane trafficking. Traffic 2020, 21, 712–719. [Google Scholar] [CrossRef]
- Chavrier, P.; Gorvel, J.-P.; Stelzer, E.; Simons, K.; Gruenberg, J.; Zerial, M. Hypervariable C-termmal domain of rab proteins acts as a targeting signal. Nature 1991, 353, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Ungermann, C. Guanine nucleotide exchange factors (GEFs) have a critical but not exclusive role in organelle localization of Rab GTPases. J. Biol. Chem. 2013, 288, 28704–28712. [Google Scholar] [CrossRef]
- Grosshans, B.L.; Ortiz, D.; Novick, P. Rabs and their effectors: Achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA 2006, 103, 11821–11827. [Google Scholar] [CrossRef]
- Guerra, F.; Bucci, C. Multiple Roles of the Small GTPase Rab7. Cells 2016, 5, 34. [Google Scholar] [CrossRef]
- Liu, Q.; Bai, Y.; Shi, X.; Guo, D.; Wang, Y.; Wang, Y.; Guo, W.-Z.; Zhang, S. High RAS-related protein Rab-7a (RAB7A) expression is a poor prognostic factor in pancreatic adenocarcinoma. Sci. Rep. 2022, 12, 17492. [Google Scholar] [CrossRef]
- Borg Distefano, M.; Hofstad Haugen, L.; Wang, Y.; Perdreau-Dahl, H.; Kjos, I.; Jia, D.; Morth, J.P.; Neefjes, J.; Bakke, O.; Progida, C. TBC1D5 controls the GTPase cycle of Rab7b. J. Cell Sci. 2018, 131, jcs216630. [Google Scholar] [CrossRef]
- Yang, M.; Chen, T.; Han, C.; Li, N.; Wan, T.; Cao, X. Rab7b, a novel lysosome-associated small GTPase, is involved in monocytic differentiation of human acute promyelocytic leukemia cells. Biochem. Biophys. Res. Commun. 2004, 318, 792–799. [Google Scholar] [CrossRef]
- Cogli, L.; Progida, C.; Thomas, C.L.; Spencer-Dene, B.; Donno, C.; Schiavo, G.; Bucci, C. Charcot-Marie-Tooth type 2B disease-causing RAB7A mutant proteins show altered interaction with the neuronal intermediate filament peripherin. Acta Neuropathol. 2013, 125, 257–272. [Google Scholar] [CrossRef]
- Fukushima, N.; Shirai, R.; Sato, T.; Nakamura, S.; Ochiai, A.; Miyamoto, Y.; Yamauchi, J. Knockdown of Rab7B, But Not of Rab7A, Which Antagonistically Regulates Oligodendroglial Cell Morphological Differentiation, Recovers Tunicamycin-Induced Defective Differentiation in FBD-102b Cells. J. Mol. Neurosci. 2023, 73, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.; Bakke, O.; Progida, C. A novel interaction between Rab7b and actomyosin reveals a dual role in intracellular transport and cell migration. J. Cell Sci. 2014, 127, jcs.155861. [Google Scholar] [CrossRef] [PubMed]
- Kjos, I.; Borg Distefano, M.; Sætre, F.; Repnik, U.; Holland, P.; Jones, A.T.; Engedal, N.; Simonsen, A.; Bakke, O.; Progida, C. Rab7b modulates autophagic flux by interacting with Atg4B. EMBO Rep. 2017, 18, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Nassari, S.; Del Olmo, T.; Jean, S. Rabs in Signaling and Embryonic Development. Int. J. Mol. Sci. 2020, 21, 1064. [Google Scholar] [CrossRef]
