Phenotype Assessment and Putative Mechanisms of Ammonium Toxicity to Plants
Abstract
1. Introduction
2. Comprehensive Evaluation System of Symptoms in Ammonium Toxicity
3. Putative Mechanisms of Ammonium Toxicity
3.1. Alleviation of Ammonium Toxicity in NO3−-Dependent Pathway
3.2. High NH4+-Induced ROS Accumulation
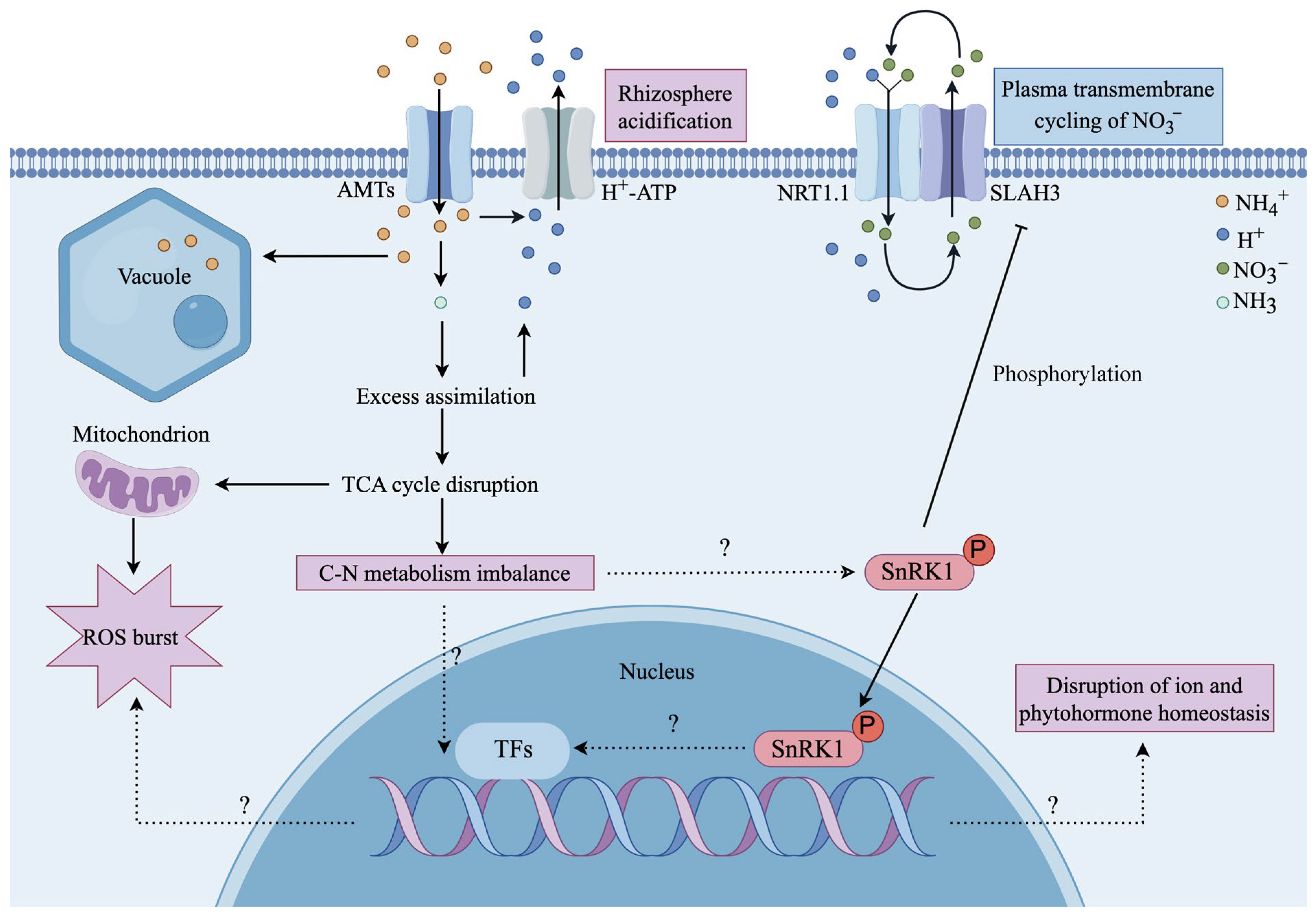
3.3. High Ammonium-Induced Ion Imbalance
3.4. High NH4+ Induced Disruption of Phytohormone Homeostasis
3.5. Rhizosphere Acidification
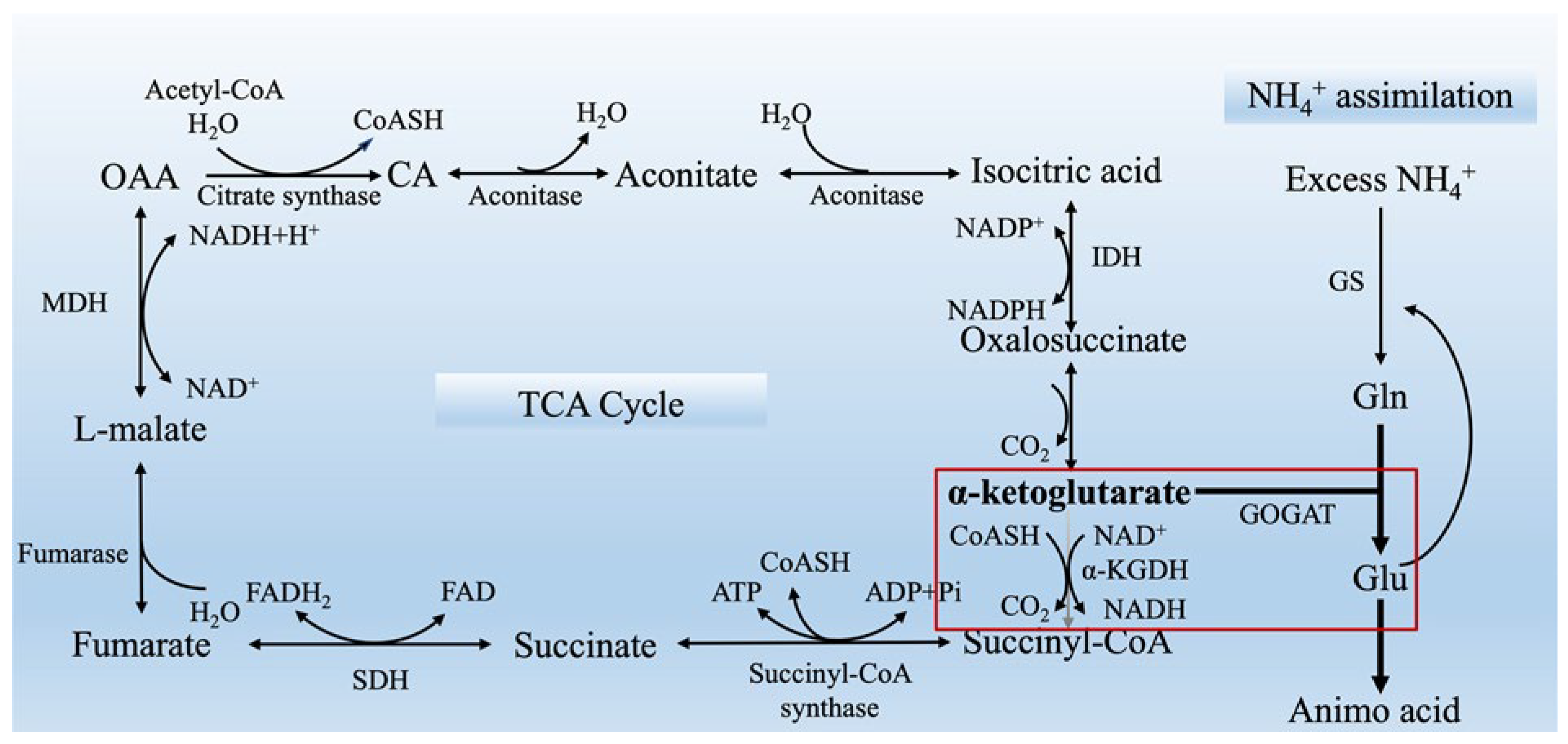
3.6. High NH4+ Induces Imbalance in C-N Metabolism
| Regulator | Pathways/Mechanisms | Species | Reference |
|---|---|---|---|
| NO3− | Nitrate interacts with NRT1.1 to promote NO3− cycling across the membrane. | Arabidopsis | [22] |
| Nitrate inhibits acidification and promotes NH4+ assimilation. | oilseed rape | [43] | |
| Nitrate modulates phytohormone pathways. | wheat | [40] | |
| ROS | ROS scavengers reduce ROS deposition in the phloem. | Arabidopsis | [24] |
| VB6 reduces H2O2 accumulation upon ammonium toxicity. | Arabidopsis | [47] | |
| Heme oxygenase OsSE5boosts the activities of ROS-scavenging enzymes. | rice | [48] | |
| Ammonium toxicity inhibits photosystems and electron transfer, thus inducing ROS accumulation. | oilseed rape | [35] | |
| Iron | The cell wall-localized ferroxidase LPR2leads to Fe and callose deposition in the phloem. | Arabidopsis | [24] |
| High NH4+-induced iron accumulation triggers excess NH4+ efflux. | Arabidopsis, lettuce | [16] | |
| The myb28,myb29 double mutant shows altered Fe accumulation and is highly hypersensitive to ammonium nutrition. | Arabidopsis | [52] | |
| Potassium | K+ competitively inhibits the uptake and accumulation of NH4+ and optimizes NH4+ metabolism. | rice | [55] |
| K+ leads to higher carbon and energy availability and improves ion homeostasis. | pea | [63] | |
| K+ supply reduces futile NH4+ cycling at the plasma membrane. | rice | [62] | |
| Increase in K+ concentration can effectively alleviate ammonium toxicity. | Arabidopsis, rice, barley | [55,56,57,58,59,60,61] | |
| Phytohormone | Ammonium toxicity decreases free IAA content in roots by inhibiting the transcription of auxin-biosynthesis genes. | rice | [64] |
| WRKY46promotes ammonium tolerance by repressing IAA-conjugating genes. | Arabidopsis | [25] | |
| High NH4+ disturbs the subcellular IAA homeostasis by upregulating the expression of PIN5. | Arabidopsis | [26] | |
| Ammonium toxicity repressing BR signaling, thus inhibiting auxin response and transport. | Arabidopsis | [65] | |
| Plants over-expressing EIN3 (a key regulator of ethylene responses) are more sensitive to NH4+toxicity. | Arabidopsis | [29] | |
| ABA signaling is required for the regulation of expression of NH4+-responsive genes. | Arabidopsis | [30] | |
| Rhizosphere pH and the TCA cycle | AMTs enhance the activity of AMT-coupled H+-ATPases to exude H+ from the root cells. | Arabidopsis, rice | [7,66,67] |
| Medium buffer MES and N,N′-dicyclohexylcarbodiimide elevate medium pH and inhibit H+-ATPase activity. | Arabidopsis | [22,36] | |
| α-ketoglutarate and 2-oxaloacetate furnish components for the GS/GOGAT cycle to promote NH4+ assimilation, thus preventing NH4+ toxicity. | Arabidopsis, Lycopersicon esculentum, Myriophyllum aquaticum | [10,68,69] | |
| Application of an alkaline solution efficiently alleviates ammonium toxicity with a concomitant reduction in shoot acidity. | Arabidopsis | [21] | |
