Implementation and One-Year Evaluation of Proenkephalin A in Critical Care
Abstract
1. Introduction
2. Results
2.1. Implementation
2.2. Baseline Characteristics
2.3. Correlation of PENK, sCr, and eGFR
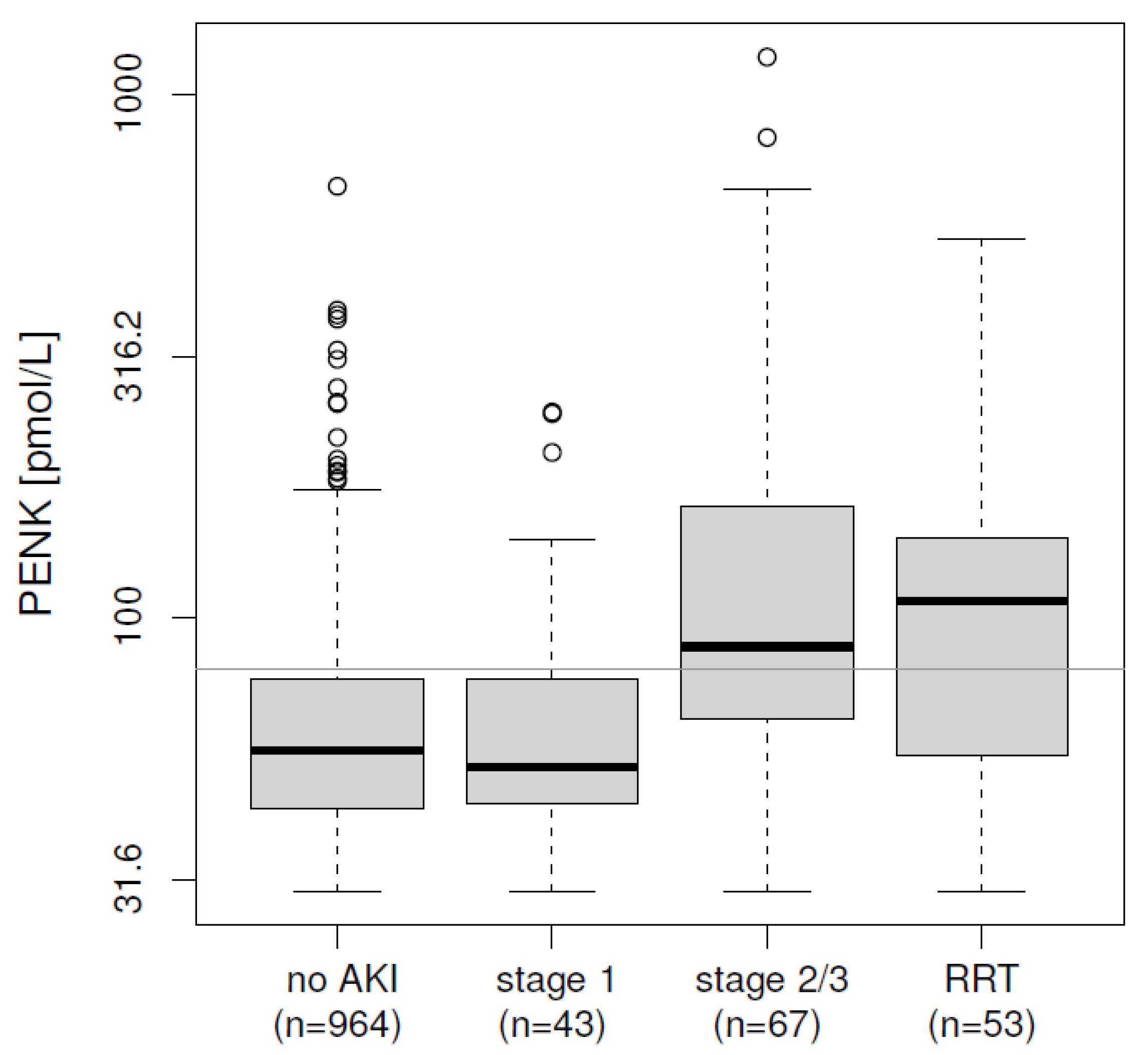
2.4. Initial Risk Stratification at ICU Admission
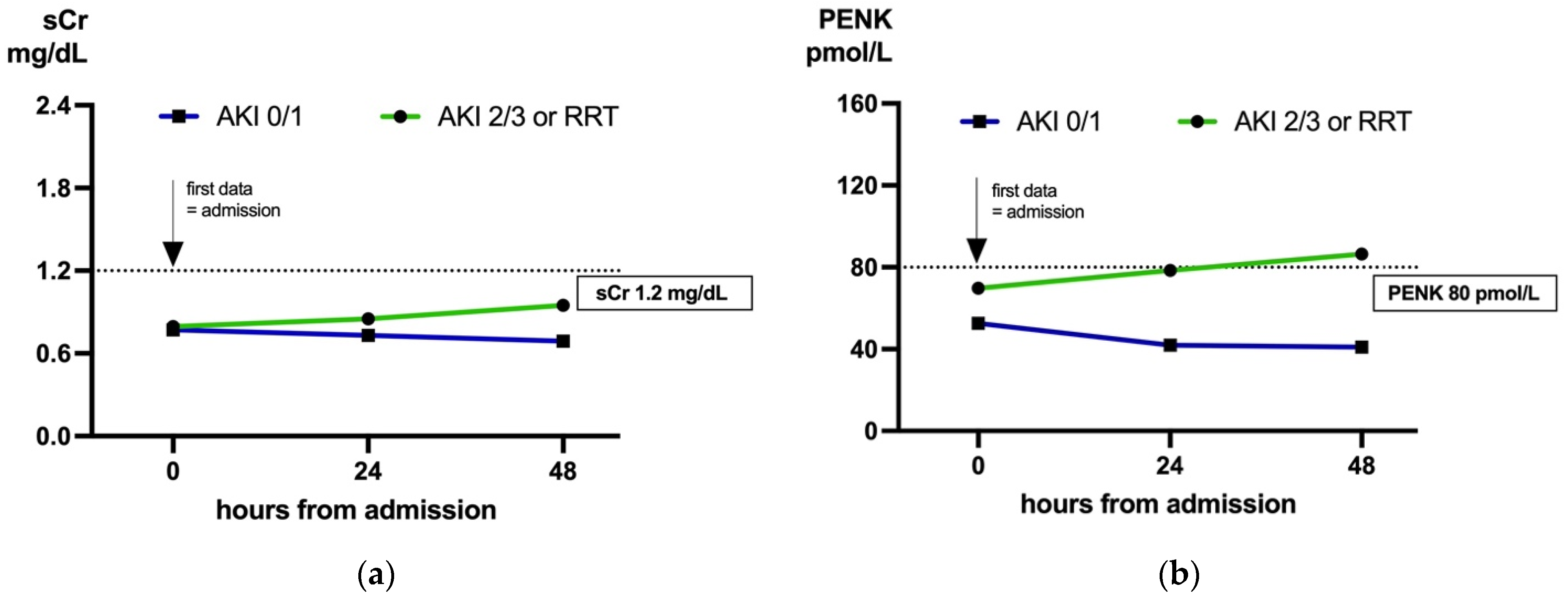
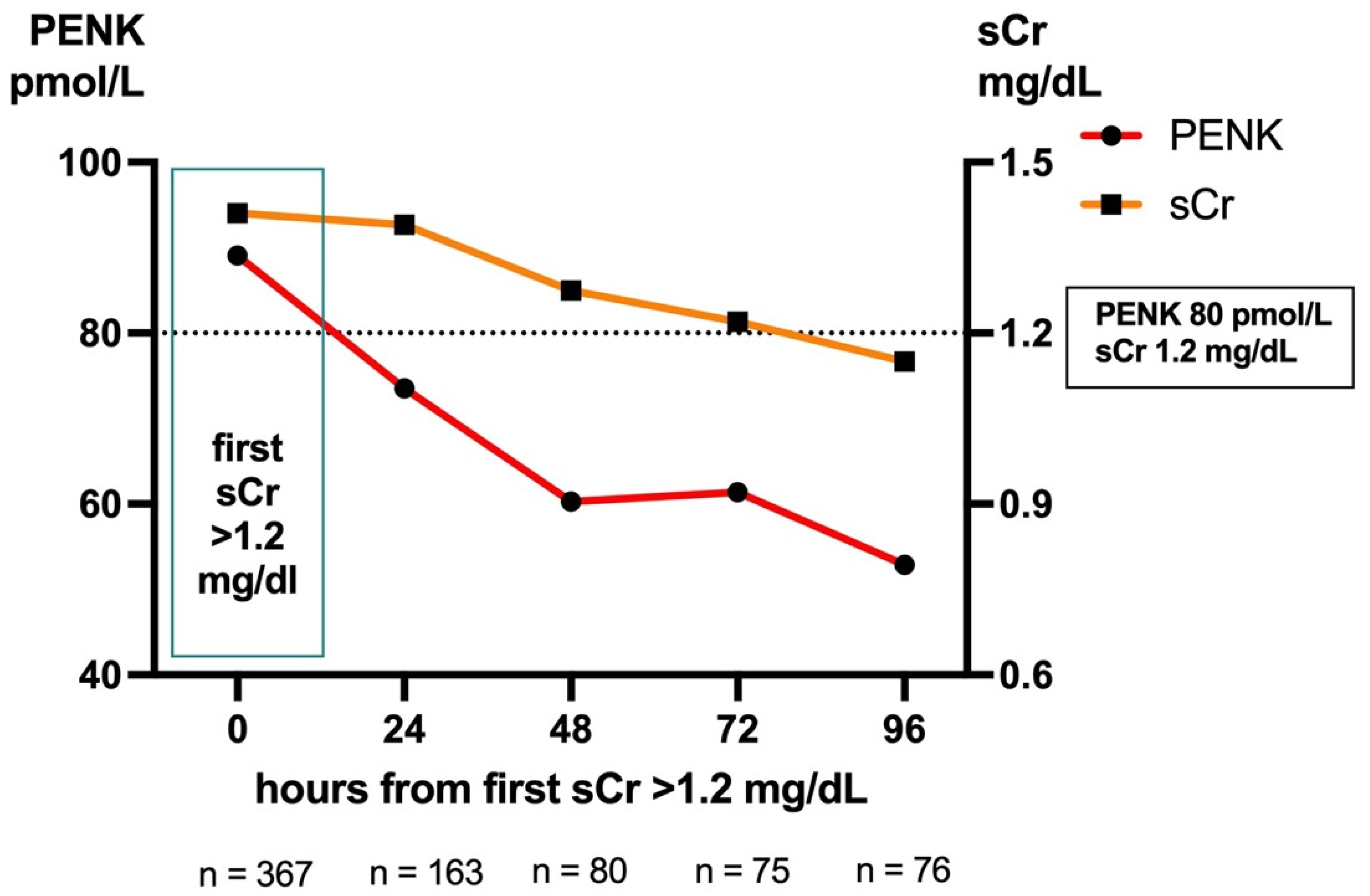
2.5. Biomarker Dynamics: Predicting AKI in Cases with Low sCr (<1.2 mg/dL) on Admission
| Admission, All Patients | Admission, sCradm < 1.2 mg/dL | 24 h, sCradm < 1.2 mg/dL | 48 h, sCradm < 1.2 mg/dL | |||||
|---|---|---|---|---|---|---|---|---|
| n (Events) | AUC [95% CI] | n (Events) | AUC [95% CI] | n (Events) | AUC [95% CI] | n (Events) | AUC [95% CI] | |
| PENK | 1127 (120) | 0.725 [0.67, 0.78] | 910 (56) | 0.65 [0.565, 0.735] | 326 (27) | 0.818 [0.719, 0.918] | 175 (22) | 0.877 [0.795, 0.959] |
| sCr | 1127 (120) | 0.716 [0.659, 0.772] | 910 (56) | 0.53 [0.447, 0.615] | 326 (27) | 0.689 [0.575, 0.803] | 175 (22) | 0.74 [0.625, 0.856] |
| eGFRCKD-EPI | 1058 (101) | 0.751 [0.697, 0.806] | 857 (45) | 0.603 [0.52, 0.687] | 293 (20) | 0.769 [0.647, 0.891] | 154 (15) | 0.796 [0.652, 0.94] |
| eGFRPENK-Crea | 1058 (101) | 0.755 [0.699, 0.811] | 857 (45) | 0.624 [0.529, 0.719] | 293 (20) | 0.83 [0.724, 0.937] | 154 (15) | 0.908 [0.842, 0.974] |
| eGFRPENK | 1058 (101) | 0.728 [0.668, 0.788] | 857 (45) | 0.638 [0.542, 0.734] | 293 (20) | 0.807 [0.684, 0.929] | 154 (15) | 0.862 [0.772, 0.952] |
2.6. Biomarker Dynamics: Trajectories Post-sCr Peak
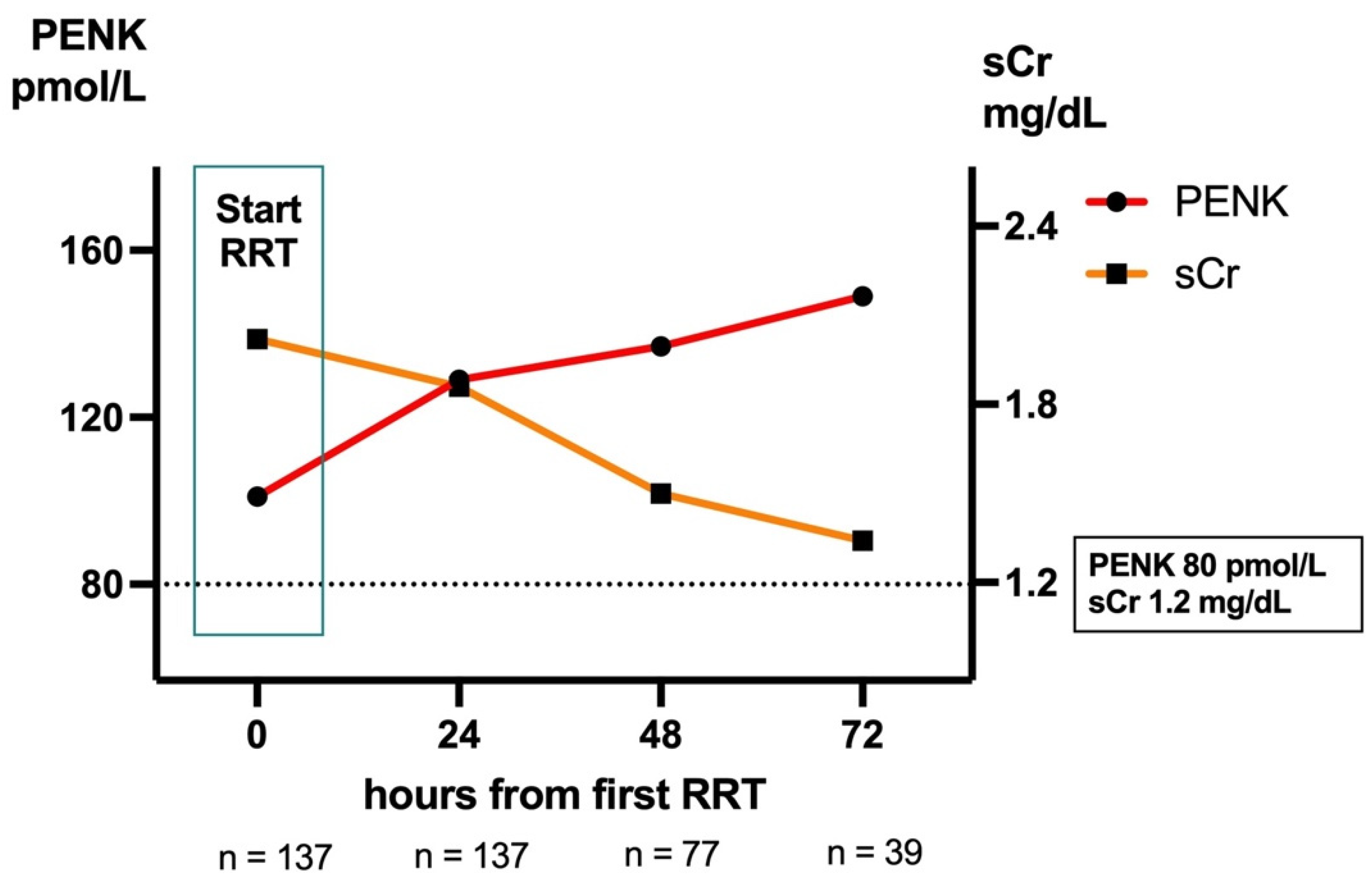
2.7. Kidney Function During RRT
2.8. Comparison of eGFR During RRT by Net Reclassification Index (NRI)-like Methods
3. Discussion
4. Materials and Methods
4.1. Patient Population
4.2. Ethics Approval and Data Collection
4.3. Implementation Process
- Unfreeze: This stage involved preparing the institution for change by addressing the need for PENK for kidney function diagnostics. The head of the department played a key role in initiating discussions, securing approvals, and ensuring alignment with institutional goals. Attending physicians were engaged early to recognize the clinical value of PENK, and initial training sessions began to educate healthcare staff. Additionally, discussions with the laboratory, IT, and procurement teams took place to assess feasibility and prepare for technical implementation.
