Anti-Colorectal Cancer Activity of Panax and Its Active Components, Ginsenosides: A Review
Abstract
1. Introduction
2. Studies of the Mechanism of Colon Cancer Treatment of the Genus Panax
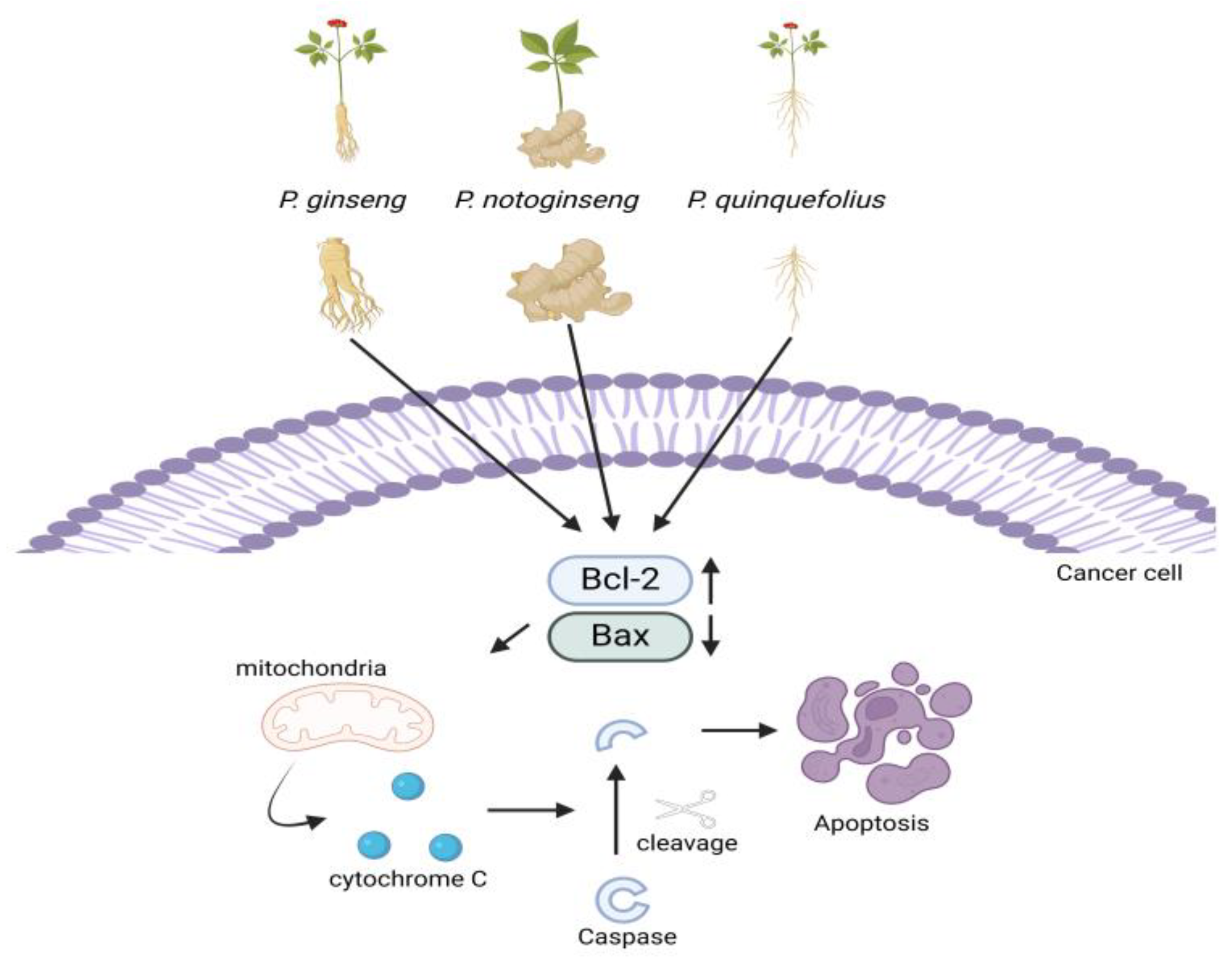
2.1. Apoptosis Pathway
2.2. Autophagy
2.3. Reducing Cancer Cachexia
2.4. Microbiota Population
3. Study on the Mechanism of Colon Cancer Treatment of Ginsenosides and Their Metabolites
3.1. Rh2
3.1.1. Anti-Inflammatory Activity
3.1.2. Arresting Cell Cycle and Apoptosis
3.1.3. Synergistic Therapy with Radiation
3.2. Rg3
3.3. Rb1
3.4. Secondary Metabolites
4. Ginsenosides with or as a Drug Carrier
4.1. 20(S)-Ginsenoside Rg3-Based Polypeptide NPs
4.2. Ginsenoside-Modified Nanostructured Lipid Carriers Containing Curcumin
4.3. Three-Layer Functional Polymer Materials with Ginsenoside
| Nanocarriers | Ginsenosides | Advantages | Mechanisms | Ref. |
|---|---|---|---|---|
| mPEG-b-P(Glu-co-Phe) NP | 20(S)-ginsenoside Rg3 | Maintenance for NPs Dissociation in acidic conditions (e.g., tumors) Effective biodistribution Slow pharmacokinetics | PCNA ↓ Caspase-3 ↑ | [138] |
| Nanostructured lipid carrier containing curcumin | Rg1, Rd, F2, protopanaxadiol, Rg3, compound K, protopanaxatriol | Bioavailability ↑ Lipid particle stabilization ↑ Cytotoxicity in tumors ↑ Uptake level ↑ | ND | [150] |
| Hydrolyzed ginsenoside | Survival rate ↑ | ND | [151] | |
| Polylactic acid polyglycolic acid | Rg3 | Induction of apoptosis Tumor weight, size ↓ | Caspase-3 ↑ CEA Bcl-2, Ki67, VEGF, ALT/AST, ↓ | [159] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| PPD | Protopanaxadiol |
| P. notoginseng | Panax notoginseng |
| CIN | Chromosomal instability |
| MSI | Microsatellite instability |
| 5-FU | Fluoropyrimidine |
| OX | Oxaliplatin |
| IRI | Irinotecan |
| FOLFOX | 5-FU + folinic acid + OX |
| FOLFIRI | 5-FU + folinic acid + IRI |
| FOLFOXIRI | 5-FU + folinic acid + OX + IRI |
| P. quinquefolium | Panax quinquefolium |
| P. ginseng | Panax ginseng |
| PPT | Protopanaxatriol |
| CK | Compound K |
| NPs | Nanoparticles |
| Bcl-2 | B-cell lymphoma-2 |
| Bax | Bcl-2-associated X protein |
| PCNA | Proliferating cell nuclear antigen |
| CEA | Carcinoembryonic antigen |
| ER | Endoplasmic reticulum |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. CpG island hypermethylation and tumor suppressor genes: A booming present, a brighter future. Oncogene 2002, 21, 5427–5440. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; de la Chapelle, A. Genetic susceptibility to non-polyposis colorectal cancer. J. Med. Genet. 1999, 36, 801–818. [Google Scholar]
- Venugopal, A.; Carethers, J.M. Epidemiology and biology of early onset colorectal cancer. Excli. J. 2022, 21, 162–182. [Google Scholar] [CrossRef]
- De’ Angelis, G.L.; Bottarelli, L.; Azzoni, C.; De’ Angelis, N.; Leandro, G.; Di Mario, F.; Gaiani, F.; Negri, F. Microsatellite instability in colorectal cancer. Acta Biomed. 2018, 89, 97–101. [Google Scholar] [CrossRef]
- Weng, J.; Li, S.; Zhu, Z.; Liu, Q.; Zhang, R.; Yang, Y.; Li, X. Exploring immunotherapy in colorectal cancer. J. Hematol. Oncol. 2022, 15, 95. [Google Scholar] [CrossRef]
- Kasi, P.B.; Mallela, V.R.; Ambrozkiewicz, F.; Trailin, A.; Liška, V.; Hemminki, K. Theranostics Nanomedicine Applications for Colorectal Cancer and Metastasis: Recent Advances. Int. J. Mol. Sci. 2023, 24, 7922. [Google Scholar] [CrossRef]
- Pretzsch, E.; Bösch, F.; Neumann, J.; Ganschow, P.; Bazhin, A.; Guba, M.; Werner, J.; Angele, M. Mechanisms of Metastasis in Colorectal Cancer and Metastatic Organotropism: Hematogenous versus Peritoneal Spread. J. Oncol. 2019, 2019, 7407190. [Google Scholar] [CrossRef]
- Shaukat, A.; Levin, T.R. Current and future colorectal cancer screening strategies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 521–531. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, P.; Marchal, J.A.; Boulaiz, H.; Carrillo, E.; Vélez, C.; Rodríguez-Serrano, F.; Melguizo, C.; Prados, J.; Madeddu, R.; Aranega, A. 5-Fluorouracil derivatives: A patent review. Expert. Opin. Ther. Pat. 2012, 22, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Park, M.Y.; Sung, M.K. 5-Fluorouracil-induced changes of intestinal integrity biomarkers in BALB/c mice. J. Cancer Prev. 2013, 18, 322–329. [Google Scholar] [CrossRef]
- Petrelli, F.; Borgonovo, K.; Cabiddu, M.; Ghilardi, M.; Lonati, V.; Maspero, F.; Sauta, M.G.; Beretta, G.D.; Barni, S. FOLFIRI-bevacizumab as first-line chemotherapy in 3500 patients with advanced colorectal cancer: A pooled analysis of 29 published trials. Clin. Colorectal. Cancer 2013, 12, 145–151. [Google Scholar] [CrossRef]
- Akdeniz, N.; Kaplan, M.A.; Uncu, D.; İnanç, M.; Kaya, S.; Dane, F.; Küçüköner, M.; Demirci, A.; Bilici, M.; Durnalı, A.G.; et al. The comparison of FOLFOX regimens with different doses of 5-FU for the adjuvant treatment of colorectal cancer: A multicenter study. Int. J. Colorectal Dis. 2021, 36, 1311–1319. [Google Scholar] [CrossRef]