| GABA limits NH4+ accumulation, inhibits increases in GS/NADH-GOGAT activity and reduces rhizosphere acidification caused by excessive NH4+. | rice | [19] | |
| Through synergistic activation of the NRT1.1-SLAH3 complex, efficient transmembrane cycling of NO3− is induced, effectively inhibiting rhizosphere acidification. | Arabidopsis | [36] | |
| C-N metabolism | Upon ammonium toxicity, increased activities of invertase, sucrose synthase, and trehalose 6-phosphate synthase leads to enhanced glycolysis and a significant energy expenditure. | rice | [73] |
| Insufficient sucrose distribution caused by impaired phloem function under high ammonium stress is the reason for the inhibition of root elongation. | Arabidopsis | [24] | |
| SnRK1.1 works upstream of SLAH3 to regulate hypocotyl growth during skotomorphogenesis in response to changes in sugar levels induced by ammonium toxicity. | Arabidopsis | [22] |
3.7. High NH4+ May Change Rhizosphere Microorganisms
4. Perspectives on Future Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xiao, C.; Fang, Y.; Wang, S.; He, K. The alleviation of ammonium toxicity in plants. J. Integr. Plant Biol. 2023, 65, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Fan, X.; Miller, A. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, B.; Chu, C.C. Nitrogen use efficiency in crops: Lessons from Arabidopsis and rice. J. Exp. Bot. 2017, 68, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, U.; Neuhäuser, B.; Dynowski, M. Molecular mechanisms of ammonium transport and accumulation in plants. FEBS Lett. 2007, 581, 2301–2308. [Google Scholar] [CrossRef]
- Situmorang, A. Elucidation of the Ammonium Major Facilitator (AMF) Family in Plants. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, 2018. [Google Scholar]
- Xu, F.; Yu, F. Sensing and regulation of plant extracellular pH. Trends Plant Sci. 2023, 28, 1422–1437. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef]
- Giordano, M.; Goodman, C.A.; Huang, F.; Raven, J.A.; Ruan, Z. A mechanistic study of the influence of nitrogen and energy availability on the NH4+ sensitivity of nitrogen assimilation in Synechococcus. J. Exp. Bot. 2022, 73, 5596–5611. [Google Scholar] [CrossRef]
- Kaviraj, M.; Kumar, U.; Snigdha, A.; Chatterjee, S. Nitrate reduction to ammonium: A phylogenetic, physiological, and genetic aspects in Prokaryotes and eukaryotes. Arch. Microbiol. 2024, 206, 297. [Google Scholar] [CrossRef]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Wang, J.; Tu, X.S.; Zhang, H.M.; Cui, J.Y.; Ni, K.; Chen, J.L.; Cheng, Y.; Zhang, J.B.; Chang, S.X. Effects of ammonium-based nitrogen addition on soil nitrification and nitrogen gas emissions depend on fertilizer-induced changes in pH in a tea plantation soil. Sci. Total Environ. 2020, 747, 141340. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.G.; Ge, H.M. Effects of nitrogen fertilizer of different forms and ratios on the growth, nitrogen absorption and utilization of young navel orange trees grafted on Poncirustrifoliata. Sci. Agr. Sin. 2015, 48, 2666–2675. [Google Scholar]
- Yang, M.; Long, Q.; Li, W.L.; Wang, Z.C.; He, X.H.; Wang, J.; Wang, X.Z.; Xiong, H.Y.; Guo, C.Y.; Zhang, G.C.; et al. Mapping the environmental cost of a typical Citrus-producing County in China: Hotspot and optimization. Sustainability 2020, 12, 1827. [Google Scholar] [CrossRef]