- Move: During this phase, the actual implementation of PENK took place. Training and education were intensified to ensure that physicians accurately interpret and utilize PENK in clinical workflows. PENK was formally integrated into standard operating procedures (SOPs) to ensure consistent application. Simultaneously, technical implementation occurred, involving assay validation in collaboration with the laboratory, integration into electronic healthcare systems by the IT department, and procurement securing necessary reagents and equipment. PENK was then actively used in clinical settings, and preliminary data collection began to monitor its effectiveness.
- Freeze: In the final stage we assessed PENK’s clinical value. Additionally, the experience in regards to feasibility from all involved departments was taken into account to evaluate and optimize the process.
4.4. Measurement of PENK and Other Variables
4.5. Statistics
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADQI | Acute Disease Quality Initiative |
| AKI | Acute kidney injury |
| AUC | Area under the receiver operating curve |
| BMI | Body mass index |
| CI | Confidence interval |
| CKD | Chronic kidney disease |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| Crea | Creatinine |
| CRRT | Continuous renal replacement therapy |
| Cys C | Cystatin C |
| EDTA | Ethylenediaminetetraacetic acid |
| eGFR | Estimated glomerular filtration rate |
| eGFRCKD-EPI | Estimated GFR based on Chronic Kidney Disease Epidemiology Collaboration |
| eGFRPENK-Crea | Estimated GFR based on Proenkephalin A 119–159, age and serum creatinine |
| eGFRPENK | Estimated GFR based on Proenkephalin A 119–159 and age |
| EK | Ethikkommittee |
| GFR | Glomerular filtration rate |
| ICCA | Intellispace Critical Care and Anesthesia |
| ICH | International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use |
| ICU | Intensive care unit(s) |
| IGFBP-7 | insulin-like growth factor-binding protein 7 |
| IQR | Interquartile ranges |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| KIM | kidney injury molecule-1 |
| MAKE | Major acute kidney events |
| MDRD | Modification of Diet in Renal Disease |
| mGFR | Measured glomerular filtration rate |
| NGAL | neutrophil gelatinase-associated lipocalin |
| NRI | Net Reclassification Index |
| PENK | Proenkephalin A 119–159 |
| ROC | Receiver operating characteristics |
| RRT | Renal replacement therapy |
| sCr | Serum creatinine |
| SOPs | Standard operating procedures |
| TIMP-2 | Tissue inhibitor metalloproteinase-2 |
References
- Gameiro, J.; Marques, F.; Lopes, J.A. Long-term consequences of acute kidney injury: A narrative review. Clin. Kidney J. 2020, 14, 789–804. [Google Scholar] [CrossRef] [PubMed]