- Stintzing, S.; Heinrich, K.; Tougeron, D.; Modest, D.P.; Schwaner, I.; Eucker, J.; Pihusch, R.; Stauch, M.; Kaiser, F.; Kahl, C.; et al. FOLFOXIRI Plus Cetuximab or Bevacizumab as First-Line Treatment of BRAF(V600E)-Mutant Metastatic Colorectal Cancer: The Randomized Phase II FIRE-4.5 (AIO KRK0116) Study. J. Clin. Oncol. 2023, 41, 4143–4153. [Google Scholar] [CrossRef]
- Livshits, Z.; Rao, R.B.; Smith, S.W. An approach to chemotherapy-associated toxicity. Emerg. Med. Clin. North. Am. 2014, 32, 167–203. [Google Scholar] [CrossRef]
- Huot, J.R.; Baumfalk, D.; Resendiz, A.; Bonetto, A.; Smuder, A.J.; Penna, F. Targeting Mitochondria and Oxidative Stress in Cancer- and Chemotherapy-Induced Muscle Wasting. Antioxid. Redox Signal 2023, 38, 352–370. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Cardaci, T.D.; McDonald, S.J.; Madero, S.S.; Unger, C.A.; Bullard, B.M.; Enos, R.T.; Velázquez, K.T.; Kubinak, J.L.; Fan, D.; et al. Obesity reduced survival with 5-fluorouracil and did not protect against chemotherapy-induced cachexia or immune cell cytotoxicity in mice. Cancer Biol. Ther. 2022, 23, 1–15. [Google Scholar] [CrossRef]
- Adebayo, A.S.; Agbaje, K.; Adesina, S.K.; Olajubutu, O. Colorectal Cancer: Disease Process, Current Treatment Options, and Future Perspectives. Pharmaceutics 2023, 15, 2620. [Google Scholar] [CrossRef]
- Shin, B.K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Shim, D.; Bak, Y.; Choi, H.G.; Lee, S.; Park, S.C. Effects of Panax species and their bioactive components on allergic airway diseases. J. Ginseng Res. 2024, 48, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Z.; Liu, J.; Guo, M.; Li, H. Panax Ginseng in the treatment of Alzheimer’s disease and vascular dementia. J. Ginseng Res. 2023, 47, 506–514. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Wang, M.; Ding, M.; Gu, S.; Xing, X.; Sun, X. Analysis of common and characteristic actions of Panax ginseng and Panax notoginseng in wound healing based on network pharmacology and meta-analysis. J. Ginseng Res. 2023, 47, 493–505. [Google Scholar] [CrossRef]
- Wang, W.; Li, K.; Xiao, W. The pharmacological role of Ginsenoside Rg3 in liver diseases: A review on molecular mechanisms. J. Ginseng Res. 2024, 48, 129–139. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Kim, J.H. Ginseng and ginsenosides on cardiovascular and pulmonary diseases; Pharmacological potentials for the coronavirus (COVID-19). J. Ginseng Res. 2024, 48, 113–121. [Google Scholar] [CrossRef]
- Hu, Y.; He, Z.; Zhang, W.; Niu, Z.; Wang, Y.; Zhang, J.; Shen, T.; Cheng, H.; Hu, W. The potential of Panax notoginseng against COVID-19 infection. J. Ginseng Res. 2023, 47, 622–626. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, X.; Xi, Z.; Li, Y.; Xu, H. Antiviral Potential of the Genus Panax: An updated review on their effects and underlying mechanism of action. J. Ginseng Res. 2023, 47, 183–192. [Google Scholar] [CrossRef]
- Yu, T.; Tang, Y.; Zhang, F.; Zhang, L. Roles of ginsenosides in sepsis. J. Ginseng Res. 2023, 47, 1–8. [Google Scholar] [CrossRef]
- You, L.; Cha, S.; Kim, M.Y.; Cho, J.Y. Ginsenosides are active ingredients in Panax ginseng with immunomodulatory properties from cellular to organismal levels. J. Ginseng Res. 2022, 46, 711–721. [Google Scholar] [CrossRef]
- Yi, Y.S. Pharmacological potential of ginseng and ginsenosides in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Ginseng Res. 2024, 48, 122–128. [Google Scholar] [CrossRef]
- Yang, S.; Han, S.B.; Kang, S.; Lee, J.; Kim, D.; Kozlova, A.; Song, M.; Park, S.H.; Lee, J. The relationship of skin disorders, COVID-19, and the therapeutic potential of ginseng: A review. J. Ginseng Res. 2023, 47, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, J.; Lan, Y.; Lei, Y.; Zeng, F.; Huang, X.; Luo, X.; Liu, R. Potential application of ginseng in sepsis:: Applications of ginseng in sepsis. J. Ginseng Res. 2023, 47, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhu, W.; Diao, Y.; Xu, G.; Wang, L.; Qu, S.; Shi, Y. Elucidation of the Mechanism of Action of Ginseng Against Acute Lung Injury/Acute Respiratory Distress Syndrome by a Network Pharmacology-Based Strategy. Front. Pharmacol. 2020, 11, 611794. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.S.; Baek, G.H.; Kim, J.W.; Kim, J.H.; Chung, E.H.; Ko, J.W.; Kwon, M.J.; Kim, S.K.; Lee, S.H.; Kim, J.S.; et al. Korean Red Ginseng alleviates dextran sodium sulfate-induced colitis through gut microbiota modulation in mice. J. Ginseng Res. 2024, 48, 581–591. [Google Scholar] [CrossRef]
- Kan, H.; Zhang, D.; Chen, W.; Wang, S.; He, Z.; Pang, S.; Qu, S.; Wang, Y. Identification of anti-inflammatory components in Panax ginseng of Sijunzi Decoction based on spectrum-effect relationship. Chin. Herb. Med. 2023, 15, 123–131. [Google Scholar] [CrossRef]
- Kim, T.H. Ginsenosides for the treatment of insulin resistance and diabetes: Therapeutic perspectives and mechanistic insights. J. Ginseng Res. 2024, 48, 276–285. [Google Scholar] [CrossRef]
- Wang, P.; Cui, J.; Du, X.; Yang, Q.; Jia, C.; Xiong, M.; Yu, X.; Li, L.; Wang, W.; Chen, Y.; et al. Panax notoginseng saponins (PNS) inhibits breast cancer metastasis. J. Ethnopharmacol. 2014, 154, 663–671. [Google Scholar] [CrossRef]