- Li, B.H.; Li, G.J.; Kronzucker, H.J.; Baluška, F.; Shi, W.M. Ammonium stress in Arabidopsis: Signaling, genetic loci, and physiological targets. Trends Plant Sci. 2014, 19, 107–114. [Google Scholar] [CrossRef]
- Li, G.J.; Zhang, L.; Wu, J.L.; Wang, Z.Y.; Wang, M.; Kronzucker, H.J.; Shi, W.M. Plant iron status regulates ammonium-use efficiency through protein N-glycosylation. Plant Physiol. 2024, 195, 1712–1727. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; p. 1535. [Google Scholar]
- Rubio-Asensio, J.S.; Bloom, A.J. Inorganic nitrogen form: A major player in wheat and Arabidopsis responses to elevated CO2. J. Exp. Bot. 2017, 68, 2611–2625. [Google Scholar] [CrossRef]
- Ma, X.L.; Zhu, C.H.; Yang, N.; Gan, L.J.; Xia, K. γ-Aminobutyric acid addition alleviates ammonium toxicity by limiting ammonium accumulation in rice (Oryza sativa) seedlings. Physiol. Plant. 2016, 158, 389–401. [Google Scholar] [CrossRef]
- Liu, Y.; von Wirén, N. Ammonium as a signal for physiological and morphological responses in plants. J. Exp. Bot. 2017, 68, 3777–3788. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, T.; Inaba, J.; Wakazaki, M.; Sato, M.; Toyooka, K.; Miyagi, A.; Kawai-Yamada, M.; Sugiura, D.; Nakagawa, T.; Kiba, T.; et al. Excessive ammonium assimilation by plastidic glutamine synthetase causes ammonium toxicity in Arabidopsis thaliana. Nat. Commun. 2021, 12, 4944. [Google Scholar] [CrossRef]
- Sun, D.; Fang, X.; Xiao, C.Z.M.; Huang, X.; Su, J.; Li, J.; Wang, J.; Wang, S.; Luan, S.; He, K. Kinase SnRK1.1 regulates nitrate channel SLAH3 engaged in nitrate-dependent alleviation of ammonium toxicity. Plant Physiol. 2021, 186, 731–749. [Google Scholar] [CrossRef]
- Zhang, L.; Song, H.; Li, B.H.; Wang, M.; Di, D.W.; Lin, X.Y.; Kronzucker, H.J.; Shi, W.M.; Li, G.J. Induction of S-nitrosoglutathione reductase protects root growth from ammonium toxicity by regulating potassium homeostasis in Arabidopsis and rice. J. Exp. Bot. 2021, 72, 4548–4564. [Google Scholar] [CrossRef]
- Liu, X.X.; Zhang, H.H.; Zhu, Q.Y.; Ye, J.Y.; Zhu, Y.X.; Jing, X.T.; Du, W.X.; Zhou, M.; Lin, X.Y.; Zheng, S.J.; et al. Phloem iron remodels root development in response to ammonium as the major nitrogen source. Nat. Commun. 2022, 13, 561. [Google Scholar] [CrossRef] [PubMed]
- Di, D.W.; Sun, L.; Wang, M.; Wu, J.J.; Kronzucker, H.J.; Fang, S.; Chu, J.F.; Shi, W.M.; Li, G.J. WRKY46 promotes ammonium tolerance in Arabidopsis by repressing NUDX9 and indole-3-acetic acid-conjugating genes and by inhibiting ammonium efflux in the root elongation zone. New Phytol. 2021, 232, 190–207. [Google Scholar] [CrossRef]
- Di, D.W.; Wu, J.J.; Ma, M.K.; Li, G.J.; Wang, M.; Kronzucker, H.J.; Shi, W.M. PIN5 is involved in regulating NH4+ efflux and primary root growth under high-ammonium stress via mediating intracellular auxin transport. Plant Soil 2023, 505, 25–40. [Google Scholar] [CrossRef]
- Li, B.H.; Li, Q.; Kronzucker, H.J.; Shi, W.M. Roles of abscisic acid and auxin in shoot-supplied ammonium inhibition of root system development. Plant Signal. Behav. 2011, 6, 1451–1453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, B.H.; Li, Q.; Su, Y.; Chen, H.; Xiong, L.M.; Mi, G.H.; Kronzucker, H.J.; Shi, W.M. Shoot-supplied ammonium targets the root auxin influx carrier AUX1 and inhibits lateral root emergence in Arabidopsis. Plant Cell Environ. 2011, 34, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Li, G.J.; Zhang, L.; Wang, M.; Di, D.W.; Kronzucker, H.J.; Shi, W.M. The Arabidopsis AMOT1/EIN3 gene plays an important role in the amelioration of ammonium toxicity. J. Exp. Bot. 2019, 70, 1375–1388. [Google Scholar] [CrossRef]