- See, E.J.; Jayasinghe, K.; Glassford, N.; Bailey, M.; Johnson, D.W.; Polkinghorne, K.R.; Toussaint, N.D.; Bellomo, R. Long-term risk of adverse outcomes after acute kidney injury: A systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019, 95, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Khadzhynov, D.; Schmidt, D.; Hardt, J.; Rauch, G.; Gocke, P.; Eckardt, K.U.; Schmidt-Ott, K.M. The Incidence of Acute Kidney Injury and Associated Hospital Mortality. Dtsch. Arztebl. Int. 2019, 116, 397–404. [Google Scholar] [CrossRef]
- Mehta, R.L.; Pascual, M.T.; Soroko, S.; Savage, B.R.; Himmelfarb, J.; Ikizler, T.A.; Paganini, E.P.; Chertow, G.M. Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int. 2004, 66, 1613–1621. [Google Scholar] [CrossRef]
- Džidić-Krivić, A.; Sher, E.K.; Kusturica, J.; Farhat, E.K.; Nawaz, A.; Sher, F. Unveiling drug induced nephrotoxicity using novel biomarkers and cutting-edge preventive strategies. Chem. Biol. Interact. 2024, 388, 110838. [Google Scholar] [CrossRef]
- Cottam, D.; Azzopardi, G.; Forni, L.G. Biomarkers for early detection and predicting outcomes in acute kidney injury. Br. J. Hosp. Med. 2022, 83, 1–11. [Google Scholar] [CrossRef]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers From the Acute Disease Quality Initiative Consensus Conference: A Consensus Statement. JAMA Netw. Open 2020, 3, e2019209. [Google Scholar] [CrossRef]
- Ragán, D.; Horváth-Szalai, Z.; Szirmay, B.; Mühl, D. Novel Damage Biomarkers of Sepsis-Related Acute Kidney Injury. eJIFCC 2022, 33, 11–22. [Google Scholar]
- Esmeijer, K.; Schoe, A.; Ruhaak, L.R.; Hoogeveen, E.K.; Soonawala, D.; Romijn, F.; Shirzada, M.R.; van Dissel, J.T.; Cobbaert, C.M.; de Fijter, J.W. The predictive value of TIMP-2 and IGFBP7 for kidney failure and 30-day mortality after elective cardiac surgery. Sci. Rep. 2021, 11, 1071. [Google Scholar] [CrossRef]
- Forni, L.G.; Joannidis, M.; Artigas, A.; Bell, M.; Hoste, E.; Joannes-Boyau, O.; Kashani, K.; Koyner, J.; Rimmele, T.; Shi, J.; et al. Characterising acute kidney injury: The complementary roles of biomarkers of renal stress and renal function. J. Crit. Care 2022, 71, 154066. [Google Scholar] [CrossRef]
- Marino, R.; Struck, J.; Hartmann, O.; Maisel, A.S.; Rehfeldt, M.; Magrini, L.; Melander, O.; Bergmann, A.; Di Somma, S. Diagnostic and short-term prognostic utility of plasma pro-enkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department. J. Nephrol. 2015, 28, 717–724. [Google Scholar] [CrossRef]