- He, S.; Lyu, F.; Lou, L.; Liu, L.; Li, S.; Jakowitsch, J.; Ma, Y. Anti-tumor activities of Panax quinquefolius saponins and potential biomarkers in prostate cancer. J. Ginseng Res. 2021, 45, 273–286. [Google Scholar] [CrossRef]
- Tao, R.; Lu, K.; Zong, G.; Xia, Y.; Han, H.; Zhao, Y.; Wei, Z.; Lu, Y. Ginseng polysaccharides: Potential antitumor agents. J. Ginseng Res. 2023, 47, 9–22. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Yu, M.H.; Cho, Y.; Jo, M.Y.; Song, K.H.; Choi, Y.H.; Kwon, T.K.; Kwak, J.Y.; Chang, Y.C. Rg3-enriched red ginseng extracts enhance apoptosis in CoCl(2)-stimulated breast cancer cells by suppressing autophagy. J. Ginseng Res. 2024, 48, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, L.; Li, H.; Bai, H.; Suo, Y.; Cui, J.; Wang, Y. Ginsenoside 20(S)-Rg3 reduces KIF20A expression and promotes CDC25A proteasomal degradation in epithelial ovarian cancer. J. Ginseng Res. 2024, 48, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, M.; Smith, E.; Yeo, K.; Tomita, Y.; Price, T.J.; Yool, A.; Townsend, A.R.; Hardingham, J.E. Differential antiangiogenic and anticancer activities of the active metabolites of ginsenoside Rg3. J. Ginseng Res. 2024, 48, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Jiang, X.L.; Zhang, Z.; Chen, W.Q.; Peng, J.J.; Liu, B.; Yung, K.K.; Zhu, P.L. 20(S)-Ginsenoside Rh2 induces apoptosis and autophagy in melanoma cells via suppressing Src/STAT3 signaling. J. Ginseng Res. 2024, 48, 559–569. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.Y. Ginseng-derived compounds as potential anticancer agents targeting cancer stem cells. J. Ginseng Res. 2024, 48, 266–275. [Google Scholar] [CrossRef]
- Hu, Q.R.; Pan, Y.; Wu, H.C.; Dai, Z.Z.; Huang, Q.X.; Luo, T.; Li, J.; Deng, Z.Y.; Chen, F. The ways for ginsenoside Rh2 to fight against cancer: The molecular evidences in vitro and in vivo. J. Ginseng Res. 2023, 47, 173–182. [Google Scholar] [CrossRef]
- Lee, D.Y.; Park, C.W.; Lee, S.J.; Park, H.R.; Kim, S.H.; Son, S.U.; Park, J.; Shin, K.S. Anti-Cancer Effects of Panax ginseng Berry Polysaccharides via Activation of Immune-Related Cells. Front. Pharmacol. 2019, 10, 1411. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, H.; Hussain, H.; Chen, X.; Park, J.H.; Kwon, S.W.; Xie, L.; Zheng, B.; Xu, X.; Wang, D.; et al. Structural analysis, anti-inflammatory activity of the main water-soluble acidic polysaccharides (AGBP-A3) from Panax quinquefolius L berry. J. Ginseng Res. 2024, 48, 454–463. [Google Scholar] [CrossRef]
- Xu, H.; Miao, H.; Chen, G.; Zhang, G.; Hua, Y.; Wu, Y.; Xu, T.; Han, X.; Hu, C.; Pang, M.; et al. 20(S)-ginsenoside Rg3 exerts anti-fibrotic effect after myocardial infarction by alleviation of fibroblasts proliferation and collagen deposition through TGFBR1 signaling pathways. J. Ginseng Res. 2023, 47, 743–754. [Google Scholar] [CrossRef]
- Kim, R.; Kim, J.W.; Choi, H.; Oh, J.E.; Kim, T.H.; Go, G.Y.; Lee, S.J.; Bae, G.U. Ginsenoside Rg5 promotes muscle regeneration via p38MAPK and Akt/mTOR signaling. J. Ginseng Res. 2023, 47, 726–734. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.H.; Kang, J.I.; Baek, J.I.; Jeon, B.M.; Kim, H.M.; Kim, S.C.; Jeong, W.I. Ginsenoside F2 Restrains Hepatic Steatosis and Inflammation by Altering the Binding Affinity of Liver X Receptor Coregulators. J. Ginseng Res. 2024, 48, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, Y.S.; Li, W.; Kwon, E.B.; Chung, H.S.; Go, Y.; Choi, J.G. Ginsenoside Rg5, a potent agonist of Nrf2, inhibits HSV-1 infection-induced neuroinflammation by inhibiting oxidative stress and NF-kappaB activation. J. Ginseng Res. 2024, 48, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Wei, R.Y.; Tang, K.; Wang, Z.; Tan, N.H. Ginsenoside Rg1 promotes neurite growth of retinal ganglion cells through cAMP/PKA/CREB pathways. J. Ginseng Res. 2024, 48, 163–170. [Google Scholar] [CrossRef]
- Jang, W.Y.; Hwang, J.Y.; Cho, J.Y. Ginsenosides from Panax ginseng as Key Modulators of NF-κB Signaling Are Powerful Anti-Inflammatory and Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 6119. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Y.; Zeng, J.Z.; Wong, A.S.T. Chemical Structures and Pharmacological Profiles of Ginseng Saponins. Molecules 2019, 24, 2443. [Google Scholar] [CrossRef]
- Kwon, H.W.; Shin, J.H.; Rhee, M.H.; Park, C.E.; Lee, D.H. Anti-thrombotic effects of ginsenoside Rk3 by regulating cAMP and PI3K/MAPK pathway on human platelets. J. Ginseng Res. 2023, 47, 706–713. [Google Scholar] [CrossRef]
- Du, G.J.; Dai, Q.; Williams, S.; Wang, C.Z.; Yuan, C.S. Synthesis of protopanaxadiol derivatives and evaluation of their anticancer activities. Anticancer. Drugs 2011, 22, 35–45. [Google Scholar] [CrossRef]
- Song, C.; Shen, T.; Kim, H.G.; Hu, W.; Cho, J.Y. 20(S)-Protopanaxadiol from Panax ginseng Induces Apoptosis and Autophagy in Gastric Cancer Cells by Inhibiting Src. Am. J. Chin. Med. 2023, 51, 205–221. [Google Scholar] [CrossRef]