- Kuchitsu, Y.; Fukuda, M. Revisiting Rab7 Functions in Mammalian Autophagy: Rab7 Knockout Studies. Cells 2018, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Lafalla Manzano, A.F.; Gil Lorenzo, A.F.; Bocanegra, V.; Costantino, V.V.; Cacciamani, V.; Benardon, M.E.; Vallés, P.G. Rab7b participation on the TLR4 (Toll-like receptor) endocytic pathway in Shiga toxin-associated Hemolytic Uremic Syndrome (HUS). Cytokine 2019, 121, 154732. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, N.; Miyamoto, Y.; Yamauchi, J. CRISPR/CasRx-Mediated Knockdown of Rab7B Restores Incomplete Cell Shape Induced by Pelizaeus-Merzbacher Disease-Associated PLP1 p.Ala243Val. Neurosci. Insights 2024, 19, 26331055241276873. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Press, B.; Wandinger-Ness, A. Rab 7: An important regulator of late endocytic membrane traffic. J. Cell Biol. 1995, 131, 1435–1452. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, B.P.; Bahr, S.J. rab7 Activity Affects Epidermal Growth Factor:Epidermal Growth Factor Receptor Degradation by Regulating Endocytic Trafficking from the Late Endosome. J. Biol. Chem. 2006, 281, 1099–1106. [Google Scholar] [CrossRef]
- Rodriguez-Furlan, C.; Borna, R.; Betz, O. RAB7 GTPases as coordinators of plant endomembrane traffic. Front. Plant Sci. 2023, 14, 1240973. [Google Scholar] [CrossRef]
- Gabe Lee, M.; Mishra, A.; Lambright, D.G. Structural Mechanisms for Regulation of Membrane Traffic by Rab GTPases. Traffic 2009, 10, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Stothard, P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. BioTechniques 2000, 28, 1102, 1104. [Google Scholar] [CrossRef]
- Progida, C.; Cogli, L.; Piro, F.; De Luca, A.; Bakke, O.; Bucci, C. Rab7b controls trafficking from endosomes to the TGN. J. Cell Sci. 2010, 123, 1480–1491. [Google Scholar] [CrossRef]
- Bucci, C.; Thomsen, P.; Nicoziani, P.; McCarthy, J.; Van Deurs, B. Rab7: A Key to Lysosome Biogenesis. Mol. Biol. Cell 2000, 11, 467–480. [Google Scholar] [CrossRef]
- Lu, L.; Hong, W. From endosomes to the trans-Golgi network. Semin. Cell Dev. Biol. 2014, 31, 30–39. [Google Scholar] [CrossRef]
- Progida, C.; Nielsen, M.S.; Koster, G.; Bucci, C.; Bakke, O. Dynamics of Rab7b-dependent transport of sorting receptors. Traffic 2012, 13, 1273–1285. [Google Scholar] [CrossRef]
- Deffieu, M.S.; Cesonyte, I.; Delalande, F.; Boncompain, G.; Dorobantu, C.; Song, E.; Lucansky, V.; Hirschler, A.; Cianferani, S.; Perez, F.; et al. Rab7-harboring vesicles are carriers of the transferrin receptor through the biosynthetic secretory pathway. Sci. Adv. 2021, 7, eaba7803. [Google Scholar] [CrossRef] [PubMed]
- Gambarte Tudela, J.; Buonfigli, J.; Luján, A.; Alonso Bivou, M.; Cebrián, I.; Capmany, A.; Damiani, M. Rab39a and Rab39b Display Different Intracellular Distribution and Function in Sphingolipids and Phospholipids Transport. Int. J. Mol. Sci. 2019, 20, 1688. [Google Scholar] [CrossRef]
- Lachance, V.; Angers, S.; Parent, J.-L. New insights in the regulation of Rab GTPases by G protein-coupled receptors. Small GTPases 2014, 5, e983872. [Google Scholar] [CrossRef]
- Pfeffer, S.R. Structural clues to Rab GTPase functional diversity. J. Biol. Chem. 2005, 280, 15485–15488. [Google Scholar] [CrossRef]
- Wittmann, J.G.; Rudolph, M.G. Crystal structure of Rab9 complexed to GDP reveals a dimer with an active conformation of switch II. FEBS Lett. 2004, 568, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, T.; Loh, E.; Hong, W.; Song, H. Structural basis for recruitment of RILP by small GTPase Rab7. EMBO J. 2005, 24, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Acosta, E.G.; Castilla, V.; Damonte, E.B. Differential requirements in endocytic trafficking for penetration of dengue virus. PLoS ONE 2012, 7, e44835. [Google Scholar] [CrossRef]