- Li, B.H.; Li, Q.; Xiong, L.M.; Kronzucker, H.J.; Krämer, U.; Shi, W.M. Arabidopsis plastid AMOS1/EGY1 integrates abscisic acid signaling to regulate global gene expression response to ammonium stress. Plant Physiol. 2012, 160, 2040–2051. [Google Scholar] [CrossRef]
- Jadid, N.; Mialoundama, A.S.; Heintz, D.; Ayoub, D.; Erhardt, M.; Mutterer, J.; Meyer, D.; Alioua, A.; Dorsselaer, A.V.; Rahier, A.; et al. Dolichol Phosphate Mannose Synthase1 mediates the biogenesis of isoprenyl-linked glycans and influences development, stress response, and ammonium hypersensitivity in Arabidopsis. Plant Cell 2011, 23, 1985–2005. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.H.; Kronzucker, H.J.; Shi, W.M. Root growth inhibition by NH4+ in Arabidopsis is mediated by the root tip and is linked to NH4+ efflux and GMPase activity. Plant Cell Environ. 2010, 33, 1529–1542. [Google Scholar] [CrossRef]
- Sarasketa, A.; González-Moro, M.B.; González-Murua, C.; Marino, D. Nitrogen source and external medium pH interaction differentially affects root and shoot metabolism in Arabidopsis. Front. Plant Sci. 2016, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; He, K.; Kleist, T.; Chen, F.; Luan, S. Anion channel SLAH3 functions in nitrate-dependent alleviation of ammonium toxicity in Arabidopsis. Plant Cell Environ. 2015, 38, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, L.; Riaz, M.; White, P.J.; Yi, C.; Wang, S.L.; Shi, L.; Xu, F.S.; Wang, C.; Cai, H.M.; et al. Integrated transcriptome and metabolome analysis reveals the physiological and molecular responses of allotetraploid rapeseed to ammonium toxicity. Environ. Exp. Bot. 2021, 189, 104550. [Google Scholar] [CrossRef]
- Xiao, C.; Sun, D.; Liu, B.; Fang, X.; Li, P.; Jiang, Y.; He, M.; Li, J.; Luan, S.; He, K. Nitrate transporter NRT1.1 and anion channel SLAH3 form a functional unit to regulate nitrate-dependent alleviation of ammonium toxicity. J. Integr. Plant Biol. 2022, 64, 942–957. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, Z.W.; Zheng, C.; Zhao, Z.Y.; Wang, Y.; Feng, L.Y.; Niu, G.; Wang, C.Q.; Wang, J.H.; Feng, H.; et al. Arabidopsis cryptochrome 1 functions in nitrogen regulation of flowering. Proc. Natl. Acad. Sci. USA 2016, 113, 7661–7666. [Google Scholar] [CrossRef]
- de Graaf, M.C.C.; Bobbink, R.; Verbeek, P.J.M.; Roelofs, J.G.M. Differential effects of ammonium and nitrate on three heathland species. Plant Ecol. 1998, 135, 185–196. [Google Scholar] [CrossRef]
- Roosta, H.R.; Schjoerring, J.K. Effects of nitrate and potassium on ammonium toxicity in cucumber plants. J. Plant Nutr. 2008, 31, 1270–1283. [Google Scholar] [CrossRef]
- Garnica, M.; Houdusse, F.; Zamarreno, A.; Garcia-Mina, J. The signal effect of nitrate supply enhances active forms of cytokinins and indole acetic content and reduces abscisic acid in wheat plants grown with ammonium. J. Plant Physiol. 2010, 167, 1264–1272. [Google Scholar] [CrossRef]
- Hachiya, T.; Watanabe, C.; Fujimoto, M.; Ishikawa, T.; Takahara, K.; Kawai-Yamada, M.; Uchimiya, H.; Uesono, Y.; Terashima, I.; Noguchi, K. Nitrate addition alleviates ammonium toxicity without lessening ammonium accumulation, organic acid depletion and inorganic cation depletion in Arabidopsis thaliana shoots. Plant Cell Physiol. 2012, 53, 577–591. [Google Scholar] [CrossRef]