- Chen, B.; Dai, W.; He, B.; Zhang, H.; Wang, X.; Wang, Y.; Zhang, Q. Current Multistage Drug Delivery Systems Based on the Tumor Microenvironment. Theranostics 2017, 7, 538–558. [Google Scholar] [CrossRef]
- Zang, X.; Zhao, X.; Hu, H.; Qiao, M.; Deng, Y.; Chen, D. Nanoparticles for tumor immunotherapy. Eur. J. Pharm. Biopharm. 2017, 115, 243–256. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Sun, Q.; Zhang, Z.; Zhao, M.; Peng, C.; Shi, S. Ginsenosides emerging as both bifunctional drugs and nanocarriers for enhanced antitumor therapies. J. Nanobiotechnol. 2021, 19, 322. [Google Scholar] [CrossRef]
- Wu, H.; Wei, G.; Luo, L.; Li, L.; Gao, Y.; Tan, X.; Wang, S.; Chang, H.; Liu, Y.; Wei, Y.; et al. Ginsenoside Rg3 nanoparticles with permeation enhancing based chitosan derivatives were encapsulated with doxorubicin by thermosensitive hydrogel and anti-cancer evaluation of peritumoral hydrogel injection combined with PD-L1 antibody. Biomater. Res. 2022, 26, 77. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, J.; Zhang, Z.; Lv, H. The combined administration of parthenolide and ginsenoside CK in long circulation liposomes with targeted tLyp-1 ligand induce mitochondria-mediated lung cancer apoptosis. Artif. Cells Nanomed. Biotechnol. 2018, 46, S931–S942. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, W.; Shen, Q.; Wang, Y.; Tseng, Y.; Luo, Z.; Wang, X.; Shi, L.; Li, C.; Liu, J. Identification and construction of a novel biomimetic delivery system of paclitaxel and its targeting therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Liang, J.; Xia, J.; Zhu, Y.; Guo, Y.; Wang, A.; Lu, C.; Ren, H.; Chen, C.; Li, S.; et al. One Stone Four Birds: A Novel Liposomal Delivery System Multi-functionalized with Ginsenoside Rh2 for Tumor Targeting Therapy. Nanomicro Lett. 2020, 12, 129. [Google Scholar] [CrossRef]
- Yang, Y.; He, P.Y.; Zhang, Y.; Li, N. Natural Products Targeting the Mitochondria in Cancers. Molecules 2020, 26, 92. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Craig, R.W. The bcl-2 gene family. Semin. Cancer Biol. 1995, 6, 35–43. [Google Scholar] [CrossRef]
- Anilkumar, U.; Prehn, J.H. Anti-apoptotic BCL-2 family proteins in acute neural injury. Front. Cell Neurosci. 2014, 8, 281. [Google Scholar] [CrossRef]
- Luo, X.; O’Neill, K.L.; Huang, K. The third model of Bax/Bak activation: A Bcl-2 family feud finally resolved? F1000Research 2020, 9, 935. [Google Scholar] [CrossRef]
- Nagata, S. Apoptotic DNA fragmentation. Exp. Cell Res. 2000, 256, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Jia, B.; Dong, K.; Li, M.; Qiu, D.; Li, L.; Xu, B.; Sun, S.; Li, C. Panax notoginseng saponins induce apoptosis in retinoblastoma Y79 cells via the PI3K/AKT signalling pathway. Exp. Eye Res. 2022, 216, 108954. [Google Scholar] [CrossRef] [PubMed]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.A.; Kim, B.R.; Kim, D.Y.; Jeong, S.; Na, Y.J.; Kim, J.L.; Yun, H.K.; Kim, B.G.; Park, S.H.; Jo, M.J.; et al. Korean Red Ginseng Extract Increases Apoptosis by Activation of the Noxa Pathway in Colorectal Cancer. Nutrients 2019, 11, 2026. [Google Scholar] [CrossRef]
- Wang, C.Z.; Wan, C.; Luo, Y.; Zhang, C.F.; Zhang, Q.H.; Chen, L.; Park, C.W.; Kim, S.H.; Liu, Z.; Lager, M.; et al. Ginseng berry concentrate prevents colon cancer via cell cycle, apoptosis regulation, and inflammation-linked Th17 cell differentiation. J. Physiol. Pharmacol. 2021, 72, 225–237. [Google Scholar] [CrossRef]
- Kang, K.A.; Yao, C.W.; Piao, M.J.; Zhen, A.X.; Fernando, P.; Herath, H.; Song, S.E.; Cho, S.J.; Hyun, J.W. Anticolon Cancer Effect of Korean Red Ginseng via Autophagy- and Apoptosis-Mediated Cell Death. Nutrients 2022, 14, 3558. [Google Scholar] [CrossRef]
- Kochan, E.; Nowak, A.; Zakłos-Szyda, M.; Szczuka, D.; Szymańska, G.; Motyl, I. Panax quinquefolium L. Ginsenosides from Hairy Root Cultures and Their Clones Exert Cytotoxic, Genotoxic and Pro-Apoptotic Activity towards Human Colon Adenocarcinoma Cell Line Caco-2. Molecules 2020, 25, 2262. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, Y.J.; Jang, M.G.; Joo, S.C.; Kwon, W.S.; Kim, S.Y.; Jung, S.K.; Yang, D.C. Investigation of ginsenosides in different tissues after elicitor treatment in Panax ginseng. J. Ginseng Res. 2014, 38, 270–277. [Google Scholar] [CrossRef]
- Choi, W.; Kim, H.S.; Park, S.H.; Kim, D.; Hong, Y.D.; Kim, J.H.; Cho, J.Y. Syringaresinol derived from Panax ginseng berry attenuates oxidative stress-induced skin aging via autophagy. J. Ginseng Res. 2022, 46, 536–542. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.J.; Woo, C.W.; Kim, S.T.; Im, M.; Park, S.K.; Kim, J.Y.; Yoo, H.J.; Woo, D.C.; Kim, J.K. Treatment of chemotherapy-induced cachexia with BST204: A multimodal validation study. Metabolomics 2021, 17, 36. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Yang, Z.; Liu, Y.; Chen, R.; Song, Z.; Pan, G.; Zhang, Y.; Guo, Z.; Ding, X.; Chen, L.; et al. Gut microbiota: A new target of traditional Chinese medicine for insomnia. Biomed. Pharmacother. 2023, 160, 114344. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shao, L.; Chen, M.Y.; Wang, L.; Yang, P.; Tan, F.B.; Zhang, W.; Huang, W.H. Panax notoginseng Alleviates Colitis via the Regulation of Gut Microbiota. Am. J. Chin. Med. 2023, 51, 107–127. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Zhang, Z.; Feng, Y. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKα/STAT3 signaling in diet-induced obesity. Theranostics 2020, 10, 11302–11323. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, M.; Tang, L.; Wang, F.; Huang, S.; Liu, S.; Lei, Y.; Wang, S.; Xie, Z.; Wang, W.; et al. TLR4 regulates RORγt(+) regulatory T-cell responses and susceptibility to colon inflammation through interaction with Akkermansia muciniphila. Microbiome 2022, 10, 98. [Google Scholar] [CrossRef]