- Seaman, M.N.J.; Harbour, M.E.; Tattersall, D.; Read, E.; Bright, N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J. Cell Sci. 2009, 122, 2371–2382. [Google Scholar] [CrossRef]
- Elbaz-Alon, Y.; Guo, Y.; Segev, N.; Harel, M.; Quinnell, D.E.; Geiger, T.; Avinoam, O.; Li, D.; Nunnari, J. PDZD8 interacts with Protrudin and Rab7 at ER-late endosome membrane contact sites associated with mitochondria. Nat. Commun. 2020, 11, 3645. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Han, C.; He, D.; Liu, H.; An, H.; Cai, Z.; Cao, X. Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood 2007, 110, 962–971. [Google Scholar] [CrossRef]
- Gutierrez, M.G.; Munafó, D.B.; Berón, W.; Colombo, M.I. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J. Cell Sci. 2004, 117, 2687–2697. [Google Scholar] [CrossRef] [PubMed]
- Maltese, W.A.; Soule, G.; Gunning, W.; Calomeni, E.; Alexander, B. Mutant Rab24 GTPase is targeted to nuclear inclusions. BMC Cell Biol. 2002, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Qi, Y.-X.; Zhang, P.; Gu, W.-T.; Yan, Z.-Q.; Shen, B.-R.; Yao, Q.-P.; Kong, H.; Chien, S.; Jiang, Z.-L. Involvement of Rab28 in NF-κB nuclear transport in endothelial cells. PLoS ONE 2013, 8, e56076. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Bucci, C. Role of the RAB7 Protein in Tumor Progression and Cisplatin Chemoresistance. Cancers 2019, 11, 1096. [Google Scholar] [CrossRef]
- Bautista, S.J.; Boras, I.; Vissa, A.; Mecica, N.; Yip, C.M.; Kim, P.K.; Antonescu, C.N. mTOR complex 1 controls the nuclear localization and function of glycogen synthase kinase 3β. J. Biol. Chem. 2018, 293, 14723–14739. [Google Scholar] [CrossRef]
- BasuRay, S.; Mukherjee, S.; Romero, E.; Wilson, M.C.; Wandinger-Ness, A. Rab7 mutants associated with Charcot-Marie-Tooth disease exhibit enhanced NGF-stimulated signaling. PLoS ONE 2010, 5, e15351. [Google Scholar] [CrossRef]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, W.H.; Liu, S.Z.; Li, A.S.R.; Liu, X.; Manolson, M.F.; Zirngibl, R.A. Evidence for Rab7b and Its Splice Isoforms Having Distinct Biological Functions from Rab7a. Int. J. Mol. Sci. 2025, 26, 2610. https://doi.org/10.3390/ijms26062610
Wong WH, Liu SZ, Li ASR, Liu X, Manolson MF, Zirngibl RA. Evidence for Rab7b and Its Splice Isoforms Having Distinct Biological Functions from Rab7a. International Journal of Molecular Sciences. 2025; 26(6):2610. https://doi.org/10.3390/ijms26062610
Chicago/Turabian StyleWong, Wing Hei, Stephanie Z. Liu, Annie Shi Ru Li, Xingyou Liu, Morris F. Manolson, and Ralph A. Zirngibl. 2025. "Evidence for Rab7b and Its Splice Isoforms Having Distinct Biological Functions from Rab7a" International Journal of Molecular Sciences 26, no. 6: 2610. https://doi.org/10.3390/ijms26062610
APA StyleWong, W. H., Liu, S. Z., Li, A. S. R., Liu, X., Manolson, M. F., & Zirngibl, R. A. (2025). Evidence for Rab7b and Its Splice Isoforms Having Distinct Biological Functions from Rab7a. International Journal of Molecular Sciences, 26(6), 2610. https://doi.org/10.3390/ijms26062610