- Du, W.; Zhang, Y.; Si, J.; Zhang, Y.; Fan, S.; Xia, H.; Kong, L. Nitrate alleviates ammonium toxicity in wheat (Triticum aestivum L.) by regulating tricarboxylic acid cycle and reducing rhizospheric acidification and oxidative damage. Plant Signal. Behav. 2021, 16, 1991687. [Google Scholar] [CrossRef]
- Li, S.; Yan, L.; Zhang, W.; Yi, C.; Haider, S.; Wang, C.; Liu, Y.; Shi, L.; Xu, F.; Ding, G. Nitrate alleviates ammonium toxicity in Brassica napus by coordinating rhizosphere and cell pH and ammonium assimilation. Plant J. 2024, 117, 786–804. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qi, B.; Hao, Y.; Liu, H.; Sun, G.; Chen, R.; Song, S. Appropriate NH4+/NO3− ratio triggers plant growth and nutrient uptake of flowering chinese cabbage by optimizing the pH value of nutrient solution. Front. Plant Sci. 2021, 12, 656144. [Google Scholar] [CrossRef]
- Coleto, I.; Marín-Peña, A.J.; Urbano-Gámez, J.A.; González-Hernández, A.I.; Shi, W.; Li, G.; Marino, D. Interaction of ammonium nutrition with essential mineral cations. J. Exp. Bot. 2023, 74, 6131–6144. [Google Scholar] [CrossRef]
- Shen, Z.; Fan, L.; Yang, S.; Yao, Y.; Chen, H.; Wang, W. Fe-based carbonitride as Fenton-like catalyst for the elimination of organic contaminants. Environ. Res. 2021, 198, 110486. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Maniero, R.; Giehl, R.; Melzer, M.; Steensma, P.; Krouk, G.; Fitzpatrick, T.; von Wiren, N. PDX1.1-dependent biosynthesis of vitamin B6 protects roots from ammonium-induced oxidative stress. Mol. Plant. 2022, 15, 820–839. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhou, H.; Zhang, J.; Guan, W.; Xu, S.; Shen, W.; Xu, G.; Xie, Y.; Foyer, C. L-cysteine desulfhydrase-related H2S production is involved in OsSE5-promoted ammonium tolerance in roots of Oryza sativa. Plant Cell Environ. 2017, 40, 1777–1790. [Google Scholar] [CrossRef]
- Kong, L.G.; Zhang, Y.X.; Zhang, B.; Li, H.W.; Wang, Z.S.; Si, J.S.; Fan, S.J.; Feng, B. Does energy cost constitute the primary cause of ammonium toxicity in plants? Planta 2022, 256, 62. [Google Scholar] [CrossRef]
- Maiber, L.; Koprivova, A.; Bender, D.; Kopriva, S.; Fischer-Schrader, K. Characterization of the amidoxime reducing components ARC1 and ARC2 from Arabidopsis thaliana. FEBS J. 2022, 289, 5656–5669. [Google Scholar] [CrossRef]
- Tejada-Jimenez, M.; Leon-Miranda, E.; Llamas, A. Chlamydomonas reinhardtii—A reference microorganism for eukaryotic molybdenum metabolism. Microorganisms 2023, 11, 1671. [Google Scholar] [CrossRef]
- Coleto, I.; Bejarano, I.; Marin-Pena, A.J.; Medina, J.; Rioja, C.; Burow, M.; Marino, D. Arabidopsis thaliana transcription factors MYB28 and MYB29 shape ammonium stress responses by regulating Fe homeostasis. New Phytol. 2021, 229, 1021–1035. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Regulation of potassium transport and signaling in plants. Curr. Opin. Plant Biol. 2017, 39, 123–128. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture-Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Szczerba, M.W.; Britto, D.T.; Ali, S.A.; Balkos, K.D.; Kronzucker, H.J. NH4+-stimulated and -inhibited components of K+ transport in rice (Oryza sativa L.). J. Exp. Bot. 2008, 59, 3415–3423. [Google Scholar] [CrossRef]