- Chen, L.; Chen, M.Y.; Shao, L.; Zhang, W.; Rao, T.; Zhou, H.H.; Huang, W.H. Panax notoginseng saponins prevent colitis-associated colorectal cancer development: The role of gut microbiota. Chin. J. Nat. Med. 2020, 18, 500–507. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Hedayati-Goudarzi, M.T.; Alizadeh, A.; Sadeghian-Nodoushan, F.; Soltaninejad, H. A review of cardioprotective effect of ginsenosides in chemotherapy-induced cardiotoxicity. Biomed. Eng. Online 2024, 23, 128. [Google Scholar] [CrossRef]
- Son, S.H.; Kang, J.; Shin, Y.; Lee, C.; Sung, B.H.; Lee, J.Y.; Lee, W. Sustainable production of natural products using synthetic biology: Ginsenosides. J. Ginseng Res. 2024, 48, 140–148. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef]
- Li, W.; Shi, H.; Wu, X. A narrative review of Panax notoginseng: Unique saponins and their pharmacological activities. J. Ginseng Res. 2024, in press. [Google Scholar] [CrossRef]
- Koo, H.; Lee, Y.S.; Nguyen, V.B.; Giang, V.N.L.; Koo, H.J.; Park, H.S.; Mohanan, P.; Song, Y.H.; Ryu, B.; Kang, K.B.; et al. Comparative transcriptome and metabolome analyses of four Panax species explore the dynamics of metabolite biosynthesis. J. Ginseng Res. 2023, 47, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ma, J.; Zhang, Y.; Zhou, Y.; Cong, X.; Hao, M. Effect of anti-skin disorders of ginsenosides- A Systematic Review. J. Ginseng Res. 2023, 47, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.W.; Ahn, K.S.; Lee, J.C.; Kim, S.H.; Chung, B.C.; Choi, M.H. Validated quantification for selective cellular uptake of ginsenosides on MCF-7 human breast cancer cells by liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2010, 396, 3017–3025. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Song, X.; Zhang, Y.; Xu, Y.; Liu, Q. Insight on structural modification, biological activity, structure-activity relationship of PPD-type ginsenoside derivatives. Fitoterapia 2022, 158, 105135. [Google Scholar] [CrossRef]
- Xu, J.; Pan, Y.; Liu, Y.; Na, S.; Zhou, H.; Li, L.; Chen, F.; Song, H. A review of anti-tumour effects of ginsenoside in gastrointestinal cancer. J. Pharm. Pharmacol. 2021, 73, 1292–1301. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Hardingham, J.E.; Palethorpe, H.M.; Tomita, Y.; Smith, E.; Price, T.J.; Townsend, A.R. Ginsenoside Rg3: Potential Molecular Targets and Therapeutic Indication in Metastatic Breast Cancer. Medicines 2019, 6, 17. [Google Scholar] [CrossRef]
- Li, H.; Huang, N.; Zhu, W.; Wu, J.; Yang, X.; Teng, W.; Tian, J.; Fang, Z.; Luo, Y.; Chen, M.; et al. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer 2018, 18, 579. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Song, Z.; Liu, Q.; Fan, D.; Song, X. Ginsenosides: A potential natural medicine to protect the lungs from lung cancer and inflammatory lung disease. Food Funct. 2023, 14, 9137–9166. [Google Scholar] [CrossRef]
- Ben-Eltriki, M.; Shankar, G.; Tomlinson Guns, E.S.; Deb, S. Pharmacokinetics and pharmacodynamics of Rh2 and aPPD ginsenosides in prostate cancer: A drug interaction perspective. Cancer Chemother. Pharmacol. 2023, 92, 419–437. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Yue, P.Y.; Mak, N.K.; Cheng, Y.K.; Leung, K.W.; Ng, T.B.; Fan, D.T.; Yeung, H.W.; Wong, R.N. Pharmacogenomics and the Yin/Yang actions of ginseng: Anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin. Med. 2007, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef]
- Castro-Castro, A.; Marchesin, V.; Monteiro, P.; Lodillinsky, C.; Rossé, C.; Chavrier, P. Cellular and Molecular Mechanisms of MT1-MMP-Dependent Cancer Cell Invasion. Annu. Rev. Cell Dev. Biol. 2016, 32, 555–576. [Google Scholar] [CrossRef] [PubMed]
- Park, M.T.; Cha, H.J.; Jeong, J.W.; Kim, S.I.; Chung, H.Y.; Kim, N.D.; Kim, O.H.; Kim, K.W. Glucocorticoid receptor-induced down-regulation of MMP-9 by ginseng components, PD and PT contributes to inhibition of the invasive capacity of HT1080 human fibrosarcoma cells. Mol. Cells 1999, 9, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Fruhwirth, G.O.; Kneilling, M.; de Vries, I.J.M.; Weigelin, B.; Srinivas, M.; Aarntzen, E. The Potential of In Vivo Imaging for Optimization of Molecular and Cellular Anti-cancer Immunotherapies. Mol. Imaging Biol. 2018, 20, 696–704. [Google Scholar] [CrossRef]