- Hollinger, A.; Wittebole, X.; François, B.; Pickkers, P.; Antonelli, M.; Gayat, E.; Chousterman, B.G.; Lascarrou, J.B.; Dugernier, T.; Di Somma, S.; et al. Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study. Kidney Int. Rep. 2018, 3, 1424–1433. [Google Scholar] [CrossRef]
- Molvin, J.; Jujic, A.; Navarin, S.; Melander, O.; Zoccoli, G.; Hartmann, O.; Bergmann, A.; Struck, J.; Bachus, E.; Di Somma, S.; et al. Bioactive adrenomedullin, proenkephalin A and clinical outcomes in an acute heart failure setting. Open Heart 2019, 6, e001048. [Google Scholar] [CrossRef]
- Emmens, J.E.; Ter Maaten, J.M.; Damman, K.; van Veldhuisen, D.J.; de Boer, R.A.; Struck, J.; Bergmann, A.; Sama, I.E.; Streng, K.W.; Anker, S.D.; et al. Proenkephalin, an Opioid System Surrogate, as a Novel Comprehensive Renal Marker in Heart Failure. Circ. Heart Fail. 2019, 12, e005544. [Google Scholar] [CrossRef]
- von Groote, T.; Albert, F.; Meersch, M.; Koch, R.; Porschen, C.; Hartmann, O.; Bergmann, D.; Pickkers, P.; Zarbock, A. Proenkephalin A 119-159 predicts early and successful liberation from renal replacement therapy in critically ill patients with acute kidney injury: A post hoc analysis of the ELAIN trial. Crit. Care 2022, 26, 333. [Google Scholar] [CrossRef]
- Smeets, N.J.L.; Hartmann, O.; Schulte, J.; Schreuder, M.F.; de Wildt, S.N. Proenkephalin A as a marker for glomerular filtration rate in critically ill children: Validation against gold standard iohexol GFR measurements. Clin. Chem. Lab. Med. 2023, 61, 104–111. [Google Scholar] [CrossRef]
- Khorashadi, M.; Beunders, R.; Pickkers, P.; Legrand, M. Proenkephalin: A New Biomarker for Glomerular Filtration Rate and Acute Kidney Injury. Nephron 2020, 144, 655–661. [Google Scholar] [CrossRef]
- Gao, J.B.; Tang, W.D.; Wang, X.; Shen, J. Prognostic value of neuropeptide proenkephalin A in patients with severe traumatic brain injury. Peptides 2014, 58, 42–46. [Google Scholar] [CrossRef]
- Chen, X.L.; Yu, B.J.; Chen, M.H. Circulating levels of neuropeptide proenkephalin A predict outcome in patients with aneurysmal subarachnoid hemorrhage. Peptides 2014, 56, 111–115. [Google Scholar] [CrossRef]
- Grill, S.; Yahiaoui-Doktor, M.; Basrai, M.; Struck, J.; Schulte, J.; Berling-Ernst, A.; Engel, C.; Ullrich, M.; Lammert, J.; Bischoff, S.C.; et al. Precursor fractions of neurotensin and enkephalin might point to molecular mechanisms of cancer risk modulation during a lifestyle-intervention in germline BRCA1/2 gene mutation carriers. Breast Cancer Res. Treat. 2021, 186, 741–752. [Google Scholar] [CrossRef]