- Kabange, N.R.; Park, S.Y.; Lee, J.Y.; Shin, D.; Lee, S.M.; Kwon, Y.; Cha, J.K.; Cho, J.H.; Duyen, D.V.; Ko, J.M.; et al. New insights into the transcriptional regulation of genes involved in the nitrogen use efficiency under potassium chlorate in rice (Oryza sativa L.). Int. J. Mol. Sci. 2021, 22, 2192. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Mi, T.W.; Gao, Y.Q.; Feng, H.Q.; Wu, W.H.; Wang, Y. STOP1 regulates LKS1 transcription and coordinates K+/NH4+ balance in Arabidopsis response to low-K+ stress. Int. J. Mol. Sci. 2021, 23, 383. [Google Scholar] [CrossRef] [PubMed]
- Aluko, O.O.; Li, C.; Yuan, G.; Nong, T.; Xiang, H.; Wang, Q.; Li, X.; Liu, H. Differential effects of ammonium (NH4+) and potassium (K+) nutrition on photoassimilate partitioning and growth of tobacco seedlings. Plants 2022, 11, 3295. [Google Scholar] [CrossRef]
- Liu, J.; Xia, H.; Gao, Y.; Pan, D.; Sun, J.; Liu, M.; Tang, Z.; Li, Z. Potassium deficiency causes more nitrate nitrogen to be stored in leaves for low-K sensitive sweet potato genotypes. Front. Plant Sci. 2022, 13, 1069181. [Google Scholar] [CrossRef]
- Li, C.; Aluko, O.O.; Shi, S.; Mo, Z.; Nong, T.; Shi, C.; Li, Z.; Wang, Q.; Liu, H. Determination of optimal NH4+/K+ concentration and corresponding ratio critical for growth of tobacco seedlings in a hydroponic system. Front. Plant Sci. 2023, 14, 1152817. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, S.; Singh, A.P. Review: Nutrient-nutrient interactions governing underground plant adaptation strategies in a heterogeneous environment. Plant Sci. 2024, 342, 112024. [Google Scholar] [CrossRef]
- Balkos, K.D.; Britto, D.T.; Kronzucker, H.J. Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72). Plant Cell Environ. 2010, 33, 23–34. [Google Scholar]
- Ariz, I.; Artola, E.; Asensio, A.C.; Cruchaga, S.; Aparicio-Tejo, P.M.; Moran, J.F. High irradiance increases NH4+ tolerance in Pisum sativum: Higher carbon and energy availability improve ion balance but not N assimilation. J. Plant Physiol. 2011, 168, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Di, D.W.; Sun, L.; Zhang, X.N.; Li, G.J.; Kronzucker, H.J.; Shi, W.M. Involvement of auxin in the regulation of ammonium tolerance in rice (Oryza sativa L.). Plant Soil. 2018, 432, 373–387. [Google Scholar] [CrossRef]
- Devi, L.L.; Pandey, A.; Gupta, S.; Singh, A.P. The interplay of auxin and brassinosteroid signaling tunes root growth under low and different nitrogen forms. Plant Physiol. 2022, 89, 1757–1773. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Deng, C.; Liu, Z.; Yin, K.; Zhang, Y.; Zhao, Z.; Zhao, R.; Zhao, N.; Zhou, X.; et al. Effect of NaCl on ammonium and nitrate uptake and transport in salt-tolerant and salt-sensitive poplars. Tree Physiol. 2024, 44, tpae020. [Google Scholar] [CrossRef] [PubMed]
- Kempinski, C.F.; Hafar, R.; Barth, C. Toward the mechanism of NH4+ sensitivity mediated by Arabidopsis GDP-mannose pyrophosphorylase. Plant Cell Environ. 2011, 34, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, S.; Sun, H.; Feng, S.; Jiang, C.; Zhou, S.; Wu, S.; Zhuang, G.; Chen, B.; Bai, Z.; et al. Complex regulatory network allows Myriophyllum aquaticum to thrive under high-concentration ammonia toxicity. Sci. Rep. 2019, 9, 4801. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, L.; Si, Y.; Zhai, Y.; Niu, M.; Han, M.; Su, T. Regulating effect of exogenous α-ketoglutarate on ammonium assimilation in poplar. Molecules 2024, 29, 1425. [Google Scholar] [CrossRef]