- Cui, Y.; Su, Y.; Deng, L.; Wang, W. Ginsenoside-Rg5 Inhibits Retinoblastoma Proliferation and Induces Apoptosis through Suppressing BCL2 Expression. Chemotherapy 2018, 63, 293–300. [Google Scholar] [CrossRef]
- Kim, J.S.; Joo, E.J.; Chun, J.; Ha, Y.W.; Lee, J.H.; Han, Y.; Kim, Y.S. Induction of apoptosis by ginsenoside Rk1 in SK-MEL-2-human melanoma. Arch. Pharm. Res. 2012, 35, 717–722. [Google Scholar] [CrossRef]
- Jiang, Z.; Deng, L.; Li, M.; Alonge, E.; Wang, Y.; Wang, Y. Ginsenoside Rg1 modulates PI3K/AKT pathway for enhanced osteogenesis via GPER. Phytomedicine 2023, 124, 155284. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Deng, J.; Ma, X.; Fan, D. Ginsenoside Rk3 is a novel PI3K/AKT-targeting therapeutics agent that regulates autophagy and apoptosis in hepatocellular carcinoma. J. Pharm. Anal. 2023, 13, 463–482. [Google Scholar] [CrossRef]
- Peng, B.; He, R.; Xu, Q.; Yang, Y.; Hu, Q.; Hou, H.; Liu, X.; Li, J. Ginsenoside 20(S)-protopanaxadiol inhibits triple-negative breast cancer metastasis in vivo by targeting EGFR-mediated MAPK pathway. Pharmacol. Res. 2019, 142, 1–13. [Google Scholar] [CrossRef]
- Zhang, H.; Yi, J.; Kim, E.; Choo, Y.; Hai, H.; Kim, K.; Kim, E.K.; Ryoo, Z.; Kim, M. 20(S)-Ginsenoside Rh2 Suppresses Oral Cancer Cell Growth by Inhibiting the Src-Raf-ERK Signaling Pathway. Anticancer. Res. 2021, 41, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chu, S.; Lin, M.; Gao, Y.; Liu, Y.; Yang, S.; Zhou, X.; Zhang, Y.; Hu, Y.; Wang, H.; et al. Anticancer property of ginsenoside Rh2 from ginseng. Eur. J. Med. Chem. 2020, 203, 112627. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, T.; Lv, X.; Zhang, J.; Liu, S. Ginsenoside Rh2 alleviates ulcerative colitis by regulating the STAT3/miR-214 signaling pathway. J. Ethnopharmacol. 2021, 274, 113997. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, W.; He, Q.; Wang, Y.; Jin, G.; Zhang, Y. Ginsenoside Rh2 suppresses colon cancer growth by targeting the miR-150-3p/SRCIN1/Wnt axis. Acta Biochim Biophys Sin 2023, 55, 633–648. [Google Scholar] [CrossRef]
- Ma, J.; Gao, G.; Lu, H.; Fang, D.; Li, L.; Wei, G.; Chen, A.; Yang, Y.; Zhang, H.; Huo, J. Reversal effect of ginsenoside Rh2 on oxaliplatin-resistant colon cancer cells and its mechanism. Exp. Ther. Med. 2019, 18, 630–636. [Google Scholar] [CrossRef]
- Lee, S.C.; Shen, C.Y.; Wang, W.H.; Lee, Y.P.; Liang, K.W.; Chou, Y.H.; Tyan, Y.S.; Hwang, J.J. Synergistic Effect of Ginsenoside Rh2 Combines with Ionizing Radiation on CT26/luc Colon Carcinoma Cells and Tumor-Bearing Animal Model. Pharmaceuticals 2023, 16, 1188. [Google Scholar] [CrossRef]
- Hu, Q.R.; Hong, H.; Zhang, Z.H.; Feng, H.; Luo, T.; Li, J.; Deng, Z.Y.; Chen, F. Methods on improvements of the poor oral bioavailability of ginsenosides: Pre-processing, structural modification, drug combination, and micro- or nano-delivery system. J. Ginseng. Res. 2023, 47, 694–705. [Google Scholar] [CrossRef]
- Hong, S.; Cai, W.; Huang, Z.; Wang, Y.; Mi, X.; Huang, Y.; Lin, Z.; Chen, X. Ginsenoside Rg3 enhances the anticancer effect of 5-FU in colon cancer cells via the PI3K/AKT pathway. Oncol. Rep. 2020, 44, 1333–1342. [Google Scholar] [CrossRef]
- Wang, N.; Yang, J.; Chen, R.; Liu, Y.; Liu, S.; Pan, Y.; Lei, Q.; Wang, Y.; He, L.; Song, Y.; et al. Ginsenoside Rg1 ameliorates Alzheimer’s disease pathology via restoring mitophagy. J. Ginseng Res. 2023, 47, 448–457. [Google Scholar] [CrossRef]
- Yao, F.D.; Yang, J.Q.; Huang, Y.C.; Luo, M.P.; Yang, W.J.; Zhang, B.; Liu, X.J. Antinociceptive effects of Ginsenoside Rb1 in a rat model of cancer-induced bone pain. Exp. Ther. Med. 2019, 17, 3859–3866. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.Q.; Xu, Y.Y.; Zhang, R.; Zhao, Q.; Zhang, Y.Q.; Huang, X.H.; Jiang, B.; Ni, M. Ginsenoside Rb1 Suppresses AOM/DSS-induced Colon Carcinogenesis. Anticancer. Agents Med. Chem. 2023, 23, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, Y.; Li, H.; Zhang, J.; Ci, Y.; Han, M. Ginsenoside Rb1 can ameliorate the key inflammatory cytokines TNF-α and IL-6 in a cancer cachexia mouse model. BMC Complement. Med. Ther. 2020, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Legoabe, L.J.; Dall’Acqua, S.; Zengin, G. Plants’ bioactive secondary metabolites in the management of sepsis: Recent findings on their mechanism of action. Front. Pharmacol. 2022, 13, 1046523. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Wang, R.; Zhao, S.; Wang, Z. Ginsenosides in Panax genus and their biosynthesis. Acta Pharm. Sin. B 2021, 11, 1813–1834. [Google Scholar] [CrossRef]
- Xiao, S.; Lin, Z.; Wang, X.; Lu, J.; Zhao, Y. Synthesis and Cytotoxicity Evaluation of Panaxadiol Derivatives. Chem. Biodivers. 2020, 17, e1900516. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.Y.; Zhang, Z.H.; Zuo, H.X.; Wang, J.Y.; Xing, Y.; Ri, M.; Jin, H.L.; Jin, C.H.; Xu, G.H.; et al. Panaxadiol inhibits programmed cell death-ligand 1 expression and tumour proliferation via hypoxia-inducible factor (HIF)-1α and STAT3 in human colon cancer cells. Pharmacol. Res. 2020, 155, 104727. [Google Scholar] [CrossRef]
- Chu, L.L.; Hanh, N.T.Y.; Quyen, M.L.; Nguyen, Q.H.; Lien, T.T.P.; Do, K.V. Compound K Production: Achievements and Perspectives. Life 2023, 13, 1565. [Google Scholar] [CrossRef]