- Melander, O.; Orho-Melander, M.; Manjer, J.; Svensson, T.; Almgren, P.; Nilsson, P.M.; Engström, G.; Hedblad, B.; Borgquist, S.; Hartmann, O.; et al. Stable Peptide of the Endogenous Opioid Enkephalin Precursor and Breast Cancer Risk. J. Clin. Oncol. 2015, 33, 2632–2638. [Google Scholar] [CrossRef]
- Donato, L.J.; Meeusen, J.W.; Lieske, J.C.; Bergmann, D.; Sparwaßer, A.; Jaffe, A.S. Analytical performance of an immunoassay to measure proenkephalin. Clin. Biochem. 2018, 58, 72–77. [Google Scholar] [CrossRef]
- Beunders, R.; Donato, L.J.; van Groenendael, R.; Arlt, B.; Carvalho-Wodarz, C.; Schulte, J.; Coolen, A.C.; Lieske, J.C.; Meeusen, J.W.; Jaffe, A.S.; et al. Assessing GFR with Proenkephalin. Kidney Int. Rep. 2023, 8, 2345–2355. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Robert, G.; Macfarlane, F.; Bate, P.; Kyriakidou, O. Diffusion of innovations in service organizations: Systematic review and recommendations. Milbank Q. 2004, 82, 581–629. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Lorenzin, A.; de Cal, M.; Perin, N.; Morisi, N.; Brendolan, A.; Lentini, P.; Zanella, M.; Ronco, C. Human Proenkephalin A 119-159 (penKid) in Extracorporeal Therapies: Ex vivo Sieving Coefficient, Diffusive Clearance, and Hemoadsorption Kinetics. Blood Purif. 2024, 53, 773–780. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B., Sr.; Steyerberg, E.W. Steyerberg, Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat. Med. 2011, 30, 11–21. [Google Scholar] [CrossRef]
- Mehta, R.; Cerdá, J.; Burdmann, E.; Tonelli, M.; Garcia Garcia, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.; et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 2015, 385, 2616–2643. [Google Scholar] [CrossRef]
- Ferreira, G.S.; Frota, M.L.; Gonzaga, M.J.D.; Vattimo, M.d.F.F.; Lima, C. The Role of Biomarkers in Diagnosis of Sepsis and Acute Kidney Injury. Biomedicines 2024, 12, 931. [Google Scholar] [CrossRef]
- Lassola, S.; Cundari, F.; Marini, G.; Corradi, F.; De Rosa, S. Advancements in Trauma-Induced Acute Kidney Injury: Diagnostic and Therapeutic Innovations. Life 2024, 14, 1005. [Google Scholar] [CrossRef]
- Hill, A.; Bergmann, D.; Schulte, J.; Zayat, R.; Marx, G.; Simon, T.-P.; Mossanen, J.; Brücken, A.; Stoppe, C. Proenkephalin A and bioactive adrenomedullin are useful for risk prognostication in cardiac surgery. Front. Cardiovasc. Med. 2023, 9, 1017867. [Google Scholar] [CrossRef]