- Poucet, T.; González-Moro, M.B.; Cabasson, C.; Beauvoit, B.; Gibon, Y.; Dieuaide-Noubhani, M.; Marino, D. Ammonium supply induces differential metabolic adaptive responses in tomato according to leaf phenological stage. J. Exp. Bot. 2021, 72, 3185–3199. [Google Scholar] [CrossRef]
- Weil, S.; Barker, A.V.; Zandvakili, O.R.; Etemadi, F. Plant growth and calcium and potassium accumulation in lettuce under different nirogen regimes of ammonium and nitrate nutrition. J. Plant Nutr. 2020, 44, 270–281. [Google Scholar] [CrossRef]
- Xie, Y.; Lv, Y.; Jia, L.; Zheng, L.; Li, Y.; Zhu, M.; Tian, M.; Wang, M.; Qi, W.; Luo, L.; et al. Plastid-localized amino acid metabolism coordinates rice ammonium tolerance and nitrogen use efficiency. Nat. Plants 2023, 9, 1514–1529. [Google Scholar] [CrossRef]
- Yang, S.; Hao, D.; Jin, M.; Li, Y.; Liu, Z.; Huang, Y.; Chen, T.; Su, Y. Internal ammonium excess induces ROS-mediated reactions and causes carbon scarcity in rice. BMC Plant Biol. 2020, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Britto, D.T.; Li, M.; Becker, A.; Kronzucker, H.J. Rapid ammonia gas transport accounts for futile transmembrane cycling under NH3/NH4+ toxicity in plant roots. Plant Physiol. 2013, 163, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Sanagi, M.; Aoyama, S.; Kubo, A.; Lu, Y.; Sato, Y.; Ito, S.; Abe, M.; Mitsuda, N.; Ohme-Takagi, M.; Kiba, T.; et al. Low nitrogen conditions accelerate flowering by modulating the phosphorylation state of FLOWERING BHLH 4 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2022942118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Fu, Y.F.; Zhou, Y.H.; Wang, C.Q.; Lan, T.; Chen, G.D.; Zeng, J.; Chen, Y.E.; Yuan, M.; Yuan, S.; et al. Nitrogen and nitric oxide regulate Arabidopsis flowering differently. Plant Sci. 2019, 284, 177–184. [Google Scholar] [CrossRef]
- Yan, J.; Niu, F.; Liu, W.Z.; Zhang, H.; Wang, B.; Lan, W.; Che, Y.; Yang, B.; Luan, S.; Jiang, Y.Q. Arabidopsis CIPK14 positively regulates glucose response. Biochem. Biophys. Res. Commun. 2014, 450, 1679–1683. [Google Scholar] [CrossRef]
- Simon, N.M.L.; Kusakina, J.; Fernández-López, Á.; Chembath, A.; Belbin, F.E.; Dodd, A.N. The energy-signaling Hub SnRK1 is important for sucrose-induced hypocotyl elongation. Plant Physiol. 2018, 176, 1299–1310. [Google Scholar] [CrossRef]
- Hamasaki, H.; Kurihara, Y.; Kuromori, T.; Kusano, H.; Nagata, N.; Yamamoto, Y.Y.; Shimada, H.; Matsui, M. SnRK1 kinase and the NAC transcription factor SOG1 are components of a novel signaling pathway mediating the low energy response triggered by ATP depletion. Front. Plant Sci. 2019, 10, 503. [Google Scholar] [CrossRef]
- Li, Q.; Chai, L.; Tong, N.; Yu, H.; Jiang, W. Potential carbohydrate regulation mechanism underlying starvation-induced abscission of tomato flower. Int. J. Mol. Sci. 2022, 23, 1952. [Google Scholar] [CrossRef]
- Wang, H.; Han, C.; Wang, J.G.; Chu, X.; Shi, W.; Yao, L.; Chen, J.; Hao, W.; Deng, Z.; Fan, M.; et al. Regulatory functions of cellular energy sensor SnRK1 for nitrate signalling through NLP7 repression. Nat. Plants 2022, 8, 1094–1107. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Durand, M.; Brehaut, V.; Hsu, F.C.; Kelemen, Z.; Texier, Y.; Krapp, A.; Tsay, Y.F. Interplay between NIN-LIKE PROTEINs 6 and 7 in nitrate signaling. Plant Physiol. 2023, 192, 3049–3068. [Google Scholar] [CrossRef]