- Pak, J.N.; Jung, J.H.; Park, J.E.; Hwang, J.; Lee, H.J.; Shim, B.S.; Kim, S.H. p53 dependent LGR5 inhibition and caspase 3 activation are critically involved in apoptotic effect of compound K and its combination therapy potential in HCT116 cells. Phytother. Res. 2020, 34, 2745–2755. [Google Scholar] [CrossRef]
- Wu, F.; Lai, S.; Feng, H.; Liu, J.; Fu, D.; Wang, C.; Wang, C.; Liu, J.; Li, Z.; Li, P. Protective Effects of Protopanaxatriol Saponins on Ulcerative Colitis in Mouse Based on UPLC-Q/TOF-MS Serum and Colon Metabolomics. Molecules 2022, 27, 8346. [Google Scholar] [CrossRef]
- Ediriweera, G.R.; Chang, Y.; Yang, W.; Whittaker, A.K.; Fu, C. Self-Assembled Protein-Polymer Nanoparticles via Photoinitiated Polymerization-Induced Self-Assembly for Targeted and Enhanced Drug Delivery in Cancer Therapy. Molecules 2025, 30, 856. [Google Scholar] [CrossRef]
- Varanko, A.; Saha, S.; Chilkoti, A. Recent trends in protein and peptide-based biomaterials for advanced drug delivery. Adv. Drug Deliv. Rev. 2020, 156, 133–187. [Google Scholar] [CrossRef] [PubMed]
- Georgilis, E.; Abdelghani, M.; Pille, J.; Aydinlioglu, E.; van Hest, J.C.; Lecommandoux, S.; Garanger, E. Nanoparticles based on natural, engineered or synthetic proteins and polypeptides for drug delivery applications. Int. J. Pharm. 2020, 586, 119537. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, Z.; Wei, S.; Ji, G.; Zheng, X.; Fu, Z.; Cheng, J. Polypeptide-based drug delivery systems for programmed release. Biomaterials 2021, 275, 120913. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, B.; Hajipour, M.J. Tumor acidic environment directs nanoparticle impacts on cancer cells. J. Colloid. Interface Sci. 2023, 634, 684–692. [Google Scholar] [CrossRef]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor pH targeting nanotechnology. J. Control. Release 2008, 132, 164–170. [Google Scholar] [CrossRef]
- Yang, J.; An, H.-W.; Wang, H. Self-assembled peptide drug delivery systems. ACS Appl. Bio Mater. 2020, 4, 24–46. [Google Scholar] [CrossRef]
- Sun, M.; Ye, Y.; Xiao, L.; Duan, X.; Zhang, Y.; Zhang, H. Anticancer effects of ginsenoside Rg3. Int. J. Mol. Med. 2017, 39, 507–518. [Google Scholar] [CrossRef]
- Qiu, R.; Qian, F.; Wang, X.; Li, H.; Wang, L. Targeted delivery of 20 (S)-ginsenoside Rg3-based polypeptide nanoparticles to treat colon cancer. Biomed. Microdevices 2019, 21, 18. [Google Scholar] [CrossRef]
- Noorafshan, A.; Ashkani-Esfahani, S. A review of therapeutic effects of curcumin. Curr. Pharm. Des. 2013, 19, 2032–2046. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 596, 105–125. [Google Scholar] [CrossRef]
- Ahmadi, N.; Hosseini, M.-J.; Rostamizadeh, K.; Anoush, M. Investigation of therapeutic effect of curcumin α and β glucoside anomers against Alzheimer’s disease by the nose to brain drug delivery. Brain Res. 2021, 1766, 147517. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, Z. The effect of curcumin on breast cancer cells. J. Breast Cancer 2013, 16, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Syng-Ai, C.; Kumari, A.L.; Khar, A. Effect of curcumin on normal and tumor cells: Role of glutathione and bcl-2. Mol. Cancer Ther. 2004, 3, 1101–1108. [Google Scholar] [CrossRef]
- Wongcharoen, W.; Phrommintikul, A. The protective role of curcumin in cardiovascular diseases. Int. J. Cardiol. 2009, 133, 145–151. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Tai, P.; Clulow, A.J.; Boyd, B.J.; Golding, M.; Singh, H.; Everett, D.W. Cholesterol stabilization of phospholipid vesicles against bile-induced solubilization. Chem. Phys. Lipids 2023, 252, 105289. [Google Scholar] [CrossRef]
- Christensen, L.P. Ginsenosides: Chemistry, biosynthesis, analysis, and potential health effects. Adv. Food Nutr. Res. 2008, 55, 1–99. [Google Scholar] [CrossRef]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Baskaran, R.; Baek, J.-H.; Sundaramoorthy, P.; Yoo, B.K. In vitro cytotoxicity and bioavailability of ginsenoside-modified nanostructured lipid carrier containing curcumin. AAPS PharmSciTech 2019, 20, 88. [Google Scholar] [CrossRef]
- Jeon, Y.; Sym, S.J.; Yoo, B.K.; Baek, J.-H. Long-term Survival, Tolerability, and Safety of First-Line Bevacizumab and FOLFIRI in Combination With Ginsenoside-Modified Nanostructured Lipid Carrier Containing Curcumin in Patients With Unresectable Metastatic Colorectal Cancer. Integr. Cancer Ther. 2022, 21, 15347354221105498. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A review on electrospun nanofibers based advanced applications: From health care to energy devices. Polymers 2021, 13, 3746. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hameed, M.M.; Mohamed Khan, S.A.P.; Thamer, B.M.; Rajkumar, N.; El-Hamshary, H.; El-Newehy, M. Electrospun nanofibers for drug delivery applications: Methods and mechanism. Polym. Adv. Technol. 2023, 34, 6–23. [Google Scholar] [CrossRef]