- Wenzl, F.A.; Wang, P.; Arrigo, M.; Parenica, J.; Jones, D.J.L.; Bruno, F.; Tarnowski, D.; Hartmann, O.; Boucek, L.; Lang, F.; et al. Proenkephalin improves cardio-renal risk prediction in acute coronary syndromes: The KID-ACS score. Eur. Heart J. 2024, 46, 38–54. [Google Scholar] [CrossRef]
- Greco, M.; Foti, D.P.; Aversa, A.; Fuiano, G.; Brunetti, A.; Simeoni, M. Cystatin C, a Controversial Biomarker in Hypothyroid Patients under Levothyroxine Therapy: THYRenal, a Pilot Cohort Observational Study. J. Clin. Med. 2020, 9, 2958. [Google Scholar] [CrossRef]
- Liang, S.; Shi, M.; Bai, Y.; Deng, Y.; Fang, M.; Li, J.; Wu, Y.; Peng, W.; Hou, Y.; Fang, H.; et al. The effect of glucocorticoids on serum cystatin C in identifying acute kidney injury: A propensity-matched cohort study. BMC Nephrol. 2020, 21, 519. [Google Scholar] [CrossRef]
- Okura, T.; Jotoku, M.; Irita, J.; Enomoto, D.; Nagao, T.; Desilva, V.R.; Yamane, S.; Pei, Z.; Kojima, S.; Hamano, Y.; et al. Association between cystatin C and inflammation in patients with essential hypertension. Clin. Exp. Nephrol. 2010, 14, 584–588. [Google Scholar] [CrossRef]
- Stevens, L.A.; Schmid, C.H.; Greene, T.; Li, L.; Beck, G.J.; Joffe, M.M.; Froissart, M.; Kusek, J.W.; Zhang, Y.; Coresh, J.; et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009, 75, 652–660. [Google Scholar] [CrossRef]
- Dépret, F.; Hollinger, A.; Cariou, A.; Deye, N.; Vieillard-Baron, A.; Fournier, M.C.; Jaber, S.; Damoisel, C.; Lu, Q.; Monnet, X.; et al. Incidence and Outcome of Subclinical Acute Kidney Injury Using penKid in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2020, 202, 822–829. [Google Scholar] [CrossRef]
- Shah, K.S.; Taub, P.; Patel, M.; Rehfeldt, M.; Struck, J.; Clopton, P.; Mehta, R.L.; Maisel, A.S. Proenkephalin predicts acute kidney injury in cardiac surgery patients. Clin. Nephrol. 2015, 83, 29–35. [Google Scholar] [CrossRef]
- Dépret, F.; Polina, A.; Amzallag, J.; Fayolle-Pivot, L.; Coutrot, M.; Chaussard, M.; Struck, J.; Hartmann, O.; Jully, M.; Fratani, A.; et al. PenKid measurement at admission is associated with outcome in severely ill burn patients. Burns 2020, 46, 1302–1309. [Google Scholar] [CrossRef]
- Beunders, R.; van Groenendael, R.; Leijte, G.P.; Kox, M.; Pickkers, P. Proenkephalin Compared to Conventional Methods to Assess Kidney Function in Critically Ill Sepsis Patients. Shock 2020, 54, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Rathnamalala, N.; Rajapakse, S. Acute kidney injury—Improving outcomes with early risk assessment and intervention. J. Ceylon Coll. Physicians 2014, 44, 22. [Google Scholar] [CrossRef]
- KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012, 2, 1–138.