- Bradáčová, K.; Sittinger, M.; Tietz, K.; Neuhäuser, B.; Kandeler, E.; Berger, N.; Ludewig, U.; Neumann, G. Maize inoculation with microbial consortia: Contrasting effects on rhizosphere activities, nutrient acquisition and early growth in different soils. Microorganisms 2019, 7, 329. [Google Scholar] [CrossRef]
- Mpanga, I.K.; Nkebiwe, P.M.; Kuhlmann, M.; Cozzolino, V.; Piccolo, A.; Geistlinger, J.; Berger, N.; Ludewig, U.; Neumann, G. The form of N supply determines plant growth promotion by P-solubilizing microorganisms in maize. Microorganisms 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Bittsánszky, A.; Pilinszky, K.; Gyulai, G.; Komives, T. Overcoming ammonium toxicity. Plant Sci. 2015, 231, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Ariz, I.; Asensio, A.C.; Zamarreño, A.M.; García-Mina, J.M.; Aparicio-Tejo, P.M.; Moran, J.F. Changes in the C/N balance caused by increasing external ammonium concentrations are driven by carbon and energy availabilities during ammonium nutrition in pea plants: The key roles of asparagine synthetase and anaplerotic enzymes. Physiol. Plant. 2013, 148, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Baena-González, E.; Rolland, F.; Thevelein, J.M.; Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 2007, 448, 938–942. [Google Scholar] [CrossRef]
- Liu, K.H.; Niu, Y.; Konishi, M.; Wu, Y.; Du, H.; Sun Chung, H.; Li, L.; Boudsocq, M.; McCormack, M.; Maekawa, S.; et al. Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature 2017, 545, 311–316. [Google Scholar] [CrossRef]
| Tissues | Symptoms | NH4+ Concentration | Species | References |
|---|---|---|---|---|
| Roots | Inhibition of primary root elongation | 5–30 mM | Arabidopsis, rice | [24,25,26,31] |
| Modifications in lateral roots, reduced root/shoot ratio and diminished fresh root weight | 5–80 mM | Arabidopsis | [21,22,27,28] | |
| Stems | Inhibition of stem growth and reduced stem fresh weight | 10–50 mM | Arabidopsis | [21,29] |
| Leaves | Leaf chlorosis | 20–60 mM | Arabidopsis, barley | [30,31,32] |
| Flowers and seeds | Reduced plant biomass and yield | 5–20 mM | Arabidopsis, oilseed rape | [21,22,33,34,35,36] |
| High NH4+-induced late flowering | 40 mM | Arabidopsis | [37] | |
| Whole plant | Whole seedling death | >1 mM | Arnica montana, Cirsium dissectum, Calluna vulgaris | [4,10,38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.-B.; Sun, L.-N.; Zhang, Z.-W.; Chen, Y.-E.; Yuan, M.; Yuan, S. Phenotype Assessment and Putative Mechanisms of Ammonium Toxicity to Plants. Int. J. Mol. Sci. 2025, 26, 2606. https://doi.org/10.3390/ijms26062606
Xie L-B, Sun L-N, Zhang Z-W, Chen Y-E, Yuan M, Yuan S. Phenotype Assessment and Putative Mechanisms of Ammonium Toxicity to Plants. International Journal of Molecular Sciences. 2025; 26(6):2606. https://doi.org/10.3390/ijms26062606
Chicago/Turabian StyleXie, Lin-Bei, Li-Na Sun, Zhong-Wei Zhang, Yang-Er Chen, Ming Yuan, and Shu Yuan. 2025. "Phenotype Assessment and Putative Mechanisms of Ammonium Toxicity to Plants" International Journal of Molecular Sciences 26, no. 6: 2606. https://doi.org/10.3390/ijms26062606
APA StyleXie, L.-B., Sun, L.-N., Zhang, Z.-W., Chen, Y.-E., Yuan, M., & Yuan, S. (2025). Phenotype Assessment and Putative Mechanisms of Ammonium Toxicity to Plants. International Journal of Molecular Sciences, 26(6), 2606. https://doi.org/10.3390/ijms26062606