- Parhi, R. Cross-linked hydrogel for pharmaceutical applications: A review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Zhou, X.; Zhai, Y.; Liu, C.; Yang, G.; Guo, J.; Li, G.; Sun, C.; Qi, X.; Li, X.; Guan, F. Sialidase NEU1 suppresses progression of human bladder cancer cells by inhibiting fibronectin-integrin α5β1 interaction and Akt signaling pathway. Cell Commun. Signal. 2020, 18, 44. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Hong, E.-H.; Lee, S.Y.; Lee, J.-Y.; Song, J.-H.; Ko, S.-H.; Shim, J.-S.; Choe, S.; Kim, D.-D.; Ko, H.-J. Boronic acid-tethered amphiphilic hyaluronic acid derivative-based nanoassemblies for tumor targeting and penetration. Acta Biomater. 2017, 53, 414–426. [Google Scholar] [CrossRef]
- Spindler, B.A.; Bergquist, J.R.; Thiels, C.A.; Habermann, E.B.; Kelley, S.R.; Larson, D.W.; Mathis, K.L. Incorporation of CEA improves risk stratification in stage II colon cancer. J. Gastrointest. Surg. 2017, 21, 770–777. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Cao, Q.; Li, J. The treatment efficacy of three-layered functional polymer materials as drug carrier for orthotopic colon cancer. Drug Deliv. 2022, 29, 2971–2983. [Google Scholar] [CrossRef]
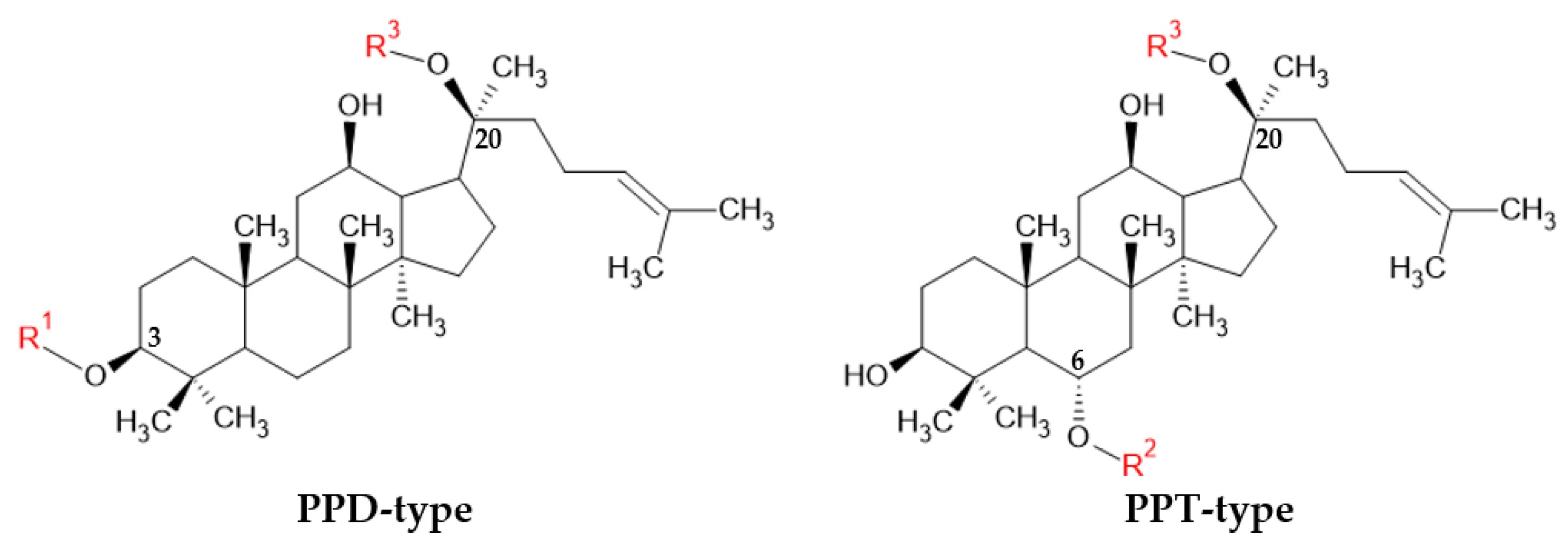

| Ginsenoside | Type | (C-3) R1 | (C-20) R3 |
|---|---|---|---|
| Rh2 | PPD type | Glc | H |
| Rg3 | Glc(2 → 1)Glc | H | |
| Rb1 | Glc(2 → 1)Glc | Glc(6 → 1)Glc | |
| CK | H | Glc | |
| PPD | H | H |
| Extract | Test Type | Dose | Mechanism | Reference |
|---|---|---|---|---|
| P. ginseng powder | In vitro (HT-29 CRC cell line) | 2.5 mg/mL (for 24 h) |
| [74] |
| P. ginseng berry | In vitro (HCT-116 and HT-29 CRC cell lines) Ex vivo (naïve CD4 cells from C57BL/6 mice spleen) | 100, 200, 300, 400, and 500 μg/mL (for 48 h) |
| [75] |
| P. ginseng root | In vitro (HCT-116 and SNU-1033 CRC cell lines) | 2 mg/mL for HCT-116 cells, 2.3 mg/mL for SNU-1033 cells (for 12, 24, and 48 h) |
| [76] |
| P. quinquefolium hairy root (elicited by methyl jasmonate) | In vitro (Caco-2 CRC cell line) | 0.017, 0.069, 0.274, 0.55, and 1.1 mg/mL (for 48 h) |
| [77] |
| BST204 (P. ginseng) | In vivo (BALB/c xenograft mouse model with 1 × 106 of CT-26 CRC cell line treated with 50 mg/kg of 5-FU) | 100 and 200 mg/kg (5 d cycles for 11 d) |
| [80] |
| P. notoginseng root | In vivo (A/J mouse CRC model with 7.5 mg/kg of azoxymethane and 1% DSS) | 30 and 90 mg/kg (for 13 weeks) |
| [86] |
| Ginsenoside | Test Type | Dose | Mechanism | Reference |
|---|---|---|---|---|
| Rh2 | In vitro (NCM460 CRC cell line) In vivo (C57BL/6J CRC mouse model with 3% DSS-induced acute colitis) | 1.25, 2.5, 5, 10, and 20 μM (for 24 h) 50 mg/kg/day (for 10 days) |
| [113] |
| In vitro (HCT-116, SW620 CRC cell line) | 10 and 20 μM (for 18 h) |
| [114] | |
| In vitro (Lovo, Lovo/L-OHP CRC cell line) | 50, 100, 200, and 250 μg/mL (for 24 h) |
| [115] | |
| In vitro (CT26/luc CRC cell line) In vivo (BALB/c xenograft mouse model with 2 × 106 CT-26 CRC cells) | 1, 20, 50, 75, 85, and 100 μM (for 24 h) 10 mg/kg (for 3 weeks, three times a week) |
| [116] | |
| Rg3 | In vitro (SW620, Lovo CRC cell line) In vivo (nude mice xenograft mouse model with 5 × 106 human CRC cells) | 0.25, 0.5, 0.75, and 1.0 mmol/L (for 48 h) 200 mg/kg (for 3 weeks) |
| [118] |
| In vitro (HCT-116 CRC cell line) | 20 μM (for 12 h) |
| [95] | |
| Rb1 | In vivo (C57BL/6 CRC mouse model with single dose of 7.5 mg/kg AOM and 2% DSS for 7 days) | No information (18 weeks, every 2 days) |
| [121] |
| In vivo (BALB/c xenograft mouse model with 1 × 106 CT-26 CRC cells) | 10.72 mg/kg (for 23 days) |
| [122] | |
| Panaxadiol derivatives | In vitro (HCT-116 CRC cell line) | 0.5–2 mM |
| [125] |
| Panaxadiol | In vitro (HCT-116, SW620, HT-29 CRC cell lines) In vivo (BALB/c nude xenograft mouse model with 5 × 107 HCT-116 CRC cells) | 1, 3, and 10 μM (for 24 and 48 h) 10 and 30 mg/kg (36 days, three times a week) |
| [126] |
| Compound K | In vitro (HCT-116 CRC cell line) | 6.25, 12,5, 25, 50, and 100 μM (24, 48 h weeks) |
| [128] |
| Protopanaxatriol | In vivo (BALB/c CRC mouse model with 3% DSS-induced acute colitis) | 25, 50, and 100 mg/kg (7 days) |
| [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.S.; Lim, H.K.; Jang, W.Y.; Cho, J.Y. Anti-Colorectal Cancer Activity of Panax and Its Active Components, Ginsenosides: A Review. Int. J. Mol. Sci. 2025, 26, 2593. https://doi.org/10.3390/ijms26062593
Kang HS, Lim HK, Jang WY, Cho JY. Anti-Colorectal Cancer Activity of Panax and Its Active Components, Ginsenosides: A Review. International Journal of Molecular Sciences. 2025; 26(6):2593. https://doi.org/10.3390/ijms26062593
Chicago/Turabian StyleKang, Han Su, Hyun Kyung Lim, Won Young Jang, and Jae Youl Cho. 2025. "Anti-Colorectal Cancer Activity of Panax and Its Active Components, Ginsenosides: A Review" International Journal of Molecular Sciences 26, no. 6: 2593. https://doi.org/10.3390/ijms26062593
APA StyleKang, H. S., Lim, H. K., Jang, W. Y., & Cho, J. Y. (2025). Anti-Colorectal Cancer Activity of Panax and Its Active Components, Ginsenosides: A Review. International Journal of Molecular Sciences, 26(6), 2593. https://doi.org/10.3390/ijms26062593