- Thau, M.R.; Bhatraju, P.K. Bhatraju, Sub-Phenotypes of Acute Kidney Injury: Do We Have Progress for Personalizing Care? Nephron 2020, 144, 677–679. [Google Scholar] [CrossRef]
- Zou, C.; Wang, C.; Lu, L. Advances in the study of subclinical AKI biomarkers. Front. Physiol. 2022, 13, 960059. [Google Scholar] [CrossRef]
- Molitoris, B.A. Measuring glomerular filtration rate in the intensive care unit: No substitutes please. Crit. Care 2013, 17, 181. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Wald, R. Strategies for the optimal timing to start renal replacement therapy in critically ill patients with acute kidney injury. Kidney Int. 2017, 91, 1022–1032. [Google Scholar] [CrossRef]
- Cove, M.E.; MacLaren, G.; Brodie, D.; Kellum, J.A. Optimising the timing of renal replacement therapy in acute kidney injury. Crit. Care 2021, 25, 184. [Google Scholar] [CrossRef]
- Klouche, K.; Gibney, R.T.N.; Forni, L.G. Can this patient be safely weaned from RRT? Intensive Care Med. 2018, 44, 639–642. [Google Scholar] [CrossRef]
- Gaudry, S.; Hajage, D.; Schortgen, F.; Martin-Lefevre, L.; Pons, B.; Boulet, E.; Boyer, A.; Chevrel, G.; Lerolle, N.; Carpentier, D.; et al. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N. Engl. J. Med. 2016, 375, 122–133. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Feng, J.; Xu, B.; Chen, Y.; Li, Z.; Guo, X.; Guan, T. Predictors for short-term successful weaning from continuous renal replacement therapy: A systematic review and meta-analysis. Ren. Fail. 2023, 45, 2176170. [Google Scholar] [CrossRef] [PubMed]
- Maynar Moliner, J.; Honore, P.M.; Sánchez-Izquierdo Riera, J.A.; Herrera Gutiérrez, M.; Spapen, H.D. Handling continuous renal replacement therapy-related adverse effects in intensive care unit patients: The dialytrauma concept. Blood Purif. 2012, 34, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Honoré, P.M.; Jacobs, R.; Joannes-Boyau, O.; De Waele, E.; Van Gorp, V.; Boer, W.; Spapen, H.D. Con: Dialy- and continuous renal replacement (CRRT) trauma during renal replacement therapy: Still under-recognized but on the way to better diagnostic understanding and prevention. Nephrol. Dial. Transplant. 2013, 28, 2723–2727; discussion 2727–2728. [Google Scholar] [CrossRef]
- von Groote, T.; Albert, F.; Meersch, M.; Koch, R.; Gerss, J.; Arlt, B.; Sadjadi, M.; Porschen, C.; Pickkers, P.; Zarbock, A. Evaluation of Proenkephalin A 119-159 for liberation from renal replacement therapy: An external, multicenter pilot study in critically ill patients with acute kidney injury. Crit. Care 2023, 27, 276. [Google Scholar] [CrossRef]
- Tichy, J.; Hausmann, A.; Lanzerstorfer, J.; Ryz, S.; Wagner, L.; Lassnigg, A.; Bernardi, M.H. Prediction of Successful Liberation from Continuous Renal Replacement Therapy Using a Novel Biomarker in Patients with Acute Kidney Injury after Cardiac Surgery-An Observational Trial. Int. J. Mol. Sci. 2024, 25, 10873. [Google Scholar] [CrossRef]
- Sezen, S.F.; Kenigs, V.A.; Kapusta, D.R. Kapusta, Renal excretory responses produced by the delta opioid agonist, BW373U86, in conscious rats. J. Pharmacol. Exp. Ther. 1998, 287, 238–245. [Google Scholar] [CrossRef]
- Grossman, A.; Besser, G.M.; Milles, J.J.; Baylis, P.H. Inhibition of vasopressin release in man by an opiate peptide. Lancet 1980, 2, 1108–1110. [Google Scholar] [CrossRef]
- Gao, S.; He, Q. Opioids and the kidney: Two sides of the same coin. Front. Pharmacol. 2024, 15, 1421248. [Google Scholar] [CrossRef]
- Lewin, K. Frontiers in Group Dynamics: Concept, Method and Reality in Social Science; Social Equilibria and Social Change. Human. Relat. 1947, 1, 5–41. [Google Scholar] [CrossRef]
- Harrison, R.; Fischer, S.; Walpola, R.L.; Chauhan, A.; Babalola, T.; Mears, S.; Le-Dao, H. Where Do Models for Change Management, Improvement and Implementation Meet? A Systematic Review of the Applications of Change Management Models in Healthcare. J. Healthc. Leadersh. 2021, 13, 85–108. [Google Scholar] [CrossRef]
| Characteristics (n = 4169 Patients Included) | Median (IQR) | |
|---|---|---|
| General | Age, years | 66 (56–75) |
| BMI, kg/m2 | 26.4 (23.9–30.1) | |
| in % | ||
| Gender | Female | 36% |
| Male | 64% | |
| Maximal AKI stadium during ICU stay | No AKI | 77.9% |
| KDIGO 1 | 5.3% | |
| KDIGO 2 or 3 | 6.8% | |
| RRT | 9.9% | |
| Length of ICU stay | Less than 2 days | 35% |
| 3–7 days | 35% | |
| 8–14 days | 13% | |
| 15–28 days | 10% | |
| >28 days or re-admitted | 7% | |
| PENK | sCr | eGFRCKD-EPI | eGFRPENK-Crea | eGFRPENK | |
|---|---|---|---|---|---|
| PENK | - | r = 0.54 (CI 0.53, −0.55) | r = −0.62 (CI −0.63, −0.61) | r = −0.85 (CI −0.85, −0.84) | r = −0.98 (CI −0.98, −0.98) |
| sCr | n = 17,900 | - | r = −0.91 (CI −0.91, −0.90) | r = −0.87 (CI −0.88, −0.87) | r = −0.55 (CI −0.56, −0.54) |
| eGFRCKD-EPI | n = 15,819 | n = 15,819 | - | r = 0.91 (CI 0.9, 0.91) | r = 0.68 (CI 0.67, 0.69) |
| eGFRPENK-Crea | n = 15,819 | n = 15,819 | n = 15,819 | - | r = 0.87 (CI 0.87, 0.88) |
| eGFRPENK | n = 15,819 | n = 15,819 | n = 15,819 | n = 15,819 | - |
| All n = 15,819 | eGFRPENK | No RRT, n = 12,120 | eGFRPENK | With RRT, n = 1945 | eGFRPENK | RRT Days, n = 1754 | eGFRPENK | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| eGFRCKD-EPI | <60 | >60 | eGFRCKD-EPI | <60 | >60 | eGFRCKD-EPI | <60 | >60 | eGFRCKD-EPI | <60 | >60 |
| <60 | 3249 | 564 | <60 | 1439 | 422 | <60 | 702 | 91 | <60 | 1108 | 51 |
| >60 | 782 | 11,224 | >60 | 343 | 9916 | >60 | 124 | 1028 | >60 | 315 | 280 |
| n | % total | n | % total | n | % total | n | % total | ||||
| higher in eGFRPENK | 564 | 3.6% | higher in eGFRPENK | 422 | 3.5% | lower in eGFRPENK | 124 | 6.4% | lower in eGFRPENK | 315 | 18.0% |
| higher in eGFRCKD-EPI | 782 | 4.9% | higher in eGFRCKD-EPI | 343 | 2.8% | lower in eGFRCKD-EPI | 91 | 4.7% | lower in eGFRCKD-EPI | 51 | 2.9% |
| Delta | 218 | 1.4% | Delta | 79 | 0.7% | Delta | 33 | 1.7% | Delta | 264 | 15.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, L.; Martin, C.; Peine, A.; Imöhl, M.; Kersten, A.; Kramann, R.; Saritas, T.; Marx, N.; Dreher, M.; Marx, G.; et al. Implementation and One-Year Evaluation of Proenkephalin A in Critical Care. Int. J. Mol. Sci. 2025, 26, 2602. https://doi.org/10.3390/ijms26062602
Martin L, Martin C, Peine A, Imöhl M, Kersten A, Kramann R, Saritas T, Marx N, Dreher M, Marx G, et al. Implementation and One-Year Evaluation of Proenkephalin A in Critical Care. International Journal of Molecular Sciences. 2025; 26(6):2602. https://doi.org/10.3390/ijms26062602
Chicago/Turabian StyleMartin, Lukas, Caren Martin, Arne Peine, Matthias Imöhl, Alexander Kersten, Rafael Kramann, Turgay Saritas, Nikolaus Marx, Michael Dreher, Gernot Marx, and et al. 2025. "Implementation and One-Year Evaluation of Proenkephalin A in Critical Care" International Journal of Molecular Sciences 26, no. 6: 2602. https://doi.org/10.3390/ijms26062602
APA StyleMartin, L., Martin, C., Peine, A., Imöhl, M., Kersten, A., Kramann, R., Saritas, T., Marx, N., Dreher, M., Marx, G., & Simon, T.-P. (2025). Implementation and One-Year Evaluation of Proenkephalin A in Critical Care. International Journal of Molecular Sciences, 26(6), 2602. https://doi.org/10.3390/ijms26062602










